Abstract
Background
Combination therapy with interferon alpha (IFN) is correlated with improved survival in patients with pancreatic ductal adenocarcinoma (PDAc) but frequently presents side effects. We designed a novel targeted adenovirus with replication restricted to cyclooxygenase 2 (Cox2)-overexpressing PDAcs and hypothesize that the locally delivered therapeutic gene IFN can augment oncolytic effects while minimizing systemic toxicity.
Methods
IFN-expressing vectors were tested in vitro with the use of 4 PDAc cell lines with cytocidal effect measured by crystal violet and colorimetrically and IFN production assayed by ELISA. Cox2 promoter activity was checked by a luciferase reporter assay. In vivo, subcutaneous tumor xenografts with 2 PDAc cell lines in nude mice were treated with a single intratumoral viral dose.
Results
All PDAc cell lines were Cox2-positive. Oncolysis from the novel Cox2-controlled virus was comparable or superior to Adwt, the wild-type virus without safety features. The absence of cytocidal effect in Cox2-negative cells with the novel virus indicated cancer specificity. In vivo, stronger tumor suppression from the novel virus was seen when compared with nonreplicating IFN-expressing vectors.
Conclusion
We demonstrated the potent therapeutic effects of a novel tumor-specific conditionally replicative IFN-expressing adenovirus. With potential to locally deliver IFN and avoid systemic toxicity, this strategy may therefore expand the application of this robust and promising therapy.
Pancreatic cancer is a highly lethal disease, with an estimated 43,140 new cases and 36,800 deaths reported in 2010.1 Of the newly diagnosed cases, approximately 85 to 90% will have inoperable disease at presentation as the result of locally advanced stage or metastases.2 Gemcitabine is currently the standard of care for adjuvant chemotherapy3,4; however, overall survival remains poor, with median of approximately 22 to 24 months in selected series.5 Recently, interferon alpha (IFN), a cytokine with direct and indirect antitumor effects,6 has shown promising improvements in survival in multimodality adjuvant therapy, but this regimen suffers from systemic side effects with an incidence as great as 95%, and more than 25% of patients cannot tolerate the systemic IFN component.7–10 This problem indicates a pressing need for the development of highly active agents for the treatment of pancreatic cancer, and IFN could be a powerful tool for the generation of such a modality. This realization, however, requires a means of limiting toxicity of IFN-based therapy.
Adenovirus (Ad) vector–based cancer gene therapy has been applied in more than 3,000 patients. This vector has high in vivo infectivity, but the conventional Ad vector is not suitable for pancreatic cancers. To overcome the weak points as cancer therapeutics, we have improved this vector system and generateda series of oncolytic Ads. We developed a conditionally replicative adenoviral system (CRAds),11 wherein viral replication is controlled by the cyclooxygenase 2 (Cox2) promoter, exploiting the knownCox2 overexpression in pancreatic tumors to drive viral replication and its lack of expression in liver, the organ of most concern for replication-related toxicity, to mitigate side effects.11–14
Modifications of the viral capsid proteins were made to improve dramatically pancreatic tumor cell infectivity over the wild-type viral structure.15,16 Work by our group and others16 has demonstrated the practicality of this approach among many tumor types, including pancreatic cancer. Investigators previously have deployed an earlier generation of IFN-expressing Ad vectors for pancreatic cancer therapy; however, all of the described vectors have been of the nonreplicating type.17–20 By combining the ability of modified, replication-competent Ad vectors to preferentially target cancer cells and to replicate within them, a therapeutic gene such as IFN can be locally delivered in massive amounts to augment the tumor-lytic viral effect while avoiding systemic toxicity. We hypothesize that a novel Cox2-controlled, selectively replicating, CRAd that expresses IFN will be highly active both in vitro and in vivo and will show superiority to nonreplicating, IFN-expressing Ad vectors previously tested.
MATERIALS AND METHODS
Cell lines and animals
The human pancreatic ductal adenocarcinoma (PDAc) cell lines MiaPaCa-2, S2O13, S2VP10, and ASPC-1; the Cox2-positive human nonsmall cell lung adenocarcinoma cell line A549; and the Cox2-negative human breast cancer cell line BT474 were obtained from the American Type Culture Collection (Manassas, VA). MiaPaCa-2, S2O13, S2VP10, A549, and ASPC-1 were maintained in Dulbecco’s modified Eagle medium (DMEM; Mediatech, Herndon, VA) with 20% fetal bovine serum (FBS) for ASPC-1 (HyClone, Logan, UT) and 5% FBS for all other cell lines, respectively. BT474 was maintained in Roswell Park Memorial Institute medium supplemented with 15% FBS and bovine insulin (0.01 mg/mL; Life Technologies, Rockville, MD). 911 cells (a kind gift of Dr. Van Der Eb, Leiden University, the Netherlands21) were maintained in DMEM supplemented with 5% FBS. All media were supplemented with penicillin (100 IU/mL) and streptomycin (100 µg/mL). Cells were grown in a humidified incubator at 37°C in a 5% CO2 atmosphere.
Female athymic nude mice (NCr-nu/nu; National Cancer Institute at Frederick, Frederick, MD) were used at 6 to 8 weeks of age for in vivo studies. All animals received humane care on the basis of the guidelines set by the American Veterinary Association. All experimental protocols involving live animals were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Adenoviral vectors
Replication-deficient Ad vectors (AdCMVLuc, AdCox2Luc) encoding the firefly luciferase reporter gene (Luc) were generated as previously described.13 The Ad5/Ad3 chimeric fiber-knob modification was incorporated into the adenoviral structure as we reported previously.15 To generate IFN-expressing vectors, an expression cassette containing adenoviral death protein (ADP), an enhancer of apoptosis and viral spread, and the gene for IFN were cloned into an E3 shuttle plasmid and introduced into the E3 region of the viral genome by homologous recombination in Escherichia coli.22,23 Cox2 promoter-controlled Ad vectors were generated by the use of homologous recombination in E. coli as previously described.11,23 All viruses were propagated in the 911 cell line and purified by double CsCl density gradient ultracentrifugation, followed by dialysis against phosphate-buffered saline (PBS) with 10% glycerol. The vectors were titrated by plaque assay, and viral particle (vp) number was measured spectrophotomectrically with absorbance at 260 nm.24 Vectors were stored at −80°C until ready for use. Viral structure was confirmed by polymerase chain reaction for Cox2 and Ad5/Ad3 fiber structure as described previously.11
In vitro analysis of infectivity and Cox2 promoter strength with Luciferase-expressing Ads
Cells (5 × 104 cells/well) were grown in 24-well plates were infected with 10 plaque-forming units (pfu)/mL for 48 h, followed by lysis with 100 mL of cell culture lysis buffer (Promega, Madison, WI), and Luc activity was determined with the Luciferase Assay System (Promega). All experiments were performed in triplicate.
In vitro quantitative analysis of cancer cell–killing ability
Cells were seeded in 96-well plates at 3000 vp/cell (1500vp/cell for S2O13, S2VP10) then infected with Ad vectors at 1vp/cell (Mia-PaCa-2) or 10 vp/cell (S2O13, S2VP10) in 100 µL of DMEM 5% medium. The cells were incubated under standard conditions, and the number of living cells was measured colorimetrically at serial time points by the use of the Cell Titer Aqueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer’s instructions. The proportion of living cells at each time point was normalized to the number of living uninfected cells. All experiments were performed in triplicate.
In vitro analysis of cytocidal effect by crystal violet staining
A total of 1 × 105 cells was plated in 24-well plates then infected with virus at 0.1 or 1 vp/cell in 1 mL of growth medium with 5% FBS. At serial time points, cells were fixed with 10% buffered formalin for 10 min then stained with 1% crystal violet in 70% ethanol for 20 min, then washed with water and dried.
In vitro IFN production by ELISA
S2O13 cells were plated in 24-well plates at 5 × 104 cells/well, then infected with virus at 1 vp/cell in 1 mL of growth medium with 5% FBS. At serial time points, cell culture supernatant was collected and centrifuged to remove cell debris. Samples were analyzed for IFN concentration by the use of a commercial human IFN ELISA kit (PBL Interferonsource, Piscataway, NJ) according to the manufacturer’s instructions.
In vivo antitumor effect in a PDAc xenograft model
MiaPaCa-2 or S2O13 cells (1 × 106 cells in 100 µL of PBS) were injected in each flank of female athymic nude mice. Groups comprised 5 animals each with 10 tumors/group. When the nodules reached a maximum diameter of 8 to 10 mm, each tumor was injected once with 1 × 1010 vp of virus or controls per 50 µL of PBS. Tumor size was measured with calipers, and tumor volume was calculated with the formula volume = width2 × length/2. Animals were euthanized in accordance with the approved institutional protocol.
Statistical methods
Statistical analysis of viral effect in vitro and in vivo was carried out with Excel (Microsoft, Redmond WA). Student t test of means was used with a 2-tailed P value of less than .05 taken to be statistically significant. Data are expressed as mean ± standard deviation of at least 3 results except where indicated.
RESULTS
Confirmation of viral structure
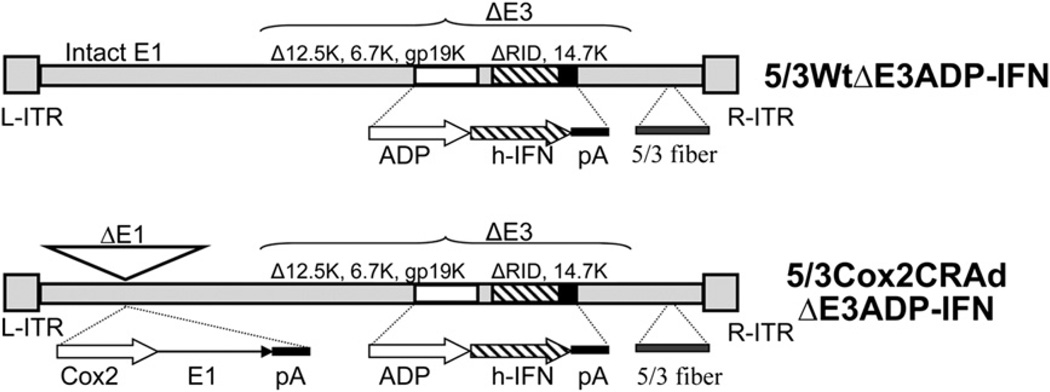
Polymerase chain reaction of viral DNA (Fig 1) was used to confirm the structure of the 5/3WtΔE3ADP-IFN with intact E1 region as well as the novel Cox-2 controlled virus 5/3Cox2CRAdΔE3ADP-IFN. An analysis was performed for both the fiber region and for Cox2 promoter status as previously described.15 The structure of all experimental viruses was validated in this manner.
Fig. 1.
Schematic of E3-deleted ADP- and IFN-expressing Ads. In both viruses, an expression cassette containing adenoviral death protein (ADP) and the human IFNα1 transgene was inserted into the viral E3 region. The Cox-2–controlled conditionally replicative Ad (CRAd) has the CoxL promoter inserted into the E1 region, whereas the wild-type transgenic Ad has an intact E1 region. Both viruses are fiber modified with the Ad 5/Ad3 fiber-knob chimera.
The 5/3 fiber modification is superior for targeting of PDAc
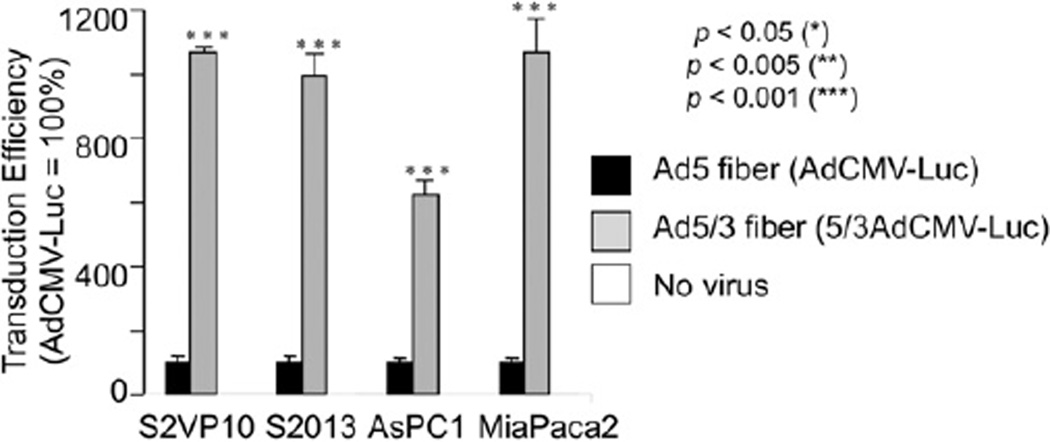
To analyze the effect of the Ad5/Ad3 fiber modification on targeting of CRAds to pancreatic cancer cells (Fig 2), 2 identical replication-incompetent viral vectors encoding the CMV promoter-driven reporter gene Luc were used: AdCMVLuc, with wild-type Ad5 fiber structure, and Ad5/3CMVLuc, expressing the Ad5/Ad3 fiber-knob chimera. Statistically significant increases of reporter gene expression (5–10 times) were detected in all 4 PDAc cell lines under investigation with the 5/3 modification as compared with the wild-type fiber.
Fig. 2.
Superiority of Ad 5/Ad 3 fiber-knob chimeric Ads for PDAc cell infection. Cancer cells were infected with a reporter vector under control of the ubiquitous CMV promoter with either the wild-type (Ad5) or Ad 5/Ad 3 fiber-knob chimeric (5/3) fiber. Results are shown as relative light units normalized to AdCMVLuc activity. The Ad5/3 fiber modification imparts significantly enhanced infectivity to all PDAc cell lines tested compared with the wild type. Asterisks indicate P <.05 for the comparison to Ad5 fiber.
Functional status of Cox2 in PDAc cell lines
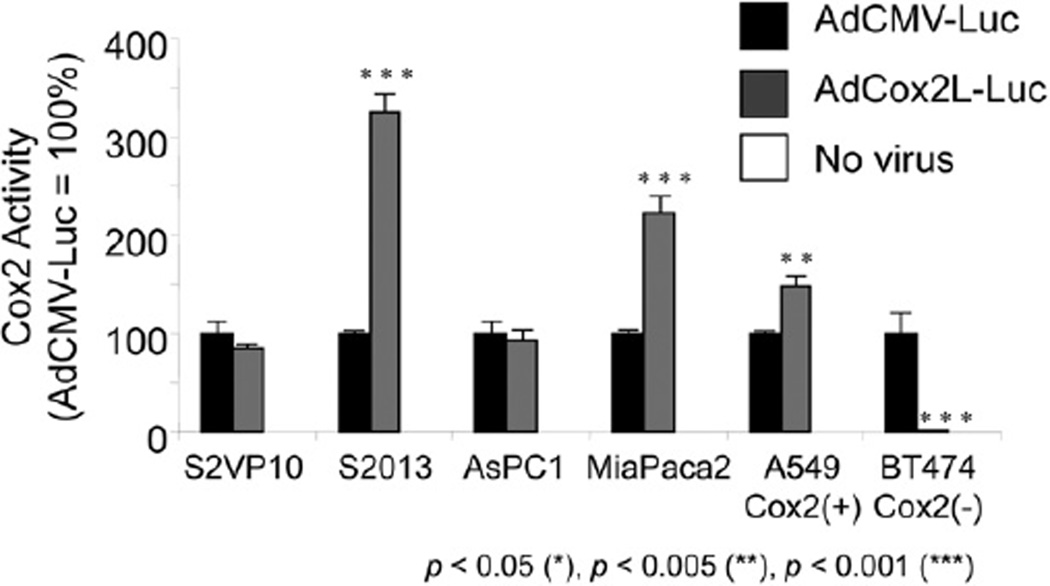
To determine the strength of the Cox2 promoter activity in PDAc cell lines (Fig 3), the human cell lines S2O13, S2VP10, ASPC-1, and MiaPaCa-2 were infected with 2 identical nonreplicating Luc-expressing vectors, AdCMVLuc, in which reporter gene expression is controlled by the ubiquitous CMV promoter, and AdCox2Luc, whereby Luc expression is dependent on cellular Cox2 promoter activity. A known Cox2 positive (A549) and negative (BT474) cell line were used as controls. All PDAc cell lines tested were positive for Cox2 promoter activity, with 2 of them (S2VP10 and ASPC-1) found to be comparable in magnitude to the strong CMV promoter-driven activity and the others (S2O13 and MiaPaCa-2) significantly greater in Cox2 activity.
Fig. 3.
Activity and selectivity of the Cox2 promoter in PDAc. Human PDAc cell lines (S2O13, S2VP10, ASPC-1, MiaPaCa-2), Cox2-positive (A549), and Cox2-negative (BT474) cell lines were infected with AdCMVLuc or Ad-Cox2Luc. Luc activity was measured 2 days after infection. Data are shown as percentages of relative light units normalized to AdCMVLuc activity. The Cox2 promoters exhibited high levels of activity in all PDAc cell lines under investigation.
Increased oncolytic efficiency of IFN-expressing Ads in vitro
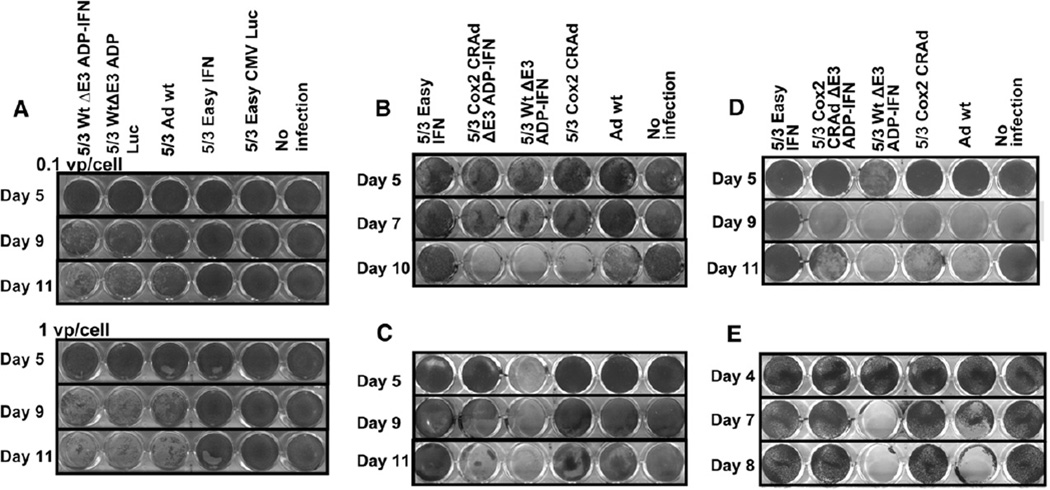
We generated Ads doubly modified for both increased cancer cell infectivity and enhanced cell–killing ability with the Ad5/Ad3 fiber modification and incorporation of the ADP-IFN expression cassette. We infected MiaPaCa-2 human PDAc cells (Fig 4, A) with 5/3WtΔE3ADP-IFN at low titers (0.1–1 vp/cell) to allow for multiple rounds of replication. Crystal violet staining showed oncolysis among replicating vectors (lanes 1–3) and minimal effect from nonreplicating vectors (lanes 4–5). The addition of ADP overexpression (5/3WtΔE3ADP-Luc) in comparison with 5/3Adwt, the infectivity-enhanced vector without ADP overexpression, showed increased oncolysis. The addition of IFN expression (5/3WtΔE3ADP-IFN) in comparison with 5/3WtΔE3ADP-Luc had the strongest effect, and furthermore the killing effect of the doubly modified virus was accelerated with a larger viral dose. A nonreplicating virus (5/3EasyIFN) with IFN expression alone showed no oncolysis upon infection with low titers.
Fig. 4.
Cytolytic effect of modified vectors in vitro. (A) MiaPaCa-2 cells were infected at 0.1 and 1 vp/cell with replication-deficient and replication-competent IFN-expressing vectors or controls, with surviving cells stained by crystal violet. Replication-incompetent viruses (lanes 4–5) show minimal effect compared to replication-competent viruses. The ADP- and IFN-expressing vector (lane 1) shows a superior killing effect in comparison with 5/3 Ad wt, the control virus with intact E3 region (lane 3), and to its identical counterpart expressing luciferase (lane 2). Furthermore, the superiority of 5/3WtΔE3ADP-IFN becomes more evident with increasing viral dose. Human PDAc cell lines MiaPaCa-2 (B), S2O13 (C), and S2VP10 (D), respectively, were infected with 1vp/cell by the use of Cox2-controlled IFN-expressing replicating vectors or controls. Across all 3 cell lines, the novel Cox2-controlled ADP- and IFN-expressing virus (lane 2) has superior potency to Ad wt, the gold standard control virus without cancer specificity (lane 5 in all), and was as good as its IFN-expressing counterpart without selectivity (lane 3 in all), as well as comparable or superior cytocidal effect to the positive control 5/3 Cox2 CRAd (lane 4 in all). (E) BT474 cells, which are known to be Cox2-negative, were infected at 1vp/cell with Cox2-controlled vectors or controls. At day 8, the novel Cox2-controlled IFN vector (lane 2) as well as the powerful 5/3 Cox2 CRAd (lane 4) showed no effect; however, the remaining 2 replicating vectors (lanes 3 and 5), which are not controlled by the Cox2 promoter, showed near-complete cytolysis.
To evaluate the effect of Cox2 replication control on cancer cell oncolysis (Fig 4, B–D), we used a similar virus incorporating both the Ad5/Ad3 chimera and ADP-IFN expression cassette, but with control of viral replication under control of the Cox2 promoter (5/3Cox2CRAdΔE3ADP-IFN). In all cell lines tested, 5/3Cox2CRAdΔE3ADP-IFN was highly potent, at a level equaling or surpassing that of Adwt, the gold standard control virus without selectivity. The novel virus was also equal or superior to 5/3Cox2CRAd, a powerful positive control,15 across all cell lines. The replication-deficient IFN-expressing Ad (5/3EasyIFN) required approximately 3 orders higher titers to successfully kill the pancreatic cancer cells when compared with our ΔE3-based replication-competent Ads producing IFN (data not shown).
In addition, to test for selective toxicity by Cox2 status (Fig 4, E), we infected human breast cancer BT474 cells, which are known to be Cox2-negative. Results of crystal violet staining at serial time points demonstrated no oncolysis from 5/3Cox2CRAdΔE3ADP-IFN or from a similar Cox2-controlled replicating virus (5/3Cox2CRAd) at day 8, and near complete oncolysis from replicating vectors without Cox2 specificity. These results indicate selective toxicity to Cox2-positive target cells with sparing of Cox2-negative cell populations.
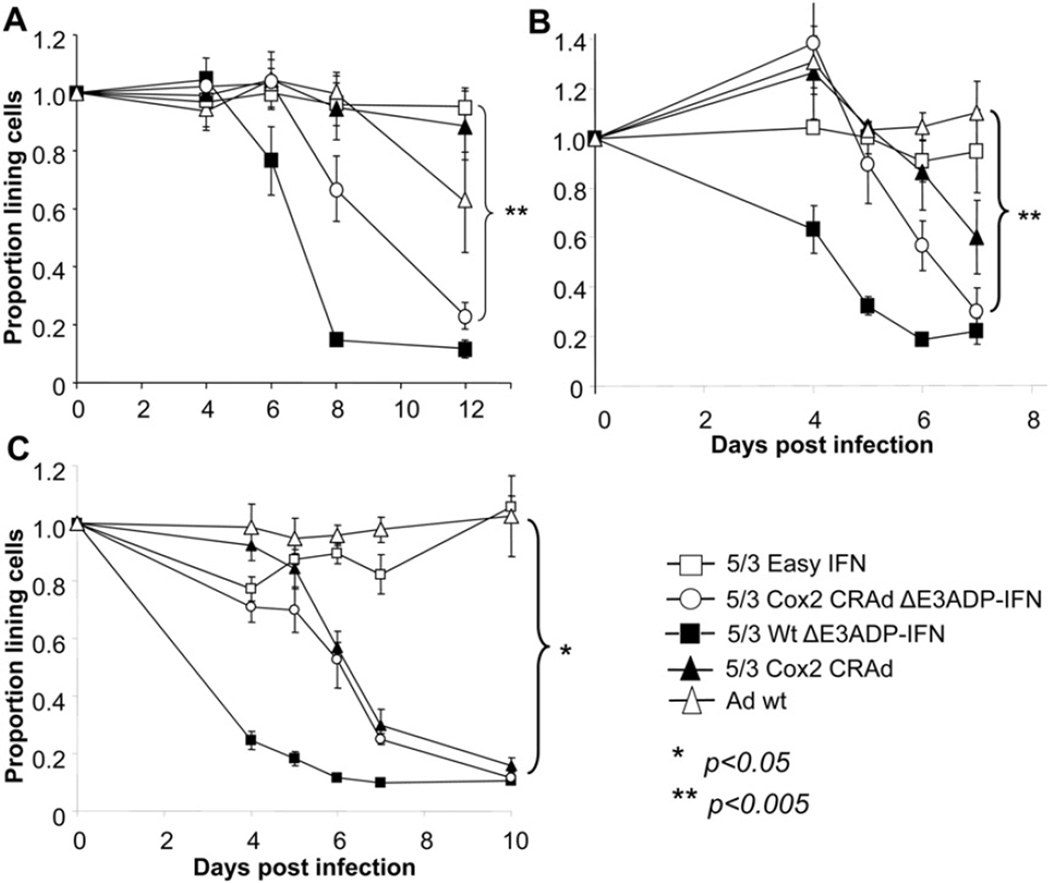
The replication and cytocidal effect of 5/3Cox2CRAdΔE3ADP-IFN were then quantitatively analyzed (Fig 5, A–C). In PDAc cell lines, when compared with 5/3WtΔE3ADP-IFN, 5/3Cox2-CRAdΔE3ADP-IFN achieved comparable levels of cancer cell killing after an early “lag” period because of its replication control. In MiaPaCa-2, S2O13, and S2VP10 cells the percentage of surviving cells at the final time point was 22.9%, 29.8%, and 11.7%, respectively, compared with that of Adwt, the gold standard control, of 62.1%, 110%, and 102.4%, respectively (P values for comparison .008, <.005, and .008).
Fig. 5.
In vitro tumor cell–killing ability of 5/3 chimeric E3-deleted, Cox2-controlled IFN-expressing Ad. Human PDAc cell lines MiaPaCa-2 (A), S2O13 (B), and S2VP10 (C), respectively, were infected at day 0. Cell viability was determined with a colorimetric cell proliferation assay. The results are shown as proportion of living cells remaining relative to unifected cells. 5/3Cox2CRAdΔE3ADP-IFN shows significantly greater cell killing ability compared with Ad wt, the gold standard control virus lacking cancer specificity, and is comparable in effect with the 5/3 chimeric ADP- and IFN-overexpressing Ad with unrestricted ability to replicate.
In addition, the killing ability of 5/3Cox2CRAdΔE3ADP-IFN and 5/3WtΔE3ADP-IFN trended toward equivalence in all cell lines and became so at the final time point in 2 of 3 cell lines (P < .005, P = NS, P = NS for MiaPaCa-2, S2O13, and S2VP10, respectively). Overall, the 5/3-modified Cox2-controlled IFN virus demonstrated a robust oncolytic effect. In S2VP10 (Fig 5, C), the difference between 5/3Cox2CRAdΔE3ADP-IFN and 5/3Cox2CRAd was observed only in earlier time points up to day 5.
IFN expression is time- and replication-dependent
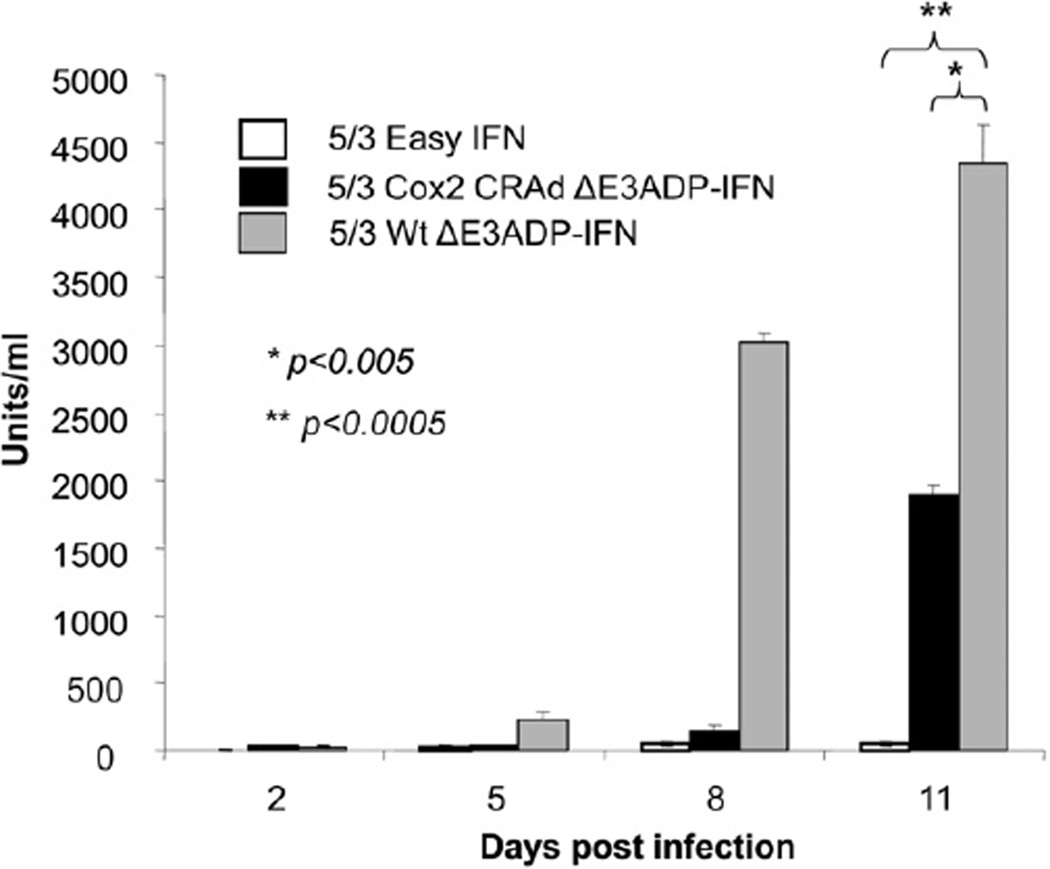
To understand the dynamics of IFN expression by 5/3Cox2CRAdΔE3ADP-IFN, IFN levels in cell culture supernatant after infection of S2O13 PDAc cells was assayed at serial time points (Fig 6). Similar to the results of the quantitative killing effect, 5/3Cox2CRAdΔE3ADP-IFN produced IFN in a lagging fashion behind 5/3WtΔE3ADP-IFN and increased in quantity in a time-dependent manner. 5/3WtΔE3ADP-IFN attained a level of 4349 units/ml at day 11 (P < .0005), compared with 1897 units/ml for 5/3Cox2CRAdΔE3ADP-IFN.At day 11, 5/3EasyIFN produced 59 units/mL.
Fig. 6.
IFN levels increase in a time- and replication-dependent fashion. IFN levels were assayed from infected cell culture supernatant. 5/3Cox2CRAdΔE3ADP-IFN shows a robust production of IFN despite its control of replication by Cox2. As expected the 5/3WtΔE3ADPIFN not under replication control by Cox2 attains greater levels of IFN production but is used for proof of principle only and is not suitable for use in vivo.
Therapeutic efficacy of replication competent IFN-producing Ads in vivo
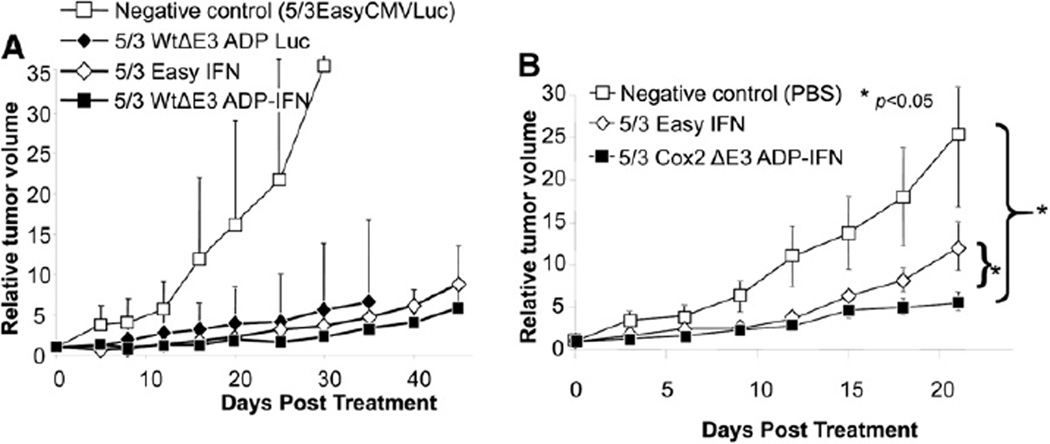
In vivo analysis of anti-tumor efficacy of replicating IFN producing viruses was performed with the use of subcutaneous xenograft models in athymic nude mice. First (Fig 7, A), the replication-competent Ad producing IFN (5/3WtΔE3ADP-IFN) was compared with an identical virus expressing luciferase (5/3WtΔE3ADP-Luc) and the replication-deficient 5/3EasyIFN. Established MiaPaCa-2 tumors were treated with a single intratumoral injection of 1010 vp of virus, and tumor size was monitored. All therapeutic viruses rapidly attained statistical significance when compared with negative control. At day 45, 5/3WtΔE3ADP-IFN showed the lowest average relative tumor volume of 5.87 mm3; however, the effect of this vector did not display a consistent statistically significant difference from 5/3EasyIFN (8.84 mm3).
Fig. 7.
Superiority of replication-competent, ADP- and IFN-expressing Ad in an in vivo human PDAc model. (A) Human MiaPaCa-2 PDAc tumor xenografts established in the flanks of nude mice were injected intratumorally with replication-competent ADP- and IFN-expressing vector or controls. Tumor size is shown as relative tumor volume compared to day 0. 5/3WtΔE3ADP-IFN showed the greatest inhibition of tumor growth and was superior to its identical counterpart, expressing luciferase as well as the nonreplicating Ad expressing the IFN transgene. (B) S2O13 xenograft nude mice were treated with a single intratumoral injection of PBS or vector. Tumor size is shown as relative tumor volume compared with day 0. Error bars indicate standard error of the mean. On day 21, the 5/3Cox2CRAdΔE3ADPIFN showed significantly stronger antitumor effect than 5/3 Easy IFN and negative control (P < .05 for both).
The subsequent experiment (Fig 7, B) tested the in vivo antitumor efficacy of 5/3Cox2CRAdΔE3ADP-IFN in a subcutaneous xenograft modelestablished with use of the aggressive metastatic-derived cell line S2O13. Established tumors were treated with a single intratumoral injection of 1010 vp of virus at day 0. At day 21, the 5/3Cox2-CRAdΔE3ADP-IFN group showed an average relative tumor volume of 5.6, compared with 12.1 with 5/3Easy IFN and 25.5 with PBS. Results demonstrate a statistically significant tumor suppression of 5/3Cox2CRAdΔE3ADP-IFN at day 21 when compared with saline and to 5/3EasyIFN (P < .05 for both).
DISCUSSION
At present, only resection can offer a possibility for the cure of pancreatic cancer; however, among the only 10% or so patients in whom complete tumor resection is possible, many will ultimately succumb to local and distant recurrence.2,5 Thus, a highly active targeted therapy that may be used alone or as an adjunct to multimodality therapy for local, advanced, and metastatic disease is acutely needed.
Adenoviral therapy offers this potential, but faces problems of low infectivity of cancer cells, inefficient viral spread within tumors, and off-target effects.16 Through rational design we have mitigated these problems to construct a novel IFN-expressing virus. In addition, this virus offers the potential of localized, high-level IFN expression in the tumor itself. This may contribute to improved tolerability of an IFN-based multimodality strategy, which offers dramatic improvement in outcome of pancreatic cancer but is constrained by treatment-limiting toxicity.7–10 IFN-expressing viruses have been used previously to good effect by other investigators19,20; however, the design of these vectors has been a nonreplicating one, which requires greater viral doses and lacks the benefit of viral persistence by replication.
The significance of our design strategy was demonstrated through analysis of infectivity and tumor specificity of our novel virus. The improved infectivity of the Ad5/Ad3 fiber-knob chimera was demonstrated by the use of different Luc expression vectors to compare infectivity with the wild-type capsid proteins to that of the Ad5/Ad3 chimera (Fig 2). Significantly increased reporter gene activity was found in all cell lines with Ad5/Ad3 indicating its superiority for tumor cell infection, and corroborating our earlier report.16
Improved cancer cell infectivity, however, does not indicate cancer specificity. This element is achieved by use of the Cox2 promoter13,14,16 to drive viral replication as well as detargeting Cox2-negative normal hepatocytes. Because most systemically administered Ad is sequestered in the liver and because the E1 gene product of early adenoviral replication is toxic to hepatocytes, restricted replication is a crucial requirement for clinical use. Again, the use of 2 reporter vectors, with one driven by Cox2 and the other a nonselective promoter, Cox2 function is shown to be great in all PDAc cell lines tested and almost completely absent in BT474, a Cox2-negative control cell line (Fig 3). This observation indicates that the desired “tumor ON/liver OFF” profile is achieved. As an additional demonstration of the Cox2-promoter-controlled specificity, we used this cell line in an assay for cytopathic effect and found no oncolysis with Cox2-controlled vectors including our novel virus, in contrast to near-complete clearing of infected cells by viruses with an intact E1 region and unrestricted replication (Fig 4, E).
Previously we reported a strategy to massively express a transgene upon Ad replication in tumor cells, which uses the gene placed into the Ad E3 region. This structure has been proven to overexpress ADP, which facilitates viral spread and leads to a more efficient intratumoral spread of the virus.22,23 On the basis of this system, we constructed an IFN- and ADP-expressing virus, incorporating the Ad5/Ad3 capsid modification, and with an intact E1 region for unrestricted replication as a proof of concept (5/3WtΔE3ADP-IFN). Because of the lack of control of replication as outlined previously, this virus is not suitable for human clinical use. In vitro testing of this virus (Fig 4, A) indicates that genetic modification of the adenoviral capsid and ADP overexpression can greatly enhance the low efficacy of conventional Ads in pancreatic cancer.
When similar in vitro testing was applied to our novel 5/3Cox2CRAdΔE3ADP-IFN (Fig 4, B–D), equal or improved potency compared with Ad5wt, the gold standard control virus without selectivity, is broadly noted, in particular in both S2O13 and S2VP10, which are derived from metastatic tumors and thought to represent a more aggressive tumor phenotype. This finding would again indicate no detrimental loss of replicating ability or killing effect imposed by Cox2 replication control and the complex viral structure in our design.
To characterize additionally the oncolytic potency of 5/3Cox2CRAdΔE3ADP-IFN, we performed quantitative cell survival assays across multiple PDAc cell lines (Fig 5, A–C). When compared with 5/3WtΔE3ADP-IFN as a positive control, a “lag phase” of cell death with 5/3Cox2CRAdΔE3ADP-IFN was repeatedly observed, which is potentially attributable to the artificial replication control imposed by the Cox2 promoter and resulting disruption of replication timing of viral genes.11 However, in all cell lines, the Cox2-controlled virus trended toward eventual equivalence in oncolytic effect with the uncontrolled IFN virus, reaching statistical equivalence in 2 of 3 experiments. Virtually no effect was seen upon low-titer infection with 5/3 Easy IFN, indicating the increased dependence of cytopathic effect in vitro on replication-dependent IFN production and ADP overexpression.
As for the separate issue of IFN expression, we analyzed levels seen after infection of a representative PDAc cell line with time (Fig 6). As expected, 5/3WtΔE3ADP-IFN produced large and increasing amounts of IFN, with a similar lagging increase produced by 5/3Cox2CRAdΔE3ADPIFN. In addition, 5/3Cox2CRAdΔE3ADP-IFN produced a significantly greater concentration of IFN than 5/3 Easy IFN, in amounts that were continuing to increase at the final time point. This virus may be expected to have both a longer-lasting and more substantial IFN production after infection of cancer cells.
To demonstrate in vivo antitumor effect, subcutaneous PDAc xenografts in nude mice were treated with the IFN vectors. In an experiment in which 5/3WtΔE3ADP-IFN was used as the experimental vector, proof of principle of superior effect of IFN and ADP coexpression compared with either alone was suggested, although the trend did not become significant. 5/3WtΔE3ADP-IFN virtually abrogated tumor growth until more than 1 month after treatment and clearly outperformed the negative control (Fig 7, A).
Next, to highlight treatment effect, we used the more aggressive S2O13 cell line, and 5/3Cox2-CRAdΔE3ADP-IFN showed superiority over the nonreplicating 5/3 Easy IFN by day 21 post treatment (Fig 7, B). Although the result is significant, the immunodeficient nature of the experimental animals limits demonstration of the IFN component’s systemic immune-stimulatory antitumor effect, which also may affect a difference seen in this system using an IFN-expressing virus.
Clearly, the ultimate goal of this work is systemic delivery of virus. Systemic delivery offers the advantages of convenience and ability to treat widespread disease, and we have previously shown with an earlier-generation virus in an orthotopic model that such a strategy does also have an antitumor effect with limited toxicity.16 The Food and Drug Administration, however, does not usually permit systemic administration of the vector before the approval of local injection protocol of the same therapeutics in the field of oncolytic Ad. The initial clinical protocol tends to be local injection (for pancreatic cancer, usually endoscopic ultrasound–guided injection). Thus, obtaining local injection data is more acute for novel structure vectors.
IFN alpha is a cytokine with pleiotropic effects. It has well-described biological properties, including inhibiting cellular proliferation by cell cycle arrest, induction of apoptosis, antiangiogenic effects, as well as a diverse immunostimulatory role. It is known to stimulate CD8+ T cells, as well as natural killer cells and monocytes, and additionally to up-regulate major histocompatibility complex expression for enhanced effector cell targeting.6 In terms of direct cellular effects, IFN acts through binding of its common receptor (composed of IFNAR-1 and -2 chains) to induce downstream tyrosine kinase signaling. However, the complexities of these processes present multiple avenues for escape mechanisms.
Differing susceptibility to IFN-alpha has been well-described, with some cancer cell types responding poorly or not at all, including some melanoma, ovarian carcinoma, and multiple myeloma; however, these cell types do respond to the related cytokine IFN-beta.6 In addition, the development of resistance to IFN has been a widely reported phenomenon. Cells that adapt to down-regulate proapoptotic IFN target genes such as TRAIL or that overexpress antiapoptotic genes display 2 such avenues of escape. A third means of IFN resistance is increased expression of epidermal growth factor receptor and its downstream targets, which actually appears to be directly stimulated by IFN as a stress response.25 It is likely that, within PDAc itself, there exists differing susceptibility to IFN treatment, which may explain differing strengths of 5/3Cox2CRAdΔE3ADP-IFN seen in vitro (Fig 4, B–D), as well as differences in IFN vector responsiveness seen in vivo between the 2 different cell lines used for tumor xenografts (Fig 7, A and B).
In addition, IFN is thought of as a cytostatic agent and best used with agents which either cause a separate tumoricidal effect such as an oncolytic virus or chemotherapy, or with complementary targeted agents such as EGFR inhibitors.25 Notably, in S2VP10 cells (Fig 5, C), the difference between 5/3Cox2 CRAdΔE3ADP-IFN and the IFN-nonexpressing virus 5/3Cox2CRAd was evident only at early time points before day 5 and this difference between these 2 vectors was less than in other cell lines. We believe this highlights the different character of this cell line and the heterogeneity that may be seen in IFN response.
Acknowledgments
Supported partly by T32CA132715 from the National Cancer Institute (SV, LA), P50CA101955 from the National Cancer Institute (SV, JD, MY), R01CA094084 from the National Cancer Institute (MY, SV, JD), and NIMHD/NIH-1P60MD003422 from the National Institute for Minority Health Disparities (JH, SV).
We thank Kazunori Aoki, MD, PhD, at National Cancer Center Research Institute of Japan, for providing interferon cDNAs.
REFERENCES
- 1.American Cancer Society. Cancer facts & figures 2010 [homepage on the Internet] Atlanta: American Cancer Society; 2010. [Accessed February 22, 2012]. Available from: http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-figures- 2010-rev. [Google Scholar]
- 2.Merchant NB, Parikh AA, Liu EH. Adjuvant chemoradiation therapy for pancreas cancer: who really benefits? Adv Surg. 2010;44:149–164. doi: 10.1016/j.yasu.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma [homepage on the Internet] National Comprehensive Cancer Network; [Accessed February 22, 2012]. Available from: http://www.nccn.org/professionals/physician_gls/PDF/pancreatic.pdf. [Google Scholar]
- 5.Katz MH, Wang H, Fleming JB, et al. Long-term survival after multidisciplinary management of resected pancreatic adenocarcinoma. Ann Surg Oncol. 2009;16:836–847. doi: 10.1245/s10434-008-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla-Sarkar M, Lindner DJ, Liu YF, et al. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 7.Nukui Y, Picozzi VJ, Traverso LW. Interferon-based adjuvant chemoradiation therapy improves survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2000;179:367–371. doi: 10.1016/s0002-9610(00)00369-x. [DOI] [PubMed] [Google Scholar]
- 8.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185:476–480. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 9.Linehan DC, Tan MC, Strasberg SM, et al. Adjuvant interferon-based chemoradiation followed by gemcitabine for resected pancreatic adenocarcinoma: a singleinstitution phase II study. Ann Surg. 2008;248:145–151. doi: 10.1097/SLA.0b013e318181e4e9. [DOI] [PubMed] [Google Scholar]
- 10.Picozzi VJ, Abrams RA, Decker PA, et al. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b-based chemoradiation: ACOSOG trial Z05031. Ann Oncol. 2010;22:348–354. doi: 10.1093/annonc/mdq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto M, Davydova J, Wang M, et al. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203–1218. doi: 10.1016/s0016-5085(03)01196-x. [DOI] [PubMed] [Google Scholar]
- 12.Yip-Schneider MT, Barnard DS, Billings SD, et al. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis. 2000;21:139–146. doi: 10.1093/carcin/21.2.139. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto M, Alemany R, Adachi Y, Grizzle WE, Curiel DT. Characterization of the cyclooxygenase-2 promoter in an adenoviral vector and its application for the mitigation of toxicity in suicide gene therapy of gastrointestinal cancers. Mol Ther. 2001;3:385–394. doi: 10.1006/mthe.2001.0275. [DOI] [PubMed] [Google Scholar]
- 14.Wesseling JG, Yamamoto M, Adachi Y, et al. Midkine and cyclooxygenase-2 promoters are promising for adenoviral vector gene delivery of pancreatic carcinoma. Cancer Gene Ther. 2001;8:990–996. doi: 10.1038/sj.cgt.7700403. [DOI] [PubMed] [Google Scholar]
- 15.Davydova J, Le LP, Gavrikova T, Wang M, Krasnykh V, Yamamoto M. Infectivity-enhanced cyclooxygenase-2–based conditionally replicative adenoviruses for esophageal adenocarcinoma treatment. Cancer Res. 2004;64:4319–4327. doi: 10.1158/0008-5472.CAN-04-0064. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez PJ, Vickers SM, Ono HA, et al. Optimization of conditionally replicative adenovirus for pancreatic cancer and its evaluation in an orthotopic murine xenograft model. Am J Surg. 2008;195:481–490. doi: 10.1016/j.amjsurg.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Hatanaka K, Suzuki K, Miura Y, et al. Interferon-alpha and antisense K-ras RNA combination gene therapy against pancreatic cancer. J Gene Med. 2004;6:1139–1148. doi: 10.1002/jgm.602. [DOI] [PubMed] [Google Scholar]
- 18.Ohashi M, Yoshida K, Kushida M, et al. Adenovirus-mediated interferon alpha gene transfer induces regional direct cytotoxicity and possible systemic immunity against pancreatic cancer. Br J Cancer. 2005;93:441–449. doi: 10.1038/sj.bjc.6602713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hara H, Kobayashi A, Yoshida K, et al. Local interferon-alpha gene therapy elicits systemic immunity in a syngeneic pancreatic cancer model in hamster. Cancer Sci. 2007;98:455–463. doi: 10.1111/j.1349-7006.2007.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narumi K, Kondoh A, Udagawa T, et al. Administration route-dependent induction of antitumor immunity by interferon-alpha gene transfer. Cancer Sci. 2010;101:1686–1694. doi: 10.1111/j.1349-7006.2010.01578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fallaux FJ, Kranenburg O, Cramer SJ, et al. Characterization of 911: A new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 22.Ono HA, Le LP, Davydova JG, Gavrikova T, Yamamoto M. Noninvasive visualization of adenovirus replication with a fluorescent reporter in the E3 region. Cancer Res. 2005;65:10154–10158. doi: 10.1158/0008-5472.CAN-05-1871. [DOI] [PubMed] [Google Scholar]
- 23.Davydova J, Gavrikova T, Brown EJ, et al. In vivo bioimaging tracks conditionally replicative adenoviral replication and provides an early indication of viral antitumor efficacy. Cancer Sci. 2010;101:474–481. doi: 10.1111/j.1349-7006.2009.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maizel JV, Jr, White DO, Scharff MD. The polypeptides of adenovirus. I. evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36:115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- 25.Caraglia M, Marra M, Tagliaferri P, et al. Emerging strategies to strengthen the anti-tumour activity of type I interferons: overcoming survival pathways. Curr Cancer Drug Targets. 2009;9:690–704. doi: 10.2174/156800909789056980. [DOI] [PubMed] [Google Scholar]