Abstract
We have examined the expression of the gene encoding the iron-protein subunit (Ip) of succinate dehydrogenase in Saccharomyces cerevisiae. The gene had been cloned by us and shown to be subject to glucose regulation (A. Lombardo, K. Carine, and I. E. Scheffler, J. Biol. Chem. 265:10419-10423, 1990). We discovered that a significant part of the regulation of the Ip mRNA levels by glucose involves the regulation of the turnover rate of this mRNA. In the presence of glucose, the half-life appears to be less than 5 min, while in glycerol medium, the half-life is greater than 60 min. The gene is also regulated transcriptionally by glucose. The upstream promoter sequence appeared to have four regulatory elements with consensus sequences shown to be responsible for the interaction with the HAP2/3/4 regulatory complex. A deletion analysis has shown that the two distal elements are redundant. These measurements were carried out by Northern (RNA) analyses of Ip mRNA transcripts as well as by assays of beta-galactosidase activity in cells carrying constructs of the Ip promoter linked to the lacZ coding sequence. These observations on the regulation of mRNA stability were also extended to the mRNA of the flavoprotein subunit of succinate dehydrogenase and in some experiments of iso-1-cytochrome c.
Full text
PDF
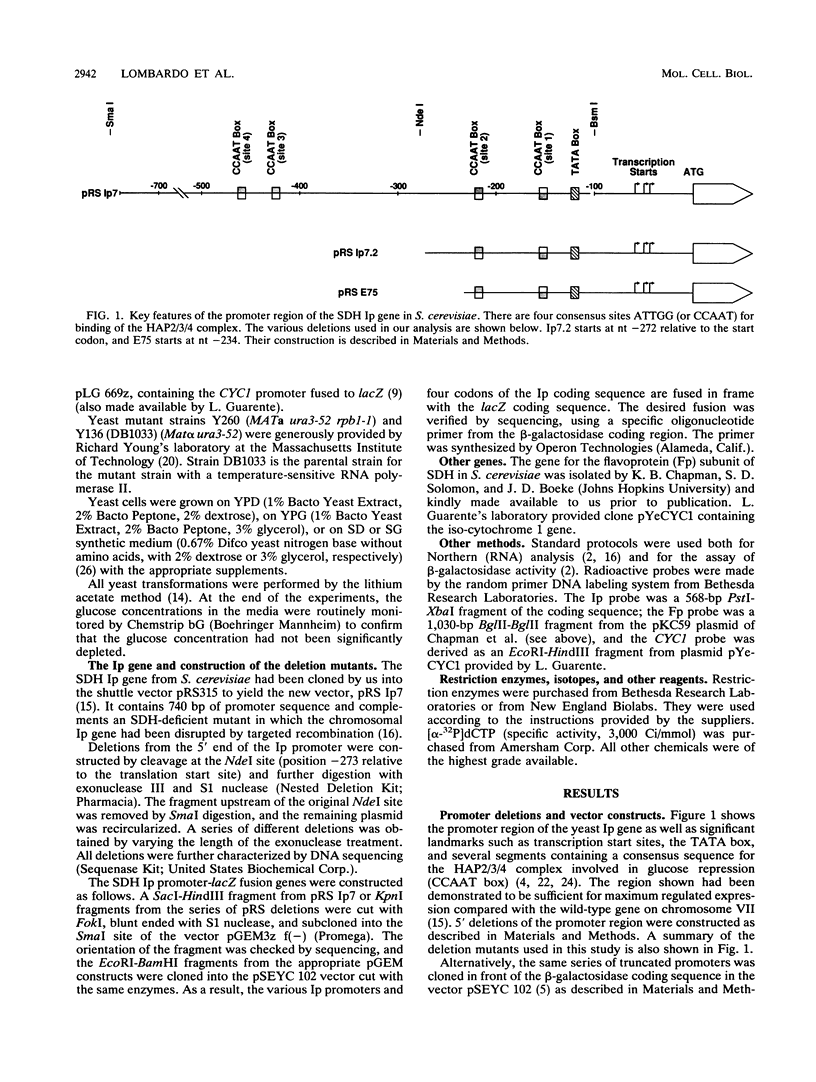






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argüelles J. C., Mbonyi K., Van Aelst L., Vanhalewyn M., Jans A. W., Thevelein J. M. Absence of glucose-induced cAMP signaling in the Saccharomyces cerevisiae mutants cat1 and cat3 which are deficient in derepression of glucose-repressible proteins. Arch Microbiol. 1990;154(2):199–205. doi: 10.1007/BF00423333. [DOI] [PubMed] [Google Scholar]
- Celenza J. L., Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986 Sep 12;233(4769):1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Olesen J., Hahn S., Baldwin A. S., Guarente L., Sharp P. A. A yeast and a human CCAAT-binding protein have heterologous subunits that are functionally interchangeable. Cell. 1988 Apr 8;53(1):25–35. doi: 10.1016/0092-8674(88)90484-9. [DOI] [PubMed] [Google Scholar]
- Emr S. D., Vassarotti A., Garrett J., Geller B. L., Takeda M., Douglas M. G. The amino terminus of the yeast F1-ATPase beta-subunit precursor functions as a mitochondrial import signal. J Cell Biol. 1986 Feb;102(2):523–533. doi: 10.1083/jcb.102.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Guarente L. Communication between mitochondria and the nucleus in regulation of cytochrome genes in the yeast Saccharomyces cerevisiae. Annu Rev Cell Biol. 1989;5:153–180. doi: 10.1146/annurev.cb.05.110189.001101. [DOI] [PubMed] [Google Scholar]
- Guarente L., Mason T. Heme regulates transcription of the CYC1 gene of S. cerevisiae via an upstream activation site. Cell. 1983 Apr;32(4):1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- Guarente L., Ptashne M. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2199–2203. doi: 10.1073/pnas.78.4.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Buratowski S., Sharp P. A., Guarente L. Identification of a yeast protein homologous in function to the mammalian general transcription factor, TFIIA. EMBO J. 1989 Nov;8(11):3379–3382. doi: 10.1002/j.1460-2075.1989.tb08501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S., Guarente L. Yeast HAP2 and HAP3: transcriptional activators in a heteromeric complex. Science. 1988 Apr 15;240(4850):317–321. doi: 10.1126/science.2832951. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Herrick D., Parker R., Jacobson A. Identification and comparison of stable and unstable mRNAs in Saccharomyces cerevisiae. Mol Cell Biol. 1990 May;10(5):2269–2284. doi: 10.1128/mcb.10.5.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo A., Carine K., Scheffler I. E. Cloning and characterization of the iron-sulfur subunit gene of succinate dehydrogenase from Saccharomyces cerevisiae. J Biol Chem. 1990 Jun 25;265(18):10419–10423. [PubMed] [Google Scholar]
- Lombardo A., Scheffler I. E. Isolation and characterization of a Saccharomyces cerevisiae mutant with a disrupted gene for the IP subunit of succinate dehydrogenase. J Biol Chem. 1989 Nov 15;264(32):18874–18877. [PubMed] [Google Scholar]
- Ma H., Bloom L. M., Walsh C. T., Botstein D. The residual enzymatic phosphorylation activity of hexokinase II mutants is correlated with glucose repression in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Dec;9(12):5643–5649. doi: 10.1128/mcb.9.12.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarse A. C., de Haan M., Bout A., Grivell L. A. Demarcation of a sequence involved in mediating catabolite repression of the gene for the 11 kDa subunit VIII of ubiquinol-cytochrome c oxidoreductase in Saccharomyces cerevisiae. Nucleic Acids Res. 1988 Jul 11;16(13):5797–5811. doi: 10.1093/nar/16.13.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marykwas D. L., Fox T. D. Control of the Saccharomyces cerevisiae regulatory gene PET494: transcriptional repression by glucose and translational induction by oxygen. Mol Cell Biol. 1989 Feb;9(2):484–491. doi: 10.1128/mcb.9.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M., Scafe C., Sexton J., Young R. Eucaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol Cell Biol. 1987 May;7(5):1602–1611. doi: 10.1128/mcb.7.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J. T., Guarente L. The HAP2 subunit of yeast CCAAT transcriptional activator contains adjacent domains for subunit association and DNA recognition: model for the HAP2/3/4 complex. Genes Dev. 1990 Oct;4(10):1714–1729. doi: 10.1101/gad.4.10.1714. [DOI] [PubMed] [Google Scholar]
- Olesen J., Hahn S., Guarente L. Yeast HAP2 and HAP3 activators both bind to the CYC1 upstream activation site, UAS2, in an interdependent manner. Cell. 1987 Dec 24;51(6):953–961. doi: 10.1016/0092-8674(87)90582-4. [DOI] [PubMed] [Google Scholar]
- Pinkham J. L., Guarente L. Cloning and molecular analysis of the HAP2 locus: a global regulator of respiratory genes in Saccharomyces cerevisiae. Mol Cell Biol. 1985 Dec;5(12):3410–3416. doi: 10.1128/mcb.5.12.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetto B., Tzagoloff A. Structure and regulation of KGD1, the structural gene for yeast alpha-ketoglutarate dehydrogenase. Mol Cell Biol. 1989 Jun;9(6):2695–2705. doi: 10.1128/mcb.9.6.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai A., Shimizu Y., Kondou S., Chibazakura T., Hishinuma F. Structure and molecular analysis of RGR1, a gene required for glucose repression of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Aug;10(8):4130–4138. doi: 10.1128/mcb.10.8.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes E. A., Sarnow P. An RNA hairpin at the extreme 5' end of the poliovirus RNA genome modulates viral translation in human cells. J Virol. 1991 Feb;65(2):913–921. doi: 10.1128/jvi.65.2.913-921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. P., Johnson M. K. The prosthetic groups of succinate dehydrogenase: 30 years from discovery to identification. FEBS Lett. 1985 Oct 14;190(2):189–198. doi: 10.1016/0014-5793(85)81282-5. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T., Yokota S., Fujiki Y. Isolation and characterization of Chinese hamster ovary cell mutants defective in assembly of peroxisomes. J Cell Biol. 1990 Mar;110(3):651–660. doi: 10.1083/jcb.110.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon A. P., Van Eijk E., Grivell L. A. Biosynthesis of the ubiquinol-cytochrome c reductase complex in yeast. Discoordinate synthesis of the 11-kd subunit in response to increased gene copy number. EMBO J. 1983;2(10):1765–1770. doi: 10.1002/j.1460-2075.1983.tb01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams F. E., Trumbly R. J. Characterization of TUP1, a mediator of glucose repression in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6500–6511. doi: 10.1128/mcb.10.12.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. M., Poyton R. O. Release of two Saccharomyces cerevisiae cytochrome genes, COX6 and CYC1, from glucose repression requires the SNF1 and SSN6 gene products. Mol Cell Biol. 1990 Mar;10(3):1297–1300. doi: 10.1128/mcb.10.3.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitomer R. S., Montgomery D. L., Nichols D. L., Hall B. D. Transcriptional regulation of the yeast cytochrome c gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3627–3631. doi: 10.1073/pnas.76.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitomer R. S., Nichols D. L. Kinetics of glucose repression of yeast cytochrome c. J Bacteriol. 1978 Jul;135(1):39–44. doi: 10.1128/jb.135.1.39-44.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]