Abstract
The extent to which T cell-mediated immune surveillance is impaired in human cancer remains a question of major importance, given its potential impact on the development of generalized treatments for advanced disease where the highest degree of heterogeneity exists. Here we report the first global analysis of immune dysfunction in patients with advanced hepatocellular carcinoma (HCC). Using multi-parameter FACS analysis, we quantified the cumulative frequency of T regulatory cells (Tregs), exhausted CD4+ helper T cells, and myeloid-derived suppressor cells (MDSC) to gain concurrent views on the overall level of immune dysfunction in these inoperable patients. We documented augmented numbers of Tregs, MDSC, PD-1+ exhausted T cells and increased levels of immunosuppressive cytokines in HCC patients, compared to normal controls, revealing a network of potential mechanisms of immune dysregulation in HCC patients. In dampening T cell-mediated anti-tumor immunity, we hypothesized that these processes may facilitate HCC progression and thwart the efficacy of immunotherapeutic interventions. In testing this hypothesis, we demonstrated that combined regimens to deplete Tregs, MDSC, and PD-1+ T cells in advanced HCC patients restored production of granzyme B by CD8+ T cells, reaching levels observed in normal controls, and also modestly increased the number of IFN-γ producing CD4+ T cells. These clinical findings encourage efforts to restore T cell function in patients with advanced stage disease, by highlighting combined approaches to deplete endogenous suppressor cell populations that can also expand effector T cell populations.
Keywords: Foxp3+ Tregs, GARP, myeloid-derived suppressor cells, hepatocellular carcinoma, dendritic cell, granzyme B
Introduction
Hepatocellular carcinoma (HCC) is the fifth-most common cancer in the world and the third highest cause of cancer-related mortality globally (1). HCC develops in patients with chronic hepatitis, either due to chronic hepatitis B or C viral infection or due to inflammation following aflatoxin ingestion, or excessive alcohol consumption. Unfortunately, most HCC patients are first diagnosed with the disease at an advanced stage or present with poor liver function, thereby preventing the use of potentially curative therapies. Thus, treatment options for patients with advanced stage disease are limited to either chemoembolization or systemic therapies, which include sorafenib an oral anti-angiogenic agent that is the current backbone of HCC therapy. Though these approaches have led to improved clinical outcomes, patients remain at high risk of disease recurrence after potentially curative surgery and ablation, and survival remains less than one year for patients with advanced stage disease. As toxic chemotherapies are often not well-tolerated by these patients due to liver dysfunction, novel immune-based therapies such as anti-tumor vaccination and adoptive transfer of tumor-specific cytotoxic T cells (CTL) hold promise; however, their impact on tumor regression remains limited (2). The lack of efficacy of such therapies implies that HCC, like many other cancers, has developed multiple strategies of escaping tumor-specific immunity (3). In order to develop efficacious immunotherapies for HCC, clinicians are faced with various challenges regarding the mechanism by which chronic viral infections and inflammation due to hepatitis and cirrhosis impacts on both tumor progression and immune cell networks. Thus, correcting the deficiencies in the basic biology of these interactions, will likely lead to a greater clinical understanding of the disease and better treatment regimens.
Immune dysfunction in a variety of cancer patients has been studied and found to include suppression of tumor-associated antigen-reactive lymphocytes by Tregs (4–7), accumulation of myeloid-derived suppressor cells (MDSC) (8–10) and dysfunctional dendritic cells (DC) (11–13). Increased numbers of CD4+CD25+ Tregs have been reported in the PBMC of HCC patients (14, 15). However, as these studies characterized Tregs merely by the surface expression of CD25, there is the possibility of inadequate discrimination of Tregs from activated CD25+ effector T cells. Depletion of Tregs or blockade of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) in vivo in experimental tumor models (16) and cancer patients (17, 18) has resulted in enhanced anti-tumor immune responses and more efficacious immunotherapy regimens.
In this study, we examined Tregs and soluble immunosuppressive factors as they have been implicated to be independent prognostic factors regardless of the etiology of HCC (5). Although molecules such as CD25, CTLA-4, CD62L and CD127 are differentially expressed on Tregs, they are also expressed during chronic T cell activation or differentiation (19), and therefore these markers may not adequately discriminate Tregs with high suppressive potential from recently activated T cells. The molecule leucine rich repeat containing 32 (LRRC32), also known as glycoprotein A repetitions predominant (GARP), has recently been reported to be a highly specific molecule for activated Foxp3+ Tregs with high suppressive function (20). As cancer patients with advanced stage disease are likely to have greater numbers of suppressive T cell subsets, we elected to evaluate GARP expression in order to distinguish highly suppressive Foxp3+ Tregs in HCC patients from Foxp3+ T cells that do not exert suppressive function.
Immunological dysfunction observed in cancer patients can also be attributed to MDSC, which are known to be elevated in chronic inflammation and malignancies (8, 9). Via diverse mechanisms, MDSC cause profound suppression of both innate and acquired anti-tumor immunity, including T cell-based immunotherapy. Considering the potential immunosuppressive role of MDSC in cancer patients, we elected to study the prevalence of this cell subset in HCC patients.
Interaction between programmed death-1 (PD-1) receptor and its ligands PD-L1 (B7-H1) and PD-L2 (B7-DC) also influences the immune response (21). Generation of T cell responses is determined by the balance of activating signals, mediated by CD28:B7-1/B7-2, or inhibitory signals produced by PD-1:B7-H1 (22). Due to its importance in suppressing T cell responses, we also compared T cell PD-1 expression levels in HCC patients and healthy controls in order to elucidate the potential role of T cell exhaustion in the immune profile of advanced stage HCC.
DCs are essential for the induction of tumor-specific T cell responses. DC differentiation, maturation and functionality are severely impaired in several human malignancies and this represents another mechanism by which potentially efficacious endogenous anti-tumor immune responses in HCC patients may be dysregulated (23). Tumor-derived factors such as TGF-β and VEGF have been shown to inhibit the differentiation of monocytes into mature DC and concomitantly skew their development towards highly suppressive MDSC (24).
The rationale of the present study was to perform a stringent multi-parameter evaluation of candidate immunosuppressive networks in conjunction with T cell subset functionality in advanced stage HCC patients in comparison to healthy controls and to evaluate the impact of chronic viral infection and prior treatment on the immune profiles of the patients. Additional relevance was provided by ascertaining the changes in T cell function that could be revealed by depletion of the suppressive cells. This represents the first study that has determined whether targeted depletion of immunosuppressive cells in advanced HCC has the potential to restore endogenous anti-tumor T cell function.
Materials and Methods
Blood samples
Heparinized peripheral blood samples were obtained from HCC patients through Data Bank and Biorepository at RPCI and from healthy donors after obtaining informed consent. Clinical therapy and baseline demographic data were recorded. Clinical characteristics were collected by chart review (Table 1) and merged with immune results that were analyzed in blinded fashion. PBMC were isolated by Ficoll-Paque™ PLUS density gradient centrifugation of blood samples (GE Healthcare) as described elsewhere (25).
Table 1.
| Patient characteristics | HCC (n=23) | Normal Healthy (n=20) |
|---|---|---|
| Gender (M:F) | 16:7 | 10:10 |
| Median age (yrs) | 64 (40–82) | |
| Child Pugh Class Liver function | A = 16 | − |
| B = 7 | ||
| C = 0 | ||
| BCLC class | A = 0 | − |
| B = 21 | ||
| C = 2 | ||
| D = 0 | ||
| Etiology of liver disease | Hepatitis B = 1 | − |
| Hepatitis C = 13 | ||
| Hepatitis B and C = 1 | ||
| Alcohol = 2 | ||
| No known risk factor = 6 | ||
| Prior therapies (some patients had more than one therapy hence numbers ≠ 23) | None = 10 | − |
| Locoregional = 8 | ||
| Chemotherapy or biologic therapy = 7 | ||
| Liver resection = 4 | ||
For the stimulation assays 8 patients were chosen. Of those patients 5 are alive, median follow up time is 15 months (range 12–25 months) and of the 3 patients who have died, median survival was 7 months (range 2–10 months). The patients had BCLC class B (n=7) or C (n=1) liver function and Child Pugh class A (n=6) or class B (n=2) liver function. The etiology of cirrhosis was chronic hepatitis C (n=4) and neither hepatitis HBV or HCV (n=4).
Tregs
FACS analysis was performed to measure peripheral blood Treg frequency using APC-H7 anti-CD3, V450 anti-CD4, PE anti-CD127, PE-Cy5 anti-CTLA-4 (BD Biosciences) and PE-Cy7 anti-PD-1, Alexa488 anti-Foxp3 (Biolegend). Detection of surface GARP levels was achieved by using mouse anti-human GARP (Enzo Life Sciences) followed by staining with PE F(ab’)2 goat anti-mouse IgG (Jackson ImmunoResearch Laboratories). Cells were incubated with normal mouse IgG for 10 min prior to surface and intracellular staining. Intracellular analysis for Foxp3 and CTLA-4 was performed after fixation and permeabilization of cells using intracellular staining kit (eBioscience) according to manufacturer’s instructions. All samples were acquired on LSRII flow cytometer (BD Biosciences) and analyzed using Flowjo Model Fit (Tree Star).
MDSC
MDSC in the peripheral blood were detected using FITC anti-CD11b (eBioscience), PE-Cy5 anti-CD33, APC anti-CD14, V450 anti-HLA-DR (BD Biosciences).
Cytokine ELISA
Plasma isolated during PBMC separation was assayed to quantify the level of IFN-γ, IL-10, and TGF-β1 using specific ELISA kits according to the manufacturer’s instructions (eBioscience).
Depletion of suppressor cells from PBMC
HCC patient PBMC depleted of GARP+, CTLA-4+, and PD-1+ T cells (gated on the CD3+CD4+ T cells) were obtained by cell sorting using a FACSAria. CD33+ MDSC were eliminated after gating on HLA-DR−CD14− cells. PBMCs with and without the depleted suppressor cells were used to measure effector T cell proliferation and cytokine production when stimulated with PHA or anti-CD3/anti-CD28 in vitro as described below.
Lymphocyte proliferation assay
Carboxyfluorescein succinimidyl ester (CFSE) staining of PBMC was performed according to the manufacturer’s instructions (Invitrogen). Briefly, 1×107 PBMCs were incubated in HBSS containing 2 mM CSFE for 10 min at 37°C and then washed three times with RPMI medium containing 10% human AB serum. Labeled cells (5×104 cells/well) were incubated in the presence or absence of 5 µg/ml PHA (Sigma) or 1 µg/ml anti-CD3 antibody and 0.5 µg/ml anti-CD28 antibody (eBioscience) in a 96 well flat bottom plate. After four days of stimulation, harvested cells were stained with APC-H7 anti-CD3, V450 anti-CD4 and V500 anti-CD8.
Intracellular cytokine-staining assay
Four hours prior to harvesting PBMCs treated with PHA or anti-CD3/anti-CD28, PMA (20 ng/ml), 1 µl of 1 mM ionomycin/ml (Sigma) and 1µg/ml of monensin (BD Biosciences) were added to the culture. Cells were washed and stained with APC-H7 anti-CD3 for 30 min at 4°C. After fixation and permeabilization, intracellular staining was performed using V450 anti-CD4, V500 anti-CD8, PE anti-IFN-γ, PE anti-granzyme B, PE isotype control and Alexa700 anti-Foxp3 (eBioscience).
Statistical analysis
Our primary objective was to compare immunophenotypes in HCC patients (n=23) and healthy controls (n=20). For each of 29 possible outcomes, the null hypothesis of no difference in the outcome distribution between the two groups was assessed using an Exact Wilcoxon Rank Sum (Mann–Whitney U) test. Per-comparison two-sided p-values less than 0.05 were considered statistically significant. With 23 patients in each group, similarly conducted experiments have 80% power to detect a minimum difference in mean expression of 0.9 standard deviations. Tests for functional responses were done in HCC patients (n=8) and healthy controls (n=8). Patients were selected on the basis of either elevated levels of Tregs/MDSC or low levels of the same. Tests of this nature have 80% power to detect a minimum difference of 1.5 standard deviations.
Post-depleted HCC and healthy control samples were also compared using the Exact Wilcoxon Rank Sum (Mann–Whitney U) test. Matched pre-depleted and post-depleted samples within the HCC patients were compared using the Wilcoxon Signed Rank Test.
Given the number of comparisons conducted, we also considered a correction for multiple testing. Methods developed by Hochberg (26) were used to identify outcome differences in the HCC and control subjects that maintained a 0.05 family-wise Type I error rate. Based on the 29 tests considered, this method identified 15 comparisons with per-comparison p-values less than 0.0027 as being interesting.
Results
Patients
Clinical characteristics of HCC patients are summarized in Table 1. At the time of this report 17/23 HCC patients have died and median survival for these patients is 7 months (range 1–21 months). For the surviving 6 patients, median follow up from time of PBMC collection is 14 months (range 12–25months). All 23 HCC patients analyzed in this study had locally advanced or metastatic disease and none had early stage surgically resectable or transplantable disease.
Cirrhosis
By radiographic criteria, 11/23 patients showed no signs of cirrhosis and 5 of them also had prior liver resection that confirmed this. Ten of these 11 patients also had Child Pugh liver cirrhosis score Class A, while one patient was a class B, confirming the limited sensitivity of radiographic methods to assess degree and or presence of cirrhosis. Of the remaining 12/23 patients had some evidence radiographically (CT, MRI, USG or more than one) of cirrhosis and none were deemed as surgical candidates, even though 5 of them had Child Pugh Class A or good liver function.
Tregs in advanced HCC exhibit a highly immunosuppressive phenotype
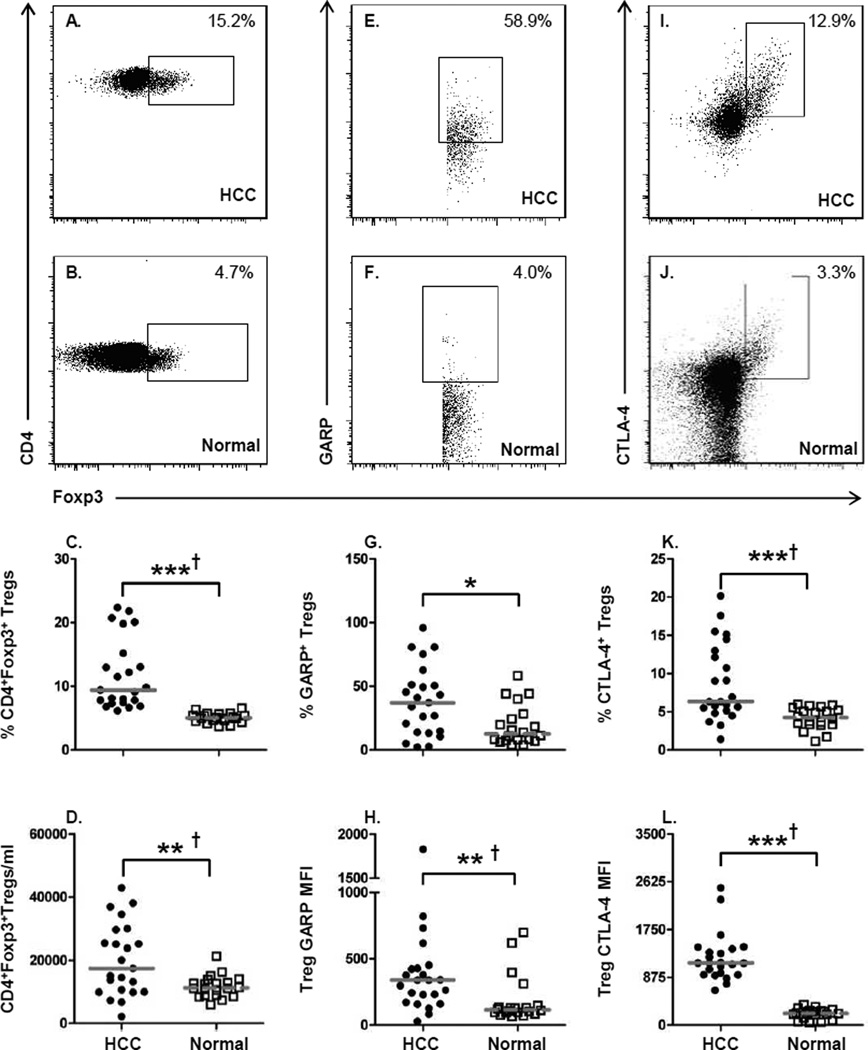
Using the baseline parameters for Tregs that other investigators have established, we also began our study by measuring the frequency of CD3+CD4+Foxp3+ Tregs. These cells were additionally identified by exclusion of CD127 expression. Representative staining and gating of CD4+Foxp3+ Tregs from one HCC patient (Fig. 1A) and one normal donor (Fig. 1B) are shown; the Tregs were present at higher frequencies in HCC patients compared to normal controls (HCC: 9.4% ± 5.4 vs. normal: 5.0% ± 0.78; P = 0.001) (Fig. 1C). In addition, the absolute number of Foxp3+ T cells in the peripheral blood was also greater in the HCC patients compared to controls (HCC: 17,440 cells/ml ± 11,343 vs. normal: 11,282 ± 3,486 cells/ml; P = 0.015) (Fig. 1D). The ratio of Foxp3 mRNA copy number was significantly increased in HCC patients as compared to controls (Supplementary Fig. 1A). A significant correlation was seen between Foxp3 gene expression and percentage of CD4+Foxp3+ T cells (Supplementary Fig. 1B), confirming that the two assays measure the same cell population. Comparison of Treg frequencies or absolute number after stratification of patients based on HCV status (Supplementary Fig. 2A, C) or having received prior treatment (Supplementary Fig. 2B, D) did not influence the level of Treg accumulation in HCC patients.
Figure 1. Increased numbers of GARP and CTLA-4 expressing Tregs in HCC patients.
Flow cytometric analysis was performed on PBMCs from HCC patients (n=23) and healthy
controls (n=20). (A, B) Representative staining from an individual HCC patient and
normal healthy donor for the frequency of CD3+CD4+Foxp3+ T cells.
(C) Frequency and (D) absolute number of cells/ml of
CD4+Foxp3+ Tregs in peripheral blood of HCC patients and normal healthy
subjects. (E, F) Representative staining of GARP on
CD3+CD4+Foxp3+ T cells. (G) Frequency of
GARP+ Tregs and (H) GARP expression levels measured by mean fluorescent
intensity (MFI) on Tregs. (I, J) Representative staining of CTLA-4 on
CD3+CD4+Foxp3+ T cells. (K) Frequency of
CTLA-4+ Tregs and (L) CTLA-4 expression levels. Each symbol represents an
individual HCC patient ( ) or normal healthy subjects (
) or normal healthy subjects ( ); lines represent median
values for the group. * P < 0.05, ** P < 0.01, ***
P < 0.001, Mann-Whitney U test; † P < 0.05
Hochberg adjustment for multiple comparison.
); lines represent median
values for the group. * P < 0.05, ** P < 0.01, ***
P < 0.001, Mann-Whitney U test; † P < 0.05
Hochberg adjustment for multiple comparison.
We expanded our analysis of Foxp3+ Tregs to include measurement of GARP (Fig. 1E, F) and CTLA-4 (Fig. 1I, J) to determine whether these cells in HCC patients exhibited a highly immunosuppressive phenotype. Evaluation of GARP and CTLA-4 expression on Tregs in advanced HCC has not been previously reported and therefore the analysis of these two markers of highly immunosuppressive Tregs is critical to understanding the nature of Treg-mediated immune suppression in HCC patients The frequency of GARP+Foxp3+ Tregs was significantly higher in HCC patients than in controls (HCC: 37.1% ± 27.1 vs. normal: 12.5% ± 15.89; P = 0.01) (Fig. 1G). The level of GARP expression was also elevated on Foxp3+ Tregs of HCC patients as compared to controls (HCC: 341 ± 368 MFI vs. normal: 116 ± 181 MFI; P = 0.001) (Fig. 1H). Neither chronic viral infection nor prior treatment of HCC patients had any impact on the profiles of GARP expression in patients (Supplementary Fig. 3A–D).
The frequency of Foxp3+ Tregs that expressed intracellular CTLA-4 was also significantly greater in HCC patients compared to controls (HCC: 6.4% ± 5.0 vs. normal: 4.2% ± 1.4; P = 0.001) (Fig. 1K). In addition, CTLA-4 expression levels were also significantly higher on Tregs from HCC patients as compared to controls (HCC: 1,137 ± 440 MFI vs. normal: 218 ± 94.4 MFI; P = 0.001) (Fig. 1L). Neither the frequency of CTLA-4+ T cells nor the CTLA-4 expression levels were influenced by chronic viral infection or prior treatment (Supplementary Fig. 3E–H). The presence of GARP+CTLA-4+ Tregs represents the first identification of a highly immunosuppressive Treg population in advanced HCC patients and that these cells may pose a significant impediment to the efficacy of anti-tumor responses elicited by immunotherapeutic or cancer vaccine approaches.
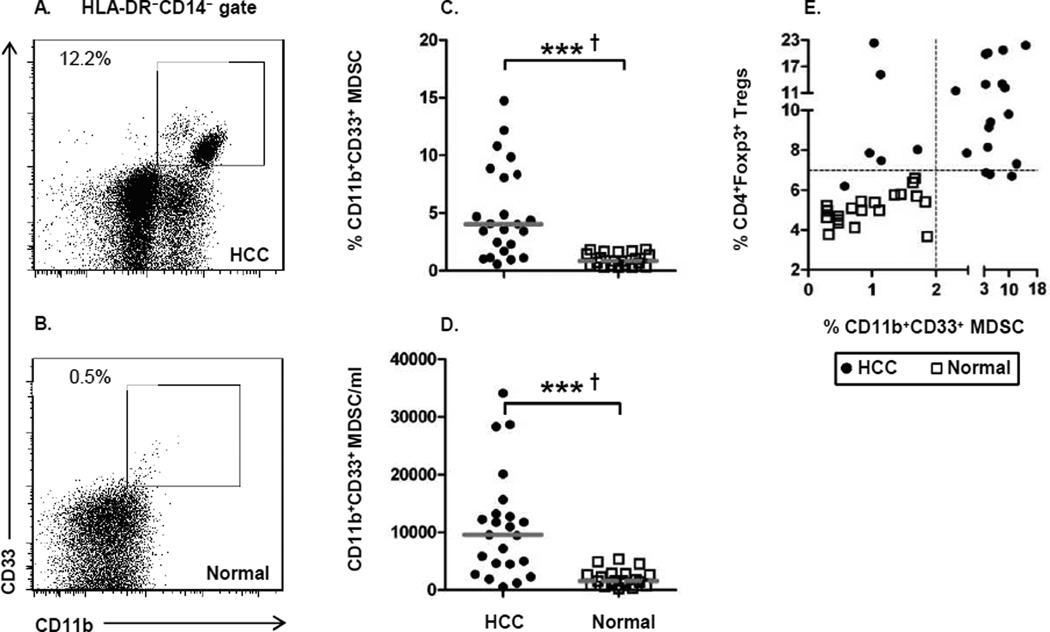
Elevated numbers of MDSC in HCC patients
Just as GARP and CTLA-4 have been understudied in advanced HCC, neither have MDSC been evaluated in this patient population despite their importance as an immunosuppressive cell in many cancers. Due to the interconnectedness of MDSC and Treg generation during malignant progression, we measured the frequency of CD14−HLA-DR−CD11b+CD33+ MDSC in each HCC patient for which we measured Treg frequency. Representative MDSC staining patterns from one HCC patient and one normal control are provided in Figure 2A and 2B. In conjunction with the elevated Tregs levels, the frequency (Fig. 2C) and absolute number of circulating MDSC (Fig. 2D) was significantly elevated in HCC patients. Additionally, the percentage of MDSC demonstrated excellent correlation with percentage of circulating Tregs (Fig. 2E). HCV infection or prior treatment did not impact MDSC frequency in HCC patients (Supplementary Fig. 4A–D). This data formally demonstrates the utility of measuring Tregs and MDSC in the same patient, as 14/23 HCC patients exhibited elevated levels of both immunosuppressive cell types. Thus, the interplay between MDSC and Tregs is likely instrumental in the establishment of the adverse immunosuppressive network in advanced HCC.
Figure 2. Accumulation of MDSCs in HCC patients.
(A, B) Representative staining of
HLA-DR−CD14−CD11b+CD33+ MDSC from one
HCC patient and one normal healthy donor. (C) Frequency and (D) absolute
number of cells/ml of MDSC in the peripheral blood of HCC patients and healthy donors.
(E) Correlation of CD11b+CD33+ MDSC frequency and
CD4+Foxp3+ Treg frequency. Each symbol represents an individual HCC patient
( ) or normal
healthy subjects (
) or normal
healthy subjects ( ); lines represent median values for the group. * P
< 0.05, ** P < 0.01, *** P < 0.001,
Mann-Whitney U test; † P < 0.05 Hochberg adjustment for multiple
comparison.
); lines represent median values for the group. * P
< 0.05, ** P < 0.01, *** P < 0.001,
Mann-Whitney U test; † P < 0.05 Hochberg adjustment for multiple
comparison.
The accumulation of MDSC does not comprise the full impact of all myeloid cells in advanced HCC, as CD11c+CD123+ pDC (Supplementary Fig. 5A–D) were also reduced in HCC patients compared to normal when measured by frequency (Supplementary Fig. 5E) or absolute number (Supplementary Fig. 5F).
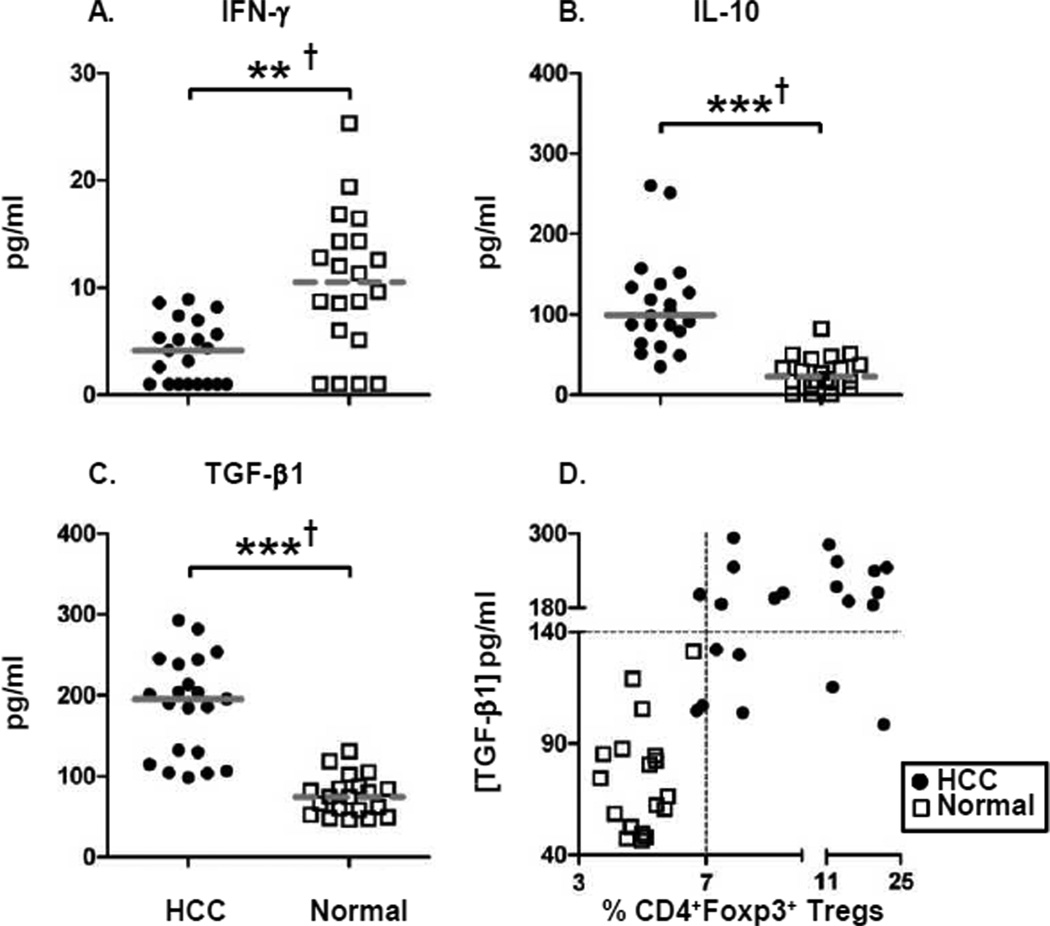
Elevated levels of immunosuppressive cytokines in HCC patients
The phenotype of augmented immunosuppression in HCC patients is additionally reflected by the diminished levels of plasma IFN-γ (Fig. 3A), a potent anti-tumor cytokine whose levels can be downregulated by the presence of Tregs. In addition to the low levels of IFN-γ, a significant increase in the levels of two Treg-generated immunosuppressive cytokines, IL-10 and TGF-β1 was found in HCC patients (Fig. 3B, C). The elevated levels of Foxp3+ Tregs in HCC patients are also associated with corresponding high plasma levels of TGF-β1 (Fig. 3D). Therefore, our results demonstrate that the cytokine milieu in which the HCC disease progresses is skewed toward an immunosuppressive phenotype and will likely adversely impact the effector function of anti-tumor immune responses while simultaneously stimulating tumor-promoting immune responses.
Figure 3. Elevated levels of immunosuppressive cytokines in HCC patients.
(A–C) Cytokine-specific sandwich ELISA of plasma from HCC patients
and healthy normal subjects were assayed in order to measure levels of circulating IFN-γ,
IL-10, and TGF-β1. (D) Correlation of TGF-β1 plasma levels and
frequency of CD4+Foxp3+ Tregs. Each symbol represents an individual HCC
patient ( )
or normal healthy subjects (
)
or normal healthy subjects ( ); lines represent median values for the group. ** P
< 0.01, *** P < 0.001, Mann-Whitney U test; †
P < 0.05 Hochberg adjustment for multiple comparison.
); lines represent median values for the group. ** P
< 0.01, *** P < 0.001, Mann-Whitney U test; †
P < 0.05 Hochberg adjustment for multiple comparison.
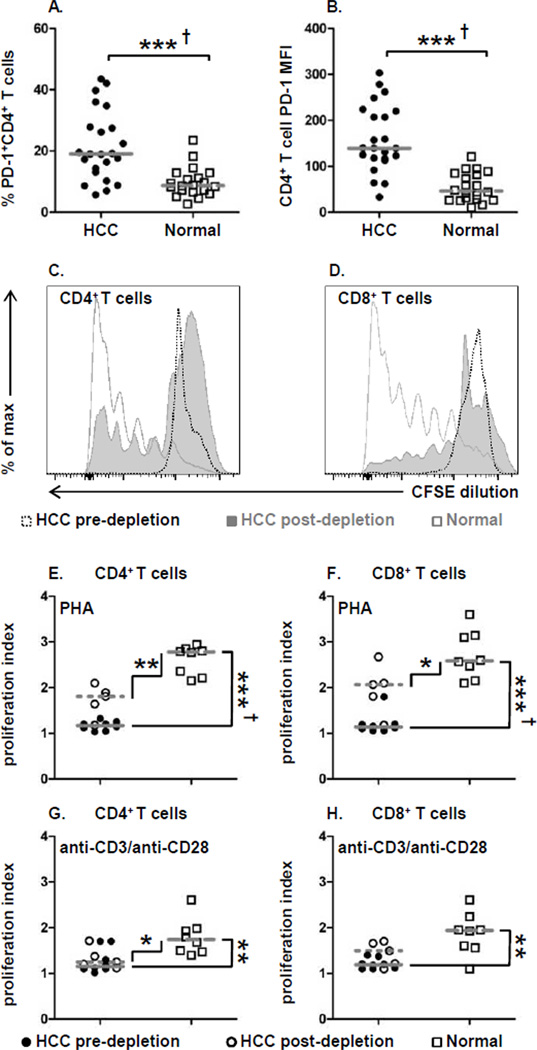
HCC patients have increased PD-1 expression on circulating CD4+ T cells
The appearance of exhausted T cells, characterized by PD-1 expression, is a hallmark of chronic viral infections such as HCV and blockade of this molecule is currently being evaluated for cancer treatment (21). Given the widespread immunosuppressive network that we found in advanced HCC, we also examined whether PD-1+ exhausted CD4+ T cells are also present as an additional indicator of diminished effector function. Both the frequency of PD-1+CD4+ T cells (HCC: 19.0% ± 11.3 vs. normal: 8.7% ± 4.8; P = 0.001) (Fig. 4A) and PD-1 expression levels (HCC: 139 ± 72 MFI vs. normal: 46 MFI ± 31; P = 0.001) (Fig. 4B) in HCC patients was significantly higher than in healthy donors. Thus, the accumulation of exhausted CD4+ T cells in HCC patients is another harbinger of immune dysregulation that must be overcome in order to elicit efficacious anti-tumor immune responses.
Figure 4. Exhausted T cells from HCC patients exhibit defective proliferation.
(A) Frequency of PD-1+CD4+ T cells and
(B) PD-1 expression levels on CD4+ T cells. (C) Representative
CD4+ T cell and (D) CD8+ T cell proliferation measured by CFSE
dilution in an HCC patient pre-depletion ( ) and post-depletion (
) and post-depletion ( ) of suppressor cells and a
normal healthy donor (
) of suppressor cells and a
normal healthy donor ( ). (E) CD4+ T cell and (F)
CD8+ T cell proliferation index for PHA and (G) CD4+ T cell and
(H) CD8+ T cell proliferation index for anti-CD3/anti-CD28 stimulation. Each
symbol represents an individual HCC patient pre-depletion (
). (E) CD4+ T cell and (F)
CD8+ T cell proliferation index for PHA and (G) CD4+ T cell and
(H) CD8+ T cell proliferation index for anti-CD3/anti-CD28 stimulation. Each
symbol represents an individual HCC patient pre-depletion ( ), post-depletion
(
), post-depletion
( ), or
normal healthy subjects (
), or
normal healthy subjects ( ); lines represent median values for the group. * P
< 0.05, ** P < 0.01, *** P < 0.001,
Mann-Whitney U test; † P < 0.05 Hochberg adjustment for multiple
comparison.
); lines represent median values for the group. * P
< 0.05, ** P < 0.01, *** P < 0.001,
Mann-Whitney U test; † P < 0.05 Hochberg adjustment for multiple
comparison.
Impaired T cell proliferation in HCC patients
The presence of an extensive immunosuppressive network undermines endogenous anti-tumor immunity by impairment of T cell function. Given the presence of exhausted T cells, we evaluated whether T cell proliferation and cytokine production were also dysregulated. CD4+ and CD8+ T cell effector function was measured with two polyclonal stimuli, the potent mitogen PHA and anti-CD3/anti-CD28, a surrogate for antigen-specific TCR-mediated stimulation. Further, to ascertain the contribution of Treg, MDSC, and PD-1+ exhausted T cell accumulation on effector T cell function these immunosuppressive cells were depleted, and the function of the remaining effector T cells was analyzed in their absence. We have for the first time directly tested whether targeted depletion of immunosuppressive cells in advanced HCC has the potential to restore endogenous anti-tumor T cell function.
Proliferation of CD4+ and CD8+ T cells assessed by CFSE dilution
revealed that both subsets from HCC patients exhibited severely impaired responses to PHA
stimulation compared to T cells from normal subjects (Fig. 4C,
D
 vs
vs
 and Fig. 4E, F
and Fig. 4E, F
 vs
vs
 ).
Additionally the depletion of the three immunosuppressive cells resulted in only moderate
improvement in PHA-mediated T cell proliferation for both CD4+ and CD8+ T
cells (Fig. 4C, D
).
Additionally the depletion of the three immunosuppressive cells resulted in only moderate
improvement in PHA-mediated T cell proliferation for both CD4+ and CD8+ T
cells (Fig. 4C, D
 and Fig. 4E, F
and Fig. 4E, F
 vs
vs
 ). Despite
the modest improvement in T cell proliferation, Treg, MDSC, and PD-1+ depletion did not
restore HCC T cell proliferation that was equivalent to that observed in normal T cells. (Fig. 4E–H
). Despite
the modest improvement in T cell proliferation, Treg, MDSC, and PD-1+ depletion did not
restore HCC T cell proliferation that was equivalent to that observed in normal T cells. (Fig. 4E–H
 vs
vs
 ).
).
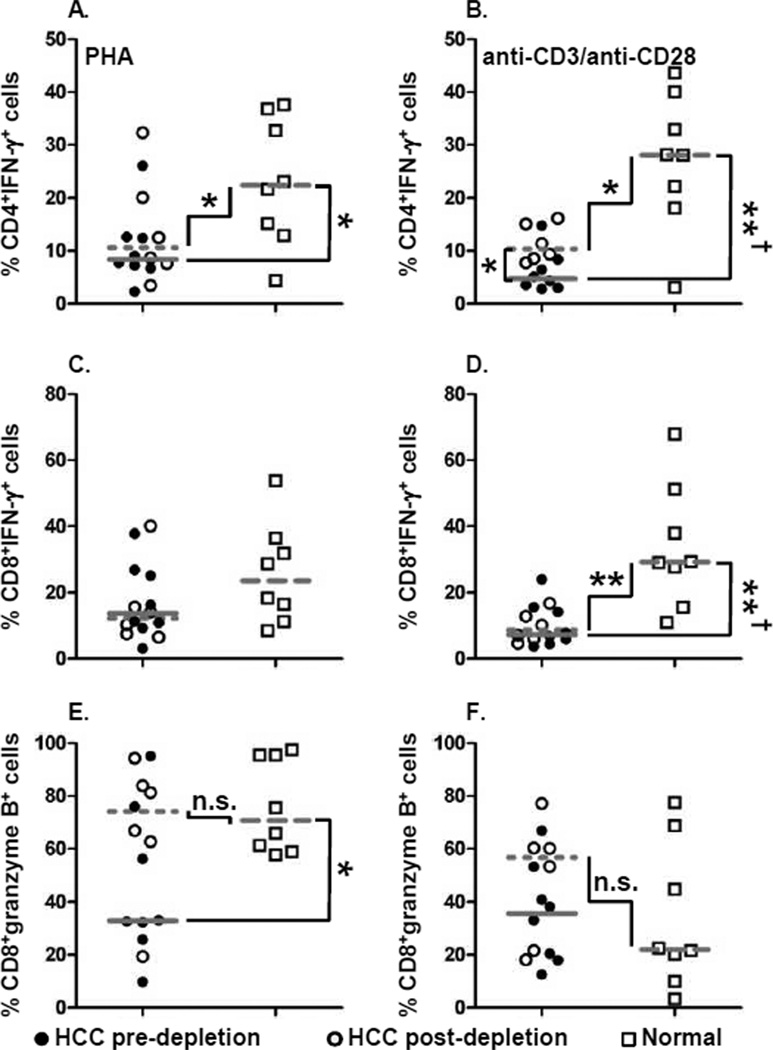
Selective restoration of T cell cytokine production upon depletion of immunosuppressive cell subsets
We measured IFN-γ and granzyme B production by CD4+ and
CD8+ T cells in the presence or absence of Tregs, MDSC, and PD-1+ exhausted T
cells (Supplementary Fig. 6). IFN-γ
producing CD4+ or CD8+ T cells from HCC patients were significantly lower than
normal subjects following both PHA and anti-CD3/anti-CD28 stimulation (Fig. 5A–D
 vs
vs
 ), once
again demonstrating the pervasiveness of immune function dysregulation in advanced HCC patients.
Importantly, the frequency of T cells producing this cytokine did not increase appreciably upon
depletion of the three immunosuppressive cells (Fig.
5A–D
), once
again demonstrating the pervasiveness of immune function dysregulation in advanced HCC patients.
Importantly, the frequency of T cells producing this cytokine did not increase appreciably upon
depletion of the three immunosuppressive cells (Fig.
5A–D
 vs
vs
 ). Failure
to restore IFN-γ production provides powerful evidence that effector T cells in advanced HCC
are unlikely to be able to overcome severe immunodysregulation by targeted depletion of Tregs,
PD-1+ T cells and MDSC alone.
). Failure
to restore IFN-γ production provides powerful evidence that effector T cells in advanced HCC
are unlikely to be able to overcome severe immunodysregulation by targeted depletion of Tregs,
PD-1+ T cells and MDSC alone.
Figure 5. Diminished IFN-γ and granzyme B production by HCC patient T cells.
Frequency of (A, B) CD4+ T cells and (C, D)
CD8+ T cells producing IFN-γ upon (A, C) PHA or (B, D)
anti-CD3/anti-CD28 stimulation. Frequency of CD8+ T cells granzyme B upon
(E) PHA or (F) anti-CD3/anti-CD28 stimulation. Each symbol represents an
individual HCC patient pre-depletion ( ), post-depletion (
), post-depletion ( ), or normal healthy
subjects (
), or normal healthy
subjects ( );
lines represent median values for the group. n.s. not significant, * P <
0.05, ** P < 0.01, Mann-Whitney U test; † P
< 0.05 Hochberg adjustment for multiple comparison.
);
lines represent median values for the group. n.s. not significant, * P <
0.05, ** P < 0.01, Mann-Whitney U test; † P
< 0.05 Hochberg adjustment for multiple comparison.
Decreased granzyme B production in CD8+ T cells was observed in
PHA-stimulated HCC patients compared to normal, but not following anti-CD3/anti-CD28 stimulation
(Fig. 5E, F
 vs
vs
 ). In
contrast to IFN-γ production, the frequency of CD8+ T cells producing granzyme B
following PHA stimulation was significantly augmented after targeted removal of the three suppressor
cells and was equivalent between normal T cells and HCC T cells post-depletion (Fig. 5E
). In
contrast to IFN-γ production, the frequency of CD8+ T cells producing granzyme B
following PHA stimulation was significantly augmented after targeted removal of the three suppressor
cells and was equivalent between normal T cells and HCC T cells post-depletion (Fig. 5E
 vs
vs
 ). In
endometrial cancer patients, an inverse relationship has been shown between the presence of Treg and
production of granzyme B expressing CD8+ T cells (27). In our studies, the depletion of highly suppressive Treg also likely accounts for the
increased numbers of granzyme B+CD8+ T cells to levels equivalent to that seen
in healthy control subjects. Thus, our findings demonstrate that the restoration of T cell responses
after depletion of suppressor cell subsets is restricted and does not ameliorate the entirety of
immune dysregulation established in these patients.
). In
endometrial cancer patients, an inverse relationship has been shown between the presence of Treg and
production of granzyme B expressing CD8+ T cells (27). In our studies, the depletion of highly suppressive Treg also likely accounts for the
increased numbers of granzyme B+CD8+ T cells to levels equivalent to that seen
in healthy control subjects. Thus, our findings demonstrate that the restoration of T cell responses
after depletion of suppressor cell subsets is restricted and does not ameliorate the entirety of
immune dysregulation established in these patients.
Discussion
Studies have demonstrated that HCC progresses even in the presence of tumor-specific immune responses in a majority of HCC patients (28), indicating that HCC utilizes multiple mechanisms to evade host anti-tumor immunity. Evasion of host anti-tumor responses can occur by the induction of Tregs, defective antigen presentation by DCs, recruitment and accumulation of MDSCs, and over-production of inhibitory cytokines such as IL-10 and TGF-β. The effect of each of these mechanisms, and other equally important processes, has been evaluated in isolation but no study to date has evaluated the contribution of the combined effect of immunosuppression on immune function in advanced HCC. Our study is the first of its kind to systematically measure key immunosuppressive processes, rather than individual subsets, to determine their collective effect on endogenous T cell effector responses. As treatment options for inoperable advanced HCC are limited, physicians are seeking options involving immunotherapies. However, there is insufficient data on the immune status in these patients to assist in determining which immunotherapies can be beneficial. Our study, which involved the simultaneous measurement of multiple mediators of immune suppression, reveals a previously un-described picture of extreme immune dysfunction in advanced HCC patients and will facilitate the rationale determination of which aspects of endogenous immunity can be exploited for treatment benefit.
Though tumor-mediated immune dysfunction can occur at several checkpoints, we have focused on the cell subsets which are currently being targeted clinically in several other cancers, namely Tregs, MDSC, and PD-1 blockade. Passive or adaptive immunotherapies are likely to succeed if tumor-mediated immunosuppressive networks are mitigated (29). Because the immunosuppressive networks are interconnected, we hypothesized that future therapies are likely to succeed if the extent of immunosuppression is accurately measured. There is excellent evidence that Tregs and MDSC are detrimental for anti-tumor immunity and that removal of either of these cell subsets greatly improves anti-tumor responses; however, there is no consensus on the method to distinguish and then deplete these cells.
CD25 has been proposed to distinguish potentially suppressive T cells, but CD4+CD25+ T cells are a heterogeneous population and only a fraction of this population are immunosuppressive (20). This method of Treg identification has shown that these cells have a high prevalence in the course of HCC disease progression (14, 15, 30), but these studies could not discriminate between activated effector T cells and immunosuppressive Tregs. Due to the limitations with CD25 as a unique marker, we have utilized Foxp3 as a marker for circulating Tregs. The elevated frequency of CD4+Foxp3+ Tregs observed in our study, are in concordance with an earlier study describing Foxp3+ Treg accumulation in HBV+ HCC patients of Chinese ancestry (30). However, reliance on Foxp3 as a specific marker for Tregs is not without its own limitations, as it is also expressed on non-suppressive TGF-β-induced Tregs (20, 31). In addition, the intracellular localization of the Foxp3 transcription factor makes it difficult to target these cells in a clinical setting with anti-Foxp3 for depletion of suppressive T cells. Thus, we have extended our Treg analysis to include surface markers that identify T cells with high suppressive potential.
We have utilized the expression of the orphan receptor GARP to identify antigen-specific Tregs with high suppressive potential. The expression of GARP dominantly controls Foxp3 via a positive feedback loop. Thus, retroviral overexpression of GARP in Th cells results in the efficient and stable re-programing of effector T cells to become Treg cells and conversely the downregulation of GARP in human Tregs significantly impaired Foxp3 expression and suppressor function (32, 33). Ours is the first study to report that this population of highly suppressive Tregs is elevated in advanced HCC. Importantly, depletion of GARP+ cells in combination with other markers was able to restore T cell function in a limited set of effector processes, namely CD8+ T cell granzyme B production. Studies that have observed no effect following CD25+ Treg depletion may not have been effectively removing these highly suppressive Tregs. The multi-factorial depletion protocol we have utilized provides a tantalizing possibility that certain aspects of T cell effector function, such as granzyme B production, can be improved by removal of GARP+ Tregs, and demonstrates that not all anti-tumor effector functions are rendered permanently refractory by the extensive immunosuppressive network.
There is a paucity of data on the extent of accumulation of MDSCs in HCC patients having elevated Treg levels. We have characterized CD14−HLA-DR−CD11b+CD33+ MDSC and report for the first time that these cells are significantly elevated in patients with advanced HCC. Cells with this same phenotype, which inhibited T cell proliferation, have been shown to be elevated in lung cancer, renal cancer and melanoma patients, and depletion of this cell subset restored T cell function (8, 34–36). Though this is finding is not altogether surprising as MDSC levels are often increased in cancer patients, we have formally demonstrated that MDSC accumulation occurs concurrently with Treg accumulation in advanced HCC. Reports in pancreatic, esophageal, and gastric cancer patients have shown that CD11b+CD33+ MDSC are associated with elevated numbers of Tregs (37). In concordance with our results of elevated numbers of both Tregs and MDSC, depletion of only one set of suppressor cells is unlikely to exert significant benefit for anti-tumor immune responses in advanced HCC. Removal of both Tregs and MDSC cells is also advantageous from the standpoint that these cells are known to induce the generation of the other (36, 38), thereby creating a feedback loop that undoubtedly hinders anti-tumor immunity. In support of this strategy, PHA-mediated proliferation and granzyme B production was improved in effector T cells stimulated in the absence of Tregs and MDSC.
Diminished granzyme B production by activated T cells of HCC patients may render their cytotoxic T cells dysfunctional. Increased prevalence of Foxp3+ Tregs in HCC patients may compromise cytolytic effector function of CD8+ T cells at least in part by inhibiting the expression of granzyme B in CD8+ T cells and therefore compromising CTL-mediated tumor cell killing. An inverse relationship between Tregs and granzyme B/perforin expressing CD8+ T cells in patients with endometrial cancer has been reported (27). However, in an earlier study comparing HCC patients and normal controls of Chinese ethnicity, no difference was reported in the granzyme B/perforin expression of circulating CD8+ T cells (30). In contrast to their findings, we observed a lower frequency of granzyme B producing CD8+ T cells; this is not surprising as we have evaluated advanced HCC patients who exhibit immune dysregulation in many compartments. Additionally, we have also demonstrated for the first time that granzyme B production by effector CD8+ T cells in advanced HCC patients can be fully restored by targeted removal of a highly suppressive Treg population. In patients with follicular lymphoma, CD8+ T cells expressing high levels of granzyme B were correlated with prolonged progression free survival after combination of rituximab and chemotherapy (39). It is tempting to speculate that restoration of CD8+ T cell granzyme B production may have similar benefits for HCC patients.
Partial restoration of T cell function in our patients indicates that anti-tumor immune effector cells in advanced disease patients may not be fully competent to combat tumor progression even when underlying tumor-associated immune dysfunction is mitigated. This needs to be further confirmed because of limited sample size availability for depletion and functional assays. We elected to measure T cell responses in an antigen-independent manner using potent polyclonal T cell stimuli. However, in spite of the strong stimulation afforded by PHA or anti-CD3/anti-CD28, only modest changes in the T cell function either before or after depletion of suppressor cells were observed. The underwhelming response to these strong polyclonal stimuli suggests that it is quite unlikely to expect robust antigen-specific responses from T cells of advanced HCC patients. Some of the effete responses could be attributed to the presence of chronic HBV and HCV infection; however, we did not observe any differences in the composition of the immunosuppressive network between HCV+ and HCV− patients. The failure to observe a difference in these two populations suggests that the malignancy itself is likely contributing to the suppression of immunity. These findings are consistent with previous studies done in patients with chronic HBV, HCV, non-viral cirrhosis and HCC (30, 40).
In conclusion, we have demonstrated that the augmented numbers of Foxp3+GARP+CTLA-4+ Tregs, MDSC, PD-1+ exhausted T cells, and increased levels of immunosuppressive cytokines represent a plethora of potential mechanisms by which HCC may foster immune dysregulation. These mediators dampen anti-tumor T cell immunity and may in fact facilitate the progression of HCC. Importantly, our study represents the first demonstration that the combined depletion of Tregs, MDSC, and PD-1+ T cells from advanced HCC patients can result in the augmentation of CD8+ T cell granzyme B production and a modest increase in the number of CD4+ T cell IFN-γ producing cells. Our findings suggest that in the clinical setting further enhancement of endogenous anti-tumor responses will have to rely on the ‘science of combination’; thus, depletion along with concomitant expansion of effector T cells may be effective in conjunction with immunotherapeutic strategies for patients with HCC.
Supplementary Material
Acknowledgements
This research was supported, in part, by the National Cancer Institute (NCI) Core Grant to Roswell Park Cancer Institute (P30 CA016056-27). Discretionary funds available to Dr. Thanavala were utilized to support this study. Dr. Iyer is supported by a grant from the American Cancer Society (MSRG -08-096-01-CCE). We gratefully acknowledge Dr. Paul Wallace and Earl Timm for their help in designing FACS experiments. The Roswell Park Cancer Institute Data Bank and Biorepository is a CCSG Shared Resource (NIH P30 CA016056-27).
Footnotes
None of the authors have any conflict of interest.
Reference List
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Yee C, Riddell SR, Greenberg PD. Prospects for adoptive T cell therapy. Curr Opin Immunol. 1997;9:702–708. doi: 10.1016/s0952-7915(97)80052-0. [DOI] [PubMed] [Google Scholar]
- 3.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 6.Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother. 2006;55:1064–1071. doi: 10.1007/s00262-005-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 8.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 9.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 10.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 11.van CH, Hoekman K, Stam AG, van den Eertwegh AJ, Kuenen BC, Scheper RJ, et al. Defective differentiation of myeloid and plasmacytoid dendritic cells in advanced cancer patients is not normalized by tyrosine kinase inhibition of the vascular endothelial growth factor receptor. Clin Dev Immunol. 2007;2007:17315. doi: 10.1155/2007/17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Della BS, Nicola S, Brambilla L, Riva A, Ferrucci S, Presicce P, et al. Quantitative and functional defects of dendritic cells in classic Kaposi's sarcoma. Clin Immunol. 2006;119:317–329. doi: 10.1016/j.clim.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Della BS, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, et al. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer. 2003;89:1463–1472. doi: 10.1038/sj.bjc.6601243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 15.Unitt E, Rushbrook SM, Marshall A, Davies S, Gibbs P, Morris LS, et al. Compromised lymphocytes infiltrate hepatocellular carcinoma: the role of T-regulatory cells. Hepatology. 2005;41:722–730. doi: 10.1002/hep.20644. [DOI] [PubMed] [Google Scholar]
- 16.Knutson KL, Dang Y, Lu H, Lukas J, Almand B, Gad E, et al. IL-2 immunotoxin therapy modulates tumor-associated regulatory T cells and leads to lasting immune-mediated rejection of breast cancers in neu-transgenic mice. J Immunol. 2006;177:84–91. doi: 10.4049/jimmunol.177.1.84. [DOI] [PubMed] [Google Scholar]
- 17.Mahnke K, Schonfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K, et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120:2723–2733. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 18.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 20.Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13439–13444. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nurieva R, Thomas S, Nguyen T, Martin-Orozco N, Wang Y, Kaja MK, et al. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25:2623–2633. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 24.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 25.Suresh K, Fraser G, Scheid E, Leber B, Gauldie J, Foley R. Generation of in vitro B-CLL specific HLA class I restricted CTL responses using autologous dendritic cells pulsed with necrotic tumor lysate. Leuk Lymphoma. 2006;47:297–306. doi: 10.1080/10428190500301231. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg Y. A Sharper Bonferroni Procedure for Multiple Tests of Significance. Biometrika Trust; 1988. pp. 800–802. [Google Scholar]
- 27.Chang WC, Li CH, Huang SC, Chang DY, Chou LY, Sheu BC. Clinical significance of regulatory T cells and CD8+ effector populations in patients with human endometrial carcinoma. Cancer. 2010;116:5777–5788. doi: 10.1002/cncr.25371. [DOI] [PubMed] [Google Scholar]
- 28.Korangy F, Ormandy LA, Bleck JS, Klempnauer J, Wilkens L, Manns MP, et al. Spontaneous tumor-specific humoral and cellular immune responses to NY-ESO-1 in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4332–4341. doi: 10.1158/1078-0432.CCR-04-0181. [DOI] [PubMed] [Google Scholar]
- 29.Sutmuller RP, van Duivenvoorde LM, van EA, Schumacher TN, Wildenberg ME, Allison JP, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 31.Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 2009;69:3995–4000. doi: 10.1158/0008-5472.CAN-08-3804. [DOI] [PubMed] [Google Scholar]
- 32.Probst-Kepper M, Geffers R, Kroger A, Viegas N, Erck C, Hecht HJ, et al. GARP: a key receptor controlling FOXP3 in human regulatory T cells. J Cell Mol Med. 2009;13:3343–3357. doi: 10.1111/j.1582-4934.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Probst-Kepper M, Kroger A, Garritsen HS, Buer J. Perspectives on Regulatory T Cell Therapies. Transfus Med Hemother. 2009;36:302–308. doi: 10.1159/000235929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients' CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2008;57:1493–1504. doi: 10.1007/s00262-008-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, et al. Population alterations of L: -arginase- and inducible nitric oxide synthase-expressed CD11b(+)/CD14 (-)/CD15 (+)/CD33 (+) myeloid-derived suppressor cells and CD8 (+) T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2009 doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 37.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–1430. doi: 10.1007/s00262-011-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finke JH, Rini B, Ireland J, Rayman P, Richmond A, Golshayan A, et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res. 2008;14:6674–6682. doi: 10.1158/1078-0432.CCR-07-5212. [DOI] [PubMed] [Google Scholar]
- 39.Laurent C, Muller S, Do C, Al-Saati T, Allart S, Larocca LM, et al. Distribution, function, and prognostic value of cytotoxic T lymphocytes in follicular lymphoma: a 3-D tissue-imaging study. Blood. 2011;118:5371–5379. doi: 10.1182/blood-2011-04-345777. [DOI] [PubMed] [Google Scholar]
- 40.Ormandy LA, Farber A, Cantz T, Petrykowska S, Wedemeyer H, Horning M, et al. Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma. World J Gastroenterol. 2006;12:3275–3282. doi: 10.3748/wjg.v12.i20.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.