Abstract
In light of the growing evidence of altered decision-making in addiction we assessed decision-making styles in drug-dependent individuals using the Melbourne Decision-Making Questionnaire (MDMQ). Consistent with the literature on laboratory tests of decision-making, we found that stimulant users reported less competent and more maladaptive decision-making styles compared with controls.
Keywords: Substance dependence, Procrastination, Self-report
1. Introduction
Drug addiction has long been associated with dysfunction of brain areas involved in higher cognitive and decision-making processes (Goldstein and Volkow, 2002). Neuropsychological studies that have been investigating decision-making abilities in chronic drug users with different experimental paradigms, have provided ample evidence for decision-making deficits in this population (Verdejo-Garcia et al., 2004; Dom et al., 2005). Different cognitive profiles of decision-making deficits in chronic drug users have emerged on some decision-making tests (Rogers et al., 1999; Ersche et al., 2005) but not on others (Clark et al., 2006; Verdejo-Garcia et al., 2007b). The neural networks subserving cognitive function are also implicated in the processes of mental abilities such as insight and self-awareness, which might result in drug users being unaware of their cognitive and decision-making deficits (Goldstein et al., 2009). We used the Melbourne Decision-Making Questionnaire (MDMQ; Mann et al., 1997) to assess chronic drug users’ own perceptions of their adaptive and maladaptive decision-making styles in a non-drug-related context. The MDMQ is based on the decision-making model of Janis and Mann (1977), which characterizes competent decision-making or vigilance as a rational, unbiased search for relevant information, the clarification of one’s goals and the careful appraisal of all alternatives prior to decision-making (e.g. “I try to be clear about my objectives before choosing”). Avoiding decisions usually leads to suboptimal outcomes and therefore is considered as being maladaptive. Avoidance includes either delaying decisions (procrastination, e.g. “I delay making decisions until it is too late”), deferring decisions to others (buck-passing, e.g.“I prefer that people who are better informed decide for me”), or quickly choosing the next available option to escape the discomfort of having to make a decision (hypervigilance, “I feel as if I am under tremendous time pressure when making decisions”). In light of drug users’ poor decision-making performance on laboratory tasks, we hypothesized that they would report less competent and more maladaptive decision-making styles compared with non-drug using healthy controls.
2. Methods
Individuals aged 20 to 56 years who either met the DSM-IV criteria (American Psychiatric Association, 2000) for dependence on opiates (n=40), cocaine, (n=51), or amphetamine dependence (n=38), or had no history of substance misuse disorder and no major psychiatric disorder (n=57). All participants were recruited from the East Anglia region of the United Kingdom; drug users through Drug Dependency Units and by word-of-mouth and controls by advertisements. Drug users were included if they were currently satisfying the DSM-IV criteria for dependence on either stimulants (i.e. amphetamines or cocaine) or on opiate drugs. Controls were included if they were in good mental and physical health and had no personal history of substance abuse. Participants had to be 18-60 years and had to be able to read and write in English at a level sufficient to complete the study-related assessment. Seven drug users did not enrol in the study as inclusion/exclusion criteria were not satisfied. The protocol was approved by the Cambridge Research Ethics Committee and written informed consent was obtained from all volunteers, who received monetary reimbursement for their participation.
Participants were screened for the exclusion criteria of co-morbid psychiatric illness, history of head injury, or neurological disorder by means of locally developed prescreening questionnaires and the Structural Clinical Interview for the DSM-IV (SCID, First et al., 2002). Diagnoses were established by the SCID and verified by medical records, if available. All participants completed the National Adult Reading Test (NART; Nelson, 1982) as an estimate of verbal IQ and the Beck Depression Inventory (BDI-II; Beck et al., 1996) to record depressive mood.
All drug users were actively using their drug of dependence, as reflected by urine analyses; all samples provided by the drug users tested positive for their drug of dependence and all samples provided by the controls were drug negative. The majority of drug users were tobacco smokers (88%) and used cannabis regularly (57%). The use of other drugs in addition to their drug of dependence was common and reasonably balanced across groups. Eleven percent of the controls were current smokers of tobacco and 36% reported recreational cannabis use in the past. Twenty-eight opiate users were enrolled in methadone-maintenance treatment, receiving on average 45 mg methadone daily (S.D.±18 mg). Ten amphetamine users received d-amphetamine on prescription as a means to control harm (average dose 38 mg, S.D.±20 mg). Six drug users (two opiate, one amphetamine, and three cocaine users) used benzodiazepines on a regular basis. Additional prescriptions were gabapentin (one cocaine user) and ibuprofen for the treatment of pain (one amphetamine user).
Decision-making styles were assessed using the MDMQ, which is a validated 22-item self-report instrument to measure patterns for coping in situations of decisional conflict (Mann et al., 1997). It consists of the four subscales: vigilance (alpha coefficient of 0.80), procrastination (alpha coefficient of 0.81), buck-passing (alpha coefficient of 0.87), and hypervigilance (alpha coefficient of 0.74). Data were analyzed using the Statistical Package for the Social Sciences 13 (SPSS Inc.). One-way analysis of variance was used to explore group differences in continuous demographic variables. Due to an unbalanced gender ratio across the groups and significantly higher levels of dysphoric mood in the drug users, gender and the BDI-II total score were included as covariates into the analyses. The MDMQ-subscales were analyzed using multivariate analysis of covariance. The Bonferroni method was used for post-hoc analyses and chi-square tests for the analysis of categorical data. All tests were two-tailed and the significance threshold was P<0.05.
3. Results
The groups were well matched on age (F31,182 =1.31, P=0.274) and verbal IQ (F3,175 =2.23, P=0.086), but there were fewer women in the drug user groups compared with the number of women in the control group (χ=13.19, P=0.004; percentage of women per group: 20% opiates, 14% cocaine, 40% amphetamines, and 39% controls). All drug users scored significantly higher on the BDI-II compared with controls (F1,182 =17.25, P<0.001).
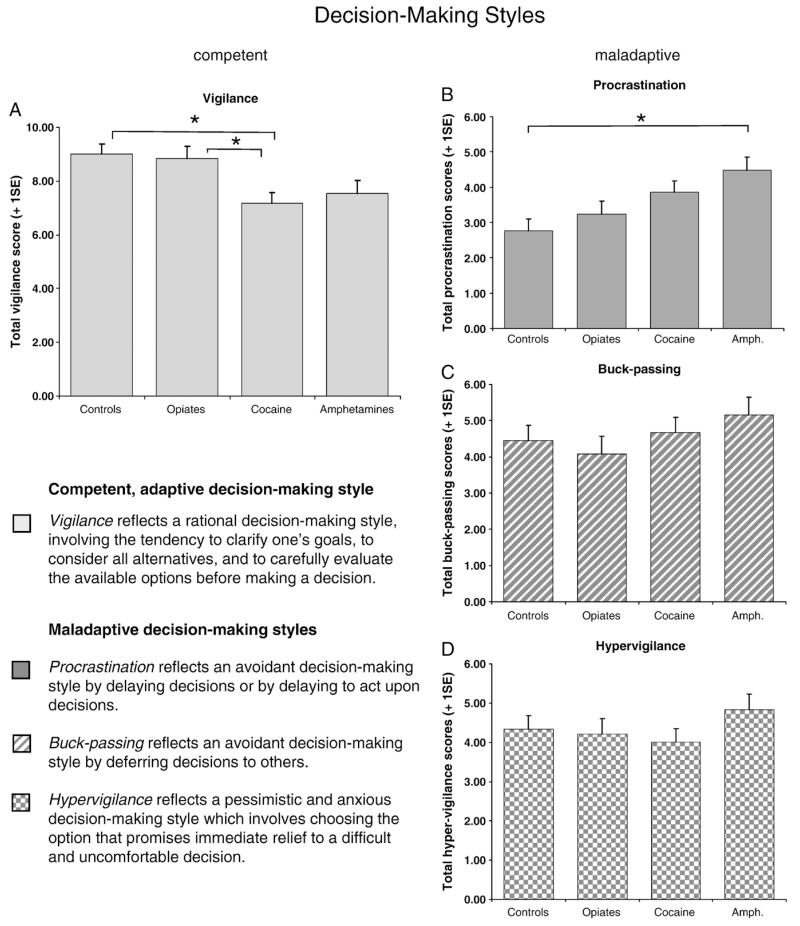
Self-reported decision-making styles differed significantly between the groups (Wilks’ Lambda F=2.16, P=0.013). As shown in Fig. 1A, there was a significant group difference on competent decision-making (F3,180 =4.68, P=0.004); cocaine users reported a less rational style of decision-making compared with controls (P=0.027) and compared with opiate users (P=0.035). With regard to maladaptive decision-making styles, there was a significant main effect on the procrastination subscale (F3,180 =4.66, P=0.004) but responses on the hypervigilance (F3,180 =0.92, P=0.434) and the buck-passing subscales (F3,180 =0.94, P=0.423) were non-significant. Post-hoc analysis revealed that amphetamine users were significantly more inclined to delay decisions than controls (P=0.004), as displayed on Fig. 1B. We further explored the relationship between procrastination (maladaptive decision-making) and vigilance (competent decision-making) scores by using partial correlation analysis to control for depression and gender. There was a negative relationship in all volunteers but in amphetamine users it reached significance (r=−0.53, P<0.01).
Fig. 1.
Decision-making styles, as assessed by the Melbourne Decision-Making Questionnaire (MDMQ), differed significantly between chronic drug users and healthy volunteers: (A) Stimulant-dependent but not opiate-dependent volunteers reported fewer rational decision-making compared with controls. (B) Amphetamine-dependent volunteers reported a significantly greater tendency to delay decisions than controls. No group differences were identified in terms of (C) buck-passing or (D) anxious (hypervigilant) decision-making styles.
Although the groups were matched for age, we explored whether the relatively wide age range of 20-56 years might have affected decision-making styles. We divided the groups by median split into young and older participants, and conducted the analyses with age as the between-subject factor. The median age in drug users and controls was 34 years (mean age in years per group: 33.9 opiates, 32.8 cocaine, 36.3 amphetamines, and controls 34.5). No group difference was observed on competent decision-making styles but a significant effect was revealed on maladaptive decision-making (Wilks’ Lambda F=2.56, P=0.040). Younger adults reported experiencing greater discomfort during decision-making than older participants (F1,182 =6.05, P=0.015). Subsequently, we included groups as a second between-subject factor in the analyses; the main effects of group on procrastination and of age on hypervigilance remained significant but no age-by-group interaction was identified, suggesting that age affected decision-making styles in the same way in drug users as in controls. We also explored the effects of gender using separate analysis with gender as the between-subject variable, but this analysis did not reveal significant group differences on any decision-making style.
4. Discussion
To the best of our knowledge, this is the first time that self-reported decision-making styles have been assessed in drug-dependent individuals using the MDMQ. As shown in Fig. 1, we identified altered decision-making styles in chronic drug users: stimulant users reported less rational and more maladaptive strategies of decision-making. The reports by stimulant users of a reduced search for information and a diminished evaluation of alternatives before making a decision, appear concurrent with their poor performance on laboratory tests of decision-making. Thus stimulant users frequently make suboptimal choices, particularly in tests where knowledge of reinforcement contingencies has to be applied (Stout et al., 2004; Verdejo-Garcia et al., 2007a; Vadhan et al., 2009) or when outcome values and probabilities are constantly changing (Rogers et al., 1999; Paulus et al., 2002, 2003). For example, on the Iowa Gambling task (Bechara et al., 1997), decision-making is assessed through the selection of cards by the participant on the basis of expected reward contingencies. Cocaine users make disadvantageous choices by preferentially selecting cards from decks that involve large rewards but also large losses (Stout et al., 2004; Verdejo-Garcia et al., 2007a). However, the type of the outcome seems to shape cocaine users’ decision-making strategies, i.e. in situations that require decisions on real monetary rewards, cocaine users make less risky decisions in situations that involve hypothetical outcomes (Vadhan et al., 2009).
Less competent decision-making styles have not only been observed in chronic cocaine users but also in chronic users of amphetamines, albeit their self-reports were only marginally significant. On behavioral tests, however, disadvantageous decision-making strategies in amphetamine users seem to be of different nature than those seen in cocaine users. For example, on the Cambridge Gamble task, participants make choices between two mutually exclusive options and place bets on the expected outcome. Chronic amphetamine users, selected the most favourable option less often but showed no signs of impaired risk adjustment (Rogers et al., 1999). A similar decision-making profile has also been identified in methamphetamine users on the prediction task, suggesting an underlying impairment in correctly estimating outcome probabilities (Paulus et al., 2002, 2003).
In the present study, amphetamine users reported the greatest tendency to avoid decisions by procrastination. Interestingly, amphetamine users’ increased tendency to postpone decisions was significantly associated with reduced competent decision-making. It is conceivable that amphetamine users delay decisions more frequently because they lack competent strategies in dealing with decisional conflicts. Although our data does not allow inferences about the underlying causes of altered decision-making styles, it is noteworthy that decisional procrastination is most prominent in individuals with cognitive deficits (Di Fabio, 2006). Cognitive function is impaired in chronic drug users (Rogers and Robbins, 2001; Verdejo-Garcia et al., 2004), and particularly impaired in amphetamine users (Scott et al., 2007; Ersche and Sahakian, 2007); it is tempting to speculate whether cognitive impairments are related to amphetamine users’ high levels of delaying decisions.
At first glance, it may seem surprising that self-reported decision-making styles in opiate-dependent individuals did not differ from controls on any MDMQ-subscale. Yet, our findings are concurrent with the literature suggesting that opiate users may be less impaired in decision-making than stimulant users (Rogers et al., 1999). Several studies indicate that decision-making impairment are only limited to subgroups of opiate users (Ersche et al., 2005; Pirastu et al., 2006; Fishbein et al., 2007). However, we also acknowledge the possibility that a lack of insight may have influenced the drug users’ self-reports. Further studies are therefore recommended to use additional ratings from family members and/or clinicians.
Although our findings have revealed new insight into decision-making styles of drug-dependent individuals, the lack of objective behavioral or neuroimaging data is a major caveat of our study. Potential relationships between decision-making styles, decisional strategies on different behavioral tests of decision-making, and the neural networks implicated in decision-making therefore remain to be investigated. Further studies should also consider including other drug-dependent groups, in particular alcohol and cannabis users, because dependencies on these drugs have also been associated with decision-making deficits (Vadhan et al., 2007; Cantrell et al., 2008). We believe that future decision-making research would benefit from using a combination of behavioral tests and self-reports.
Acknowledgements
The authors would like to thank all volunteers for their contributions. The study was approved by the Cambridge Research Ethics Committee and has been performed in accordance with the ethical standards laid down in the Declaration of Helsinki. This work was carried out within the University of Cambridge Behavioural and Clinical Neuroscience Institute, supported by a joint award from the Medical Research Council and the Wellcome Trust. KD Ersche holds the Betty Behrens Research Fellowship from Clare Hall College, Cambridge (U.K.) and receives grant support from the Medical Research Council.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders4th Edition. American Psychiatric Association; Washington, DC: 2000. Text Revision. [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Cantrell H, Finn PR, Rickert ME, Lucas J. Decision making in alcohol dependence: Insensitivity to future consequences and comorbid disinhibitory psychopathology. Alcoholism, Clinical and Experimental Research. 2008;32:1398–1407. doi: 10.1111/j.1530-0277.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in chronic and former substance users. Biological Psychiatry. 2006;60:512–522. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Di Fabio A. Decisional procrastination correlates: Personality traits, self-esteem or perception of cognitive failure? International Journal for Educational and Vocational Guidance. 2006;6:109–122. [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, Van den Brink W. Substance use disorders and the orbitofrontal cortex—Systematic review of behavioural decision-making and neuroimaging studies. The British Journal of Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Clark L, London M, Robbins TW, Sahakian BJ. Punishment induces risky decision-making in methadone-maintained opiate users but not in heroin users or healthy volunteers. Neuropsychopharmacology. 2005;30:2115–2124. doi: 10.1038/sj.npp.1300812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Sahakian BJ. The neuropsychology of amphetamine and opiate dependence: implications for treatment. Neuropsychology Review. 2007;17:317–336. doi: 10.1007/s11065-007-9033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) Biometrics Research. New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fishbein DH, Krupitsky E, Flannery BA, Langevin DJ, Bobashev G, Verbitskaya E, Augustine CB, Bolla KI, Zvartau E, Schech B, Egorova V, Bushara N, Tsoy M. Neurocognitive characterizations of Russian heroin addicts without a significant history of other drug use. Drug and Alcohol Dependence. 2007;90:25–38. doi: 10.1016/j.drugalcdep.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. The American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends in Cognitive Sciences. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janis IL, Mann L. Decision-making: a psychological analysis of conflict, choice, and commitment. The Free Press; New York: 1977. [Google Scholar]
- Mann L, Burnett P, Radford M, Ford S. The Melbourne Decision Making Questionnaire: An instrument for measuring patterns for coping with decisional conflict. Journal of Behavioral Decision Making. 1997;10:1–19. [Google Scholar]
- Nelson HE. National adult reading test manual. NFER-Nelson; Windsor (UK): 1982. [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biological Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Pirastu R, Fais R, Messina M, Bini V, Spiga S, Falconieri D, Diana M. Impaired decision-making in opiate-dependent subjects: Effect of pharmacological therapies. Drug and Alcohol Dependence. 2006;83:163–168. doi: 10.1016/j.drugalcdep.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JFW, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: Evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Current Opinion in Neurobiology. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: A critical review and meta-analysis. Neuropsychology Review. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin AL, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychonomic Bulletin & Review. 2004;11:742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, van Gorp WG, Gunderson EW, Haney M, Foltin RW. Acute effects of smoked marijuana on decision making, as assessed by a modified gambling task, in experienced marijuana users. Journal of Clinical and Experimental Neuropsychology. 2007;29:357–364. doi: 10.1080/13803390600693615. [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Hart CL, Haney M, van Gorp WG, Foltin RW. Decision-making in long-term cocaine users: Effects of a cash monetary contingency on gambling task performance. Drug and Alcohol Dependence. 2009;102:95–101. doi: 10.1016/j.drugalcdep.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lopez-Torrecillas F, Gimenez CO, Perez-Garcia M. Clinical implications and methodological challenges in the study of the neuropsychological correlates of cannabis, stimulant, and opioid abuse. Neuropsychology Review. 2004;14:1–41. doi: 10.1023/b:nerv.0000026647.71528.83. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug and Alcohol Dependence. 2007a;90:2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addictive Behaviors. 2007b;32:950–966. doi: 10.1016/j.addbeh.2006.06.032. [DOI] [PubMed] [Google Scholar]