Abstract
Background
Helicobacter pylori-infection associated gastritis is known to be a significant risk factor of gastric cancer. Serum levels of Gastrin-17 and Pepsinogen1which are respectively biomarkers of gastric antral and corpus mucosal activity are well known parameters of atrophic gastritis.
Objectives
To determine the prevalence of Helicobacter pylori and atrophic gastritis amongst dyspeptic patients and to compare the production of PGI and G-17 in the various atrophic stages.
Methods
A total of 139 dyspeptic patients aged 46.68±15.50 years [females 106 aged47.23±15.51years, males 33 aged 44.48±14.62] were included during the one year period, March 2008–april 2009 at the district hospital Tombel. The degree of atrophy was determined by the levels of serum pepsinogen1, and gastrin-17 and the presence of Helicobacter pylori antibodies detected by an enzyme immunoassay.
Results
The prevalence of Helicobacter pylori was 79.82% and that for atrophic gastritis was 6.6%. A decrease in mean serum levels of gastin-17 along with increasing antral atrophy was observed; the mean serum levels of pepsinogen1 were reduced during progression of corpus atrophy.
Conclusion
A weak reverse correlation(r =-0.036) was found between Gastrin-17 and Helicobacter pylori antibodies.
Key words: Helicobacter pylori, atrophic gastritis, gastrin, pepsinogen
Introduction
Gastritis, the inflammation of the mucosa of the stomach still remains a serious medical problem for many people world wide. Complications such as peptic ulcer and gastric cancer will result from untreated gastritis1. Peptic ulcer and gastric cancer were associated with increased acid production, stress and anxiety, however, after the discovery of Helicobacter pylori (a spiral gram negative rod shaped bacterium that colonizes the human stomach) by two Australian doctors Robin J. Warren and Barry J. Marshall in 1982, there is authentic evidence to show an association between H pylori -infection and the development of gastric cancer2. The organism is found in the mucous layer of the stomach overlying the gastric epithelium. Among the possible precancerous diseases, long term chronic atrophic gastritis is considered to be important. According to the statements of the Maastricht 2000 consensus, atrophic gastritis is an indication for the eradication of H pylori3. Measurements of serum levels of pepsinogen 1(S-PG1) or the ratio of serum pepsinogen 1 to pepsinogen II (PGI/PGII) are well known non edoscopic tools for diagnosing atrophic gastritis of the gastric corpus, and assays for gastrin (particularly gastrin-17) could be used as an indicator of the morphological status of the antral mucosa. Local antral and corpus gastritis are accompanied respectively by a down torn in levels of serum gastrin-17 and serum pepsinogen I1,4–6.
It is not uncommon to find people of all age groups presenting with dyspepsia and other symptoms of gastritis. The limited resources for endoscopy, poverty, poor health, and the unnecessary intake of drugs and antibiotics could account for the high incidence of symptoms of gastritis in the society. Little or no data exist on previous studies of gastritis in Cameroon.
The main purpose of the present investigation was to determine the prevalence of Helicobacter pylori-infection and atrophic gastritis among patients with dyspepsia in Tombel Central using a test panel. The specific objective of the investigation was to compare the production of serum pepsinogenI, and gastrin-17in the various atrophic stages.
Methods
Study design, population and setting
The study was a cross-sectional approach and consisted of 139 patients presenting various dyspeptic symptoms in the district hospital of Tombel, south west region of Cameroon from March 2008 to April 2009. Helicobacter pylori positivity was defined by the serologic presence of HpAb>30EIU. The patients were aged 18–82 years with a male/female ratio of 33/106.
Sample collection and handling
Blood samples (5mls) for measurements of PGI, PGII, G-17, and IgG class of antibodies to Helicobacter pylori were drawn after an overnight fast in EDTA anticoagulated tubes. Patients name, birth day, time and date of sample collection was recorded. The samples were centrifuged at 1500g for 10 minutes and the plasma samples were stored at 28oc for two days until analyzed. Plasma samples were transported to the St Albert's clinic laboratory in Buea for analysis in ice bags.
Laboratory tests
Gastrin-17, Pepsinogen1, PepsinogenII, and IgA/IgG class antibodies to Helicobacter pylori were determined using specific enzyme immuno assay (EIA )tests (G-17 EIA test kit, PG1 EIA test kit, PGII EIA test kit, and Helicobacter pylori IgA/IgG EIA test kit, Biohit plc) and were performed in batches of 30 samples on a microplate according to the instructions of the manufacturer. All EIA techniques were based on measuring the absorbance after a peroxidation reaction at 450nm. Between the reaction steps, the plates were washed manually. The absorbances were measured using a micro well plate reader (Bp 400). For determination of PG1, PGII and G-17 values, second order fits on standard concentrations were used to interpolate/extrapolate unknown sample concentrations. The IgG antibodies to Helicobacter pylori were expressed as enzyme immuno units (EIU) according to instructions from the company. EIU of 30 and above were considered positive for Helicobacter pylori.
Diagnosis of Atrophic gastritis
In classification of patients with different topographic types of atrophic gastritis with the blood test panel, an algorithm and a computer program (gastrosoft, Biohit plc) were used. For the interpretation and classification of patients into normal stomach (N), atrophic gastritis in the antrum (A), atrophic gastritis in the corpus(C), atrophic gastritis in both antrum and corpus (AC).These criteria for the best discrimination of gastritis of different types were obtained from the Biohit Company. The algorithm and the different topographic types of atrophic gastritis are presented in figure 1. According to applied instructions, serum concentrations of PG1<25µg/l were considered as markers of atrophic gastritis in the corpus; serum concentrations of G-17<2.5pmol/l were considered as markers of atrophic gastritis in the antrum, and G-17<5pmol/L if PG1<50µg/l as markers of mild atrophic gastritis in the corpus. Interpretation of results of IgG class of antibodies to Helicobacter pylori (HpAb) detection was carried out as follows; HpAb titers<30EIU-negative result, ≥30EIU-positive result.
Figure 1.
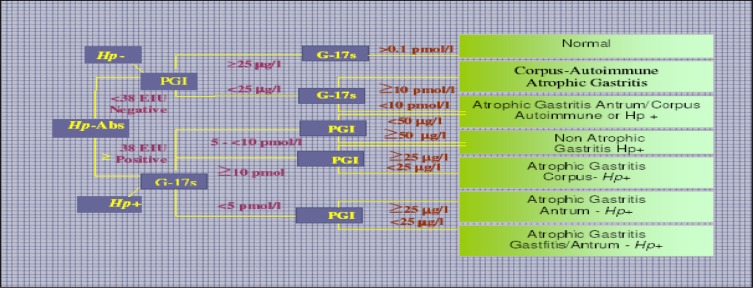
Algorithm (decision tree) for classification of patients into different categories of atrophic gastritis by the Helicobacter pylori antibody titre (HpAb), and serum levels of PG1 andG-17fastThe absence of evidence of Helicobacter pylori-infection is considered to indicate an autoimmune origin of gastritis
Data collection and analysis
The purpose of the study was explained to all participants before recruiting and taking their blood samples. Patients presenting with dyspepsia, heartburns, nausea, vomiting, and other epigastric complaints were consecutively recruited in the study from March to July 2008. A questionnaire on demographic factors containing the date of birth, sex, ethnic group, marital status and employment states were administered to participants and all patients signed a written consent form before enrolment into the study. The study was accepted by the ethical committees of the; Tombel District Hospital, Faculty of Health Sciences of the University of Buea, and Biohit, p.l.c. Helsinki, Finland. Assay analysis was done using the Biohit Gastrosoft software, which is an algorithm used to classify the gastritis. The statistics, significance, prevalence and mean values were calculated by means of the computer program SPSS12.0 software at 95% confidence interval.
The only operation carried out to patients was the blood sampling. The maximum amount of venous blood taking was 5mls. All information related to patient health and laboratory results were handled confidentially. Patients were informed about their results after testing, and an explanation of the meaning of the results was done.
Ethical consideration
Clearance was obtained from the national ethics committee.
Results
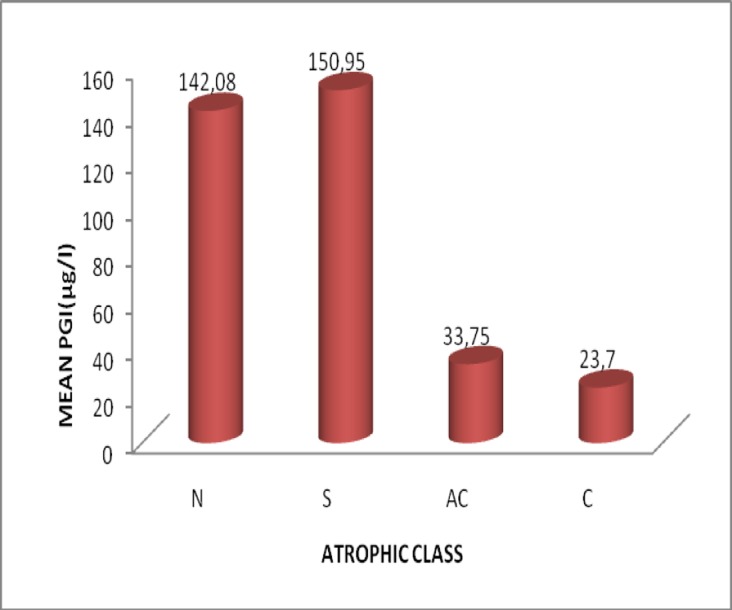
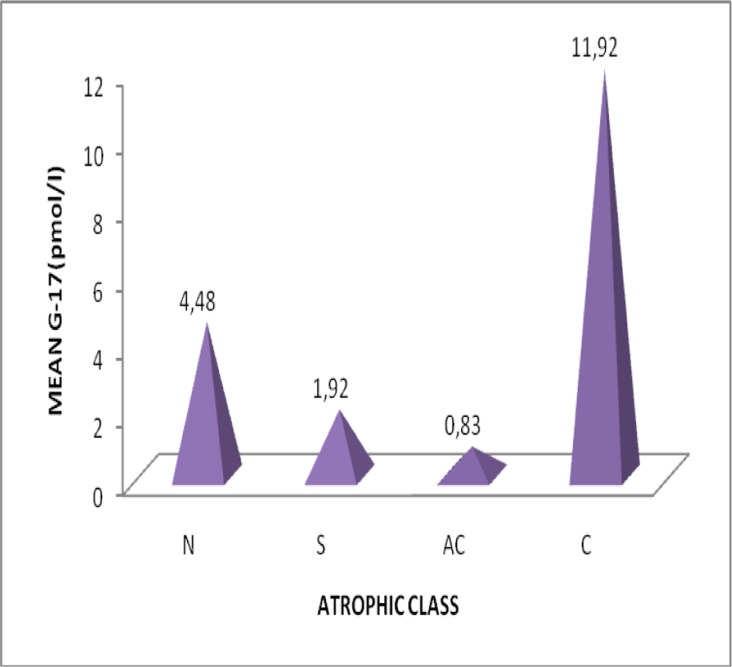
The study population consisted of 139 dyspeptic patients in the period March to July 208. [sex ratio M/F=0.311, aged 18-82 (46.68±15.40) years, females 106 (aged 47.23±15.51) years, males33 (aged 44.48±14.62) years] in the District Hospital Tombel, South West Region, Cameroon from March 2008April 2009]. 79.86% were Helicobacter positive in serology (HpAbe≥30EIU) and prevalence of atrophic gastritis was 6.6%. The algorithm and Gastrosoft revealed that: 106 patients had Non atrophic (superficial) gastritis, 5 had atrophic corpus gastritis, 4 atrophic gastritis antrum/corpus and 24 were normal with healthy mucosa. In patients with non-atrophic antrum gastritis compared with those with normal gastric mucosa, the fasting G-17 and PG1 levels in serum were 1.92±3.62pmol/l versus4.48±14.18pmol/l and 150.95±78.68µg/l versus142.08±107.32 µg/l respectively. There was a weak reverse correlation(r=-0.036) between G-17 and HpAb. The mean PG1 levels decreased with increasing grade of atrophy in the corpus mucosa. Correspondingly, G-17 levels decreased with increasing grade of atrophic antrum gastritis. There was a significant difference in the mean values of PG1, G-17, and HpAb, Fcal> Ftab, @=0.05. There were no significant differences in the prevalence of Helicobacter pylori among sex, age, marital status, profession, and educational status of studied participants.
Discussion
Helicobacter pylori-infection -infection causes acute gastritis in most infected individuals. In a certain number of patients with persistent infection, chronic active gastritis develops, leading finally to glandular atrophy, a risk factor for gastric adenoma and cancer7 . Correa 19928 postulated the paradigm of gastric carcinogenesis that chronic gastritis progresses into gastric atrophy and intestinal metaplasia. The prevalence of Helicobacter pylori-infection was 79.82%. The results are consistent with those of Biohit 20054 that Helicobacter pylori-infection accounts for 70–90% amongst gastritis patients in Africa. The prevalence of atrophic gastritis was 6.6% and is similar with what was obtained in many other countries4.
The mean levels of G-17 and PG1 decreased as the degree of atrophic gastritis increased respectively in the antrum and corpus areas. This is certainly based on loss of normal mucosa glands and cells in the antrum and corpus mucosa9. G-17 is synthesized and secreted almost solely from antral G-cells and they disappear with the loss of antral glands and with the extension of intestinal metaplasia in the antrum4,10. G-17 levels are also influenced by the presence of Helicobacter pylori in the stomach, intragastric acidity and physiological stimuli10. Serum pepsinogen has been reported to be a marker of atrophic gastritis and eradication of Helicobacter pylori. Kiyohira et al in 20039 investigating the variation of serum pepsinogen concentrations in relation to histologic features in Helicobacter pylori infected persons concluded that at least three factors influence serum pepsinogen concentrations including; atrophy, inflammation, and Helicobacter pylori-infection in the gastric mucosa. There was no significant difference in the prevalence of Helicobacter pylori infection amongst sex, marital status, profession, level of education, and age of the study participants. There was a significant difference in the production of pepsinogen 1, gastrin-17 and antibodies to Helicobacter pylori this probably due the fact that they require different stimuli for production and are produced by different cells10.
According to the Maastricht III concensus statement, the gold standard for the diagnosis of atrophic gastritis is biopsy examination during endoscopy while that for Helicobacter pylori infection is the Carbon-13 urea breath and the stool antigen tests. However, many studies have reported high sensitivity and specificity with the gastropanel test. Väänänen et al in 20037 and Nicolin et al in 200211 suggested that the overall accuracy of the blood test panel in the diagnosis of atrophic gastritis is over 80% when compared with diagnosis from endoscopy and biopsies.
Similarly, Suovaniemi et al in 200512 showed that the simultaneous detection of PGI. G-17 and HpAb is a suitable tool for non invasive screening and diagnosis of atrophic gastritis from a blood test. We measured the IgG antibody levels to Helicobacter pylori which could remain high for up to six months even after treatment. This study could not really differentiate between patients with recent or past Helicobacter pylori infection. More studies need to be done in Cameroon, to correlate Gastropanel biomarkers with endoscopy and related biopsy examination and with other tests such as the Carbon13 breath test, stool antigen test, culture etc.
Conclusion
This study comes to inform and educate the population that Helicobacter pylori is a causative agent of gastritis and a risk factor, for peptic ulcer and gastric cancer formerly believed to be caused by stress and spicy foods, and that gastritis and that atrophic gastritis and Helicobacter pylori infection can be diagnosed with a blood test. In conjunction with the gastrosoft computer, the test panel can be regarded as gold standards for the effective and reliably identification of Helicobacter pylori infection and atrophic gastritis and the monitoring of a successful treatment of Helicobacter pylori infection and the healing of atrophic gastritis.
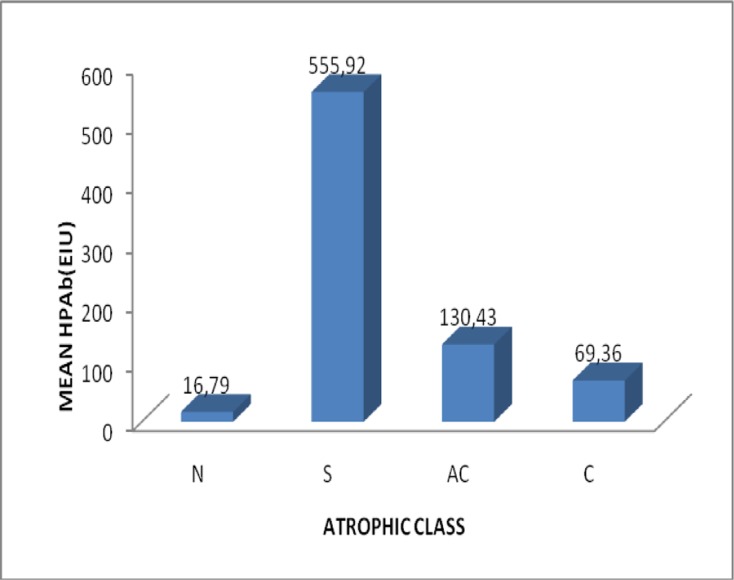
Figure 2.
Variation of mean HPAb with atrophic class
Figure 3.
Variation of mean pepsinogen I with atrophic class
Figure 4.
Variation of mean gastrin-17 with atrophic class
Table 1.
Prevalence rates of Helicobacter pylori among dyspeptic patients based on demographic factors. At 95% confidence interval
| Parameter | Number (%) | HpAbe≥30EIU | HpAb<30EIU | X^2cal | X^2tab | P |
| Sex | ||||||
| Males | 33(23.74%) 27(81.82%) | 06(18.18%) | ||||
| Females | 106(76.26%) | 84(79.25%) | 22(20.75%) | 0.216 | 0.823 | 0.65 |
| Age | ||||||
| 18–28 | 17(12.23%) 15(88.24%) | 2(11.76%) | ||||
| 29–39 | 34(24.46%) | 28(82.35%) | 6(17.64%) | |||
| 40–50 | 36(25.90%) | 28(77.78%) | 8(22.22%) | 2.70 | 11.07 | 0.746 |
| 51–61 | 23(16.55%) | 18(78.26%) | 5(21.74%) | |||
| 62–72 | 21(15.11%) | 17(80.95%) | 4(19.05%) | |||
| 73–82 | 08(5.76%) | 5(62.50%) | 3(37.50%) | |||
| Marital status | ||||||
| Married | 80(57.55%) | 61(76.25%) | 19(23.75%) | |||
| Single | 28(20.14%) | 26(92.86%) | 2(7.14%) | 6.32 | 7.81 | 0.097 |
| Widow(er) | 28(20.14%) | 21(75.00%) | 7(25.00%) | |||
| Divorce | 3(02.16%) | 3(100.00 %) | 00(00.00%) | |||
| Profession | ||||||
| Farming | 25(17.99%) | 19(76.00%) | 6(24.00%) | |||
| Trade | 13(09.35%) | 12(92.31%) | 1(7.69%) | 3.64 | 9.48 | 0.45 |
| Student | 6(04.22%) | 5(88.33%) | 1(16.67%) | |||
| Employed | 16(11.51%) | 14(87.50%) | 2(12.50%) | |||
| None | 79(56.83%) | 61(77.22%) | 18(22.78%) |
Table 2.
Serologic markers of atrophy in patients with Helicobacter pylori
| Grade of atrophy | Parameter | mean±SD | |
| Atrophic gastritis in the antrum | 0, n=24 | HpAb | 16.79±6.39 |
| G-17 | 4.48±14.18 | ||
| 1,n=106 | HpAb | 555.92±1326.36 | |
| G-17 | 1.92±3.62 | ||
| Corpus atrophic gastritis | 0, n=24 | HpAb | 16.79±6.39 |
| predominated atrophy | G-17 | 4.48±14.18 | |
| PG1 | 142.08±107.32 | ||
| 1,n=1062,n=5 | HpAb | 555.92±1326.36 | |
| G-17 | 1.92±3.62 | ||
| PG1 | 150.95±78.68 | ||
| HpAb | 69.36±119.07 | ||
| G-17 | 11.92±17.55 | ||
| PG1 | 23.70±18.74 | ||
| Atrophic gastritis in corpus and | 2,n=4 | HpAb | 130.43±115.40 |
| antrum | G-17 | 0.83±0.85 | |
| PG1 | 33.75±22.63 |
Grade: 0= Normal and healthy mucosa 1=Non atrophic gastritis 2=Atrophic gastritis
Acknowledgements
It is with deep satisfaction that I acknowledge the support of the Biohit P.L.C Finland for their efforts towards the success of this work. Sincere appreciations go to Mr. Phillip Ngole Ngwesse, Nhon Dr.Ntoko Mekolle Epie, Nhon Ajebe Elias Ebong, Mr. Amadou Zoumba, Mr. Mbong Michael, Mr. Mbulle Valentine, Prof. Alobwede Epie Esambe, and TACUDA Yaoundé Branch for their financial assistance.
Special gratitude to Mrs. Florence Njitone, Mr. Alobwede Daniel, Dr Njanjo Georges, Dr.Edie Ngolle Francis, Mr Kome Lucas Ebong, Mr Robert Nyirgchu, Mr Fobisong Kajetan, for their coordination.
References
- 1.Di Mario F, Franzè A, Cavallaro LG. Noninvasive approach to diagnosis of uppergastrointestinal diseases. AREA QUALITÀ srl. 2008;1:5–64. [Google Scholar]
- 2.Härkönen M, Laxen F, Nikulin M, Nilson I, Sande N, Sipponen P, Suovaniemi O, Wadstrom T. Increased risk of developing atrophic gastritis in patients infected with Cag+ Helicobacter pylori. Scand J Gastroenterol. 2001;36:928–933. doi: 10.1080/003655201750305431. [DOI] [PubMed] [Google Scholar]
- 3.2011. May 15, WWW.biohit.com/diagnostics-servicelaboratory/ www.biohit.com/gastropanel-gastropanel research applications.
- 4.Biohit plc, author. Diagnostics-Literature. Helsinki, Finland: 2002–2005. [Google Scholar]
- 5.Ivaskin V. Lapina Report the final on the reliability of ELISA test panel of serum level of PGI, G-17 and H. pylori antibodies in patients with peptic ulcer and gastritis. Russian J Gastroenterol. 2004:1–4. [Google Scholar]
- 6.Bergeret M, Dupont C, Kalach N, Legoede J, Raymond J, Wanne A. Serum levels of pepsinogen 1, pepsinogenII and gastrin -17 in the course of Helicobacter pylori gastritis in pediatrics. Letters to the editor. J Pediatric Gastroenterol Nutr. 2004;39:568–570. doi: 10.1097/00005176-200411000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Väänänen H, Vauhkonne M, Helske T, Kääriänen I, Rasmussen M, Tunturi H, Koskenpato J, Sotkha M, Turunen M, Sandströmme R, Ristikankare M, Jussila A, Sipponen P. Non endoscopic diagnosis of atrophic gastritis with a blood test . Correlation between gastric histology and serum levels of gastrin -17 and pepsinogen 1: A multicenter study. Lippincott &Wilkins; 2003. pp. 1–4. [DOI] [PubMed] [Google Scholar]
- 8.Correa P. Human gastric carcinogens: A multistep and multifactorial process. First American cancer society award lecture on cancer epidemiology and prevention. Cancer. 1992;52:6735–6742. [PubMed] [Google Scholar]
- 9.Kiyohira K, Yoshihara M, Iio M, Haruma K, Tanaka S, Chayama K. Serum pepsinogen concentrations as a marker of Helicobacter pylori-infection and histologic grade of gastritis; evaluation of gastric mucosa by serum pepsinogen levels. J Gastroenterol. 2003;38:332–338. doi: 10.1007/s005350300060. [DOI] [PubMed] [Google Scholar]
- 10.Pasechnikov Victor D, Sergey Chukov Z, Sergey Kotelevets M, Alexander Mostovov N, Varvara Mernova P, Polyakova Maria. Invasive and Non-invasive diagnosis of Helicobacter pylori associated atrophic gastritis: A comparative study. Scand J Gastroenterol. 2005;40:297–301. doi: 10.1080/00365520410010607. [DOI] [PubMed] [Google Scholar]
- 11.Nicolin G, Zagari R, Pozzato P, Lunedei V, Delura L, Antonini F. Diagnosis of atrophic gastritis based upon a combination of three noninvasive tests. Preliminary results of the Loiano-Monghidoro project. J Gastroenterol Hepathol. 2002;A264 Dig Liver Dis. 2001;33. [Google Scholar]
- 12.Suovaniemi O, Harkonen M, Paloheimo L, Sipponen P. Gastro panel: Diagnosing atrophic gastritis from serum providing a tool for evidenced-based medicine. Business Briefing Global Health Care. 2003:1–4. www.biohit.com. [Google Scholar]