Abstract
A female patient in her 60s presented with a history of malaise, chills, headache and vomiting. She was in shock on presentation with a high haematocrit and a low albumin with evidence of rhabdomyolysis. Severe limb and truncal oedema developed with worsening hypotension leading to intensive care unit admission for multiple organ support. Extensive radiological, microbiological and immunological work up was negative with the exception of a monoclonal gammopathy. A review of patient investigations led to a diagnosis of Clarkson's disease. Treatment with high-dose methylprednisolone and intravenous immunoglobulins led to a rapid decline in the creatine kinase (CK) level and vasopressor requirements. The patient was discharged home on long-term terbutaline and has made a good recovery.
Background
Systemic capillary leak syndrome (Clarkson's disease) is considered to be a rare disease, but our review of the literature suggests that it may be under-reported. Systemic capillary leak syndrome should be included in the differential diagnosis of hypovolaemic and vasodilatory shock and considered when there is an inappropriately raised haematocrit and low albumin level on admission. Its recognition is important as it is a potentially reversible condition amenable to immunosuppression which can lead to rapid resolution of symptoms.
Case presentation
A female patient in her 60s presented to the emergency department of our hospital with a 3-day history of coryzal symptoms, malaise, fatigue, headache, chills and vomiting. The patient reported reduced urine output for the last 2 days. The only medical history was migraines for which she was on no regular medication.
On examination the patient was alert and orientated with cold peripheries. The peripheral pulse was difficult to palpate and was 128 bpm, blood pressure 109/70 mm Hg with core temperature of 35.6°C and respiratory rate 28 breaths/min. The SpO2 was 95% breathing oxygen at 10 l/min. Examination of the heart, chest and abdomen was unremarkable. There was no neck stiffness, joint swelling, rash or inflamed fauces.
Investigations and treatment
Urinalysis revealed 1+ of protein and a trace of glucose. A full blood count revealed a white cell count of 30.75×109/mm3, the platelet count was 120×109/mm3, the haemoglobin was 17.6 g/dl and the haematocrit was 0.543. Blood film showed a neutrophil leucocytosis with no left shift or toxic granulation. Blood biochemistry revealed sodium 131 mmol/l, potassium 3.6 mmol/l, urea 16.9 mmol/l; creatine 192 μmol/l, albumin 22 g/l, glucose 17.9 mmol/l and creatine kinase 4014 mg/dl. The arterial blood gas showed pH 7.05, bicarbonate 12.3 mmol/l, lactate of 14 mmol/l and a base deficit of 17.9 mEq/l. Chest radiograph and 12 lead ECG were normal. A summary of investigations performed is included in table 1.
Table 1.
Summary of investigations
| Variable | Reference range (adults) | Day 1 | Day 3 | Day 7 |
|---|---|---|---|---|
| Haematocrit (%) | 0.36–0.46 | 0.543 | 0.429 | 0.231 |
| Haemoglobin (g/dl) | 11.5–16.0 | 17.6 | 14.2 | 7.7 |
| WCC (×109/l) | 4.0–10.5 | 30.75 | 25.77 | 20.91 |
| Differential count (×109/l) | ||||
| Neutrophils | 1.8–7.5 | 24.81 | 21.38 | 18.80 |
| Lymphocytes | 1.3–4.0 | 3.44 | 1.82 | 0.88 |
| Monocytes | 0.2–0.8 | 2.31 | 2.56 | 1.21 |
| Eosinophils | 0.02–0.4 | 0.14 | 0.01 | 0.00 |
| Basophils | 0.0–0.20 | 0.06 | 0.01 | 0.03 |
| Platelet count (×109/l) | 145–400 | 120 | 60 | 65 |
| Mean corpuscular volume (fl) | 80.0–101.0 | 96.5 | 95.1 | 96.2 |
| Lactate dehydrogenase (IU/l) | 313–618 | 1284 | ||
| Sodium (mmol/l) | 134–145 | 128 | 133 | 131 |
| Potassium (mmol/l) | 3.6–5.3 | 3.6 | 4.9 | 4.4 |
| Urea (mmol/l) | 2.8–7.0 | 16.9 | 10.9 | 13.4 |
| Creatine (umol/l) | 44–80 | 192 | 108 | 98 |
| Creatine kinase (IU/l) | < 135 | 4014 | 12113 | |
| Glucose (mmol/l) | 2.7–11.0 | 20.7 | ||
| Corrected calcium (mmol/l) | 2.1–2.55 | 2.27 | 2.60 | |
| Thyroid-stimulating hormone (mU/l) | 0.27–4.2 | 1.39 | ||
| Albumin (g/l) | 35–49 | 22 | 16 | 19 |
| Amylase (IU/l) | 30–110 | 236 | ||
| Lactate (mmol/l) | 0.5–1.6 | 12.0 | ||
| Rheumatological tests | ||||
| Serum κ light chains | High levels | |||
| Serum immunofixation | Presence of an IgG κ paraprotein | |||
| C3 | Low | |||
| C4 | Normal | |||
| C4 esterase inhibitor | Normal | |||
| Mast cell tryptase | Sample lost in transport to reference laboratory | |||
| Antinuclear antibody | Negative | |||
| Myeloperoidase | Negative | |||
| Proteinase 3 | Negative | |||
| ANCAIF | Negative | |||
| Antiglomerular basement membrane antibody | Negative | |||
| Rheumatoid factor | Negative | |||
A total of 4000 ml of crystalloid fluid resuscitation (Plasmalyte, Baxter Healthcare Ltd, Berkshire) was given together with intravenous tazocin and clarithomycin with a presumptive diagnosis of septic shock. To exclude occult infection and bowel ischaemia a CT scan of the chest, abdomen and pelvis was performed which was unremarkable. Despite fluid resuscitation the patient developed worsening hypotension and was transferred to the ICU for vasopressor support with norepinephrine.
Cardiac output monitoring was used to guide a total of 14 litres of fluids in the first 24 h. Despite this the blood pressure remained low despite high doses of norepinephrine. An echocardiogram revealed good left ventricular systolic function and no gross valvular abnormalities. Low-dose hydrocortisone was started.
On day 2 the results of microbiology were negative for blood cultures, urine cultures, legionella and pneumococcal antigen and non-directed bronchiolar lavage. It was noted that the patient had developed tense periorbital, chest, abdominal wall and four limb oedema. The CK had increased from 4014 mg/dl on admission to 14 212 mg/dl (see figure 1). In spite of the severe oedema the patient remained conscious and showed no signs of pulmonary oedema.
Figure 1.
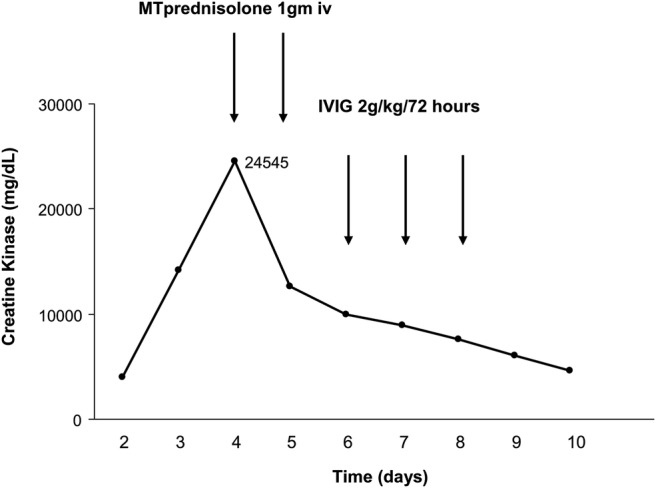
Temporal trend in creatine kinase levels and the response to treatment. MT, methylprednisolone; IVIG, intravenous immunoglobulin.
In light of the negative microbiology, rapidly rising CK and severe peripheral oedema we revisited the diagnosis of sepsis. Rheumatological investigations are also summarised in table 1. An open lateral rectus muscle biopsy showed no evidence of an inflammatory myopathy. A repeat CT of the chest, abdomen and pelvis failed to demonstrate any occult collection.
Further serological tests showed evidence of a IgG-κ monoclonal gammopathy and negative cocksackie and enterovirus. The increasing CK, monoclonal gammopathy, negative microbiology tests, severe peripheral oedema and refractory shock was consistent with a diagnosis of idiopathic systemic capillary leak syndrome (SCLS) and so the patient was given 1 g intravenous methylprednisolone on days 4 and 5 and started intravenous aminophylline. Antibiotics were discontinued. There was a rapid reduction in the CK (see figure 1); however, the vasopressor requirement remained high and so intravenous immunoglobulin (IVIG) was administered on day 6 following which there was a sustained reduction in vasopressor requirements (figure 2).
Figure 2.
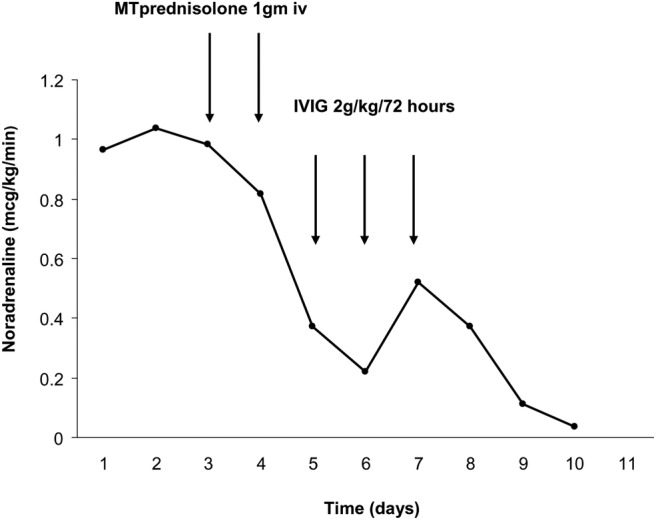
Temporal trend in norepinephrine use and the response to treatment. MT, methylprednisolone; IVIG, intravenous immunoglobulin.
Outcome and follow-up
As the oedema resolved it was apparent that the patient had developed a severe neuromyopathy for which she required a period of weaning from mechanical ventilation via a tracheostomy for further 9 days. She was subsequently discharged home on terbutaline 2.5 mg three times a day as long-term prophylaxis. She was reviewed in our ICU follow-up and rheumatology clinic at 6 months and has had no further episodes of unexplained oedema and her myopathy was recovering.
Discussion
Idiopathic SCLS was first described by Bayard Clarkson in 1960 in a young woman who developed cyclical oedema around the time of her menses.1 It is a rare disorder of presumed endothelial dysfunction that leads to increased capillary permeability, resulting in hypovolaemia due to plasma extravasation. There have been less than 170 cases described in the literature but it may be under-reported due to its rarity, non-specific presentation and high mortality. The diagnosis is made from the triad of haemoconcentration, hypotension and hypoalbuminaemia in the absence of other causes of shock, making it a diagnosis of exclusion. Shock refractory to fluid resuscitation coupled with facial, truncal and limb oedema are characteristic with a haematocrit that may be greater than 0.6. Exclusion of sepsis, toxic shock, anaphylaxis and other causes of systemic inflammatory response syndrome are essential. Both a relapsing and remitting course have been identified as well as a more chronic low-grade disorder.2
Its pathophysiology is unclear although electron micrographs of muscle biopsy specimens show endothelial cell ‘blebbing’ and apoptosis suggesting a breach in endothelial integrity.2 In total, 80% of cases are associated with a monoclonal gammopathy of uncertain significance (MGUS) adding weight to the concept of an immune-mediated mechanism.2 It bears similarities to acquired angioedema where autoantibodies bind with C1 inhibitor leading to increased complement activation and increased levels of permeability factors.
Vascular endothelial growth factor (VEGF) is involved in endothelial homeostasis and permeability and there is evidence of a relationship between serum VEGF and the clinical course of SCLS.3 In this respect, SCLS has similarities to the POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, myeloma and skin abnormalities) which is mediated through VEGF and is associated with plasma cell disorders. This data would also add support to the hypothesis that the pathophysiology of SCLS is mediated through the endothelium.4
Different treatments have been tried targeting putative pathways in the pathogenesis of the acute leak stage of the illness with specific emphasis on immune-mediated effects on the endothelium. These include prostacyclin analogues5 and immunomodulatory agents such as monoclonal antibodies to tumour necrosis factor and VEGF. Bevacizumab, which is a monoclonal antibody to VEGF, has shown a variable response in clinical outcome in two cases.4 6 Because of the prominence of monoclonal gammopathy in SCLS melphalan and prednisolone have been tried to reduce immunoglobulin load with little effect.7 8 Although steroids have been used to maintain remission in the chronic form of SCLS there is limited evidence of effect in the acute setting although methylprednisolone has been used in SCLS associated with anaplastic lymphoma with good effect9 and prednisolone 1 mg/kg appeared to lead to an improvement in symptoms where plasmaphoresis and IVIG had not.10
Maintenance therapies tried for SCLS include β-adrenergic agonists and phosphodiesterase inhibitors in an attempt to stabilise the endothelial membrane although the data to guide long-term treatment is limited.11 12
Learning points.
Systemic capillary leak syndrome is a rare but potentially reversible cause of shock and should be considered in a patient presenting with shock associated with a raised haematocrit and low albumin.
Timely intervention with immunosuppression can lead to a rapid resolution in symptoms.
Further work is needed to improve our understanding of the pathogenesis of this disease as data to guide treatment for both the acute phase and long-term maintenance therapies to prevent relapse is limited.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Clarkson B, Thompson D, Horwith M, et al. Cyclical oedema and shock due to increased capillary permeability. Am J Med 1960;2013:193–216 [DOI] [PubMed] [Google Scholar]
- 2.Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med 2010;2013:90–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinoshita Y, Kasaoka S, Fujita M, et al. Synchronized changes in serum vascular endothelial growth factor during the clinical course of chronic systemic capillary leak syndrome. Intern Med (Tokyo, Japan);2013:791–4 [DOI] [PubMed] [Google Scholar]
- 4.Lesterhuis WJ, Rennings AJ, Leenders WP, et al. Vascular endothelial growth factor in systemic capillary leak syndrome. Am J Med 2009;2013:e5–7 [DOI] [PubMed] [Google Scholar]
- 5.Kao NL, Richmond GW, Luskin AT. Systemic capillary leak syndrome. Chest 1993;2013:1637–8 [DOI] [PubMed] [Google Scholar]
- 6.Yabe H, Yabe M, Koike T, et al. Rapid improvement of life threatening capillary leak syndrome after stem cell transplantation by Bevacizumab. Blood 2013:2723–4 [DOI] [PubMed] [Google Scholar]
- 7.Hiraoka E, Matsushima Y, Inomoto-Naribayashi Y, et al. Systemic capillary leak syndrome associated with multiple myeloma of IgG kappa type. Intern Med 1995;2013:1220–4 [DOI] [PubMed] [Google Scholar]
- 8.Beermann W, Horstrup KA, Will R. Systemic capillary leak syndrome. Am J Med 1998;2013:554. [DOI] [PubMed] [Google Scholar]
- 9.Lourdes LS, Al-Quran SZ, Dang NH, et al. Systemic capillary leak syndrome as an initial presentation of ALK-negative anaplastic large cell lymphoma. Case Rep Hematol 2012;2013:954201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Airaghi L, Montori D, Santambrogio L, et al. Chronic systemic capillary leak syndrome. Report of a case and review of the literature. J Intern Med 2000;2013:731–5 [DOI] [PubMed] [Google Scholar]
- 11.Droder RM, Kyle RA, Greipp PR. Control of systemic capillary leak syndrome with aminophylline and terbutaline. Am J Med 1992;2013:523–6 [DOI] [PubMed] [Google Scholar]
- 12.Tahirkheli NK, Greipp PR. Treatment of the systemic capillary leak syndrome with terbutaline and theophylline. A case series. Ann Intern Med 1999;2013:905–9 [DOI] [PubMed] [Google Scholar]


