Abstract
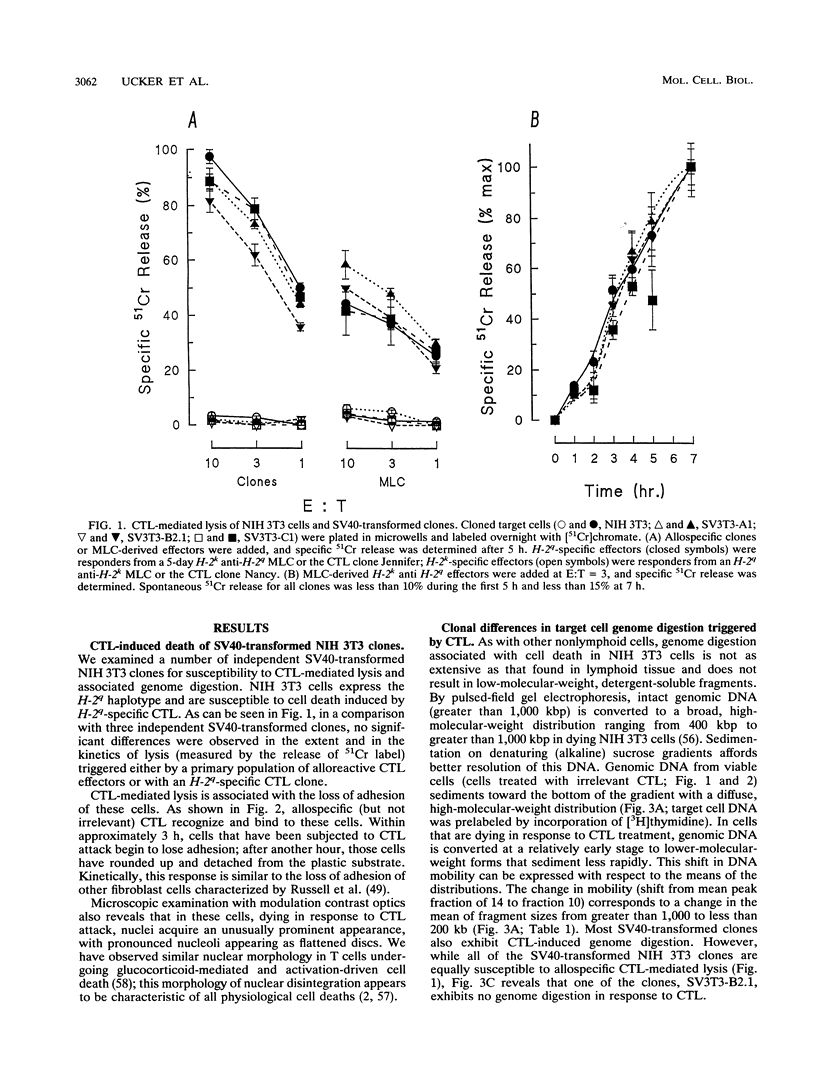
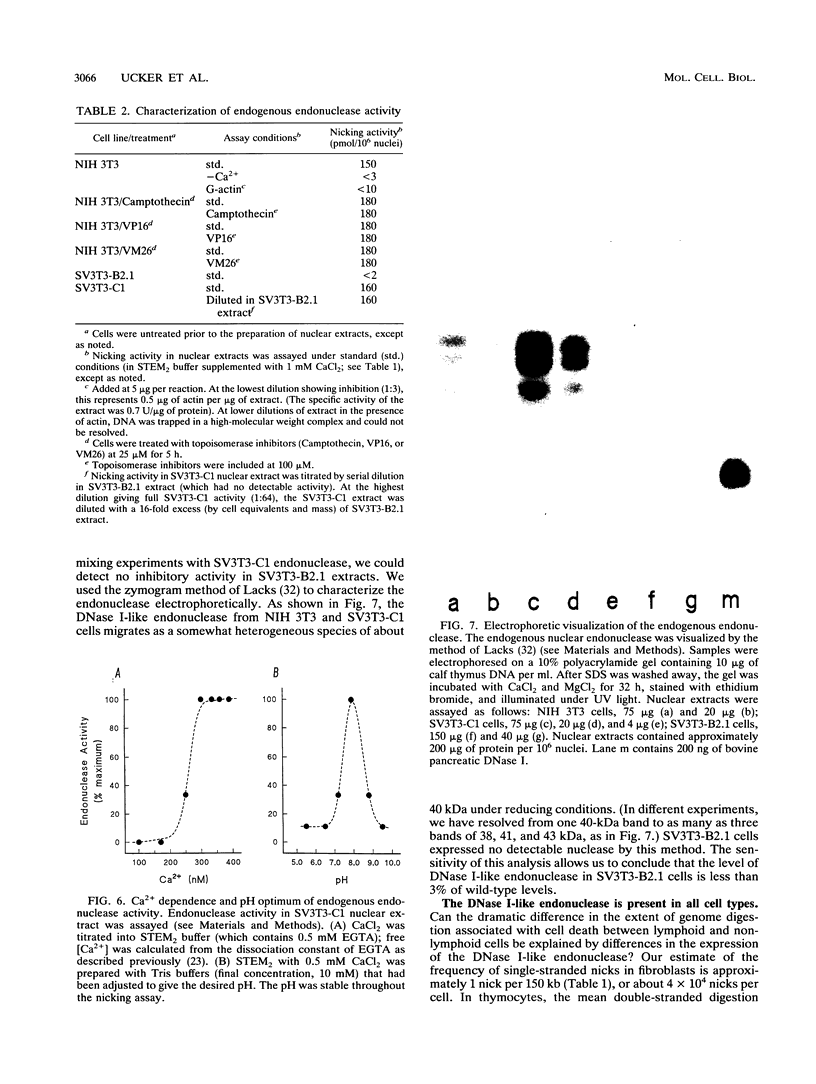
We examined virally transformed murine fibroblast clones as targets for cytotoxic T lymphocyte (CTL)-triggered lysis and genome digestion. Strikingly, while all clones were essentially equivalent in the ability to be lysed, one clone, SV3T3-B2.1, failed to exhibit genome digestion associated with CTL attack. Other aspects of the physiological cell death process, including loss of adhesion and nuclear envelope breakdown (lamin phosphorylation and solubilization), were not altered in this clone. The absence of genome digestion associated with CTL-induced cell death correlated with the absence of endodeoxyribonuclease activity in the nuclei of that clone. Characterization of the activity affected identifies a calcium-dependent, DNase I-like endonuclease of approximately 40 kDa, normally present constitutively in all cell nuclei, as the enzyme responsible for genome digestion associated with CTL-mediated cell death. These observations indicate that neither genome digestion per se nor its consequences [such as activation of poly(ADP-ribose) polymerase] are essential for cell death resulting from the triggering of this cell suicide process.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnemri E. S., Litwack G. Glucocorticoid-induced lymphocytolysis is not mediated by an induced endonuclease. J Biol Chem. 1989 Mar 5;264(7):4104–4111. [PubMed] [Google Scholar]
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Bachvaroff R. J., Ayvazian J. H., Skupp S., Rapaport F. T. Specific restriction endonuclease degradation of DNA as a consequence of immunologically mediated cell damage. Transplant Proc. 1977 Mar;9(1):807–812. [PubMed] [Google Scholar]
- Berger N. A. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985 Jan;101(1):4–15. [PubMed] [Google Scholar]
- Berger N. A., Sikorski G. W., Petzold S. J., Kurohara K. K. Association of poly(adenosine diphosphoribose) synthesis with DNA damage and repair in normal human lymphocytes. J Clin Invest. 1979 Jun;63(6):1164–1171. doi: 10.1172/JCI109410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Campbell V. W., Jackson D. A. The effect of divalent cations on the mode of action of DNase I. The initial reaction products produced from covalently closed circular DNA. J Biol Chem. 1980 Apr 25;255(8):3726–3735. [PubMed] [Google Scholar]
- Campisi J., Morreo G., Pardee A. B. Kinetics of G1 transit following brief starvation for serum factors. Exp Cell Res. 1984 Jun;152(2):459–466. doi: 10.1016/0014-4827(84)90647-5. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Seto S., Wasson D. B., Carrera C. J. DNA strand breaks, NAD metabolism, and programmed cell death. Exp Cell Res. 1986 Jun;164(2):273–281. doi: 10.1016/0014-4827(86)90028-5. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Compton M. M., Cidlowski J. A. Identification of a glucocorticoid-induced nuclease in thymocytes. A potential "lysis gene" product. J Biol Chem. 1987 Jun 15;262(17):8288–8292. [PubMed] [Google Scholar]
- Dealtry G. B., Naylor M. S., Fiers W., Balkwill F. R. DNA fragmentation and cytotoxicity caused by tumor necrosis factor is enhanced by interferon-gamma. Eur J Immunol. 1987 May;17(5):689–693. doi: 10.1002/eji.1830170517. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke R. C., Cohen J. J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986 Fall;5(4):289–299. [PubMed] [Google Scholar]
- Duke R. C., Persechini P. M., Chang S., Liu C. C., Cohen J. J., Young J. D. Purified perforin induces target cell lysis but not DNA fragmentation. J Exp Med. 1989 Oct 1;170(4):1451–1456. doi: 10.1084/jem.170.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan B., Cooke C. A., Heck M. M., Liu L. F. Topoisomerase II is a structural component of mitotic chromosome scaffolds. J Cell Biol. 1985 May;100(5):1706–1715. doi: 10.1083/jcb.100.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flieger D., Riethmüller G., Ziegler-Heitbrock H. W. Zn++ inhibits both tumor necrosis factor-mediated DNA fragmentation and cytolysis. Int J Cancer. 1989 Aug 15;44(2):315–319. doi: 10.1002/ijc.2910440221. [DOI] [PubMed] [Google Scholar]
- Gaido M. L., Cidlowski J. A. Identification, purification, and characterization of a calcium-dependent endonuclease (NUC18) from apoptotic rat thymocytes. NUC18 is not histone H2B. J Biol Chem. 1991 Oct 5;266(28):18580–18585. [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gray L. S., Gnarra J. R., Russell J. H., Engelhard V. H. The role of K+ in the regulation of the increase in intracellular Ca2+ mediated by the T lymphocyte antigen receptor. Cell. 1987 Jul 3;50(1):119–127. doi: 10.1016/0092-8674(87)90668-4. [DOI] [PubMed] [Google Scholar]
- Gromkowski S. H., Brown T. C., Cerutti P. A., Cerottini J. C. DNA of human Raji target cells is damaged upon lymphocyte-mediated lysis. J Immunol. 1986 Feb 1;136(3):752–756. [PubMed] [Google Scholar]
- Gromkowski S. H., Brown T. C., Masson D., Tschopp J. Lack of DNA degradation in target cells lysed by granules derived from cytolytic T lymphocytes. J Immunol. 1988 Aug 1;141(3):774–778. [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hedgecock E. M., Sulston J. E., Thomson J. N. Mutations affecting programmed cell deaths in the nematode Caenorhabditis elegans. Science. 1983 Jun 17;220(4603):1277–1279. doi: 10.1126/science.6857247. [DOI] [PubMed] [Google Scholar]
- Hevelone J., Hartman P. S. An endonuclease from Caenorhabditis elegans: partial purification and characterization. Biochem Genet. 1988 Aug;26(7-8):447–461. doi: 10.1007/BF02399412. [DOI] [PubMed] [Google Scholar]
- Hinshaw D. B., Armstrong B. C., Burger J. M., Beals T. F., Hyslop P. A. ATP and microfilaments in cellular oxidant injury. Am J Pathol. 1988 Sep;132(3):479–488. [PMC free article] [PubMed] [Google Scholar]
- Howell D. M., Martz E. Intracellular reovirus survives cytotoxic T lymphocyte-mediated lysis of its host cell. J Gen Virol. 1987 Nov;68(Pt 11):2899–2907. doi: 10.1099/0022-1317-68-11-2899. [DOI] [PubMed] [Google Scholar]
- Howell D. M., Martz E. Nuclear disintegration induced by cytotoxic T lymphocytes. Evidence against damage to the nuclear envelope of the target cell. J Immunol. 1988 Feb 1;140(3):689–692. [PubMed] [Google Scholar]
- Howell D. M., Martz E. The degree of CTL-induced DNA solubilization is not determined by the human vs mouse origin of the target cell. J Immunol. 1987 Jun 1;138(11):3695–3698. [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Isaacs J. T. Activation of a Ca2+-Mg2+-dependent endonuclease as an early event in castration-induced prostatic cell death. Prostate. 1988;13(2):103–117. doi: 10.1002/pros.2990130203. [DOI] [PubMed] [Google Scholar]
- Lacks S. A. Deoxyribonuclease I in mammalian tissues. Specificity of inhibition by actin. J Biol Chem. 1981 Mar 25;256(6):2644–2648. [PubMed] [Google Scholar]
- Landon C., Nowicki M., Sugawara S., Dennert G. Differential effects of protein synthesis inhibition on CTL and targets in cell-mediated cytotoxicity. Cell Immunol. 1990 Jul;128(2):412–426. doi: 10.1016/0008-8749(90)90037-r. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopardi E., Friend D. S., Rosenau W. Target cell lysis: ultrastructural and cytoskeletal alterations. J Immunol. 1984 Dec;133(6):3429–3436. [PubMed] [Google Scholar]
- Lewis C. D., Laemmli U. K. Higher order metaphase chromosome structure: evidence for metalloprotein interactions. Cell. 1982 May;29(1):171–181. doi: 10.1016/0092-8674(82)90101-5. [DOI] [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Identification of a factor in conventional muscle actin preparations which inhibits actin filament self-association. Biochem Biophys Res Commun. 1980 Sep 16;96(1):18–27. doi: 10.1016/0006-291x(80)91175-4. [DOI] [PubMed] [Google Scholar]
- Martz E., Howell D. M. CTL: virus control cells first and cytolytic cells second? DNA fragmentation, apoptosis and the prelytic halt hypothesis. Immunol Today. 1989 Mar;10(3):79–86. doi: 10.1016/0167-5699(89)90231-4. [DOI] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation kills immature thymocytes. FASEB J. 1989 May;3(7):1843–1849. doi: 10.1096/fasebj.3.7.2497041. [DOI] [PubMed] [Google Scholar]
- Millard P. J., Henkart M. P., Reynolds C. W., Henkart P. A. Purification and properties of cytoplasmic granules from cytotoxic rat LGL tumors. J Immunol. 1984 Jun;132(6):3197–3204. [PubMed] [Google Scholar]
- Nicholson M. L., Young D. A. Effect of glucocorticoid hormones in vitro on the structural integrity of nuclei in corticosteroid-sensitive and -resistant lines of lymphosarcoma P1798. Cancer Res. 1978 Nov;38(11 Pt 1):3673–3680. [PubMed] [Google Scholar]
- Peter M., Nakagawa J., Dorée M., Labbé J. C., Nigg E. A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990 May 18;61(4):591–602. doi: 10.1016/0092-8674(90)90471-p. [DOI] [PubMed] [Google Scholar]
- Poenie M., Tsien R. Y., Schmitt-Verhulst A. M. Sequential activation and lethal hit measured by [Ca2+]i in individual cytolytic T cells and targets. EMBO J. 1987 Aug;6(8):2223–2232. doi: 10.1002/j.1460-2075.1987.tb02494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Polzar B., Mannherz H. G. Nucleotide sequence of a full length cDNA clone encoding the deoxyribonuclease I from the rat parotid gland. Nucleic Acids Res. 1990 Dec 11;18(23):7151–7151. doi: 10.1093/nar/18.23.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzar B., Nowak E., Goody R. S., Mannherz H. G. The complex of actin and deoxyribonuclease I as a model system to study the interactions of nucleotides, cations and cytochalasin D with monomeric actin. Eur J Biochem. 1989 Jun 15;182(2):267–275. doi: 10.1111/j.1432-1033.1989.tb14826.x. [DOI] [PubMed] [Google Scholar]
- Russell J. H. Internal disintegration model of cytotoxic lymphocyte-induced target damage. Immunol Rev. 1983;72:97–118. doi: 10.1111/j.1600-065x.1983.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Russell J. H., Masakowski V., Rucinsky T., Phillips G. Mechanisms of immune lysis. III. Characterization of the nature and kinetics of the cytotoxic T lymphocyte-induced nuclear lesion in the target. J Immunol. 1982 May;128(5):2087–2094. [PubMed] [Google Scholar]
- Russell J. H., Musil L., McCulley D. E. Loss of adhesion. A novel and distinct effect of the cytotoxic T lymphocyte-target interaction. J Immunol. 1988 Jan 15;140(2):427–432. [PubMed] [Google Scholar]
- Scanlon M., Laster S. M., Wood J. G., Gooding L. R. Cytolysis by tumor necrosis factor is preceded by a rapid and specific dissolution of microfilaments. Proc Natl Acad Sci U S A. 1989 Jan;86(1):182–186. doi: 10.1073/pnas.86.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hyslop P. A., Hinshaw D. B., Spragg R. G., Sklar L. A., Cochrane C. G. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Polyomavirus DNA is damaged in target cells during cytotoxic T-lymphocyte-mediated killing. J Virol. 1989 Feb;63(2):572–578. doi: 10.1128/jvi.63.2.572-578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley W. U., Baker A. R., Colten H. R. DNA degradation in mammalian cells following complement-mediated cytolysis. J Immunol. 1971 Feb;106(2):576–579. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Swingle K. F., Cole L. J. Radiation-induced free polydeoxyribonucleotides in lymphoid tissues: a product of the action of neutral deoxyribonuclease (DNase 1). Radiat Res. 1967 Jan;30(1):81–95. [PubMed] [Google Scholar]
- Ucker D. S. Death by suicide: one way to go in mammalian cellular development? New Biol. 1991 Feb;3(2):103–109. [PubMed] [Google Scholar]
- Ucker D. S., Yamamoto K. R. Early events in the stimulation of mammary tumor virus RNA synthesis by glucocorticoids. Novel assays of transcription rates. J Biol Chem. 1984 Jun 25;259(12):7416–7420. [PubMed] [Google Scholar]
- Vanderbilt J. N., Bloom K. S., Anderson J. N. Endogenous nuclease. Properties and effects on transcribed genes in chromatin. J Biol Chem. 1982 Nov 10;257(21):13009–13017. [PubMed] [Google Scholar]