Abstract
Cells of Candida albicans WO-1 spontaneously switch between a white and opaque CFU, and this phase transition involves a dramatic change in cellular phenotype. By using a differential hybridization screen, an opaque-specific cDNA, Op1a, which represents the transcript of a gene regulated by switching, has been isolated. The gene for Op1a is transcribed by opaque but not by white cells. The nucleotide sequence of the Op1a cDNA reveals over 99% base homology with an acid protease gene of C. albicans, and the predicted amino acid sequence demonstrates that the product of this gene is a member of the family of pepsinogens, which possess a hydrophobic leader sequence for secretion and two catalytic aspartate domains. Southern blots of both genomic DNA digested with 14 different endonucleases and electrophoretically separated chromosomes were probed with the Op1a cDNA. No polymorphisms were detected in either case between white and opaque cells, suggesting that no genomic reorganization occurs in the proximity of the gene during the white-opaque transition. Although transcription of Op1a correlates with the high levels of extracellular protease activity in opaque cell cultures and the absence of activity in white cell cultures, stimulation of extracellular protease activity by addition of serum albumin is not accompanied by Op1a transcription in cultures of WO-1 white cells or cultures of two additional clinical isolates of C. albicans, suggesting that expression of one or more other protease genes is stimulated in these cases. The results demonstrate that transcription of the Op1a gene is under the rigid control of switching in strain WO-1.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agatensi L., Franchi F., Mondello F., Bevilacqua R. L., Ceddia T., De Bernardis F., Cassone A. Vaginopathic and proteolytic Candida species in outpatients attending a gynaecology clinic. J Clin Pathol. 1991 Oct;44(10):826–830. doi: 10.1136/jcp.44.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
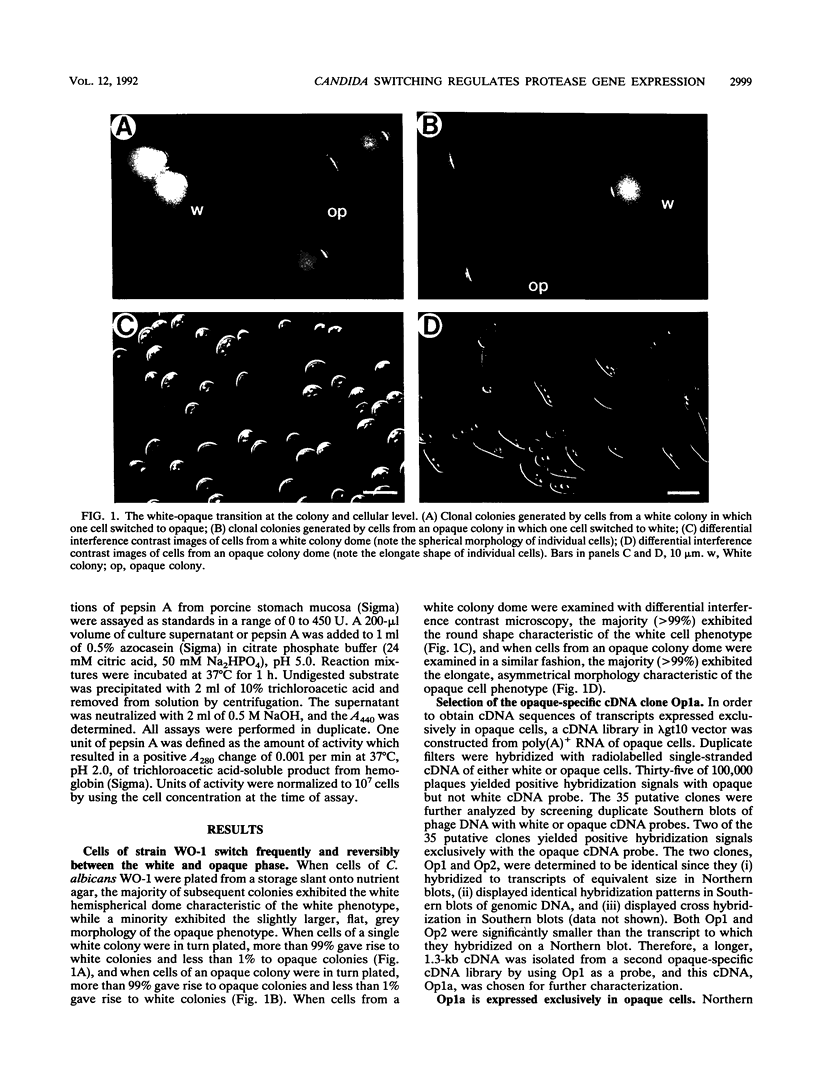
- Anderson J. M., Soll D. R. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987 Dec;169(12):5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Cundiff L., Schnars B., Gao M. X., Mackenzie I., Soll D. R. Hypha formation in the white-opaque transition of Candida albicans. Infect Immun. 1989 Feb;57(2):458–467. doi: 10.1128/iai.57.2.458-467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Mihalik R., Soll D. R. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J Bacteriol. 1990 Jan;172(1):224–235. doi: 10.1128/jb.172.1.224-235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell G. W., Soll D. R. Effects of low concentrations of zinc on the growth and dimorphism of Candida albicans: evidence for zinc-resistant and -sensitive pathways for mycelium formation. Infect Immun. 1979 Oct;26(1):348–354. doi: 10.1128/iai.26.1.348-354.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen M. S., Voss E., Soll D. R. Switching at the cellular level in the white-opaque transition of Candida albicans. J Gen Microbiol. 1990 Oct;136(10):1925–1936. doi: 10.1099/00221287-136-10-1925. [DOI] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Buffo J., Herman M. A., Soll D. R. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984 Mar 15;85(1-2):21–30. doi: 10.1007/BF00436698. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman K. D., Rosen N. L., Newman P. J., Montgomery R. R. Enzymatic amplification of specific cDNA inserts from lambda gt11 libraries. Nucleic Acids Res. 1988 Sep 12;16(17):8718–8718. doi: 10.1093/nar/16.17.8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruit J., Cailliez J. C., Odds F. C., Poulain D. Expression of an epitope by surface glycoproteins of Candida albicans. Variability among species, strains and yeast cells of the genus Candida. J Med Vet Mycol. 1990;28(3):241–252. doi: 10.1080/02681219080000301. [DOI] [PubMed] [Google Scholar]
- Ghannoum M. A., Swairjo I., Soll D. R. Variation in lipid and sterol contents in Candida albicans white and opaque phenotypes. J Med Vet Mycol. 1990;28(2):103–115. [PubMed] [Google Scholar]
- Hube B., Turver C. J., Odds F. C., Eiffert H., Boulnois G. J., Köchel H., Rüchel R. Sequence of the Candida albicans gene encoding the secretory aspartate proteinase. J Med Vet Mycol. 1991;29(2):129–132. [PubMed] [Google Scholar]
- Kennedy M. J., Rogers A. L., Hanselmen L. R., Soll D. R., Yancey R. J., Jr Variation in adhesion and cell surface hydrophobicity in Candida albicans white and opaque phenotypes. Mycopathologia. 1988 Jun;102(3):149–156. doi: 10.1007/BF00437397. [DOI] [PubMed] [Google Scholar]
- Kolotila M. P., Diamond R. D. Effects of neutrophils and in vitro oxidants on survival and phenotypic switching of Candida albicans WO-1. Infect Immun. 1990 May;58(5):1174–1179. doi: 10.1128/iai.58.5.1174-1179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz M. B., Cortelyou M. W., Kirsch D. R. Integrative transformation of Candida albicans, using a cloned Candida ADE2 gene. Mol Cell Biol. 1986 Jan;6(1):142–149. doi: 10.1128/mcb.6.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Lehman D., Good C., Magee P. T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985 Sep;49(3):571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker B. A., Carle G. F., Kobayashi G. S., Medoff G. Comparison of the separation of Candida albicans chromosome-sized DNA by pulsed-field gel electrophoresis techniques. Nucleic Acids Res. 1989 May 25;17(10):3783–3793. doi: 10.1093/nar/17.10.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Inducible proteinase of Candida albicans in diagnostic serology and in the pathogenesis of systemic candidosis. J Med Microbiol. 1980 Aug;13(3):423–435. doi: 10.1099/00222615-13-3-423. [DOI] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Virulence for mice of a proteinase-secreting strain of Candida albicans and a proteinase-deficient mutant. J Gen Microbiol. 1983 Feb;129(2):431–438. doi: 10.1099/00221287-129-2-431. [DOI] [PubMed] [Google Scholar]
- Olaiya A. F., Sogin S. J. Ploidy determination of Canadida albicans. J Bacteriol. 1979 Dec;140(3):1043–1049. doi: 10.1128/jb.140.3.1043-1049.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomés R., Gil C., Nombela C. Genetic analysis of Candida albicans morphological mutants. J Gen Microbiol. 1985 Aug;131(8):2107–2113. doi: 10.1099/00221287-131-8-2107. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggsby W. S., Torres-Bauza L. J., Wills J. W., Townes T. M. DNA content, kinetic complexity, and the ploidy question in Candida albicans. Mol Cell Biol. 1982 Jul;2(7):853–862. doi: 10.1128/mcb.2.7.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikkerink E. H., Magee B. B., Magee P. T. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J Bacteriol. 1988 Feb;170(2):895–899. doi: 10.1128/jb.170.2.895-899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross I. K., De Bernardis F., Emerson G. W., Cassone A., Sullivan P. A. The secreted aspartate proteinase of Candida albicans: physiology of secretion and virulence of a proteinase-deficient mutant. J Gen Microbiol. 1990 Apr;136(4):687–694. doi: 10.1099/00221287-136-4-687. [DOI] [PubMed] [Google Scholar]
- Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981 May 14;659(1):99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scherer S., Magee P. T. Genetics of Candida albicans. Microbiol Rev. 1990 Sep;54(3):226–241. doi: 10.1128/mr.54.3.226-241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky B., Buffo J., Soll D. R. High-frequency switching of colony morphology in Candida albicans. Science. 1985 Nov 8;230(4726):666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Bedell G., Thiel J., Brummel M. The dependency of nuclear division on volume in the dimorphic yeast Candida albicans. Exp Cell Res. 1981 May;133(1):55–62. doi: 10.1016/0014-4827(81)90356-6. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Galask R., Isley S., Rao T. V., Stone D., Hicks J., Schmid J., Mac K., Hanna C. Switching of Candida albicans during successive episodes of recurrent vaginitis. J Clin Microbiol. 1989 Apr;27(4):681–690. doi: 10.1128/jcm.27.4.681-690.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R. High-frequency switching in Candida albicans. Clin Microbiol Rev. 1992 Apr;5(2):183–203. doi: 10.1128/cmr.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Staebell M., Langtimm C., Pfaller M., Hicks J., Rao T. V. Multiple Candida strains in the course of a single systemic infection. J Clin Microbiol. 1988 Aug;26(8):1448–1459. doi: 10.1128/jcm.26.8.1448-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R. The regulation of cellular differentiation in the dimorphic yeast Candida albicans. Bioessays. 1986 Jul;5(1):5–11. doi: 10.1002/bies.950050103. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staib F. Serum-proteins as nitrogen source for yeastlike fungi. Sabouraudia. 1965 Oct;4(3):187–193. doi: 10.1080/00362176685190421. [DOI] [PubMed] [Google Scholar]
- Tang J., Wong R. N. Evolution in the structure and function of aspartic proteases. J Cell Biochem. 1987 Jan;33(1):53–63. doi: 10.1002/jcb.240330106. [DOI] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W. Resolution of DNA molecules greater than 5 megabases by contour-clamped homogeneous electric fields. Nucleic Acids Res. 1987 Oct 12;15(19):7865–7876. doi: 10.1093/nar/15.19.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]