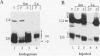Abstract
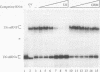
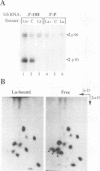
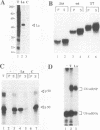
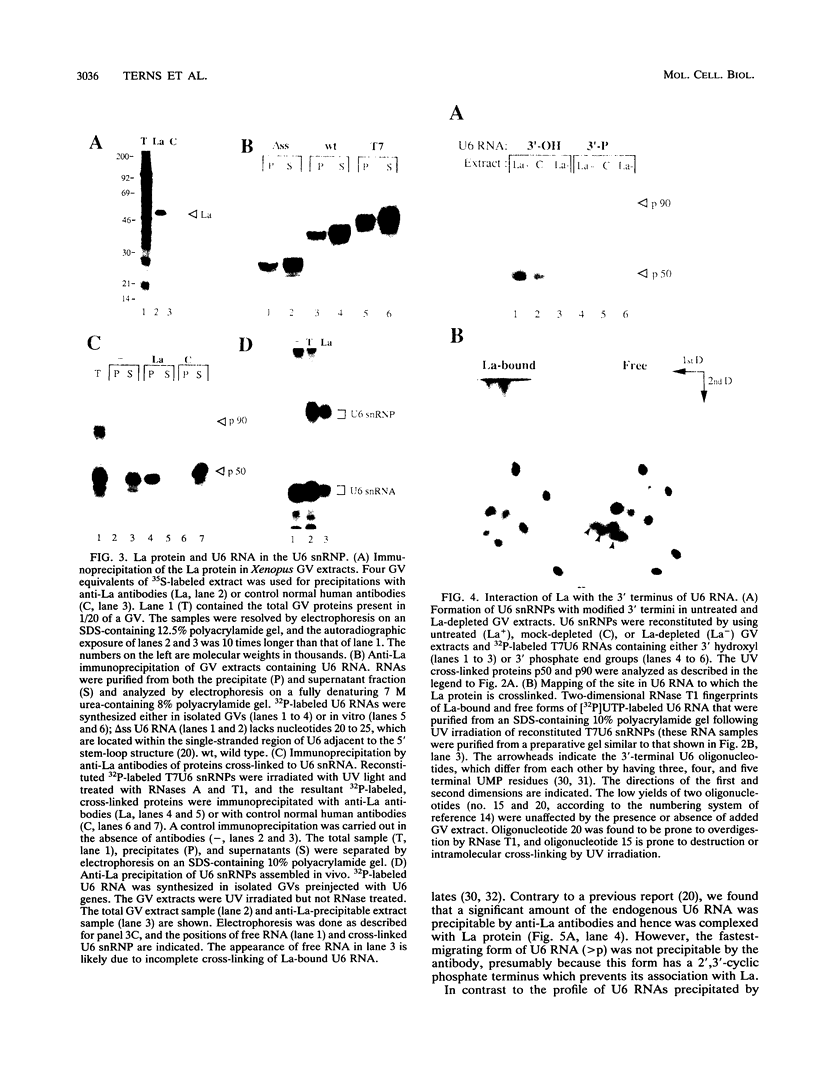
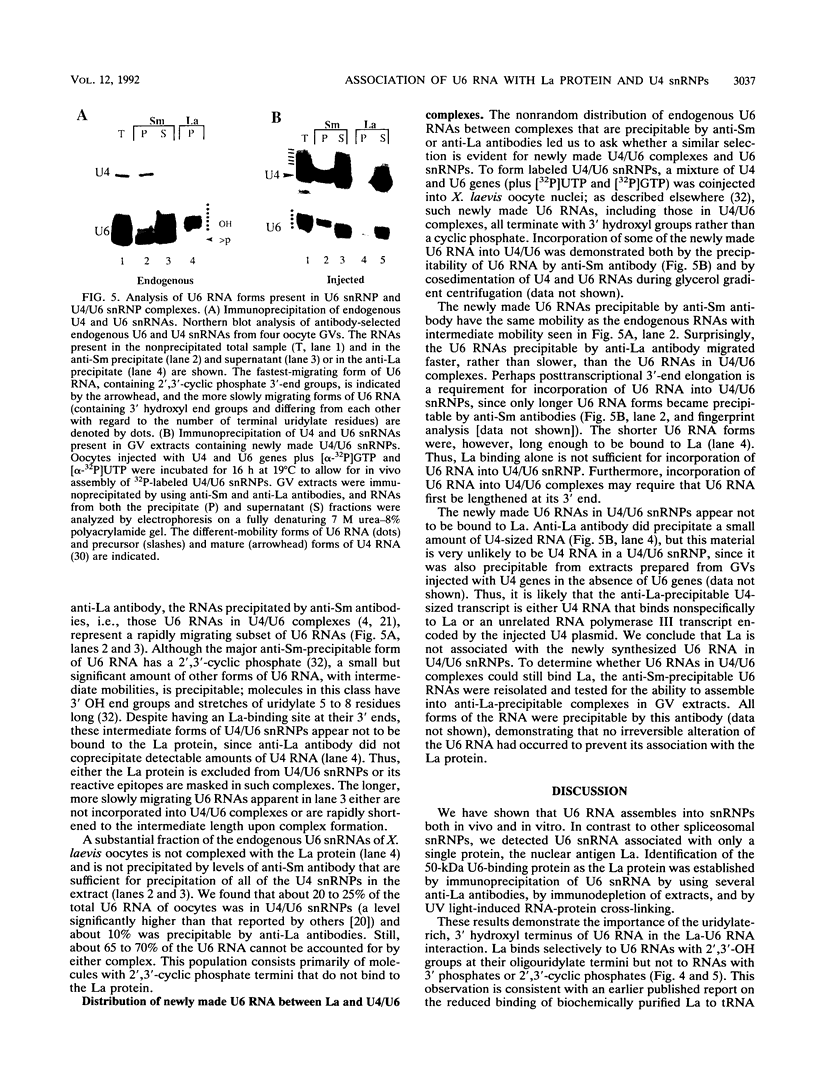
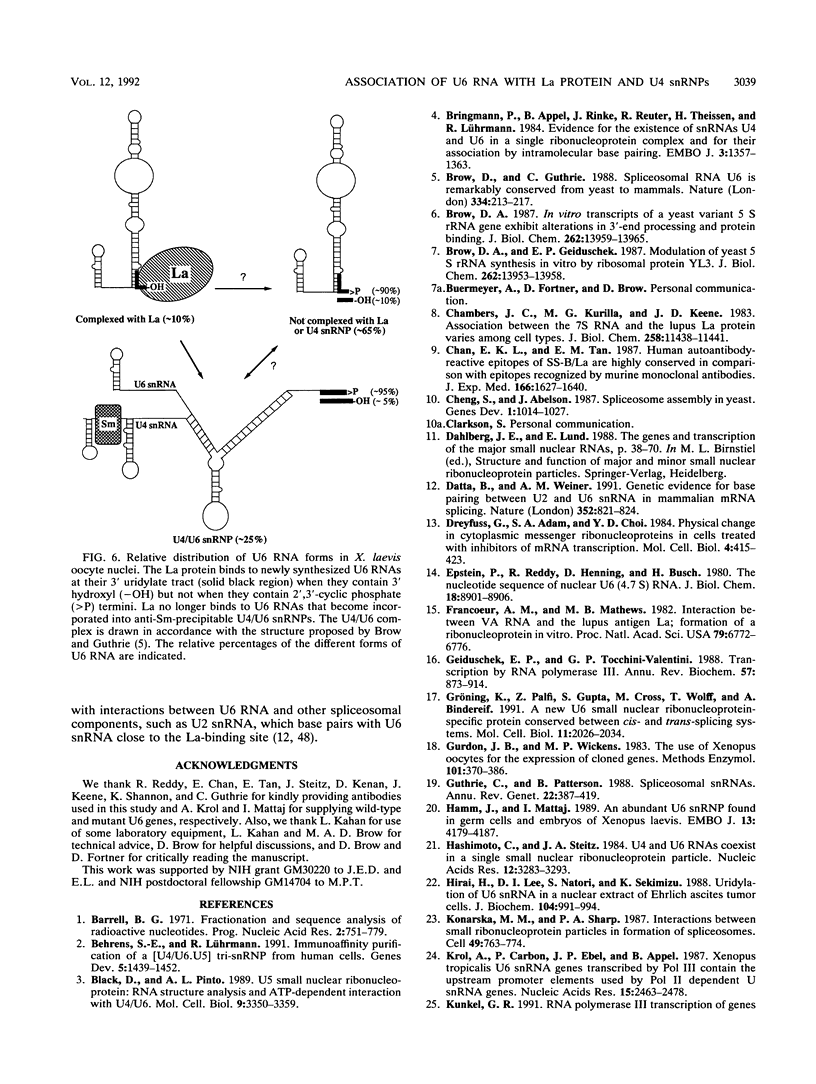
We have identified and characterized a U6 small nuclear (sn) ribonucleoprotein particle (RNP) present in the nuclei of Xenopus laevis oocytes. The structure of this U6 snRNP was investigated by native gel shift analysis and a combination of RNA-protein UV cross-linking, RNase T1 fingerprinting, and immunoprecipitation assays. These analyses demonstrate that certain forms of U6 snRNA associate with the 50-kDa nuclear antigen La both in vivo and in vitro. The La protein binds the stretch of uridylates at the 3' hydroxyl end of newly synthesized U6 snRNA. La does not bind to mature U6 snRNAs that have 2',3'-cyclic phosphate (greater than p) groups at their 3' ends (E. Lund and J. E. Dahlberg, Science 255:327-330, 1992) or to U6 snRNAs in anti-Sm-precipitable U4/U6 snRNPs. We propose that 3'-end modification, including posttranscriptional UMP addition, modulates the binding of La protein to U6 snRNA which, in turn, may affect the function of this RNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens S. E., Lührmann R. Immunoaffinity purification of a [U4/U6.U5] tri-snRNP from human cells. Genes Dev. 1991 Aug;5(8):1439–1452. doi: 10.1101/gad.5.8.1439. [DOI] [PubMed] [Google Scholar]
- Black D. L., Pinto A. L. U5 small nuclear ribonucleoprotein: RNA structure analysis and ATP-dependent interaction with U4/U6. Mol Cell Biol. 1989 Aug;9(8):3350–3359. doi: 10.1128/mcb.9.8.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann P., Appel B., Rinke J., Reuter R., Theissen H., Lührmann R. Evidence for the existence of snRNAs U4 and U6 in a single ribonucleoprotein complex and for their association by intermolecular base pairing. EMBO J. 1984 Jun;3(6):1357–1363. doi: 10.1002/j.1460-2075.1984.tb01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brow D. A., Geiduschek E. P. Modulation of yeast 5 S rRNA synthesis in vitro by ribosomal protein YL3. A possible regulatory loop. J Biol Chem. 1987 Oct 15;262(29):13953–13958. [PubMed] [Google Scholar]
- Brow D. A., Guthrie C. Spliceosomal RNA U6 is remarkably conserved from yeast to mammals. Nature. 1988 Jul 21;334(6179):213–218. doi: 10.1038/334213a0. [DOI] [PubMed] [Google Scholar]
- Brow D. A. In vitro transcripts of a yeast variant 5 S rRNA gene exhibit alterations in 3'-end processing and protein binding. J Biol Chem. 1987 Oct 15;262(29):13959–13965. [PubMed] [Google Scholar]
- Chambers J. C., Kurilla M. G., Keene J. D. Association between the 7 S RNA and the lupus La protein varies among cell types. J Biol Chem. 1983 Oct 10;258(19):11438–11441. [PubMed] [Google Scholar]
- Chan E. K., Tan E. M. Human autoantibody-reactive epitopes of SS-B/La are highly conserved in comparison with epitopes recognized by murine monoclonal antibodies. J Exp Med. 1987 Dec 1;166(6):1627–1640. doi: 10.1084/jem.166.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. C., Abelson J. Spliceosome assembly in yeast. Genes Dev. 1987 Nov;1(9):1014–1027. doi: 10.1101/gad.1.9.1014. [DOI] [PubMed] [Google Scholar]
- Datta B., Weiner A. M. Genetic evidence for base pairing between U2 and U6 snRNA in mammalian mRNA splicing. Nature. 1991 Aug 29;352(6338):821–824. doi: 10.1038/352821a0. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Adam S. A., Choi Y. D. Physical change in cytoplasmic messenger ribonucleoproteins in cells treated with inhibitors of mRNA transcription. Mol Cell Biol. 1984 Mar;4(3):415–423. doi: 10.1128/mcb.4.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P., Reddy R., Henning D., Busch H. The nucleotide sequence of nuclear U6 (4.7 S) RNA. J Biol Chem. 1980 Sep 25;255(18):8901–8906. [PubMed] [Google Scholar]
- Francoeur A. M., Mathews M. B. Interaction between VA RNA and the lupus antigen La: formation of a ribonucleoprotein particle in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6772–6776. doi: 10.1073/pnas.79.22.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E. P., Tocchini-Valentini G. P. Transcription by RNA polymerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- Gröning K., Palfi Z., Gupta S., Cross M., Wolff T., Bindereif A. A new U6 small nuclear ribonucleoprotein-specific protein conserved between cis- and trans-splicing systems. Mol Cell Biol. 1991 Apr;11(4):2026–2034. doi: 10.1128/mcb.11.4.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Wickens M. P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzymol. 1983;101:370–386. doi: 10.1016/0076-6879(83)01028-9. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Patterson B. Spliceosomal snRNAs. Annu Rev Genet. 1988;22:387–419. doi: 10.1146/annurev.ge.22.120188.002131. [DOI] [PubMed] [Google Scholar]
- Hamm J., Mattaj I. W. An abundant U6 snRNP found in germ cells and embryos of Xenopus laevis. EMBO J. 1989 Dec 20;8(13):4179–4187. doi: 10.1002/j.1460-2075.1989.tb08603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C., Steitz J. A. U4 and U6 RNAs coexist in a single small nuclear ribonucleoprotein particle. Nucleic Acids Res. 1984 Apr 11;12(7):3283–3293. doi: 10.1093/nar/12.7.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H., Lee D. I., Natori S., Sekimizu K. Uridylation of U6 RNA in a nuclear extract in Ehrlich ascites tumor cells. J Biochem. 1988 Dec;104(6):991–994. doi: 10.1093/oxfordjournals.jbchem.a122597. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987 Jun 19;49(6):763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- Krol A., Carbon P., Ebel J. P., Appel B. Xenopus tropicalis U6 snRNA genes transcribed by Pol III contain the upstream promoter elements used by Pol II dependent U snRNA genes. Nucleic Acids Res. 1987 Mar 25;15(6):2463–2478. doi: 10.1093/nar/15.6.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner E. A., Lerner M. R., Janeway C. A., Jr, Steitz J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc Natl Acad Sci U S A. 1981 May;78(5):2737–2741. doi: 10.1073/pnas.78.5.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Cyclic 2',3'-phosphates and nontemplated nucleotides at the 3' end of spliceosomal U6 small nuclear RNA's. Science. 1992 Jan 17;255(5042):327–330. doi: 10.1126/science.1549778. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. Differential accumulation of U1 and U4 small nuclear RNAs during Xenopus development. Genes Dev. 1987 Mar;1(1):39–46. doi: 10.1101/gad.1.1.39. [DOI] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. In vitro synthesis of vertebrate U1 snRNA. EMBO J. 1989 Jan;8(1):287–292. doi: 10.1002/j.1460-2075.1989.tb03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Paine P. L. Nonaqueous isolation of transcriptionally active nuclei from Xenopus oocytes. Methods Enzymol. 1990;181:36–43. doi: 10.1016/0076-6879(90)81110-g. [DOI] [PubMed] [Google Scholar]
- Lührmann R., Kastner B., Bach M. Structure of spliceosomal snRNPs and their role in pre-mRNA splicing. Biochim Biophys Acta. 1990 Nov 30;1087(3):265–292. doi: 10.1016/0167-4781(90)90001-i. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Francoeur A. M. La antigen recognizes and binds to the 3'-oligouridylate tail of a small RNA. Mol Cell Biol. 1984 Jun;4(6):1134–1140. doi: 10.1128/mcb.4.6.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., Slobbe R. L., van Venrooij W. J. Analysis of protein--RNA interactions within Ro ribonucleoprotein complexes. Nucleic Acids Res. 1991 Oct 11;19(19):5173–5180. doi: 10.1093/nar/19.19.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Henning D., Das G., Harless M., Wright D. The capped U6 small nuclear RNA is transcribed by RNA polymerase III. J Biol Chem. 1987 Jan 5;262(1):75–81. [PubMed] [Google Scholar]
- Reddy R., Henning D., Tan E., Busch H. Identification of a La protein binding site in a RNA polymerase III transcript (4.5 I RNA). J Biol Chem. 1983 Jul 10;258(13):8352–8356. [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Association of the lupus antigen La with a subset of U6 snRNA molecules. Nucleic Acids Res. 1985 Apr 11;13(7):2617–2629. doi: 10.1093/nar/13.7.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke J., Steitz J. A. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982 May;29(1):149–159. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Shannon K. W., Guthrie C. Suppressors of a U4 snRNA mutation define a novel U6 snRNP protein with RNA-binding motifs. Genes Dev. 1991 May;5(5):773–785. doi: 10.1101/gad.5.5.773. [DOI] [PubMed] [Google Scholar]
- Singh R., Gupta S., Reddy R. Capping of mammalian U6 small nuclear RNA in vitro is directed by a conserved stem-loop and AUAUAC sequence: conversion of a noncapped RNA into a capped RNA. Mol Cell Biol. 1990 Mar;10(3):939–946. doi: 10.1128/mcb.10.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Terns M. P., Jacob S. T. Potential role of poly(A) polymerase in the assembly of polyadenylation-specific RNP complexes. Nucleic Acids Res. 1991 Jan 25;19(2):343–351. doi: 10.1093/nar/19.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankan P., McGuigan C., Mattaj I. W. Domains of U4 and U6 snRNAs required for snRNP assembly and splicing complementation in Xenopus oocytes. EMBO J. 1990 Oct;9(10):3397–3404. doi: 10.1002/j.1460-2075.1990.tb07541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. A., Manley J. L. Base pairing between U2 and U6 snRNAs is necessary for splicing of a mammalian pre-mRNA. Nature. 1991 Aug 29;352(6338):818–821. doi: 10.1038/352818a0. [DOI] [PubMed] [Google Scholar]