Abstract
Although neurotrophic factors have long been recognized as potent agents for protecting against neuronal degeneration, clinical success in treating Parkinson’s disease and other neurodegenerative disorders has been hindered by difficulties in delivery of trophic factors across the blood brain barrier (BBB). Bone marrow hematopoietic stem cell-based gene therapy is emerging as a promising tool for overcoming drug delivery problems, as myeloid cells can cross the BBB and are recruited in large numbers to sites of neurodegeneration, where they become activated microglia that can secrete trophic factors. We tested the efficacy of bone marrow-derived microglial delivery of neurturin (NTN) in protecting dopaminergic neurons against neurotoxin-induced death in mice. Bone marrow cells were transduced ex vivo with lentivirus expressing the NTN gene driven by a synthetic macrophage-specific promoter. Infected bone marrow cells were then collected and transplanted into recipient animals. Eight weeks after transplantation, the mice were injected with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropuridine (MPTP) for seven days to induce dopaminergic neurodegeneration. Microglia-mediated NTN delivery dramatically ameliorated MPTP-induced degeneration of tyrosine hydroxylase (TH)-positive neurons of the substantia nigra and their terminals in the striatum. Microglia-mediated NTN delivery also induced significant recovery of synaptic marker staining in the striatum of MPTP-treated animals. Functionally, NTN treatment restored MPTP-induced decline in general activity, rearing behavior, and food intake. Thus, bone marrow-derived microglia can serve as cellular vehicles for sustained delivery of neurotrophic factors capable of mitigating dopaminergic injury.
Keywords: Parkinson’s disease, Gene therapy, Neurodegenerative disease, Neurotrophic factors, Dopamine
1. Introduction
Parkinson’s disease (PD) is an increasingly common neurodegenerative disease with profound motor, cognitive, and behavioral dysfunction, caused primarily by the death of dopaminergic neurons in the substantia nigra [6]. Symptoms of PD are delayed, appearing only after about 70–80% of striatal dopamine and half of nigral dopamine neurons have been lost [1,8,19]. Current treatments focus on dopamine replacement, which has a limited window of efficacy. A long-sought but elusive therapeutic goal is to prevent progressive dopaminergic neuronal loss in early symptomatic patients. Although neurotrophic factors such as glial cell line-derived neurotrophic factor (GDNF) and neurturin (NTN) are currently recognized as potent survival factors for the nigrostriatal dopaminergic neurons that degenerate in PD, attempts to develop a neurotrophic factor-based therapy have been disappointing due to the difficulty of delivering it to the brain. Because of their large molecular size, peripherally administered GDNF or NTN cannot effectively cross the blood–brain barrier.
Bone marrow-derived microglia (BMDM) comprise a promising vehicle for delivery of therapeutic gene products to the central nervous system (CNS), as they are capable of crossing the BBB (as monocytes) and then differentiating into microglia [12,18]. Activated microglia are effectively recruited from circulating monocytes to sites of neurodegeneration [2,16,21]. Using a synthetic macrophage-specific promoter (MSP) that restricts transgene expression to the monocyte/macrophage lineage, we previously demonstrated a proof-of-principle for the therapeutic use of BMDM for sustained delivery of GDNF to sites of MPTP-induced dopaminergic neurodegeneration in mice [2]. To validate further the utility of BMDM as a cellular vehicle for PD gene therapy, we tested the efficacy of bone marrow-derived microglia-mediated delivery of NTN to regions of PD-associated dopaminergic injury in the murine MPTP model. NTN is a member of the GDNF family of ligands that has been shown to promote survival of nigrostriatal dopaminergic neurons in animal models of PD [9,13,14,20]. The amino acid sequence of mature NTN shares about 42% similarity with GDNF [15]. Both molecules signal through GDNF family receptors (GFR).
The efficacy of BMDM-mediated delivery of NTN, in conjunction with our previous report on BMDM-mediated delivery of GDNF [2], makes a strong case for the utility of bone marrow transplantation-based gene therapy for the treatment of neurodegenerative disorders. It is important to compare different neurotrophic factors in terms of therapeutic benefits, as well as side effect profiles.
2. Materials and methods
2.1. Animals
Male C57BL/6J mice, 6–8 weeks old at the time of transplantation, were group-housed in a 12/12 h light/dark cycle and in accordance with institutional requirements for animal care, National Institutes of Health Guide for the Care and Use of Laboratory Animals, and Society for Neuroscience guidelines.
2.2. Construction of NTN lentiviral plasmid and macrophage-specific synthetic promoter
We developed a highly active macrophage-specific synthetic promoter (MSP) that directs transgene expression predominantly to this cellular lineage [2,11]. A lentiviral vector incorporating mouse NTN cDNA driven by MSP was created using standard molecular procedures. The expression construct contained only the fully mature NTN sequence, without the pre-pro region of the wild-type transcript, because this results in a significant increase in the secretion of active NTN [9]. The final construct was sequenced to verify the site of insertion, as well as the integrity of the NTN cDNA. A similar lentiviral vector carrying the cDNA that encodes GFP, also driven by the MSP, was generated for use as a control vector. Viral stocks were generated by transient transfection of 293T cells with the lentiviral vector together with three separate packaging plasmids (pMDLg/pRRE, pRSV Rev, and pMD.G; PlasmidFactory GmbH & Co. KG, Bielefeld, Germany).
2.3. Lentiviral transduction and transplantation of bone marrow cells
Bone marrow harvest and lentiviral transduction were performed as described previously [2]. Following transduction, 1 × 106 cells, without selection, were injected via the tail vein into recipient mice previously treated with 950 cGy of total body irradiation. Mice that received bone marrow cells transduced with a lentiviral vector in which the expression of NTN was driven by the macrophage-specific synthetic promoter are designated MSP-NTN mice, whereas control mice that received bone marrow cells transduced with the lentiviral vector in which the expression of GFP was driven by the same promoter are designated MSP-GFP mice.
2.4. MPTP treatment
MPTP–HCl (Sigma, St. Louis, MO) dissolved in physiological saline was injected subcutaneously into MSP-NTN and MSP-GFP mice as follows: 15 mg/kg free base MPTP on day 1, 25 mg/kg on day 2, and 30 mg/kg daily on days 3–7 [2]. Control mice were treated with saline following the same regimen.
2.5. Behavioral tests
The open field test was performed as described before [7] at 4 weeks after the last dose of MPTP, so as to avoid any potential influence on behavior of peripheral toxicity associated with MPTP intoxication. Mice were assessed for, body weight, food intake, and allodynia as previously described [2,4].
2.6. Tissue processing and immunohistochemistry
The procedure was performed as described previously [2]. Sections were incubated with either rabbit anti-tyrosine hydroxylase (TH; Chemicon International, Billerica, MA) at 1:2000 dilution or mouse anti-synaptophysin (Sigma, St. Louis, MO) at 1:1000 dilution for 48 h at 4 °C. For double-color immunofluorescence, the sections were first incubated with TH antibody followed by anti-Iba1 antibody (1:2000; Wako, Osaka, Japan).
2.7. Stereology and optical density measurement
The total number of Nissl-positive/TH-immunoreactive neurons in substantia nigra pars compacta and optical density of the TH and synaptophysin-positive fibers in the striatum was estimated as described before [2].
2.8. Statistical analysis
Statistical significance was determined using Student’s t-test or ANOVA at the 95% confidence level and was followed by Tukey–Kramer multiple comparison test. Results are expressed as mean ± s.e.m and considered significant at P < 0.05. The body weight data at different time points were analyzed by repeated measures ANOVA.
3. Results
3.1. NTN expression in bone marrow cells transduced with MSP-NTN lentiviral vector
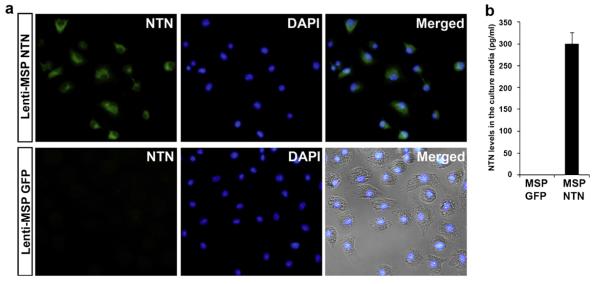
Bone marrow cells from donor mice were genetically modified using lentiviral vectors encoding either NTN or GFP cDNA. These constructs were driven by the MSP, thereby favoring enhancement of both the therapeutic efficacy and safety of neurotrophic factor gene therapy by restricting the expression of the transgene to microglial precursor cells (monocyte/macrophage) [2]. To confirm the expression of NTN in the transduced marrow cells aliquots of MSP-NTN and MSP-GFP transduced cells were cultured in macrophage medium [17] for seven days to transform them into macrophages. Immunocytochemical staining revealed that >95% of the MSP-NTN transduced macrophages expressed NTN in their cytoplasm, whereas no NTN staining was observed in MSP-GFP transduced macrophages (Fig. 1).
Fig. 1.
NTN expression in marrow-derived macrophages in vitro. (a) Immunocytochemical localization of NTN in macrophages. (b) NTN levels by ELISA in the culture medium.
3.2. Neuroprotective effect of microglia-mediated NTN delivery against MPTP-mediated nigrostriatal dopaminergic degeneration
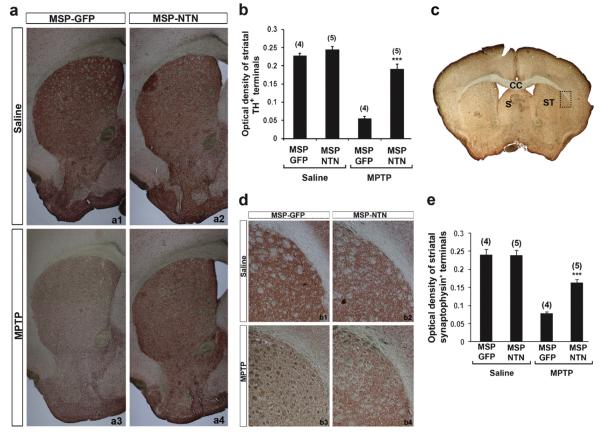
Nine weeks after the last injection of either MPTP or saline, recipient mice were sacrificed and the neuroprotective effect of microglia-mediated NTN delivery on MPTP-induced nigrostriatal dopaminergic degeneration was assessed by quantitative analysis of TH-positive neurons in the substantia nigra pars compacta (SNpc), as well as the density of TH-positive axon terminals in the striatum. Stereological analysis demonstrated up to a 55% loss of TH-positive neurons in the SNpc of MSP-GFP mice following MPTP treatment, compared with saline-treated animals (Fig. 2). The TH-positive dendritic fiber networks in the substantia nigra pars reticulata (SNpr) were also dramatically reduced after MPTP treatment in MSP-GFP mice (Fig. 2a3). In contrast, there was only 18–24% MPTP-induced loss of TH-positive neurons in the SNpc of MSP-NTN mice, compared with saline-treated mice (Fig. 2b). Moreover, the density of the SNpr TH-positive dendritic fiber network in the MSP-NTN mice was largely preserved in the face of MPTP treatment, relative to saline treatment (Fig. 2a4). Similar results were observed for dopamine fiber terminals in the striatum. Relative to the controls, there was an average 76% loss of the TH staining intensity in MSP-GFP mice, whereas the loss in MSP-NTN mice was only ~21% (Fig. 3a and b). Quantification of synapses in parallel sections of the striatum was achieved by immunohistochemistry with synaptophysin antibody to distinguish real axonal protection/regeneration from enhanced TH secretion following microglia-mediated NTN delivery (Fig. 3d). Synaptophysin immunostaining was significantly higher in MPTP-treated MSP-NTN mice than in MPTP-treated MSP-GFP mice (Fig. 3d and e). To assess whether the observed protective effect of microglia-mediated NTN delivery on the nigrostriatal dopaminergic system was due to accumulation of gene-modified microglia, we attempted to count the total number of Iba1-positive, as well as NTN-positive, cells in the SNpc of MSP-NTN mice. However, due to high background staining of NTN it was quite challenging to count NTN-positive microglia in tissue sections of MSP-NTN mice after immunohistochemical localization. Therefore, we examined the relative proportion of bone marrow-derived microglia in the SNpc of MSP-GFP mice. GFP is not secreted and can easily be visualized without further staining. Moreover, GFP-positive cells are characterized as being macrophages/microglia, due to being derived from the transplanted bone marrow cells and GFP expression is driven by the same MSP as NTN. In MSP-GFP mice nine weeks after the last injection of MPTP, 47% of the total microglia (Iba1-positive) in the nigra were bone marrow-derived, whereas the proportion of Iba-1 positive bone marrow-derived microglia in the saline-treated MSP-GFP mice was only 14% (Fig. 4a and b). Most importantly, genetically modified Iba1-positive bone marrow-derived microglia were seen in close proximity to TH-positive neurons (Fig. 4c), providing additional evidence that therapeutic neurotrophic factors secreted by microglia are accessible to diseased neurons. The influence of whole-body irradiation on BBB integrity and the possibility that such damage permitted enhanced recruitment of marrow derived microglia to the brain cannot be excluded.
Fig. 2.
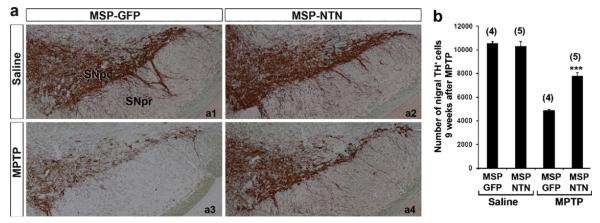
Effect of microglia-mediated NTN delivery on MPTP-induced degeneration of nigral dopaminergic neurons. (a) Midbrain sections of MSP-GFP and MSP-NTN mice showing TH-immunostaining in the substantia nigra. (b) Plots of stereologic data illustrating the protective effect of NTN delivery at 9 weeks post MPTP treatment (***P < 0.001). The number of animals used in each group is shown in parentheses.
Fig. 3.
Effect of microglia-mediated NTN delivery on MPTP-induced degeneration of dopaminergic terminals in the striatum. (a) TH-positive terminals in the striatum of MSP-GFP and MSP-NTN mice. (b) Plots of optical density measurement of TH-positive terminals in the striatum (***P < 0.001). (c) Low power photomicrograph of the pre-commissural striatum. (d) Representative high power photomicrograph of synaptophysin staining in the dorso-lateral striatum (represents dotted rectangle in (c)) of MSP-GFP and MSP-NTN mice. (e) Quantification of the synaptophysin immunoreactivity in the dorso-lateral striatum of MSP-GFP and MSP-NTN mice 9 weeks post MPTP treatment (***P < 0.001). CC, corpus callosum; S, septum; ST, straitum.
Fig. 4.
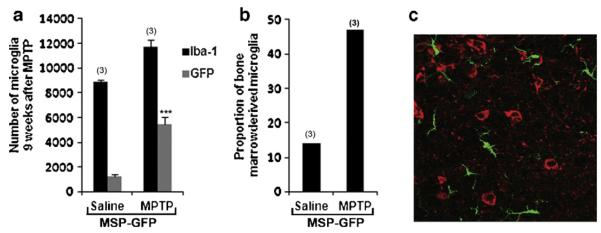
Recruitment of bone marrow-derived microglia to the nigra nine weeks after the last injection of either MPTP or saline. (a) Total number of Iba1-and GFP-positive cells in the nigra of MSP-GFP mice. (b) Proportion of marrow-derived (GFP-positive) microglia in the nigra of MSP-GFP mice (n = 3 in each group). (c) Section of MPTP-treated MSP-GFP mice showing marrow-derived microglia (green) in close proximity to TH-positive neurons (red). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. Effect of microglia-mediated NTN delivery on MPTP-induced motor impairment
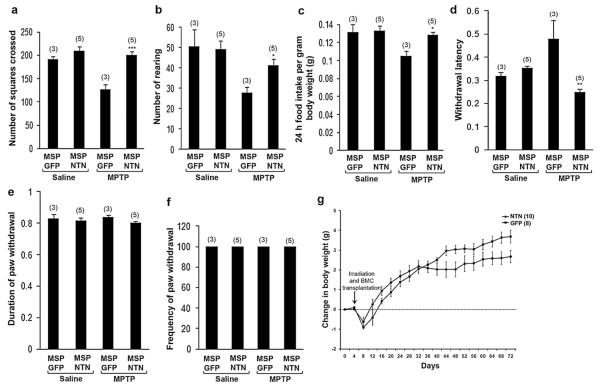
Mice were subjected to tests of general locomotor activity and rearing behavior in the open field maze. Relative to control mice, MPTP treatment significantly reduced activity levels (Fig. 5a) and rearing behavior (Fig. 5b) in MSP-GFP mice. In contrast, the activity and rearing behavior of MPTP-treated MSP-NTN mice were similar to those of control mice. Consistent with lower activity levels, MSP-GFP mice treated with MPTP exhibited reduced food intake normalized for body weight (Fig. 5c). This effect was reversed in MSP-NTN mice treated with MPTP.
Fig. 5.
Microglia-mediated NTN delivery improved functional recovery without major side effects. Total activity (a, ***P < 0.001) and rearing behavior (b, *P < 0.05) assessed by open field test (repeated four times). (c) 24 h food intake normalized for body weight (repeated three times, *P < 0.05). Allodynia, as assessed by the latency (d), duration (e) and frequency (f) of the paw withdrawal response (repeated three times). (g) Mean change from initial body weight is expressed ± standard error. All the behavior tests were performed 8 weeks post MPTP treatment.
3.4. Assessment of side effects of microglia-mediated NTN delivery
Direct brain infusion of GDNF, a neurotrophic factor closely related to NTN, is associated with significant adverse effects, including allodynia and weight loss [13]. In our study, no animals demonstrated signs of allodynia, as determined by paw withdrawal duration (Fig. 5e) or frequency (Fig. 5f) in response to the application of acetone on the mid-plantar surface of the hind paw. However, the latency to paw withdrawal after acetone application was relatively higher in the MSP-GFP mice treated with MPTP, and this effect was significantly reversed in MSP-NTN mice treated with MPTP (Fig. 5d). Body weight was recorded every four days for the duration of the experiment and expressed as mean change from initial body weight. Although both the MSP-GFP and MSP-NTN groups lost weight acutely after whole body irradiation and transplantation, they quickly resumed a trajectory of increasing weight (Fig. 5g). Over time, MSP-GFP mice gained significantly more weight than MSP-NTN mice.
4. Discussion
We demonstrate here the effectiveness of gene-modified bone marrow-derived microglia for sustained delivery of NTN to regions associated with MPTP-induced neurodegeneration in mice. The results corroborate our previous proof-of-principle for using microglia for sustained delivery of GDNF to particular sites of neurodegeneration [2] and provide further support for bone marrow transplantation-based neurotrophic factor gene therapies for PD. Neuroprotective effects of BMDM-mediated delivery of NTN on dopaminergic neurons in the nigra and its terminals in the striatum were comparable to those of GDNF, indicating that BMDM-mediated delivery NTN, as well as GDNF, will ultimately be effective in PD management, each having some advantages and thereby providing options for individual PD patients.
The slow but steady pace of progressive neuronal loss renders PD amenable to treatment strategies that protect remaining neurons. Given this progressive nature of PD, an important goal of NTN therapy should be continuous delivery over months or years in order to maintain dopamine neuron survival and function. In this context, the use of microglia to deliver NTN is particularly attractive, given the fact that these cells are derived from a defined pool of stem cells in the bone marrow, and are capable of crossing the BBB, providing effective sustained local delivery of NTN over a prolonged period. In support of this is the experience in bone marrow gene therapy for X-linked severe combined immunodeficiency patients, who exhibit sustained immune reconstitution for 10 years or more [10].
Using gene-modified microglia, neurotrophic factors can be delivered effectively over large portions of the brain. Whereas the small brain size of rodents may allow for direct focal delivery, even with advanced techniques it would be difficult to cover the entire striatum of human brain, approximately 700–1000 times larger by weight than rodent brain. Preclinical studies in monkeys using direct delivery have indicated that bioavailability of GNDF might be limited to a small portion (2–9%) of the human putamen [22]. In contrast, microglia-mediated delivery is unaffected by the size of the brain or any particular nucleus within the brain. The larger the target size of the degenerating area, the more microglia will be recruited to the region.
When antibody-based clearance of hematopoietic stem cell niches becomes a clinical reality [5,23], microglia-mediated NTN gene therapy can be achieved using techniques no more invasive than peripheral venipuncture. Hematopoietic stem cells can be collected from a patient’s own peripheral blood via apheresis, transduced ex vivo, and then re-infused back to the patient for PD treatment. Recent success in treating X-linked adrenoleukodystrophy supports this view [3]. In that study, autologous CD34+ cells were harvested from the patients, genetically modified ex vivo with a lentiviral vector, and then re-infused after the patients had received myeloablative treatment. Beginning 14–16 months after transplantation, progressive cerebral demyelination in the patients abated, a clinical benefit that was attributed to recruitment of bone marrow-derived cells as precursors to CNS macrophages/microglia [3].
5. Conclusion
Although the prospects of gene therapy have been viewed by some with skepticism, improved safety features and clear clinical benefits achieved with bone marrow-based gene therapy in the last few years [3,10], have helped to overcome earlier con0cerns for safety. Our previous report on bone marrow-derived microglia-based delivery of GDNF [2] and the current report on microglia-mediated NTN delivery substantiate the utility of microglia as natural cellular vehicles for delivering large therapeutic proteins across the blood–brain barrier and suggest that neurotrophic factor delivery based on transplantation of genetically modified bone marrow stem cells is a promising avenue for developing neuroprotective strategies for PD.
HIGHLIGHTS.
▶ Efficacy of microglial NTN delivery for Parkinson’s disease gene therapy is tested.
▶ Gene modified bone marrow-derived microglia accumulate in diseased sites.
▶ Microglial NTN delivery dramatically reduces degeneration of dopaminergic neurons.
▶ Protected dopaminergic terminals in the striatum, and restored motor deficits.
▶ No apparent major side effects were observed.
Acknowledgments
This work was supported by NIH grants (NS046004 and AG024579), a Merit Review Grant from the Veterans Health Administration, and a NIH Clinical and Translational Science Award (UL1 TR0000149) Pilot Project grant awarded to S.L.
References
- [1].Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. Journal of the Neurological Sciences. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- [2].Biju K, Zhou Q, Li G, Imam SZ, Roberts JL, Morgan WW, Clark RA, Li S. Macrophage-mediated GDNF delivery protects against dopaminergic neurodegeneration: a therapeutic strategy for Parkinson’s disease. Molecular Therapy. 2010;18:1536–1544. doi: 10.1038/mt.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrere F, Blanche S, Audit M, Payen E, Leboulch P, l’Homme B, Bougneres P, von KC, Fischer A, Cavazzana-Calvo M, Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- [4].Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- [5].Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- [7].Dunnett SB, Carter RJ, Watts C, Torres EM, Mahal A, Mangiarini L, Bates G, Morton AJ. Striatal transplantation in a transgenic mouse model of Huntington’s disease. Experimental Neurology. 1998;154:31–40. doi: 10.1006/exnr.1998.6926. [DOI] [PubMed] [Google Scholar]
- [8].Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- [9].Fjord-Larsen L, Johansen J, Kusk P, Tornoe J, Gronborg M, Rosenblad C, Wahlberg LU. Efficient in vivo protection of nigral dopaminergic neurons by lentiviral gene transfer of a modified neurturin construct. Experimental Neurology. 2005;195:49–60. doi: 10.1016/j.expneurol.2005.03.006. [DOI] [PubMed] [Google Scholar]
- [10].Hacein-Bey-Abina S, Hauer J, Lim A, Picard C, Wang GP, Berry CC, Martinache C, Rieux-Laucat F, Latour S, Belohradsky BH, Leiva L, Sorensen R, Debre M, Casanova JL, Blanche S, Durandy A, Bushman FD, Fischer A, Cavazzana-Calvo M. Efficacy of gene therapy for X-linked severe combined immunodeficiency. New England Journal of Medicine. 2010;363:355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].He W, Qiang M, Ma W, Valente AJ, Quinones MP, Wang W, Reddick RL, Xiao Q, Ahuja SS, Clark RA, Freeman GL, Li S. Development of a synthetic promoter for macrophage gene therapy. Human Gene Therapy. 2006;17:949–959. doi: 10.1089/hum.2006.17.949. [DOI] [PubMed] [Google Scholar]
- [12].Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- [13].Hoane MR, Gulwadi AG, Morrison S, Hovanesian G, Lindner MD, Tao W. Differential in vivo effects of neurturin and glial cell-line-derived neurotrophic factor. Experimental Neurology. 1999;160:235–243. doi: 10.1006/exnr.1999.7175. [DOI] [PubMed] [Google Scholar]
- [14].Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, III, Gasmi M, Bartus RT. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Annals of Neurology. 2006;60:706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- [15].Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM, Jr., Milbrandt J. Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature. 1996;384:467–470. doi: 10.1038/384467a0. [DOI] [PubMed] [Google Scholar]
- [16].Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends in Neurosciences. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- [17].Li G, Biju KC, Xu X, Zhou Q, Chen C, Valente AJ, He W, Reddick RL, Freeman GL, Ahuja SS, Clark RA, Li S. Macrophage LXRalpha gene therapy ameliorates atherosclerosis as well as hypertriglyceridemia in LDLR(−/−) mice. Gene Therapy. 2011;18:835–841. doi: 10.1038/gt.2011.29. [DOI] [PubMed] [Google Scholar]
- [18].Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- [19].McGeer PL, Itagaki S, Akiyama H, McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Annals of Neurology. 1988;24:574–576. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- [20].Ramaswamy S, Soderstrom KE, Kordower JH. Trophic factors therapy in Parkinson’s disease. Progress in Brain Research. 2009;175:201–216. doi: 10.1016/S0079-6123(09)17514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rodriguez M, varez-Erviti L, Blesa FJ, Rodriguez-Oroz MC, Arina A, Melero I, Ramos LI, Obeso JA. Bone-marrow-derived cell differentiation into microglia: a study in a progressive mouse model of Parkinson’s disease. Neurobiology of Disease. 2007;28:316–325. doi: 10.1016/j.nbd.2007.07.024. [DOI] [PubMed] [Google Scholar]
- [22].Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z, Gerhardt GA, Gash DM. Point source concentration of GDNF may explain failure of phase II clinical trial. Experimental Neurology. 2006;202:497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- [23].Straathof KC, Rao K, Eyrich M, Hale G, Bird P, Berrie E, Brown L, Adams S, Schlegel PG, Goulden N, Gaspar HB, Gennery AR, Landais P, Davies EG, Brenner MK, Veys PA, Amrolia PJ. Haemopoietic stem-cell transplantation with antibody-based minimal-intensity conditioning: a phase 1/2 study. Lancet. 2009;374:912–920. doi: 10.1016/S0140-6736(09)60945-4. [DOI] [PubMed] [Google Scholar]