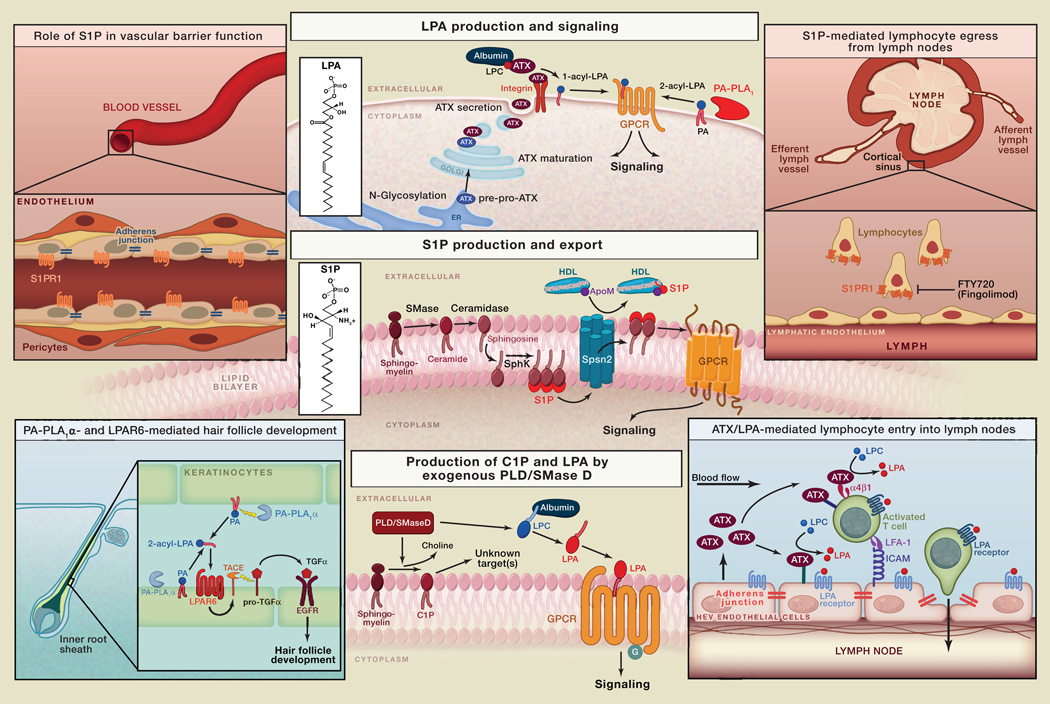
Lysophosphatidic acid (LPA; monoacyl-glycerol-3-phosphate) and sphingosine 1-phosphate (S1P) are bioactive lysophospholipids that regulate critical biological functions and disease processes. LPA and S1P act on distinct G protein-coupled receptors (GPCRs) that initiate multiple signaling cascades in many cell types. There are six known LPA receptors (LPAR1–6) and five for S1P (S1PR1–5). LPAR1-3 and S1PR1–5 constitute the Edg subfamily of GPCRs, whereas LPAR4–6 are more closely related to purinergic receptors. LPA signaling is critical to such diverse processes as vascular and neural development, lymphocyte homing (Kanda et al., 2008), and hair follicle development (Inoue et al., 2011) and is implicated in pulmonary fibrosis (Tager et al., 2008), neuropathic pain, pruritus, fetal hydrocephalus, and tumor progression. S1P signaling is essential for vascular development and endothelial integrity and plays key roles in the immune, central nervous, and cardiovascular systems (Blaho and Hla, 2011; Rivera et al., 2008).
Though LPA and S1P use similar signaling pathways, their biosynthetic routes are quite distinct. Bioactive LPA is produced extracellularly from more complex phospholipids by specific exo/ectophospholipases. In contrast, S1P is mostly synthesized intracellularly by sphingosine kinases and then exported across the plasma membrane. LPA and S1P are present in physiologically relevant concentrations in the circulation; they are rapidly turned over by cell-associated lipid phosphate phosphatases (LPPs). Plasma S1P regulates vascular barrier function; the role of plasma LPA is less well understood. This SnapShot summarizes the LPA/S1P biosynthetic pathways and highlights selected biological actions of either lipid.
LPA
LPA comprises various molecular species that vary in the length and degree of saturation of their fatty acid chain, which is esterified at the sn-1 or sn-2 position of the glycerol backbone. Ether-linked 1-alkyl-LPA and 1-alkenyl-LPA species also exist but are much less abundant. All six LPA receptors (LPAR1–6) can be stimulated by 1-acyl-LPA, albeit with different potencies. Some LPA receptors (LPAR3 and LPAR6) prefer unsaturated 2-acyl-LPA species, whereas LPAR5 exhibits a clear preference for ether-linked 1-alkyl-LPA.
Autotaxin
The major route of LPA production occurs through the hydrolysis of extracellular lysophosphatidylcholine (LPC; carrier-bound or membrane-derived) by a secreted lysophospholipase D (lysoPLD) named autotaxin (ATX). ATX, or ENPP2, is a unique member of the ectonucleotide pyrophosphatase/phosphodiesterase (ENPP) family. ATX is synthesized as a pre-pro-enzyme and, after N-glycosylation and proteolytic maturation, secreted as an active lysoPLD. It is present in the circulation and accounts for LPA production in plasma. Secreted ATX can interact with target cells by directly binding to activated integrins and possibly to cell surface heparan sulfate proteoglycans, which facilitates the production and delivery of LPA close to its receptors (Moolenaar and Perrakis, 2011).
ATX-LPA signaling regulates many biological processes, including the entry of lymphocytes into lymph nodes at special blood vessels, the high endothelial venules (HEVs). ATX secreted from HEV endothelium binds to chemokine-activated T cells via α4β1 integrins, and possibly to the endothelial cells themselves, to generate LPA from LPC. LPA, in turn, stimulates T cell motility and may open up endothelial cell junctions, thus promoting T cell migration across the HEV endothelium into lymph nodes (Kanda et al., 2008).
PA-Specific Phospholipases A1
A second but less common route of LPA production involves the hydrolysis of phosphatidic acid (PA) in the outer leaflet of the plasma membrane by membrane-associated ectophospholipases A1 (PA-PLA1α and PA-PLA1β; also known as LIPH and LIPI, respectively). The resulting unsaturated 2-acyl-LPA acts preferentially on LPAR6 (formerly P2RY5) and LPAR3. The PLA1α/LIPH-LPAR6 signaling axis regulates hair follicle development via transactivation of EGF receptors in keratinocytes of the inner root sheath (Inoue et al., 2011). Loss-of-function mutations in LIPH and LPAR6/P2RY5 underlie familial hair growth disorders.
Exogenous Phospholipases D
Receptor-active LPA can also be produced by exogenous phospholipases D (sphingomyelinases D), notably those from spider (Loxosceles) venom and certain pathogenic corynebacteria (van Meeteren et al., 2004). These unique PLDs hydrolyze both sphingomyelin (SM) and LPC to produce ceramide-1-phosphate (C1P, acting on unknown targets) and bioactive LPA, respectively, which leads to local dermonecrosis and intravascular coagulation.
S1P
S1P is produced from SM hydrolysis, involving the sequential actions of sphingomyelinase (SMase; type C), ceramidase, and either of two sphingosine kinases (SphK1 and SphK2). SMase and ceramidase are secreted enzymes; an ectoceramidase also exists. SphK1 and SphK2 have different tissue distribution and subcellular localization patterns. SphK1 is cytosolic, whereas SphK2 is also found in the nucleus. Upon cell activation, SphK1 transiently associates with the plasma membrane to phosphorylate sphingosine into S1P. A small fraction of SphK1 is secreted and detected in plasma. In the ER membrane, S1P is degraded by S1P lyase into hexadecenal and phosphoethanolamine. Basal activity of the metabolic enzymes maintains intracellular sphingolipid flux, which is coupled to other phospholipid biosynthetic pathways. Thus, intracellular S1P and dihydro-S1P fulfill metabolic roles as they are degraded by S1P lyase to provide metabolites for phospholipid biosynthesis and membrane homeostasis. Newly synthesized S1P is exported by a specific transporter termed Spsn2 (Kawahara et al., 2009). About 65% of circulating S1P is bound to Apolipoprotein M in HDL particles; a second S1P pool is bound to albumin and likely undergoes rapid turnover (Blaho and Hla, 2011). Plasma S1P (0.5–2 µM) is derived from multiple sources; red blood cells are thought to be the primary source, but endothelial cells may also contribute (Pappu et al., 2007). Lymphatic endothelial cells maintain S1P levels in lymph fluid. S1P levels in interstitial fluids of tissues such as the thymus and lymphoid organs are very low (nM), thus contributing to the formation of a systemic or vascular S1P gradient. High activity of S1P-degrading enzymes may help to maintain the S1P concentration gradient. The S1P gradient is used to promote the egress of immune cells from the thymus and secondary lymphoid organs (Rivera et al., 2008). Egress of hematopoietic progenitor cells from peripheral tissues also relies on the S1P gradient. Interference with the S1P gradient by S1P lyase inhibition or inhibition of the egress-promoting S1PR1 receptor results in profound perturbation of immune cell trafficking. This mechanism likely underlies the therapeutic efficacy of Fingolimod (Gilenya), a functional S1PR1 antagonist, in suppressing autoreactive T cell trafficking into the central nervous system. Fingolimod/Gilenya (code name: FTY720) has been approved for the treatment of relapsing multiple sclerosis (Brinkmann et al., 2010). In vascular endothelial cells, S1PR1 helps to maintain the barrier function of the endothelium by promoting the formation of adherens junctions and focal contacts. S1PR1 is also required for embryonic vasculogenesis (Blaho and Hla, 2011).
Abbreviations
- ApoM
apolipoprotein M
- ATX
autotaxin
- C1P
ceramide-1-phosphate
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- GPCR
G protein-coupled receptor
- HDL
high-density lipoprotein
- HEV
high endothelial venule
- LPA
lysophosphatidic acid
- LPC
lysophosphatidylcholine
- PA
phosphatidic acid
- PLA1
phospholipase A1
- PLD
phospholipase D
- S1P
sphingosine 1-phoshate
- SphK
sphingosine kinase
- SM
sphingomyelin
- SMase
sphingomyelinase
- TACE
tumor necrosis factor-alpha-converting enzyme
- TGFα
transforming growth factor alpha
REFERENCES
- Blaho VA, Hla T. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem. Rev. 2011;111:6299–6320. doi: 10.1021/cr200273u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Inoue A, Arima N, Ishiguro J, Prestwich GD, Arai H, Aoki J. LPA-producing enzyme PA-PLA1α regulates hair follicle development by modulating EGFR signalling. EMBO J. 2011;30:4248–4260. doi: 10.1038/emboj.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat. Immunol. 2008;9:415–423. doi: 10.1038/ni1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH, Perrakis A. Insights into autotaxin: how to produce and present a lipid mediator. Nat. Rev. Mol. Cell Biol. 2011;12:674–679. doi: 10.1038/nrm3188. [DOI] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008;8:753–763. doi: 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- van Meeteren LA, Frederiks F, Giepmans BN, Pedrosa MF, Billington SJ, Jost BH, Tambourgi DV, Moolenaar WH. Spider and bacterial sphingomyelinases D target cellular lysophosphatidic acid receptors by hydrolyzing lysophosphatidylcholine. J. Biol. Chem. 2004;279:10833–10836. doi: 10.1074/jbc.C300563200. [DOI] [PubMed] [Google Scholar]