Abstract
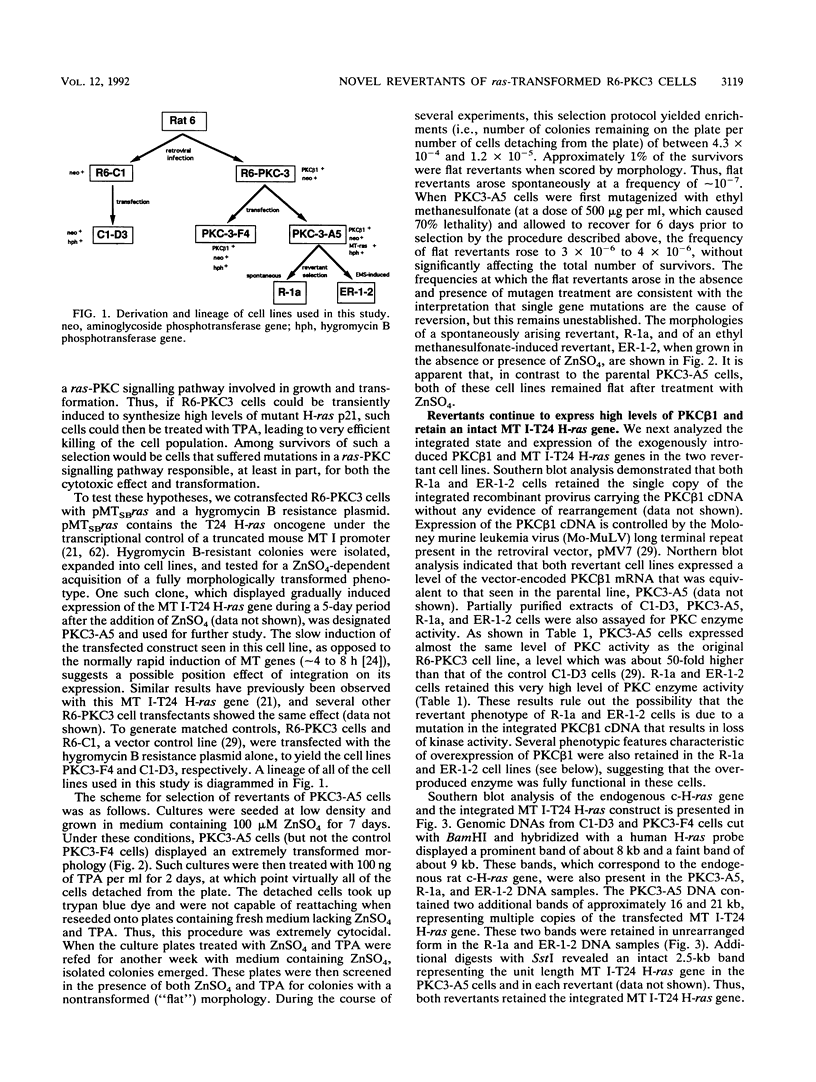
Rat 6 fibroblasts that overproduce protein kinase C beta 1 (R6-PKC3 cells) are hypersensitive to complete transformation by the T24 H-ras oncogene; yet T24 H-ras-transformed R6-PKC3 cells are killed when exposed to 12-O-tetradecanoylphorbol-13-acetate (TPA) (W.-L. W. Hsiao, G. M. Housey, M. D. Johnson, and I. B. Weinstein, Mol. Cell. Biol. 9:2641-2647, 1989). Treatment of an R6-PKC3 subclone that harbors a T24 H-ras gene under the control of an inducible mouse metallothionein I promoter with ZnSO4 and TPA is extremely cytocidal. This procedure was used to isolate rare revertants that are resistant to this toxicity. Two revertant lines, R-1a and ER-1-2, continue to express very high levels of protein kinase C enzyme activity but, unlike the parental cells, do not grow in soft agar. Furthermore, these revertants are resistant to the induction of anchorage-independent growth by the v-src, v-H-ras, v-raf, and, in the case of the R-1a line, v-fos oncogenes. Both revertant lines, however, retain the ability to undergo morphological alterations when either treated with TPA or infected with a v-H-ras virus, thus dissociating anchorage independence from morphological transformation. The revertant phenotype of both R-1a and ER-1-2 cells is dominant over the transformed phenotype in somatic cell hybridizations. Interestingly, the revertant lines no longer induce the metallothionein I-T24 H-ras construct or the endogenous metallothionein I and II genes in response to three distinct agents: ZnSO4, TPA, and dexamethasone. The reduction in activity of metallothionein promoters seen in these revertants may reflect defects in signal transduction pathways that control the expression of genes mediating specific effects of protein kinase C and certain oncogenes in cell transformation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Birren B. W., Ganz T., Piletz J. E., Herschman H. R. Molecular cloning of the rat metallothionein 1 (MT-1) mRNA sequence. DNA. 1983;2(1):15–22. doi: 10.1089/dna.1.1983.2.15. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Bacher N., Zisman Y., Berent E., Livneh E. Isolation and characterization of PKC-L, a new member of the protein kinase C-related gene family specifically expressed in lung, skin, and heart. Mol Cell Biol. 1991 Jan;11(1):126–133. doi: 10.1128/mcb.11.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M. Complexities of the protein kinase C pathway. Mol Carcinog. 1991;4(5):339–344. doi: 10.1002/mc.2940040502. [DOI] [PubMed] [Google Scholar]
- Borner C., Filipuzzi I., Weinstein I. B., Imber R. Failure of wild-type or a mutant form of protein kinase C-alpha to transform fibroblasts. Nature. 1991 Sep 5;353(6339):78–80. doi: 10.1038/353078a0. [DOI] [PubMed] [Google Scholar]
- Choi P. M., Tchou-Wong K. M., Weinstein I. B. Overexpression of protein kinase C in HT29 colon cancer cells causes growth inhibition and tumor suppression. Mol Cell Biol. 1990 Sep;10(9):4650–4657. doi: 10.1128/mcb.10.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletta G., Cirafici A. M., Consiglio E., Vecchio G. Forskolin and a tumor promoter are able to induce c-fos and c-myc expression in normal, but not in a v-ras-transformed rat thyroid cell line. Oncogene Res. 1987 Sep-Oct;1(4):459–466. [PubMed] [Google Scholar]
- Contente S., Kenyon K., Rimoldi D., Friedman R. M. Expression of gene rrg is associated with reversion of NIH 3T3 transformed by LTR-c-H-ras. Science. 1990 Aug 17;249(4970):796–798. doi: 10.1126/science.1697103. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Gerald P. S. Improved techniques for the induction of mammalian cell hybridization by polyethylene glycol. Somatic Cell Genet. 1976 Mar;2(2):165–176. doi: 10.1007/BF01542629. [DOI] [PubMed] [Google Scholar]
- Diaz-Laviada I., Larrodera P., Diaz-Meco M. T., Cornet M. E., Guddal P. H., Johansen T., Moscat J. Evidence for a role of phosphatidylcholine-hydrolysing phospholipase C in the regulation of protein kinase C by ras and src oncogenes. EMBO J. 1990 Dec;9(12):3907–3912. doi: 10.1002/j.1460-2075.1990.tb07611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Eldar H., Zisman Y., Ullrich A., Livneh E. Overexpression of protein kinase C alpha-subtype in Swiss/3T3 fibroblasts causes loss of both high and low affinity receptor numbers for epidermal growth factor. J Biol Chem. 1990 Aug 5;265(22):13290–13296. [PubMed] [Google Scholar]
- Fleischman L. F., Chahwala S. B., Cantley L. ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science. 1986 Jan 24;231(4736):407–410. doi: 10.1126/science.3001936. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Gauthier-Rouvière C., Fernandez A., Lamb N. J. ras-induced c-fos expression and proliferation in living rat fibroblasts involves C-kinase activation and the serum response element pathway. EMBO J. 1990 Jan;9(1):171–180. doi: 10.1002/j.1460-2075.1990.tb08093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988 Apr;2(4):394–402. doi: 10.1101/gad.2.4.394. [DOI] [PubMed] [Google Scholar]
- Glass D. J., Rees-Jones R. W., Goff S. P. Isolation and characterization of flat revertant cell lines from A-MuLV-transformed fibroblasts. Oncogene Res. 1990;5(3):175–185. [PubMed] [Google Scholar]
- Godwin A. K., Lieberman M. W. Early and late responses to induction of rasT24 expression in Rat-1 cells. Oncogene. 1990 Aug;5(8):1231–1241. [PubMed] [Google Scholar]
- Guadagno S. N., Borner C., Weinstein I. B. Altered regulation of a major substrate of protein kinase C in rat 6 fibroblasts overproducing PKC beta I. J Biol Chem. 1992 Feb 5;267(4):2697–2707. [PubMed] [Google Scholar]
- Guerrero I., Pellicer A., Burstein D. E. Dissociation of c-fos from ODC expression and neuronal differentiation in a PC12 subline stably transfected with an inducible N-ras oncogene. Biochem Biophys Res Commun. 1988 Feb 15;150(3):1185–1192. doi: 10.1016/0006-291x(88)90754-1. [DOI] [PubMed] [Google Scholar]
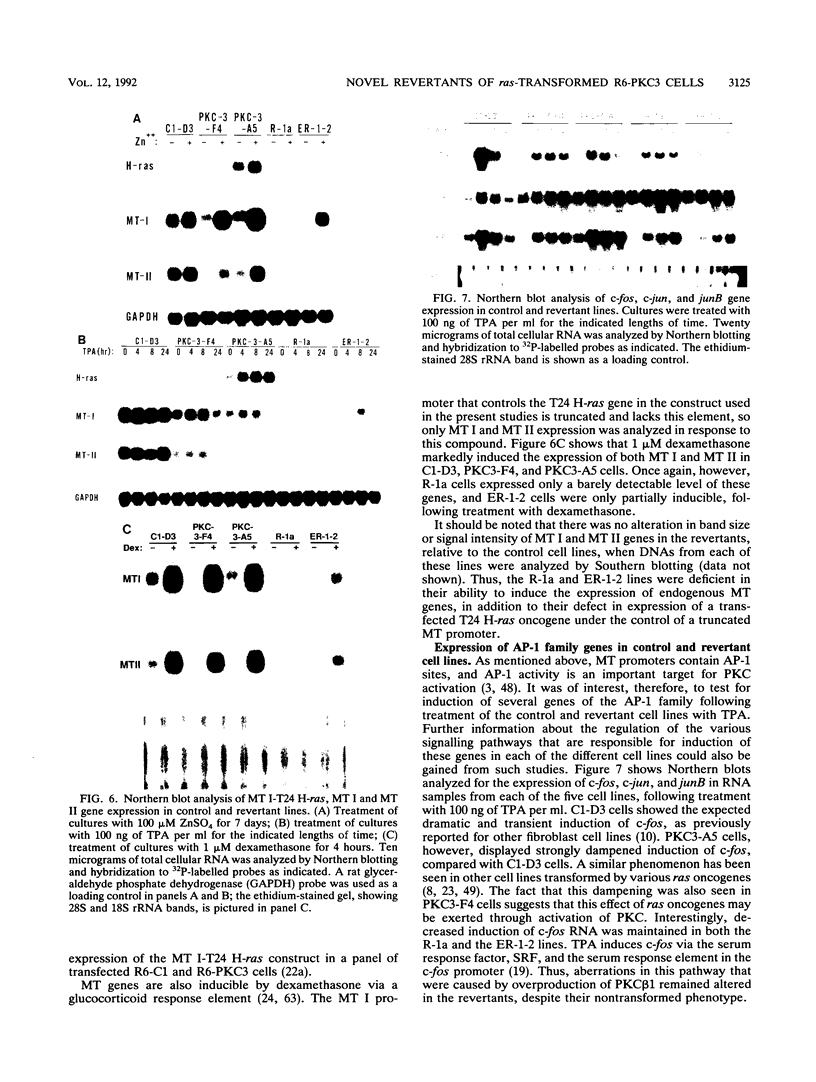
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hartman S. C., Mulligan R. C. Two dominant-acting selectable markers for gene transfer studies in mammalian cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoemann C. D., Zarbl H. Use of revertant cell lines to identify targets of v-fos transformation-specific alterations in gene expression. Cell Growth Differ. 1990 Dec;1(12):581–590. [PubMed] [Google Scholar]
- Hong H. J., Hsu L. C., Gould M. N. Molecular cloning of rat Krev-1 cDNA and analysis of the mRNA levels in normal and NMU-induced mammary carcinomas. Carcinogenesis. 1990 Jul;11(7):1245–1247. doi: 10.1093/carcin/11.7.1245. [DOI] [PubMed] [Google Scholar]
- Hoshina S., Ueffing M., Weinstein I. B. Growth factor-induced DNA synthesis in cells that overproduce protein kinase C. J Cell Physiol. 1990 Nov;145(2):262–267. doi: 10.1002/jcp.1041450210. [DOI] [PubMed] [Google Scholar]
- Housey G. M., Johnson M. D., Hsiao W. L., O'Brian C. A., Murphy J. P., Kirschmeier P., Weinstein I. B. Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell. 1988 Feb 12;52(3):343–354. doi: 10.1016/s0092-8674(88)80027-8. [DOI] [PubMed] [Google Scholar]
- Hsiao W. L., Housey G. M., Johnson M. D., Weinstein I. B. Cells that overproduce protein kinase C are more susceptible to transformation by an activated H-ras oncogene. Mol Cell Biol. 1989 Jun;9(6):2641–2647. doi: 10.1128/mcb.9.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao W. L., Wu T., Weinstein I. B. Oncogene-induced transformation of a rat embryo fibroblast cell line is enhanced by tumor promoters. Mol Cell Biol. 1986 Jun;6(6):1943–1950. doi: 10.1128/mcb.6.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Chida K., Kamata N., Nose K., Kato M., Homma Y., Takenawa T., Kuroki T. Enhancement of inositol phospholipid metabolism and activation of protein kinase C in ras-transformed rat fibroblasts. J Biol Chem. 1988 Dec 5;263(34):17975–17980. [PubMed] [Google Scholar]
- Imbra R. J., Karin M. Metallothionein gene expression is regulated by serum factors and activators of protein kinase C. Mol Cell Biol. 1987 Apr;7(4):1358–1363. doi: 10.1128/mcb.7.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler J. L., Schatz C., Wasylyk C., Chatton B., Wasylyk B. A Harvey-ras responsive transcription element is also responsive to a tumour-promoter and to serum. Nature. 1988 Mar 17;332(6161):275–278. doi: 10.1038/332275a0. [DOI] [PubMed] [Google Scholar]
- Kamata T., Kung H. F. Modulation of maturation and ribosomal protein S6 phosphorylation in Xenopus oocytes by microinjection of oncogenic ras protein and protein kinase C. Mol Cell Biol. 1990 Mar;10(3):880–886. doi: 10.1128/mcb.10.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata T., Sullivan N. F., Wooten M. W. Reduced protein kinase C activity in a ras-resistant cell line derived from Ki-MSV transformed cells. Oncogene. 1987 Mar;1(1):37–46. [PubMed] [Google Scholar]
- Karin M., Imbra R. J., Heguy A., Wong G. Interleukin 1 regulates human metallothionein gene expression. Mol Cell Biol. 1985 Oct;5(10):2866–2869. doi: 10.1128/mcb.5.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Sherr C. J. A dominant suppressive mutation in a cellular gene restores the nontransformed phenotype to v-fms-transformed mink cells. Oncogene. 1991 May;6(5):687–693. [PubMed] [Google Scholar]
- Kitayama H., Sugimoto Y., Matsuzaki T., Ikawa Y., Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989 Jan 13;56(1):77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- Krauss R. S., Housey G. M., Johnson M. D., Weinstein I. B. Disturbances in growth control and gene expression in a C3H/10T1/2 cell line that stably overproduces protein kinase C. Oncogene. 1989 Aug;4(8):991–998. [PubMed] [Google Scholar]
- Krauss R. S., Weinstein I. B. A novel, plasmid-based system for studying gene rearrangements in mammalian cells. Mol Cell Biol. 1991 Aug;11(8):3915–3924. doi: 10.1128/mcb.11.8.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Cuadrado A., Jones J. E., Trotta R., Burstein D. E., Thomson T., Pellicer A. Regulation of protein kinase C activity in neuronal differentiation induced by the N-ras oncogene in PC-12 cells. Mol Cell Biol. 1990 Jun;10(6):2983–2990. doi: 10.1128/mcb.10.6.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Fleming T. P., Warren B. S., Blumberg P. M., Aaronson S. A. Involvement of functional protein kinase C in the mitogenic response to the H-ras oncogene product. Mol Cell Biol. 1987 Nov;7(11):4146–4149. doi: 10.1128/mcb.7.11.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Moscat J., Aaronson S. A. Novel source of 1,2-diacylglycerol elevated in cells transformed by Ha-ras oncogene. Nature. 1987 Nov 19;330(6145):269–272. doi: 10.1038/330269a0. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., de la Peña P., Moscat J., Garcia-Barreno P., Anderson P. S., Aaronson S. A. Rapid stimulation of diacylglycerol production in Xenopus oocytes by microinjection of H-ras p21. Science. 1987 Oct 23;238(4826):533–536. doi: 10.1126/science.2821623. [DOI] [PubMed] [Google Scholar]
- Lamph W. W., Wamsley P., Sassone-Corsi P., Verma I. M. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature. 1988 Aug 18;334(6183):629–631. doi: 10.1038/334629a0. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lin A. H., Groppi V. E., Gorman R. R. Platelet-derived growth factor does not induce c-fos in NIH 3T3 cells expressing the EJ-ras oncogene. Mol Cell Biol. 1988 Nov;8(11):5052–5055. doi: 10.1128/mcb.8.11.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. C., Paterson H. F., Morris J. D., Hall A., Marshall C. J. p21H-ras-induced morphological transformation and increases in c-myc expression are independent of functional protein kinase C. EMBO J. 1989 Apr;8(4):1099–1104. doi: 10.1002/j.1460-2075.1989.tb03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. D., Price B., Lloyd A. C., Self A. J., Marshall C. J., Hall A. Scrape-loading of Swiss 3T3 cells with ras protein rapidly activates protein kinase C in the absence of phosphoinositide hydrolysis. Oncogene. 1989 Jan;4(1):27–31. [PubMed] [Google Scholar]
- Murphy A. J., Efstratiadis A. Cloning vectors for expression of cDNA libraries in mammalian cells. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8277–8281. doi: 10.1073/pnas.84.23.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The Albert Lasker Medical Awards. The family of protein kinase C for signal transduction. JAMA. 1989 Oct 6;262(13):1826–1833. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Selinger Z., Scolnick E. M., Bassin R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nori M., Shawver L. K., Weber M. J. A Swiss 3T3 variant cell line resistant to the effects of tumor promoters cannot be transformed by src. Mol Cell Biol. 1990 Aug;10(8):4155–4162. doi: 10.1128/mcb.10.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada S., Mizuno K., Saido T. C., Akita Y., Suzuki K., Kuroki T., Ohno S. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J Biol Chem. 1990 Dec 25;265(36):22434–22440. [PubMed] [Google Scholar]
- Persons D. A., Wilkison W. O., Bell R. M., Finn O. J. Altered growth regulation and enhanced tumorigenicity of NIH 3T3 fibroblasts transfected with protein kinase C-I cDNA. Cell. 1988 Feb 12;52(3):447–458. doi: 10.1016/s0092-8674(88)80037-0. [DOI] [PubMed] [Google Scholar]
- Price B. D., Morris J. D., Marshall C. J., Hall A. Stimulation of phosphatidylcholine hydrolysis, diacylglycerol release, and arachidonic acid production by oncogenic ras is a consequence of protein kinase C activation. J Biol Chem. 1989 Oct 5;264(28):16638–16643. [PubMed] [Google Scholar]
- Qureshi S. A., Joseph C. K., Rim M., Maroney A., Foster D. A. v-Src activates both protein kinase C-dependent and independent signaling pathways in murine fibroblasts. Oncogene. 1991 Jun;6(6):995–999. [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Voulalas P. J., Franza B. R., Jr, Curran T. Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev. 1988 Dec;2(12B):1687–1699. doi: 10.1101/gad.2.12b.1687. [DOI] [PubMed] [Google Scholar]
- Reynolds V. L., Lebovitz R. M., Warren S., Hawley T. S., Godwin A. K., Lieberman M. W. Regulation of a metallothionein-rasT24 fusion gene by zinc results in graded alterations in cell morphology and growth. Oncogene. 1987;1(3):323–330. [PubMed] [Google Scholar]
- Richards R. I., Heguy A., Karin M. Structural and functional analysis of the human metallothionein-IA gene: differential induction by metal ions and glucocorticoids. Cell. 1984 May;37(1):263–272. doi: 10.1016/0092-8674(84)90322-2. [DOI] [PubMed] [Google Scholar]
- Ryder K., Lanahan A., Perez-Albuerne E., Nathans D. jun-D: a third member of the jun gene family. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1500–1503. doi: 10.1073/pnas.86.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Der C. J., Verma I. M. ras-induced neuronal differentiation of PC12 cells: possible involvement of fos and jun. Mol Cell Biol. 1989 Aug;9(8):3174–3183. doi: 10.1128/mcb.9.8.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Searle P. F., Chen H. Y., Brinster R. L., Palmiter R. D. A 12-base-pair DNA motif that is repeated several times in metallothionein gene promoters confers metal regulation to a heterologous gene. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7318–7322. doi: 10.1073/pnas.81.23.7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartmann M., Jans D. A., Parker P. J., Nagamine Y., Hemmings B. A., Jaken S., Eppenberger U., Fabbro D. Overexpression of the alpha-type protein kinase (PK) C in LLC-PK1 cells does not lead to a proportional increase in the induction of two 12-O-tetradecanoylphorbol-13-acetate-inducible genes. Cell Regul. 1991 Jun;2(6):491–502. doi: 10.1091/mbc.2.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom R., Verma I. M. Revertants of v-fos-transformed rat fibroblasts: suppression of transformation is dominant. Mol Cell Biol. 1990 Nov;10(11):5626–5633. doi: 10.1128/mcb.10.11.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]
- Yamada H., Omata-Yamada T., Wakabayashi-Ito N., Carter S. G., Lengyel P. Isolation of recessive (mediator-) revertants from NIH 3T3 cells transformed with a c-H-ras oncogene. Mol Cell Biol. 1990 Apr;10(4):1822–1827. doi: 10.1128/mcb.10.4.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Ciardiello F., Talbot N., McGeady M. L., Cooper H., Benade L., Salomon D. S., Bassin R. H. Isolation of a new class of 'flat' revertants from ras-transformed NIH3T3 cells using cis-4-hydroxy-L-proline. Oncogene. 1990 Aug;5(8):1179–1186. [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Latreille J., Jolicoeur P. Revertants of v-fos-transformed fibroblasts have mutations in cellular genes essential for transformation by other oncogenes. Cell. 1987 Nov 6;51(3):357–369. doi: 10.1016/0092-8674(87)90632-5. [DOI] [PubMed] [Google Scholar]
- de Groot R. P., Auwerx J., Karperien M., Staels B., Kruijer W. Activation of junB by PKC and PKA signal transduction through a novel cis-acting element. Nucleic Acids Res. 1991 Feb 25;19(4):775–781. doi: 10.1093/nar/19.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]