Abstract
Human actinomycosis is a rare soft tissue infection caused by Gram-positive, anaerobic bacteria Actinomyces israelii, a commensal of the oral cavity. The major clinical forms of actinomycosis are cervicofacial, thoracic, abdominal and pelvic forms. The cervicofacial region is most commonly affected. Actinomycosis is sometimes difficult to diagnose and it should be borne in mind in the differential diagnosis of numerous infectious and non-infectious diseases. We report a patient who came with tooth pain and extra-oral swelling which later on presented as multiple draining sinuses. Our initial suspicion was dento-alveolar abscess or osteomyelitis. However, a culture of the discharge and subsequent biopsy revealed actinomycetes, confirming cervicofacial actinomycosis, but presenting itself not as the typical ‘lumpy jaw’. The patient was successfully treated conservatively with a short but intensive antibiotic course.
Background
Actinomycosis was first described as a clinical entity over 100 years ago.1 Actinomycosis is a subacute to chronic bacterial infection caused by anaerobic and microaerophilic Gram-positive bacteria that are often also normally found in the oral cavity, the gastrointestinal tract and the female genital tract.2 Known to occur in cattle since the early 19th century, the disease was first described as a clinical entity in humans by Israel in 1878.3 It has a worldwide distribution and most frequent clinical form is cervicofacial actinomycosis.4 The infection is characterised by contiguous spread, suppurative and granulomatous inflammation, and formation of multiple abscesses and sinus tracts that may discharge ‘sulfur granules’.5 Previously a common and ultimately fatal disease, its prognosis has improved vastly since the introduction of antimicrobial agents.6 Actinomycosis can mimic numerous infectious and non-infectious diseases. Its rarity and fewer typical advanced presentations add to the diagnostic difficulties.7 We report one such unusual case.
Case presentation
A 45-year-old man presented to the Department of Oral Medicine, Diagnosis and Radiology, with the history of slowly progressive swelling on the left side of the face since 3 months, and subsequent multiple draining sinuses since 4 weeks. Patient also complained of a tooth pain on the left side of the jaw since few months.
Physical examination revealed a diffuse swelling on the left side of the face, slightly tender woody hard multiple nodular lesions, mostly located around the left angle of mandible, side of the neck and behind the left corner of the eye. These multiple abscesses were reddish purple in colour and showed draining sinuses that opened onto the perimandibular region (figure 1). However, there was absence of lymphadenopathy and fever but patient exhibited mild trismus.
Figure 1.
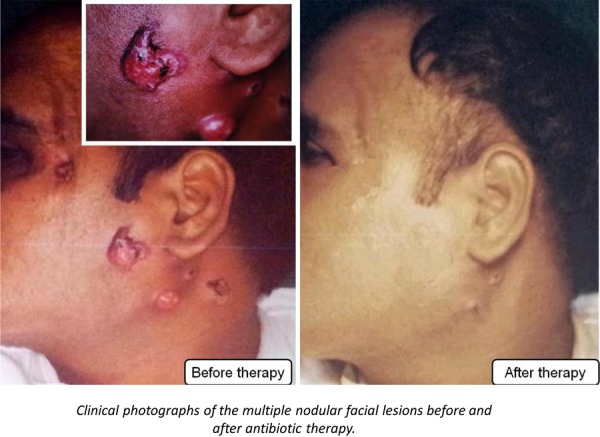
Clinical photographs of lesions, preantibiotic and postantibiotic treatments.
Investigations
Orthopantomograph x-ray evaluation revealed a badly carious mandibular left third molar tooth with a peri-apical infection and an infected maxillary left third molar root stumps (figure 2).
Figure 2.
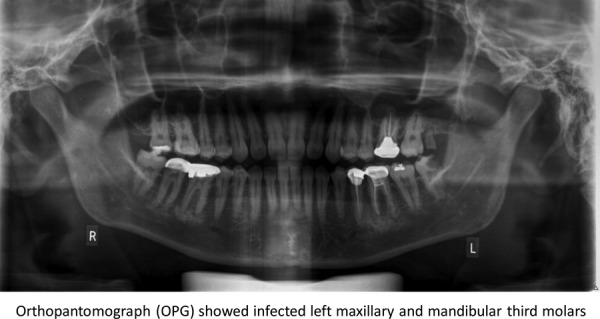
Orthopantomograph showed infected left maxillary and mandibular third molars.
Laboratory studies revealed mild leukocytosis, and elevated levels of erythrocyte sedimentation rate and C reactive protein.
The microbiologic culture of the discharge revealed the presence of Fresobacterium nucleatum, Porphyromonas asaccharolytica, Staphylococcus aureus and some actinomycetes.
Surgical excision of one of the nodules with discharge was performed under local anaesthesia and the biopsied specimen was sent for histopathologic evaluation.
H&E stained sections showed colonies of filamentous actinomyces bacteria with peripherally situated, radiating hyaline eosinophilic material representing the ‘Splendore-Hoeppli’ phenomenon. There was dense polymorphonuclear infiltrate surrounded by chronic granulation tissue (figure 3). Splendore-Hoeppli phenomenon is evident in varied infections ranging from fungal (sporotrichosis, candidiasis, aspergillosis), bacterial (botryomycosis, nocardiosis, actinomycosis) and parasitic infections (schistosomiasis, pythiosis, cutaneous larva migrans).8
Figure 3.
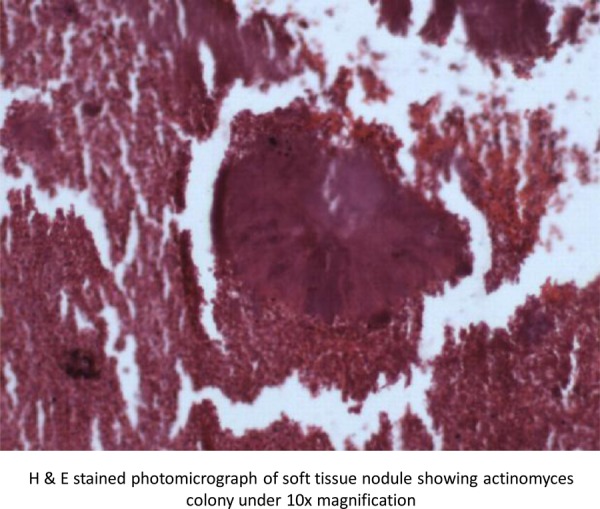
H&E stained photomicrograph of soft tissue nodule showing actinomyces colony under ×10 magnification.
A special Gram staining was therefore done, which confirmed actinomycetes infection.
Differential diagnosis
The diagnosis of the cervicofacial form of actinomycosis, on clinical examination alone is difficult at the onset, unless the physician is aware of it, but becomes easy once the granules are discharged.
A number of inflammatory, infectious and non-infectious diseases were considered in the clinical differential diagnosis. Abscesses and fistulae due to pyogenic bacteria were thought to be unlikely because of the gradual onset of the swelling, his preserved general health and the absence of fever, lymphadenopathy or leucocytosis.
Osteomyelitis of jaws too was unlikely in the radiographic absence of osteolysis of bone with irregular borders, the absence of radiopaque sequestra/radiolucent cloacae or any periosteal reaction.
Tertiary syphilis, subcutaneous tuberculosis with fistulae or scrofuloderma and various benign and malignant tumours of the cervicofacial region were excluded by the histological findings. The absence of cervical lymphadenopathy too supported actinomycosis as it usually does not spread by the lymphatic system due to the size of the bacterium.
As a rule, we suggest that any soft tissue swelling on the face and neck should be investigated for actinomycosis.
Treatment
The infected left third molars were extracted under local anaesthesia and concurrently high-dose penicillin G (1 million units per day) was given intravenously for 4 weeks, which substantially resolved the facial lesions (figure 1). The patient was subsequently put on oral penicillin for a further period of 3 months to achieve complete remission and decrease the probability of recurrence.
Outcome and follow-up
Our patient was treated successfully with intravenous penicillin G, initially for 4 weeks, which reduced the lesions and trismus substantially. The patient was later put on oral systemic amoxycillin and clavulinic acid combination drug, Augmentin for few weeks, to achieve complete remission. A weekly monitoring was also done to see the patient's response to therapy.
The patient was advised to maintain good oral hygiene and come for regular dental visits, to prevent recurrence.
Discussion
Actinomycosis occurs worldwide and can affect people of all ages, but the majority of cases are reported in young to middle-aged adults (aged 20–50 years). No racial predilection exists. For unknown reasons, men are affected more commonly than women, with the exception of pelvic actinomycosis.9
The common clinical presentations are cervicofacial, thoracic and abdominal.10 Actinomycosis is also seen as a genital infection in women, particularly those with a history of intrauterine contraceptive device usage.11 Apart from these sites, the infection has also been reported in the testis12 and liver.13 Cranial and intracranial actinomycosis have also been reported by Sundaram et al.14
Cervicofacial actinomycosis is the most common manifestation, comprising 50–70% of reported cases. Infection typically occurs following oral surgery or in patients with poor dental hygiene.4 This form of actinomycosis is characterised in the initial stages by soft tissue swelling of the perimandibular area. Direct spread into the adjacent tissues occurs over time, along with development of fistulas (sinus tracts) that discharge purulent material containing yellow sulphur granules.
Complications depend on the site of infection, from the skin of the face, it may spread to nearby parts, such as scalp or ears. From the oral cavity, it may spread to the tongue,15 larynx,16 trachea, salivary glands and the tubes that connect the throat to the nose. If the infection spreads to the brain via cranial invasion or bloodstream, a brain abscess could develop.
Actinomyces produce extensive fibrotic reactions with central necrotic lesion, resulting in hypovascular tissue with low potential for oxygen reduction and limited antibiotic penetration.
Consequently, treatment requires high-dose, intensive antibiotic therapy.
Antibiotics that possess no activity against actinomyces species include metronidazole, aminoglycosides, aztreonam, co-trimoxazole, penicillinase-resistant penicillins (methicillin, nafcillin, oxacillin, cloxacillin) and cephalexins. The data concerning the fluoroquinolones (ciprofloxacin, levofloxacin, moxifloxacin) are limited; however, success has been cited in case reports.17 18
High-dose penicillin administered over a prolonged period (6 months to 1 year) is the cornerstone of therapy for actinomycosis. Success with shorter courses of therapy (less than 6 months) has been reported, especially in cervicofacial actinomycosis.19 Our patient too was successfully treated with short but intensive course of penicillin G antibiotic.
The risk of actinomycetes developing penicillin resistance appears to be minimal. Lack of a clinical response to penicillin usually indicates the presence of resistant companion bacteria, which may require modification of the antibiotic regimen.
Ultimately, the treatment duration should be tailored to the individual patient based on clinical and radiological response. Patients should be monitored more closely if shorter treatment durations are considered.
Complete recovery is expected in 90% of patients with cervicofacial actinomycosis,20 as was achieved in our case too.
Learning points.
Actinomycosis should be included in the differential diagnosis of multiple draining sinuses in the cervical region, besides osteomyelitis, tuberculous lymphadenitis and other granulomatous diseases. As a rule, we suggest that any soft tissue swelling on the face and neck should be investigated for actinomycosis.
Excellent prognosis if diagnosed early and treated with appropriate antibiotic therapy drug of choice is intravenous penicillin G antibiotic for minimum of 4 weeks.
Lack of clinical response to penicillin usually indicates the presence of resistant companion bacteria which may require modification of antibiotic regimen.
Complications may include osteomyelitis of mandible, invasion of the cranium or the bloodstream if the disease is left untreated.
Maintenance of good oral hygiene and adequate regular dental care are important.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Israel J. Neue Beobactungen auf dem Bebiete der Mykosen des Menshen. Virchows Arch Pathol Anat 1878;2013:15–53 [Google Scholar]
- 2.Oostman O, Smego RA. Cervicofacial actinomycosis: diagnosis and management. Curr Infect Dis Rep 2005;2013:170–4 [DOI] [PubMed] [Google Scholar]
- 3.Grigoriu D, Delacrétaz J, Borelli D. Actinomycosis: medical mycology. Basle, Switzerland: Editiones Roche, 1987:425–32 [Google Scholar]
- 4.Bassi E, Ortonne N, Revuz J, et al. Cervicofacial actinomycosis. G Ital Dermatol Venereol 2012;2013:325–6 [PubMed] [Google Scholar]
- 5.De Montpreville VT, Nashashibi N, Dulmet EM. Actinomycosis and other bronchopulmonary infections with bacterial granules. Ann Diagn Pathol 1999;2013:67–74 [DOI] [PubMed] [Google Scholar]
- 6.Haldane DJM. Community acquired pneumonia. Medicine (Springer US) 2007;2013:827–40 [Google Scholar]
- 7.Weese WC, Smith IM. A study of 57 cases of actinomycosis over a 36-year period. A diagnostic ‘failure’ with good prognosis after treatment. Arch Intern Med 1975;2013:1562–8 [PubMed] [Google Scholar]
- 8.Hussein MR. Muco-cutaneous Splendore-Hoeppli phenomenon. J Cutan Pathol 2008;2013:979–88 [DOI] [PubMed] [Google Scholar]
- 9.Lippes J. Pelvic actinomycosis: a review and preliminary look at prevalence. Am J Obstet Gynecol 1999;2013:265–9 [DOI] [PubMed] [Google Scholar]
- 10.Mcneil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev 1994;2013:357–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hager WD, Majumdar B. Pelvic actinomycosis in women using intra uterine contraceptive devices. Am J Obstet Gynecol 1979;2013:60–3 [DOI] [PubMed] [Google Scholar]
- 12.Lin CV, Jwo SC, Lin CC. Primary testicular actinomucosis mimicking metastatic tumor. Int J Urol 2005;2013:519–21 [DOI] [PubMed] [Google Scholar]
- 13.Lai AT, Lam CM, Ng KK, et al. Hepatic actinomycosis presenting as a liver tumor: case report and literature review. Asian J Surg 2004;2013:345–7 [DOI] [PubMed] [Google Scholar]
- 14.Sundaram C, Purohit AK, Prasad VS, et al. Cranial and intracranial actinomycosis. Clin Neuropathol 2004;2013:173–7 [PubMed] [Google Scholar]
- 15.Kurtaran H, Ugur KS, Ark N, et al. Tongue abscess with actinomycosis. J Craniofac Surg 2011;2013:1107–9 [DOI] [PubMed] [Google Scholar]
- 16.Schumann R, Lorenz KJ, Tisch M, et al. Laryngeal and pharyngeal actinomycosis. HNO 2010;2013:867–71 [DOI] [PubMed] [Google Scholar]
- 17.Macfarlane DJ, Tucker LG, Kemp RJ. Treatment of recalcitrant actinomycosis with ciprofloxacin. J Infect 1993;2013:177–80 [DOI] [PubMed] [Google Scholar]
- 18.Ferreira Dde F, Amado J, Neves S, et al. Treatment of pulmonary actinomycosis with levofloxacin. J Bras Pneumol 2008;2013:245–8 [DOI] [PubMed] [Google Scholar]
- 19.Sudhakar SS, Ross JJ. Short-term treatment of actinomycosis: two cases and a review. Clin Infect Dis 2004;2013:444–7 [DOI] [PubMed] [Google Scholar]
- 20.Katz BJ, Kalter DC, Bruce S. Subcutaneous nodules in a man diagnosed as having tuberculosis. Arch Dermatol 1988;2013:121–122, 124–125 [PubMed] [Google Scholar]


