Abstract
The mechanisms that regulate the expression of the H chain of the iron storage protein ferritin in Friend erythroleukemia cells (FLCs) after exposure to hemin (ferric protoporphyrin IX), protoporphyrin IX, and ferric ammonium citrate (FAC) have been investigated. Administration of hemin increases the steady-state level of ferritin mRNA about 10-fold and that of ferritin protein expression 20-fold. Experiments with the transcriptional inhibitor actinomycin D and transfection studies demonstrate that the increment in cytoplasmic mRNA content results from enhanced transcription of the ferritin H-chain gene and cannot be attributed to stabilization of preexisting mRNAs. In addition to transcriptional effects, translational regulation induces the recruitment of stored mRNAs into functional polyribosomes after hemin and FAC administration, resulting in a further increase in ferritin synthesis. Administration of protoporphyrin IX to FLCs produces divergent transcriptional and translational effects. It increases transcription but appears to suppress ferritin mRNA translation. FAC treatment increases the mRNA content slightly (about twofold), and the ferritin levels rise about fivefold over the control values. We conclude that in FLCs, hemin induces ferritin H-chain biosynthesis by multiple mechanisms: a transcriptional mechanism exerted also by protoporphyrin IX and a translational one, not displayed by protoporphyrin IX but shared with FAC.
Full text
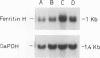
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986 Jan 24;14(2):915–927. doi: 10.1093/nar/14.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistini A., Coccia E. M., Marziali G., Bulgarini D., Scalzo S., Fiorucci G., Romeo G., Affabris E., Testa U., Rossi G. B. Intracellular heme coordinately modulates globin chain synthesis, transferrin receptor number, and ferritin content in differentiating Friend erythroleukemia cells. Blood. 1991 Oct 15;78(8):2098–2103. [PubMed] [Google Scholar]
- Battistini A., Marziali G., Albertini R., Habetswallner D., Bulgarini D., Coccia E. M., Fiorucci G., Romeo G., Orsatti R., Testa U. Positive modulation of hemoglobin, heme, and transferrin receptor synthesis by murine interferon-alpha and -beta in differentiating Friend cells. Pivotal role of heme synthesis. J Biol Chem. 1991 Jan 5;266(1):528–535. [PubMed] [Google Scholar]
- Beaumont C., Dugast I., Renaudie F., Souroujon M., Grandchamp B. Transcriptional regulation of ferritin H and L subunits in adult erythroid and liver cells from the mouse. Unambiguous identification of mouse ferritin subunits and in vitro formation of the ferritin shells. J Biol Chem. 1989 May 5;264(13):7498–7504. [PubMed] [Google Scholar]
- Beaumont C., Jain S. K., Bogard M., Nordmann Y., Drysdale J. Ferritin synthesis in differentiating Friend erythroleukemic cells. J Biol Chem. 1987 Aug 5;262(22):10619–10623. [PubMed] [Google Scholar]
- Boyd D., Vecoli C., Belcher D. M., Jain S. K., Drysdale J. W. Structural and functional relationships of human ferritin H and L chains deduced from cDNA clones. J Biol Chem. 1985 Sep 25;260(21):11755–11761. [PubMed] [Google Scholar]
- Cairo G., Bardella L., Schiaffonati L., Arosio P., Levi S., Bernelli-Zazzera A. Multiple mechanisms of iron-induced ferritin synthesis in HeLa cells. Biochem Biophys Res Commun. 1985 Nov 27;133(1):314–321. doi: 10.1016/0006-291x(85)91877-7. [DOI] [PubMed] [Google Scholar]
- Chen J. J., London I. M. Hemin enhances the differentiation of mouse 3T3 cells to adipocytes. Cell. 1981 Oct;26(1 Pt 1):117–122. doi: 10.1016/0092-8674(81)90039-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cox T. C., Bawden M. J., Martin A., May B. K. Human erythroid 5-aminolevulinate synthase: promoter analysis and identification of an iron-responsive element in the mRNA. EMBO J. 1991 Jul;10(7):1891–1902. doi: 10.1002/j.1460-2075.1991.tb07715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar T., Stripecke R., Gray N. K., Goossen B., Constable A., Johansson H. E., Hentze M. W. Identification of a novel iron-responsive element in murine and human erythroid delta-aminolevulinic acid synthase mRNA. EMBO J. 1991 Jul;10(7):1903–1909. doi: 10.1002/j.1460-2075.1991.tb07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. L., Richter G. W. Isoferritins in rat Kupffer cells, hepatocytes, and extrahepatic macrophages. Biosynthesis in cell suspensions and cultures in response to iron. Lab Invest. 1981 Dec;45(6):567–574. [PubMed] [Google Scholar]
- Drysdale J. W. Human ferritin gene expression. Prog Nucleic Acid Res Mol Biol. 1988;35:127–172. doi: 10.1016/s0079-6603(08)60612-1. [DOI] [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- Eisenstein R. S., Garcia-Mayol D., Pettingell W., Munro H. N. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):688–692. doi: 10.1073/pnas.88.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossen B., Caughman S. W., Harford J. B., Klausner R. D., Hentze M. W. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990 Dec;9(12):4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso J. A., Hillis T. J., Mooney-Frank J. A. Ferritin is not a required intermediate for iron utilization in heme synthesis. Biochim Biophys Acta. 1984 Feb 14;797(2):247–255. doi: 10.1016/0304-4165(84)90128-4. [DOI] [PubMed] [Google Scholar]
- Greiser-Wilke I., Ostertag W., Goldfarb P., Lang A., Furusawa M., Conscience J. F. Inducibility of spleen focus-forming virus by BrdUrd is controlled by the differentiated state of the cell. Proc Natl Acad Sci U S A. 1981 May;78(5):2995–2999. doi: 10.1073/pnas.78.5.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. M., Treffry A., Lilley T. H. Ferritin as an iron-storage protein: mechanisms of iron uptake. J Inorg Biochem. 1986 Aug;27(4):287–293. doi: 10.1016/0162-0134(86)80068-x. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Casey J. L., Koeller D. M., Rouault T. A., Harford J. B., Klausner R. D. A model for the structure and functions of iron-responsive elements. Gene. 1988 Dec 10;72(1-2):201–208. doi: 10.1016/0378-1119(88)90145-x. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., Klausner R. D. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec 11;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Keim S., Papadopoulos P., O'Brien S., Modi W., Drysdale J., Leonard W. J., Harford J. B., Klausner R. D. Cloning, characterization, expression, and chromosomal localization of a human ferritin heavy-chain gene. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7226–7230. doi: 10.1073/pnas.83.19.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Caughman S. W., Dancis A., Harford J. B., Klausner R. D. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagus R. Characterization of in vitro translation products. Methods Enzymol. 1987;152:296–304. doi: 10.1016/0076-6879(87)52034-1. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Daniels-McQueen S., Patino M. M., Gaffield L., Walden W. E., Thach R. E. Derepression of ferritin messenger RNA translation by hemin in vitro. Science. 1990 Jan 5;247(4938):74–77. doi: 10.1126/science.2294594. [DOI] [PubMed] [Google Scholar]
- Louache F., Testa U., Pelicci P., Thomopoulos P., Titeux M., Rochant H. Regulation of transferrin receptors in human hematopoietic cell lines. J Biol Chem. 1984 Sep 25;259(18):11576–11582. [PubMed] [Google Scholar]
- Mattia E., Josic D., Ashwell G., Klausner R., van Renswoude J. Regulation of intracellular iron distribution in K562 human erythroleukemia cells. J Biol Chem. 1986 Apr 5;261(10):4587–4593. [PubMed] [Google Scholar]
- Mattia E., den Blaauwen J., Ashwell G., van Renswoude J. Multiple post-transcriptional regulatory mechanisms in ferritin gene expression. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1801–1805. doi: 10.1073/pnas.86.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattia E., den Blaauwen J., van Renswoude J. Role of protein synthesis in the accumulation of ferritin mRNA during exposure of cells to iron. Biochem J. 1990 Apr 15;267(2):553–555. doi: 10.1042/bj2670553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi E., Testa U., Louache F., Thomopoulos P., Salvo G., Samoggia P., Peschle C. Expression of transferrin receptors in phytohemagglutinin-stimulated human T-lymphocytes. Evidence for a three-step model. J Biol Chem. 1986 Mar 5;261(7):3036–3042. [PubMed] [Google Scholar]
- Reuben R. C., Rifkind R. A., Marks P. A. Chemically induced murine erythroleukemic differentiation. Biochim Biophys Acta. 1980 Sep 22;605(3):325–346. doi: 10.1016/0304-419x(80)90015-3. [DOI] [PubMed] [Google Scholar]
- Rhyner K., Taetle R., Bering H., To D. Transferrin receptor regulation is coupled to intracellular ferritin in proliferating and differentiating HL60 leukemia cells. J Cell Physiol. 1985 Dec;125(3):608–612. doi: 10.1002/jcp.1041250333. [DOI] [PubMed] [Google Scholar]
- Rittling S. R., Woodworth R. C. The synthesis and turnover of ferritin in rat L-6 cells. Rates and response to iron, actinomycin D, and desferrioxamine. J Biol Chem. 1984 May 10;259(9):5561–5566. [PubMed] [Google Scholar]
- Rogers J., Munro H. Translation of ferritin light and heavy subunit mRNAs is regulated by intracellular chelatable iron levels in rat hepatoma cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2277–2281. doi: 10.1073/pnas.84.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Dancis A., Caughman W., Harford J. B., Klausner R. D. Influence of altered transcription on the translational control of human ferritin expression. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6335–6339. doi: 10.1073/pnas.84.18.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S. Sequential induction of heme pathway enzymes during erythroid differentiation of mouse Friend leukemia virus-infected cells. J Exp Med. 1976 Feb 1;143(2):305–315. doi: 10.1084/jem.143.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theil E. C. Ferritin: structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- Wagstaff M., Worwood M., Jacobs A. Properties of human tissue isoferritins. Biochem J. 1978 Sep 1;173(3):969–977. doi: 10.1042/bj1730969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden W. E., Daniels-McQueen S., Brown P. H., Gaffield L., Russell D. A., Bielser D., Bailey L. C., Thach R. E. Translational repression in eukaryotes: partial purification and characterization of a repressor of ferritin mRNA translation. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9503–9507. doi: 10.1073/pnas.85.24.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Transferrin receptors of human fibroblasts. Analysis of receptor properties and regulation. Biochem J. 1982 Oct 15;208(1):19–26. doi: 10.1042/bj2080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. P., Bailey-Wood R., Jacobs A. The effect of chelating agents on cellular iron metabolism. Clin Sci Mol Med. 1976 Mar;50(3):145–152. doi: 10.1042/cs0500145. [DOI] [PubMed] [Google Scholar]
- White K., Munro H. N. Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem. 1988 Jun 25;263(18):8938–8942. [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]