Abstract
Objective
To demonstrate the feasibility of a placebo-controlled trial of antipsychotics for delirium in the intensive care unit (ICU) and to test the hypothesis that antipsychotics would improve days alive without delirium or coma.
Design
Randomized, double-blind, placebo-controlled trial.
Setting
Six tertiary care medical centers in the United States.
Patients
101 mechanically ventilated medical and surgical ICU patients.
Intervention
Patients were randomly assigned to receive haloperidol or ziprasidone or placebo every 6 hours for up to 14 days. Frequency of administration was adjusted twice daily according to delirium status, level of sedation, and side effects.
Measurements and Main Outcomes
The primary end point was the number of days patients were alive without delirium or coma. During the 21-day study period, patients in the haloperidol group spent a similar number days alive without delirium or coma (median [IQR], 14.0 [6.0–18.0] days) as did patients in the ziprasidone (15.0 [9.1–18.0] days) and placebo groups (12.5 [1.2–17.2] days) (p = 0.66). No differences were found in secondary clinical outcomes, including ventilator-free days (p = 0.25), hospital length of stay (p = 0.68), and mortality (p = 0.81). Ten (29%) patients in the haloperidol group reported symptoms consistent with akathisia, compared with 6 (20%) patients in the ziprasidone group and 7 (19%) patients in the placebo group (p = 0.60), and a global measure of extrapyramidal symptoms was similar between treatment groups (p = 0.46).
Conclusions
A randomized, placebo-controlled trial of antipsychotics for delirium in mechanically ventilated ICU patients is feasible. Treatment with antipsychotics in this limited pilot trial did not improve the number of days alive without delirium or coma nor did it increase adverse outcomes. Thus, a large trial is needed to determine whether use of antipsychotics for ICU delirium is appropriate.
Keywords: delirium, intensive care units, antipsychotic agents, haloperidol, ziprasidone, clinical trial
Delirium is an acute disorder of consciousness and cognition that occurs in 60% to 80% of mechanically ventilated intensive care unit (ICU) patients (1–3). Among these patients, this manifestation of acute brain dysfunction conveys profound risk for morbidity and mortality, as it is associated with self-extubation (4), prolonged hospital stays (5, 6), and increased mortality (1, 6, 7). It is estimated, in addition, that ICU delirium is associated with health care costs ranging between $6 to $20 billion annually in the United States alone (8).
Despite its numerous deleterious effects, no placebo-controlled clinical trials exist to support the use of pharmacologic agents solely intended to treat delirium in the ICU. In fact, the majority of delirious patients receive no specific treatment for this neuropsychiatric syndrome, in large part because it frequently remains unrecognized in clinical practice (9, 10). When diagnosed, delirium should first prompt the identification and management of potential underlying causes. Then, to prevent harmful sequelae, the Society of Critical Care Medicine (SCCM) has recommended the use of haloperidol for the treatment of delirium in critically ill patients (11), despite its unproven efficacy and the absence of Food and Drug Administration (FDA) approval for this indication. In keeping with this recommendation, haloperidol is currently the medication most widely used to treat ICU delirium (12). In recent years, however, newer “atypical” antipsychotics (e.g., risperidone, olanzapine, quetiapine, and ziprasidone) have also gained popularity (13), though only olanzapine has been studied in the ICU (14).
Given the lack of compelling evidence supporting the use of antipsychotics for delirium in critically ill patients and the potential adverse effects associated with these medications, including torsades de pointes and sudden cardiac death (15, 16), hypotension (17), neuroleptic malignant syndrome (18), and extrapyramidal symptoms (19), placebo-controlled clinical trials are greatly needed to evaluate the efficacy of antipsychotic medications in reducing the burden of delirium in the ICU. Thus, to demonstrate the feasibility of a randomized, placebo-controlled trial of antipsychotics in mechanically ventilated ICU patients, we conducted the Modifying the INcidence of Delirium (MIND) Trial, a multicenter, randomized, double-blind, placebo-controlled trial. We hypothesized that both haloperidol and ziprasidone would be efficacious and safe for the treatment of ICU delirium. This work has been presented in abstract form (20).
METHODS
Patient Population
Study personnel screened all adult (>18 years of age) mechanically ventilated medical and surgical ICU patients who had an abnormal level of consciousness or were receiving sedative or analgesic medications in six tertiary care medical centers: Vanderbilt University Medical Center, Saint Thomas Hospital, and the Department of Veterans Affairs Medical Center in Nashville, TN; University of North Carolina Hospitals in Chapel Hill, NC; University of Iowa Hospitals in Iowa City, IA; and Moses H. Cone Memorial Hospital in Greensboro, NC. The institutional review boards at each participating center approved the study protocol, and we obtained informed consent from study participants or their authorized surrogates. In response to our Investigational New Drug Application for this investigator-initiated trial, the FDA designated the trial exempt.
We excluded patients from enrollment for the following reasons: pregnancy, continuous mechanical ventilation >60 hours prior to screening, no plan for gastric access within 48 hours, moribund state and/or withdrawal of life support, admission after drug overdose or suicide attempt, ongoing outpatient neuroleptic use, allergy to haloperidol or ziprasidone or history of neuroleptic malignant syndrome, ongoing seizures, stroke in the past 2 weeks, high risk for ventricular dysrhythmias (ongoing treatment with drugs known to prolong the QT interval [listed in the Study Protocol section] or baseline QTc ≥500 ms in the absence of a bundle branch block, history of torsades de pointes, clinically significant ventricular tachycardia during the current hospital stay, myocardial infarction in the past 2 weeks, uncompensated stage IV heart failure, refractory hypokalemia [<3.0 mg/dL] or hypomagnesemia [<1.8 mg/dL]), or previously diagnosed neurologic disease (suspected anoxic brain injury, acute traumatic brain injury, or moderate to severe dementia). Dementia was identified using the medical record and two validated surrogate questionnaire, the modified Blessed dementia rating scale (mBDRS) (21) and the Informant Questionnaire of Cognitive Dysfunction in the Elderly (IQCODE) (22). We excluded patients with an mBDRS score ≥4 or an IQCODE score ≥4.0, in keeping with prior literature showing that these cutoffs identify patients with moderate to severe dementia (21, 23).
Randomization
After informed consent was obtained, we randomly assigned patients in a 1:1:1 manner to treatment with haloperidol, ziprasidone, or placebo, using a computer-generated, permuted-block randomization scheme stratified according to study center. A coordinating center biostatistician designated treatment group assignments on a list that was provided only to the investigational pharmacists at each study center, who referred to their unique list after each patient was enrolled to determine group assignment. Except for the pharmacist, neither study personnel nor patients were aware of treatment group assignment.
Study Protocol and Assessments
Immediately after enrollment, patients received one of the three colorless, odorless, and tasteless study drugs: 5 mg haloperidol (as a solution containing 1 mg/mL), 40 mg ziprasidone (as a solution containing 8 mg/mL), or matching placebo (as a 5 mL solution). Alternatively, patients in any treatment group who were temporarily without gastric access received blinded study drug per group assignment via a 0.5 mL intramuscular injection. No more than 8 total doses could be given intramuscularly. The second dose of study drug was administered 12 hours after the first dose as long as the QTc interval remained <500 ms; subsequent doses were administered every 6 hours until clinical changes prompted a change in this frequency.
Trained study personnel evaluated patients twice daily for acute brain dysfunction, diagnosing delirium with the Confusion Assessment Method for the ICU (CAM-ICU) (24–26) and assessing level of arousal with the Richmond Agitation-Sedation Scale (RASS) (27, 28). Study drug frequency was reduced to every 8 hours when patients were CAM-ICU negative (i.e., delirium/coma-free) on two consecutive assessments, reduced to every 12 hours when patients were delirium/coma-free on three consecutive assessments, and discontinued when patients were delirium/coma-free on four consecutive assessments (i.e., >48 consecutive hours without delirium). Additionally, study drug frequency was reduced in a similar manner when patients were oversedated—diagnosed when observed RASS was ≥2 levels deeper than target RASS (determined by the managing ICU team)—despite discontinuation of sedatives and sedating analgesics. Study drug was restarted (or dosing frequency was increased) when oversedation resolved. Likewise, if patients who were delirium-free had recurrent delirium, study drug was restarted if previously discontinued or dosing frequency was increased to the previously effective frequency. Regardless of clinical status, all study drug was discontinued on study day 14.
Every day that study drug was administered, research personnel assessed patients for extrapyramidal symptoms using a modified Simpson-Angus Scale (29). The original Simpson-Angus Scale consists of 10 items: gait, arm dropping, shoulder shaking, elbow rigidity, wrist rigidity, leg pendulousness, head dropping, glabella tap, tremor, and salivation. Because some of these items cannot be assessed reliably in ICU patients, we used a modified scale that included elbow rigidity, wrist ridigity, glabella tap, tremor, and salivation as well as dystonia. Subjective akathisia was assessed on days that patients were not comatose or delirious using a 10 cm visual analog scale; we considered any result >0 to indicate akathisia. Patients were also assessed daily for QT interval prolongation and other adverse events. Study drug was discontinued if extrapyramidal symptoms (≥3 points on 3 or more categories of the modified Simpson-Angus Scale) or QTc prolongation >500 ms was observed and restarted when the adverse effect resolved. Also, study drug was discontinued during any period that the following drugs known to prolong the QT interval were administered: class Ia or III antiarrhythmics, pimozide, gatifloxacin, moxifloxacin, pentamidine, cotrimoxazole, tacrolimus, and dolasetron. Finally, study drug was permanently discontinued if life-threatening drug-related adverse events occurred, including neuroleptic malignant syndrome, torsades de pointes, or other ventricular tachycardia requiring treatment, or if the patient developed dystonia unresponsive to anticholinergic treatment.
Other treatments, including approaches to sedation, were determined by the managing ICU team. Each of the participating ICUs utilized patient-targeted sedation as part of usual practice; bedside nurses regularly monitored level of sedation using validated sedation scales and titrated sedative doses up or down to achieve the targeted level of sedation specified in the physicians’ orders, which were updated as needed according to changes in the clinical scenario. Daily spontaneous awakening trials (i.e., daily interruption of sedatives) were common but not yet protocolized or mandated in all participating ICUs. Open-label antipsychotic administration was strongly discouraged during the trial but could be employed if the ICU team considered it necessary for breakthrough delirium and agitation. None of the participating ICUs employed formalized non-pharmacologic interventions to prevent or treat delirium.
All adverse events were reported to an independent Data Safety Monitoring Board (DSMB). The DSMB reviewed two interim analyses of adverse events after enrollment of 30 and 60 patients; no interim analysis of efficacy end points was conducted.
To evaluate whether the enterally-administered study drug was reaching systemic circulation, blood was collected from each patient within 48 hours of study drug initiation. At the Nathan Kline Institute (Orangeburg, New York), haloperidol plasma concentration was measured by radioimmunoassay (30), and ziprasidone plasma concentration was measured by liquid chromatographic assay using fluorescence detection (31).
End Points and Follow-Up
The primary efficacy end point was the number of days patients were alive without delirium or coma (i.e., delirium/coma-free days) during the 21-day study period. We chose this end point because the sedating effects of antipsychotics could increase coma duration, thereby confounding an analysis of delirium duration. We sought to assess the effect of antipsychotics on the duration of “normal” brain function in the ICU, hypothesizing that treatment would increase the number of “normal,” or delirium/coma-free, days by reducing delirium duration (while coma duration remained unchanged). Secondary efficacy end points included daily delirium risk, duration of delirium, duration of coma, the number of days patients were alive and breathing without assistance during the 21-day study period (ventilator-free days) (32), time to ICU and hospital discharge, and all-cause 21-day survival.
Each study day prior to ICU discharge or death, patients who responded to physical but not to verbal stimulation [RASS -4] or did not respond at all to verbal or physical stimulation [RASS -5] were classified as comatose. Since delirium cannot be diagnosed in the setting of coma, patients who were not comatose but were CAM-ICU positive were classified as delirious. Those without coma or delirium were classified as delirium/coma-free. We assumed all patients who were discharged during the 21-day study period to be delirium/coma-free after discharge.
Accuracy of sedation was determined each study day by comparing observed RASS with target RASS (determined by the managing ICU team). A patient was considered accurately sedated on days that the observed RASS was within 1 point of the target RASS.
Statistical Analysis
Based on pilot data, we anticipated that patients in the placebo group would have a mean±SD of 10.02±8.48 delirium/coma-free days and a median (interquartile range [IQR]) of 12 [0–18] days. Because this variable is skewed, we transformed delirium/coma-free days for the power analysis. At a 2-sided significance level of 2.5%, after Bonferonni adjustment for pairwise comparisons of three groups, a trial with 29 patients in each group would have 80% analytical power to detect a 25% increase (i.e., improvement) in delirium/coma-free days by the intervention.
We analyzed all data using an intention-to-treat approach. Continuous data are described using median and IQR and categorical data using frequencies and proportions. Except for analyses of repeated measures, we used the Kruskal-Wallis test to compare continuous variables between the three treatment groups and the chi-square test to compare categorical variables. To compare the effects of the two study drugs and placebo on ICU and hospital length of stay, we used time-to-event analyses; Kaplan-Meier analyses were used to determine medians and interquartile ranges, and log-rank tests were used to assess the effect of the treatments. To examine the effect of treatment group on daily delirium risk, we used a Markov regression model with generalized estimating equations (GEE) to analyze the probability of being delirious on each day following receipt of study drug according to treatment group, adjusting for mental status at the time study drug was administered (33). We analyzed in the model all 24-hour periods of observation (from 7:00 am to 6:59 am the following day) that included study drug administration (days 1–14) and were followed by a delirium assessment; 24-hour periods ending in death or coma were excluded. To assess for an interaction between current mental status and the effect of study drug on delirium risk, we included an interaction term in the Markov regression model. When analyzing repeated observations from the same patient, we used methods that account for data clustering (34). Specifically, we compared daily RASS targets between the treatment groups using proportional odds logistic regression with GEE and daily doses of sedatives between the treatment groups using linear mixed models. No subgroup analyses were performed. We used R (version 2.7.2 Patched, www.r-project.org) for all statistical analyses (35).
RESULTS
Enrollment and Baseline Characteristics
From February 2005 to July 2007, 3,297 patients who met inclusion criteria were screened for enrollment. Of these, we enrolled 103 patients. Figure 1 displays the reasons for exclusion and follow-up for the study population per CONSORT guidelines (36). All 101 patients from whom any outcomes data were obtained were included in the analyses. Two patients who were enrolled and randomized to treatment with ziprasidone were withdrawn from the trial due to ventricular tachycardia prior to ever receiving study drug. No outcomes data for analysis were obtained from these two patients.
Figure 1. CONSORT flow diagram showing enrollment, randomization, and follow-up of the study population.
Abbreviations: NMS, neuroleptic malignant syndrome
*Two patients were excluded after randomization, before study drug was administered, due to ventricular tachycardia. No outcomes data could be collected for these two patients after their withdrawal.
The three groups were similar at baseline (Table 1). Severity of illness was high at enrollment, and most patients had symptoms of acute brain dysfunction; 49% of all patients were delirious at enrollment, and 36% were comatose. Only seven patients (7%) had received haloperidol prior to study enrollment, and no patient had received ziprasidone.
Table 1.
Baseline characteristics
| Characteristica | Haloperidol (n=35) |
Ziprasidone (n=30) |
Placebo (n=36) |
|---|---|---|---|
| Age, years | 51 [35–59] | 54 [47–66] | 56 [43–68] |
| Female, n (%) | 15 (43) | 9 (30) | 14 (39) |
| APACHE II score | 26 [21–31] | 26 [23–32] | 26 [21–32] |
| SOFA score | 11 [10–13] | 10 [9–12] | 11 [9–13] |
| Charlson comorbidity index | 0 [0–1] | 1 [0–2] | 1 [0–2] |
| ICU type, n (%) | |||
| Medical | 20 (57) | 20 (67) | 23 (64) |
| Surgical | 8 (23) | 6 (20) | 8 (22) |
| Trauma | 7 (20) | 4 (13) | 5 (14) |
| ICU admission diagnosis, n (%) | |||
| Sepsis/acute respiratory distress syndrome | 10 (29) | 10 (33) | 9 (25) |
| Chronic obstructive pulmonary disease/asthma | 3 (9) | 2 (7) | 4 (11) |
| Other pulmonaryb | 6 (17) | 7 (23) | 3 (8) |
| Myocardial infarction/congestive heart failure | 2 (6) | 0 (0) | 1 (3) |
| Other medicalc | 6 (17) | 4 (13) | 9 (25) |
| Traumatic injury | 7 (20) | 4 (13) | 5 (14) |
| Surgeryd | 1 (3) | 3 (10) | 5 (14) |
| Brain dysfunction on first study day, n (%) | |||
| Coma | 12 (35) | 9 (32) | 14 (40) |
| Delirium | 16 (47) | 15 (54) | 17 (49) |
| Antipsychotics prior to enrollment, n (%) | |||
| Haloperidol | 1 (3) | 2 (7) | 4 (11) |
| Ziprasidone | 0 (0) | 0 (0) | 0 (0) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; ICU, intensive care unit.
Median [interquartile range] unless otherwise noted
Other pulmonary included respiratory failure due to pulmonary hypertension, pulmonary fibrosis, pulmonary embolism, hemoptysis, and cystic fibrosis
Other medical included altered mental status, gastrointestinal hemorrhage, hepatic failure, malignancy, and renal failure
Surgery included colonic, gastric, head/neck, hepatobiliary, pancreatic, and vascular surgery
Antipsychotic Therapy
Duration and volume of study drug administered were similar among the three treatment groups (Table 2); the majority of patients in each group received at least 4 days of study drug. On days that study drug was administered, patients in the haloperidol group received 15.0 [10.8–17.0] mg/day and patients in the ziprasidone group received 113.3 [81.0–140.0] mg/day. All but 9 doses of study drug were administered as an enteral solution; three patients (one in the ziprasidone group and two in the placebo group) received three intramuscular doses each.
Table 2.
Study Drug Delivery and Other Antipsychotics
| Druga | Haloperidol (n=35) |
Ziprasidone (n=30) |
Placebo (n=36) |
p value |
|---|---|---|---|---|
| Study drug | ||||
| Days on drug | 7 [4–10] | 4 [3–10] | 5 [3–7] | 0.23 |
| Volume, mL | 110 [45–158] | 56 [28–141] | 82 [35–120] | 0.41 |
| Total Dose, mg | 110 [45–158] | 450 [220–1130] | NA | NA |
| Average Daily Dose, mg | 15 [11–17] | 113 [81–140] | NA | NA |
| Additional haloperidolb | ||||
| Patients treated, n (%) | 6 (17) | 9 (30) | 14 (39) | 0.13 |
| Total Dose, mg | 4.5 [2.9–23.8] | 10.0 [5.0–20.0] | 12.5 [5.5–50.2] | 0.30 |
| Average Daily Dose, mg | 4.5 [2.9–12.5] | 5.7 [5.0–10.0] | 5.0 [10.0–11.9] | 0.65 |
| Additional atypical antipsychoticsb | ||||
| Patients treated, n (%) | 1 (3) | 2 (7) | 4 (11) | 0.39 |
Abbreviations: NA, not applicable.
Median [interquartile range] unless otherwise noted
Administered by the ICU team in addition to study drug for breakthrough delirium and agitation.
In addition to study drug, at least one dose of antipsychotic medication was administered by the ICU team to one-third of all patients, almost entirely as open-label haloperidol (Table 2). In the placebo group, 15 (42%) patients received an antipsychotic in addition to study drug compared with 7 (20%) patients in the haloperidol group and 10 (33%) patients in the ziprasidone group (p = 0.14). These open-label antipsychotics were typically given as single doses; only 7 (19%) patients in the placebo group received an open-label antipsychotic on more than one day compared with 2 (6%) patients in the haloperidol group and 4 (13%) patients in the ziprasidone group. Among these patients, 3 (8%) in the placebo group, 1 (3%) in the haloperidol group, and 1 (3%) in the ziprasidone group received open-label haloperidol at doses comparable to those administered per protocol to patients in the haloperidol group (i.e., 15 mg/day or more). Thus, compared to the study drug doses, the total doses of open-label haloperidol were small; a median [IQR] dose of 4.5 [2.9–23.8] mg was administered to 6 patients in the haloperidol group over the course of the trial, 10.0 [5.0–20.0] mg was administered to 9 patients in the ziprasidone group, and 12.5 [5.5–50.2] mg was administered to 14 patients in the placebo group.
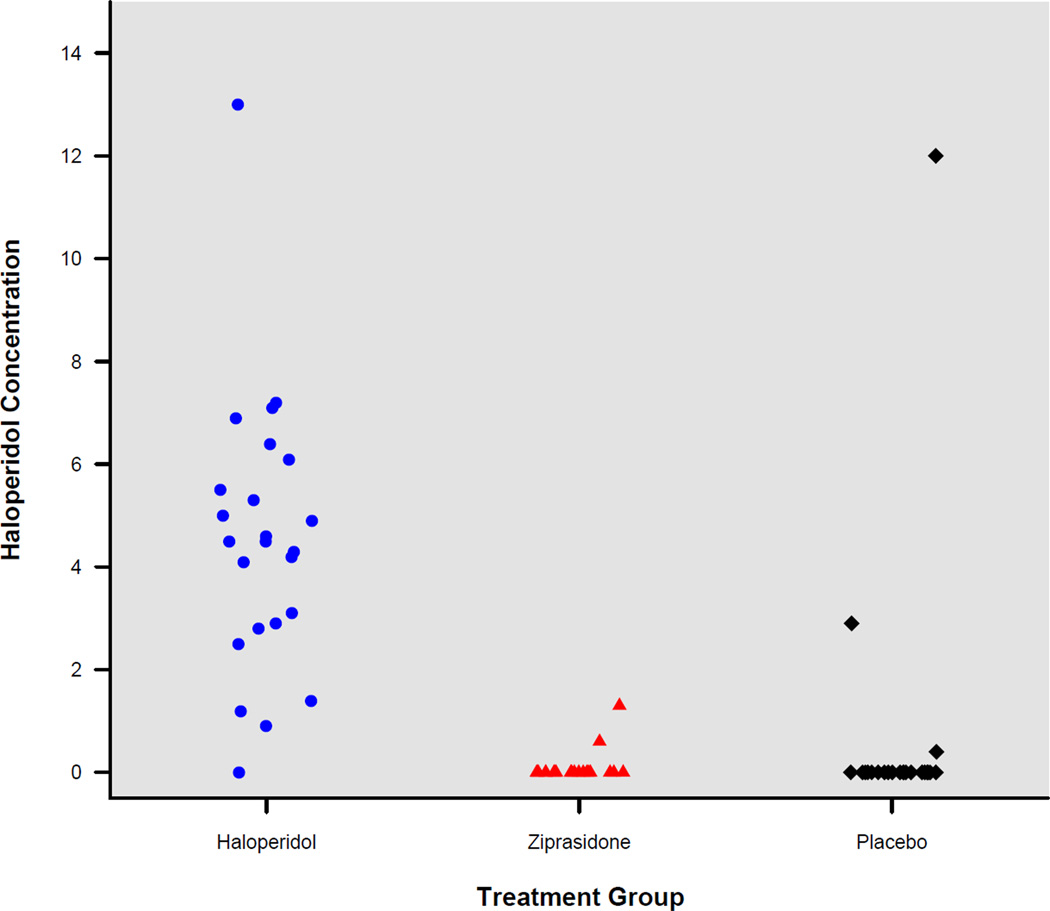
Forty-eight hours after study drug initiation, only patients in the haloperidol group had circulating concentrations of haloperidol in the range considered therapeutic for schizophrenia (Figure 2); a therapeutic range for ICU delirium has not been defined. The median [IQR] haloperidol plasma concentration was 4.5 [2.85–5.8] ng/mL in the haloperidol group compared with 0 [0–0] ng/mL in the ziprasidone group and 0 [0–0] ng/mL in the placebo group (p = 0.0001). Patients in the ziprasidone group had therapeutic circulating concentrations of ziprasidone 48 hours after study drug initiation (median 67 ng/mL, IQR 18.5–138 ng/mL).
Figure 2. Haloperidol plasma concentrations on study day two according to study group.
For patients in the haloperidol group, the median [interquartile range] haloperidol plasma concentration was 4.5 [2.85–5.8] ng/mL. Alternatively, all but two patients in the ziprasidone group and three in the placebo group had undetectable haloperidol plasma concentrations.
Neurologic and Other Outcomes
Neither haloperidol nor ziprasidone significantly increased the number of days patients were alive without delirium or coma, compared with placebo, and the duration of both delirium and coma was similar among treatment groups (Figure 3 and Table 3). Additionally, daily delirium risk was no different for those treated with haloperidol (odds ratio [OR] for delirium 1.2, 95% confidence interval [CI] 0.6 to 2.2) or ziprasidone (OR 1.1, 95% CI 0.5 to 2.2) compared with placebo (p = 0.80); no interaction was noted between current mental status and the effect of study drug on delirium risk.
Figure 3. Line graph of percentage of patients alive without delirium or coma during the 21-day study period according to treatment group.
Acute brain dysfunction, in the form of delirium or coma, was common at enrollment in all treatment groups and resolved gradually throughout the course of the trial. Treatment with antipsychotics did not significantly affect the number of days patients were alive without delirium or coma; the median [interquartile range] delirium/coma-free days was 14.0 [6.0–18.0] days in the haloperidol group vs. 15.0 [9.1–18.0] days in the ziprasidone group vs. 12.5 [1.2–17.2] days in the placebo group) (p = 0.66).
Table 3.
Clinical Outcomes
| Outcomea | Haloperidol (n=35) |
Ziprasidone (n=30) |
Placebo (n=36) |
p value |
|---|---|---|---|---|
| Delirium/coma-free daysb | 14.0 [6.0–18.0] | 15.0 [9.1–18.0] | 12.5 [1.2–17.2] | 0.66 |
| Delirium days | 4 [2–7] | 4 [2–8] | 4 [2–6] | 0.93 |
| Resolution of delirium on study drug, n (%)c | 24 (69) | 23 (77) | 21 (58) | 0.28 |
| Coma days | 2 [0–4] | 2 [0–4] | 2 [0–5] | 0.90 |
| % of days accurately sedatedd | 70 [56–83] | 64 [50–94] | 71 [53–92] | 0.91 |
| Ventilator-free days | 7.8 [0–15.0] | 12.0 [0–18.6] | 12.5 [0–23.3] | 0.25 |
| Length of stay, days | ||||
| ICU | 11.7 [4.6–15.7] | 9.6 [3.8–14.5] | 7.3 [4.7–12.3] | 0.70 |
| Hospital | 13.8 [9.4-NA] | 13.5 [9.3-NA] | 15.4 [8.9-NA] | 0.68 |
| 21-day mortality, n (%) | 4 (11) | 4 (13) | 6 (17) | 0.81 |
| Average extrapyramidal symptoms scoree | 0 [0–0.2] | 0 [0–0] | 0 [0–0] | 0.56 |
Median [interquartile range] unless otherwise noted
The number of days patients were alive without delirium or coma during the 21-day study period
Patients without resolution of delirium on study drug included those who died or were discharged with delirium, those who had delirium >21 days, and those with resolution of delirium after study drug was discontinued for other reasons, e.g., at study day 14.
Percentage of days while on study drug that patients were either at the level of sedation targeted by the ICU team or within 1 RASS point of the stated goal.
Measured using a modified Simpson-Angus Scale (29), composed of six items scored from 0 (normal) to 4 (extreme)
Daily sedation goals (i.e., target RASS) were similar in the three groups throughout the study (p = 0.52); on study day 1, the median target RASS was -2 (light sedation) in all groups, rising to RASS -1 (drowsy) by study day 3 and RASS 0 (alert and calm) by study day 5 in all groups. To achieve these sedation goals, patients in the three treatment groups received similar doses of sedative medications (Figure 4), and accurate sedation was achieved on the majority of days for patients in all three groups (Table 3).
Figure 4. Sedative exposure according to treatment group and study day.
During the trial, patients in the three treatment groups received similar doses per day of benzodiazepines (p = 0.10), opiates (p = 0.87), and propofol (p = 0.16). These graphs display the median dose of these sedatives received by patients in each treatment group according to study day. Benzodiazepine doses are displayed in lorazepam equivalents; opiate doses are displayed in fentanyl equivalents. For each class of sedative, the number of patients in each group who received any sedative is also shown according to study day.
Abbreviations: HAL, haloperidol; ZIP, ziprasidone; PBO, placebo
Other clinical outcomes were similarly unaffected by antipsychotic treatment, including duration of ICU and hospital stay, time spent alive and breathing without assistance, and mortality during the study period.
Safety
No serious adverse event occurred during the trial; none of the patients developed signs or symptoms of neuroleptic malignant syndrome, and no ventricular arrhythmias occurred in patients after initiation of the study drug.
Ten (29%) patients in the haloperidol group reported symptoms consistent with akathisia, compared with 6 (20%) patients in the ziprasidone group and 7 (19%) patients in the placebo group (p = 0.60). Among patients who reported akathisia, severity of symptoms (as determined by the maximum score reported by each patient on a visual analog scale) was not significantly different according to treatment group (median [IQR], 7.5 [2.6–8.0] in the haloperidol group vs. 4.0 [2.0–8.5] in the ziprasidone group vs. 5.0 [3.5–9.4] in the placebo group; p = 0.65). In contrast to akathisia, other extrapyramidal symptoms were experienced by fewer patients according to the modified Simpson-Angus Scale; 4 (11%) patients in the haloperidol group vs. 2 (7%) patients in the ziprasidone group vs. 6 (17%) patients in the placebo group were observed to have these extrapyramidal symptoms (p = 0.46). Only one patient, who was in the placebo group, had extrapyramidal symptoms that prompted treatment with benztropine.
Ten patients had prolongation of the QTc >500 ms while receiving study drug, usually within 48 hours of study drug receipt. Two of these patients were in the haloperidol group, five were in the ziprasidone group, and three were in the placebo group (p = 0.31). None of the patients developed a ventricular arrhythmia.
DISCUSSION
In the MIND Trial, the first reported randomized, placebo-controlled trial to evaluate the efficacy of antipsychotics for delirium in the ICU (20), we found no evidence to suggest that either haloperidol or ziprasidone effectively treats delirium in mechanically ventilated ICU patients. Patients treated with these medications spent no more time free of delirium and coma during the trial than did patients treated with placebo. Similarly, the adverse effects classically associated with antipsychotics were not significantly more likely to affect patients in the treatment groups compared with those receiving placebo. In light of the current widespread use of haloperidol as well as atypical antipsychotics for the management of ICU delirium, these results strongly suggest that a much larger, multicenter, placebo-controlled trial is needed to definitively determine whether or not continued use of antipsychotics in the ICU is warranted.
Whereas some claim that it is not feasible to randomize this difficult population of critically ill patients to treatment with antipsychotics or placebo due to frequent use of these medications in the ICU, the MIND Trial demonstrates that a double-blind, placebo-controlled trial of antipsychotics for ICU delirium is feasible. Not only did the institutional review boards of multiple medical centers approve the trial as ethically sound, ICU clinicians and surrogate decision makers similarly agreed that patients’ participation in the MIND Trial was appropriate, and the patients themselves nearly uniformly reconsented once capable. Withdrawal from the trial was minimal as was discontinuation of study drug due to adverse effects. In addition, patients were assessed easily throughout the trial for delirium using a validated, reliable instrument, and objective tools were available to facilitate monitoring for adverse effects, including extrapyramidal symptoms.
Though the pathogenesis of delirium in critically ill patients remains relatively unproven, hypotheses generated by research outside of the ICU propose that delirium results from a decrease in acetylcholine and increase in dopamine in the brain (37), both of which may occur in response to factors promoting cerebral oxidative stress during critical illness. Antipsychotics, which exert their affects by altering concentrations of a variety of neurotransmitters in the central nervous system, have therefore been recommended as potentially effective pharmacologic therapies for delirium. Haloperidol, for instance, works primarily via potent antagonism of the dopamine D-2 receptor (38), whereas ziprasidone (likewise a dopamine D-2 antagonist) also has important action as an antagonist of serotonin 5-HT2A receptors, thereby reducing the extrapyramidal side effects caused by dopamine blockade (39). In addition to these neurotransmitter-mediated effects, animal and in vitro models suggests that typical and atypical antipsychotics also have immunomodulatory and neuroprotective effects that may serve to reduce the CNS derangements resulting from critical illness (40, 41).
Two clinical trials have found that antipsychotics, compared with placebo, hasten the resolution of delirium in hospitalized patients without critical illness. Hu et al. (42) found that haloperidol and olanzapine each led to earlier improvements in Delirium Rating Scales scores among 175 elderly patients, compared with placebo. Similarly, Kalisvaart and colleagues (43) found that haloperidol reduced the severity and duration of delirium among elderly hip-surgery patients, compared with placebo. In the only clinical trial published to date that evaluated antipsychotics for delirium in the ICU, Skrobik et al. (14) observed that Delirium Index scores fell over time for patients treated with haloperidol and those treated with olanzapine, but no placebo group was studied.
Despite the findings of these trials and a sound scientific rationale, our results do not support the hypothesis that antipsychotics definitively treat delirium during intense critical illness. Several possible explanations for these results exist. First, this pilot investigation, which was designed primarily to demonstrate the feasibility of a double-blind, placebo-controlled trial of antipsychotics for ICU delirium, was likely significantly underpowered to demonstrate the potential efficacy of these medications for many outcomes, such as length of stay and survival. Second, antipsychotics may effectively treat some “positive” symptoms of hyperactive delirium, e.g., psychomotor agitation and hallucinations, without reducing “negative” symptoms of hypoactive delirium, including inattention, disordered cognition, and a depressed level of consciousness. Third, changes in drug absorption due to critical illness may have diminished circulating concentrations of the study drugs. Our measurements suggested therapeutic concentrations were achieved, but due to limited resources only one measurement (48 hours post-enrollment) was made per patient. Finally, delirium in the ICU may be less responsive to focused pharmacologic therapies than delirium in hospitalized patients without critical illness. Whereas the latter population frequently develops delirium when one or two precipitating risk factors are encountered, critically ill patients are exposed on average to eleven different risk factors that may contribute to the development and persistence of delirium in the ICU (4, 5). Antipsychotics may be effective in treating delirium due to some risk factors (e.g., hypoxia due to cardiogenic pulmonary edema) but not delirium due to other risk factors (such as inflammation-driven coagulopathy due to sepsis). Or, treatment with antipsychotics without concomitant resolution of the majority of precipitating risk factors may be ineffective. Definitive treatment of ICU delirium undoubtedly requires the identification and treatment of underlying somatic causes, efforts which were undertaken by the ICU teams caring for patients in all three of the treatment groups in this trial.
Antipsychotic medications, however, may be beneficial when somatic causes of delirium are treated and modifiable delirium risk factors are optimized. Non-pharmacologic interventions targeting risk factors for delirium, for example, have been evaluated in non-ICU cohorts (44), though no data exists about the efficacy of these approaches in the ICU. Of the pharmacologic strategies intended to reduce the burden of delirium in the ICU, sedation with the α2-adrenergic receptor agonist dexmedetomidine has been shown to reduce the duration of delirium as well as coma in the ICU as compared with more traditional sedation using benzodiazepines (45, 46).
Important strengths of the MIND Trial include randomization, inclusion of a placebo group, use of validated and reliable instruments for the diagnosis of delirium and coma, and blinding of ICU and research personnel as well as patients to treatment allocation (though blinding required delivery of study drug enterally rather than intravenously, as haloperidol is most often delivered in the ICU, since the FDA did not permit intravenous administration of ziprasidone). A placebo group is essential in delirium trials, since the symptoms of this form of brain dysfunction resolve over time for the majority of patients without any specific treatment.
Limitations of the trial include the small sample size, lack of enforcement by study-personnel of a standardized sedation protocol, and the exposure of some patients in the ziprasidone and placebo groups to open-label haloperidol. The sample size was small by design, since our primary objective was to demonstrate feasibility. Ten percent of patients enrolled did not develop delirium; to enroll patients as early as possible so that study drug would be administered on all days that patients were delirious, we did not require a diagnosis of delirium at enrollment but instead enrolled patients at high risk. Thus, inclusion of a small number of non-delirious patients likely reduced our statistical power when comparing outcomes. Our results may not apply to some ICU patients who might normally be treated with antipsychotics, including patients transferred from another hospital after 5 days of mechanical ventilation, those without gastric access, those with severe dementia, and patients post-suicide attempt. Though clinicians in all participating ICUs utilize validated sedation scales and protocols as well as daily interruption of sedation, it is possible that not all patients were treated with the same approach to sedation. Daily sedation goals, however, (as measured by RASS targets determined by the ICU teams) were similar for patients in the three treatment groups as were daily doses of benzodiazepines, opiates, and propofol. Though strongly discouraged by study personnel, ICU teams occasionally treated patients with intravenous haloperidol despite receipt of study drug, primarily due to agitation. As shown, doses of open-label haloperidol were typically given to such patients on a single study day; only a very small number of patients were treated on more than one study day with doses comparable to the doses of haloperidol given per study protocol to patients in the haloperidol group, but these instances may have biased the results against showing a difference between treatment groups. Future trials should work to restrict open-label antipsychotic administration and track agitation as a distinct outcome.
CONCLUSIONS
The MIND Trial demonstrates that a randomized, placebo-controlled trial of antipsychotics for delirium in mechanically ventilated ICU patients is feasible. In this double-blind, placebo-controlled, multicenter clinical trial, neither haloperidol nor ziprasidone significantly reduced the duration of delirium compared with placebo. Patients in the three treatment groups spent a similar number of days alive without delirium or coma. This pilot investigation, however, was not designed to definitively determine whether antipsychotics are effective for ICU delirium. In light of the current widespread use of antipsychotics for the treatment of delirium in the ICU, a much larger multicenter placebo-controlled trial is needed to decisively and carefully determine whether or not continued use of antipsychotics in the ICU is appropriate.
ACKNOWLEDGEMENTS
We thank the MIND Trial study personnel at Vanderbilt University Medical Center, University of North Carolina Hospitals, University of Iowa Hospitals, Moses H. Cone Memorial Hospital, Saint Thomas Hospital, and the Department of Veterans Affairs Medical Center in Nashville, TN; members of the Data and Safety Monitoring Board (Arthur P. Wheeler, M.D., Jonathan Schildcrout, Ph.D., and Tina V. Hartert, M.D., M.P.H.); and the ICU staff at Vanderbilt University Medical Center, University of North Carolina Hospitals, University of Iowa Hospitals, Moses H. Cone Memorial Hospital, Saint Thomas Hospital, and the Department of Veterans Affairs Medical Center in Nashville, TN for their invaluable participation in the trial.
Funding: This investigator-initiated study was aided by receipt of study drug from Pfizer, Inc., who had no role in the design or conduct of the trial; in the collection, analysis, or interpretation of the data; or in the preparation, review, approval, or publication strategy of this manuscript. Dr. Girard received support from the National Institutes of Health (HL007123), the Hartford Geriatrics Health Outcomes Research Scholars Award Program, the Vanderbilt Physician Scientist Development Program, and the VA Tennessee Valley Geriatric Research, Education and Clinical Center (GRECC). Dr. Pandharipande received support from the VA Clinical Science Research and Development Service (VA Career Development Award), the ASCCA-FAER-Abbott Physician Scientist Award, and the Vanderbilt Physician Scientist Development Program. Dr. Ely received support from the VA Clinical Science Research and Development Service (VA Merit Review Award), the VA Tennessee Valley GRECC, and the National Institutes of Health (AG027472).
Footnotes
MIND TRIAL INVESTIGATORS
Vanderbilt University School of Medicine, Nashville, TN: Timothy D Girard, MD, MSCI, Pratik P Pandharipande, MD, MSCI, Brenda T Pun, MSN, Bryan Cotton, MD, Russell R. Miller, MD, MPH, Jennifer L Thompson, MPH, Ayumi K Shintani, PhD, MPH, Herbert Y Meltzer, MD, Paul W. Ragan, MD, Sharlet Anderson, MS, Joyce Okahashi, RN, Gordon R Bernard, MD, Robert S Dittus, MD, MPH, and E Wesley Ely, MD, MPH; University of North Carolina, Chapel Hill, NC: Shannon S Carson, MD, Peter Rock, MD, Lydia H Chang, MD, and Kayvon Salimi, MD; University of Iowa Carver College of Medicine, Iowa City, IA: Gregory A Schmidt, MD; Moses H. Cone Memorial Hospital, Greensboro, NC: Patrick E Wright, MD; Saint Thomas Hospital, Nashville, TN: Angelo E Canonico, MD; and the Department of Veterans Affairs Medical Center, Tennessee Valley Healthcare System, Nashville, TN: Timothy D Girard, MD, MSCI, Pratik P Pandharipande, MD, MSCI, Bryan A Cotton, MD, Robert S Dittus, MD, MPH, and E Wesley Ely, MD, MPH.
REFERENCES
- 1.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 2.Micek ST, Anand NJ, Laible BR, et al. Delirium as detected by the CAM-ICU predicts restraint use among mechanically ventilated medical patients. Crit Care Med. 2005;33:1260–1265. doi: 10.1097/01.ccm.0000164540.58515.bf. [DOI] [PubMed] [Google Scholar]
- 3.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois MJ, Bergeron N, Dumont M, et al. Delirium in an intensive care unit: a study of risk factors. Intensive Care Med. 2001;27:1297–1304. doi: 10.1007/s001340101017. [DOI] [PubMed] [Google Scholar]
- 5.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ouimet S, Kavanagh BP, Gottfried SB, et al. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33:66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 7.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32:2254–2259. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 8.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32:955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 9.Inouye SK, Foreman MD, Mion LC, et al. Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161:2467–2473. doi: 10.1001/archinte.161.20.2467. [DOI] [PubMed] [Google Scholar]
- 10.Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39:248–253. doi: 10.1067/mem.2002.122057. [DOI] [PubMed] [Google Scholar]
- 11.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Ely EW, Stephens RK, Jackson JC, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med. 2004;32:106–112. doi: 10.1097/01.CCM.0000098033.94737.84. [DOI] [PubMed] [Google Scholar]
- 13.Patel RL, Gambrell MA, Speroff T, et al. Delirium and sedation in the intensive care unit (ICU): survey of behaviors and attitudes of 1,384 healthcare professionals. Crit Care Med. 2009;37:825–832. doi: 10.1097/CCM.0b013e31819b8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skrobik YK, Bergeron N, Dumont M, et al. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444–449. doi: 10.1007/s00134-003-2117-0. [DOI] [PubMed] [Google Scholar]
- 15.Sharma ND, Rosman HS, Padhi ID, et al. Torsades de Pointes associated with intravenous haloperidol in critically ill patients. Am J Cardiol. 1998;81:238–240. doi: 10.1016/s0002-9149(97)00888-6. [DOI] [PubMed] [Google Scholar]
- 16.Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23:215–228. doi: 10.2165/00002018-200023030-00004. [DOI] [PubMed] [Google Scholar]
- 18.Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome. Br J Anaesth. 2000;85:129–135. doi: 10.1093/bja/85.1.129. [DOI] [PubMed] [Google Scholar]
- 19.Tandon R, Jibson MD. Extrapyramidal side effects of antipsychotic treatment: scope of problem and impact on outcome. Ann Clin Psychiatry. 2002;14:123–129. doi: 10.1023/a:1016811222688. [DOI] [PubMed] [Google Scholar]
- 20.Girard TD, Carson SS, Pandharipande PP, et al. The modifying the incidence of delirium (MIND) trial: a randomized controlled trial of the feasibility, efficacy, and safety of antipsychotics for the prevention and treatment of ICU delirium. Am J Respir Crit Care Med. 2008;177:A817. [Google Scholar]
- 21.Kay DWK. The epidemiology and identification of brain deficit in the elderly. In: Eisdorfer C, Friedel RO, editors. Cognitive and Emotional Disturbance in the Elderly: Clinical Issues. Chicago, IL: Year Book Medical Publishers, Inc.; 1977. pp. 11–26. [Google Scholar]
- 22.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 23.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16:275–293. doi: 10.1017/s1041610204000390. [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 25.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Ely EW, Pun BT. The Confusion Assessment Method for the ICU (CAM-ICU) training manual. [Accessed September 20, 2006]; Available online at: http://wwwicudeliriumorg/delirium/CAM-ICUTraininghtml. [Google Scholar]
- 27.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 28.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 29.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 30.Devanand DP, Marder K, Michaels KS, et al. A randomized, placebo-controlled dose-comparison trial of haloperidol for psychosis and disruptive behaviors in Alzheimer's disease. Am J Psychiatry. 1998;155:1512–1520. doi: 10.1176/ajp.155.11.1512. [DOI] [PubMed] [Google Scholar]
- 31.Suckow RF, Fein M, Correll CU, et al. Determination of plasma ziprasidone using liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;799:201–208. doi: 10.1016/j.jchromb.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 32.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Zeger SL, Liang KY. An overview of methods for the analysis of longitudinal data. Stat Med. 1992;11:1825–1839. doi: 10.1002/sim.4780111406. [DOI] [PubMed] [Google Scholar]
- 35.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 36.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med. 2001;134:657–662. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 37.Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Seminars in Clinical Neuropsychiatry. 2000;5:132–148. doi: 10.153/SCNP00500132. [DOI] [PubMed] [Google Scholar]
- 38.Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- 39.Seeger TF, Seymour PA, Schmidt AW, et al. Ziprasidone (CP-88,059): a new antipsychotic with combined dopamine and serotonin receptor antagonist activity. J Pharmacol Exp Ther. 1995;275:101–113. [PubMed] [Google Scholar]
- 40.Song C, Lin A, Kenis G, et al. Immunosuppressive effects of clozapine and haloperidol: enhanced production of the interleukin-1 receptor antagonist. Schizophr Res. 2000;42:157–164. doi: 10.1016/s0920-9964(99)00116-4. [DOI] [PubMed] [Google Scholar]
- 41.Schetz JA, Perez E, Liu R, et al. A prototypical Sigma-1 receptor antagonist protects against brain ischemia. Brain Res. 2007;1181:1–9. doi: 10.1016/j.brainres.2007.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu H, Deng W, Yang H. A prospective random control study comparison of olanzapine and haloperidol in senile delirium. Chongqing Med J. 2004;8:1234–1237. [Google Scholar]
- 43.Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658–1666. doi: 10.1111/j.1532-5415.2005.53503.x. [DOI] [PubMed] [Google Scholar]
- 44.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 45.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 46.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]