Abstract
This study examined the effects of a virtual reality distraction intervention on chemotherapy-related symptom distress levels in 16 women aged 50 and older. A cross-over design was used to answer the following research questions: (1) Is virtual reality an effective distraction intervention for reducing chemotherapy-related symptom distress levels in older women with breast cancer? (2) Does virtual reality have a lasting effect? Chemotherapy treatments are intensive and difficult to endure. One way to cope with chemotherapy-related symptom distress is through the use of distraction. For this study, a head-mounted display (Sony PC Glasstron PLM—S700) was used to display encompassing images and block competing stimuli during chemotherapy infusions. The Symptom Distress Scale (SDS), Revised Piper Fatigue Scale (PFS), and the State Anxiety Inventory (SAI) were used to measure symptom distress. For two matched chemotherapy treatments, one pre-test and two post-test measures were employed. Participants were randomly assigned to receive the VR distraction intervention during one chemotherapy treatment and received no distraction intervention (control condition) during an alternate chemotherapy treatment. Analysis using paired t-tests demonstrated a significant decrease in the SAI (p = 0.10) scores immediately following chemotherapy treatments when participants used VR. No significant changes were found in SDS or PFS values. There was a consistent trend toward improved symptoms on all measures 48 h following completion of chemotherapy. Evaluation of the intervention indicated that women thought the head mounted device was easy to use, they experienced no cybersickness, and 100% would use VR again.
INTRODUCTION
One out of every eight women will develop breast cancer in her lifetime, and 211,000 women annually are diagnosed with invasive breast cancer.1 Seventy-seven percent of new cases and 84% of breast cancer deaths occur in women aged 50 and older.2 The incidence of breast cancer is highest among the 75–79 age bracket.2 Chemotherapy is a recommended course of treatment for breast cancer in older women. It is prescribed either prior to or after surgery in an attempt to diminish tumor mass, eradicate occult micrometastatic disease, and increase disease-free survival. However, as few as 30% of affected elderly women receive some chemotherapy treatment,3 in part because concerns about treatment toxicity and tolerability outweigh concerns about survival. In addition, once prescribed, women often have difficulty adhering to the prescribed schedule because of chemotherapy-related symptoms. Nonetheless, chemotherapy treatment is an effective treatment for older women,4,5 and the chances for survival are greatly enhanced if women receive all of the recommended chemotherapy treatments. Women who successfully complete chemotherapy have a greater chance of non-recurrence and long-term quality of life.6 Thus, helping older women tolerate needed treatments is critical to their survival.
Symptoms associated with chemotherapy treatments represent a major stressor for older people. Interventions that reduce treatment-related symptom distress should enhance a person’s ability to cope with the disease, not only by eliminating an important stressor, but perhaps also by helping to engender a sense of mastery over the illness. Effective coping can lead to increased adherence to treatment regimens and, ultimately, improved survival. One way to decrease symptom distress is through the use of distraction interventions, which have proven effective because they divert the focus of attention away from unpleasant symptoms.7,8 Techniques such as relaxation, music, and imagery are distraction interventions, and research has demonstrated that they relieve chemotherapy symptoms such as pain, anxiety, nausea, fatigue, and stress.9,10 Virtual reality (VR) has unique features that may allow it to be a more effective distractor than other interventions.
VR is immersive and interactive, engaging several senses simultaneously.11,12 This technology allows an individual to hear and feel stimuli that correspond with a visual image. The person wears a head-mounted device that projects an image with the corresponding sounds. The sense of touch is involved through use of a computer mouse that allows manipulation of the image. VR does not require practice prior to use in the clinical setting. The headset provides an enclosed visual environment and supplies the person with distracting images.13-15 VR may be an ideal distractor for the person who copes by focusing on a problem and who has difficulty directing their attention away from a stressful situation.
There have been few tests of VR as a distraction intervention. Among those few, Kozarek et al.16 explored the feasibility of using a virtual vision head-mounted display and travelogue tape as a distraction intervention for 50 adults undergoing routine gastric laboratory procedures. An improved tolerance to the procedure, measured using a visual analog scale, was noted in 85% of the patients. Ratings by nurses using the same scale confirmed that the distraction intervention was effective.16 This project did not employ a control condition.
Wint et al., using a randomized control design with 30 subjects, reported no significant differences in visual analog scale (VAS) pain scores between adolescents with cancer who used virtual reality as a distraction intervention during lumbar punctures and those who did not receive this intervention.15 However, there was an improvement in VAS score in the intervention group, and 77% of subjects who used the head-mounted display reported that virtual reality was an effective distractor.15
Schneider and Workman reported improvements in symptom distress when children aged 10–17 used a VR distraction intervention during outpatient chemotherapy treatments for leukemia or lymphoma.13,17 Their findings were reproduced in a sample of adult women, aged 18–55, receiving chemotherapy for breast cancer. Symptom Distress Scale scores and Piper Fatigue Scale values were significantly lower following chemotherapy treatments when women used the VR intervention.14,18 In Japan, Oshuga et al.19 developed the “bedside wellness system,” which allows patients to take a virtual walk through the forest while in bed. Images are portrayed on bedside screens with corresponding sensory sensations (bird sounds, cool breezes) produced by the system. Foot devices enhance movement of the lower extremities and control movement of the image. A preliminary study with 20 healthy subjects suggested that the intervention helped people to relax, and further research is now being conducted with adult cancer patients.19
A study by Hoffman et al.20 found that virtual reality was a more effective distractor than Nintendo video games in controlling burn pain during dressing changes in a sample of 12 adults and children. Subjects reported that the environment created by the head mounted device was more engaging than the flat screen Nintendo images. In follow-up research using a sample of seven adults and children, pain ratings were compared during range-of-motion exercises. Pain ratings were significantly lower when patients used the VR than when they had no distraction. Further, pain continued to be reduced even with repeated use of VR, suggesting that the intervention was a true distractor, not just a novel experience.21
Conceptual framework
Lazarus and Folkman’s stress and coping framework was used to guide the study. In this framework, coping is defined as the “constantly changing cognitive and behavioral efforts to manage specific external and/or internal demands that are appraised as taxing or exceeding the resources of the person.”22 Coping is a process involving appraisal of a stressful situation and attempts to manage the stressful situation. Coping responses reflect the various thoughts and activities people use to manage stressful situations.22
Lazarus and Folkman theorize that two types of strategies with distinct coping functions exist: those directed at (a) managing or altering the problem, or problem-focused coping, and (b) regulating the emotional response to the problem, or emotion-focused coping. Lazarus and Folkman state that individuals turn to emotion-focused coping when they perceive that nothing can be done to change the threatening condition. Distraction therapies are an emotion-focused coping strategy.22 In this study, the premise being tested is that distraction will enhance adaptation to chemotherapy-related symptoms.
MATERIALS AND METHODS
Research questions and design
This pilot study investigated the use of virtual reality equipment in a population of older women, aged 50–77, with breast cancer. A cross-over design was used to answer the following questions: (1) Is virtual reality an effective distraction intervention for reducing chemotherapy-related symptom distress levels in older women with breast cancer? (2) Does virtual reality have a lasting effect?
Sample
The population for this study was a group of older women, aged 50–77, who were scheduled to receive intravenous chemotherapy as part of their treatment plan. A convenience sample of 16 women with breast cancer was selected. Inclusion criteria for the study participants include (1) diagnosis of breast cancer, (2) first diagnosis of cancer, (3) age 50 years or older, (4) requiring at least two matched cycles of intravenous chemotherapy, (5) able to read and write English, (6) without clinical evidence of primary or metastatic disease to the brain, (7) without history of seizures, (8) no history of motion sickness, and (9) a score of 24 or greater on the Mini Mental Status Exam. For sample demographics, see Table 1.
Table 1.
Demographics of Sample (N = 16)
| Age | |
| 50–77, M = 57.7, SD = 6.8 years | |
| Diagnosis | |
| Adenocarcinoma | 16 (100%) |
| Ethnic identification | |
| Caucasian | 15 |
| African American | 1 |
85% participation rate.
Instruments and equipment
Virtual reality technology
VR is a computer-simulated technique, which allows an individual to hear and feel stimuli that correspond with a visual image. The individual wears an 8-oz head-mounted device, which projects an image with the corresponding sounds. The sense of touch is involved through use of a computer mouse that allows for the manipulation of the image. For this study, a commercially available headset (Sony PC Glasstron PLM- S700) was used (Fig. 1). Participants wore the head-mounted device during their intravenous chemotherapy treatment. Participants chose from three CD-ROM based scenarios; (Oceans Below®, A World of Art®, or Titanic: Adventure Out of Time®). Each scenario could last several hours.
FIG. 1.
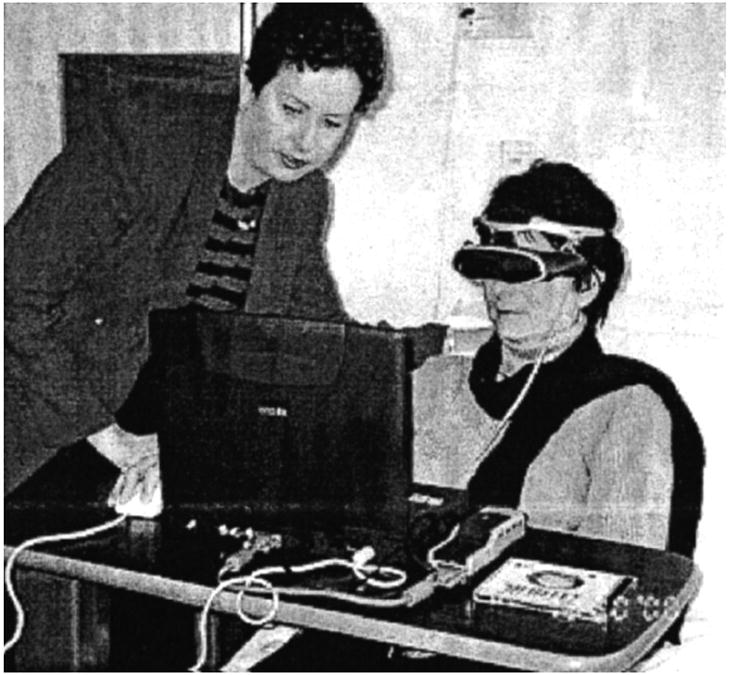
Patient using virtual reality in clinic setting.
Mini Mental Status Exam (MMSE)
The MMSE was used as a screening test to exclude those with cognitive impairment.23 The reliability and validity of the instrument are well established.23 The instrument was used to screen potential participants in order to prevent exposing individuals with limited cognitive abilities to equipment that they may find frustrating. All eligible participants achieved a score of 24 or higher.
Demographic questionnaire
An investigator-developed demographic questionnaire included items about age, gender, race, and computer experience. Data regarding disease, chemotherapy treatments and antiemetics were collected from the medical record.
Revised Piper Fatigue Scale (PFS)
The PFS is composed of 22 items scaled from 0 to 10 which measures fatigue.24 The coefficient alpha for the PFS with a population of breast cancer patients was 0.97. Concurrent validity is supported by significant correlations with the Profile of Mood States25 and the Fatigue Symptom Checklist.26 Coefficient alpha for the instrument in this study was 0.98.
State-Anxiety Inventory for Adults (SAI)
The SAI measures transitory anxiety states in adults, which includes anxiety induced by stressful procedures.27 Respondents rate each item on a scale from 0 to 3, with half of the items being reverse scored. A total score ranging from 0 to 60 is obtained by adding the weighted score for each item. The reliability and validity of this instrument are well established. Alpha reliability in a sample of women with breast cancer who used guided imagery during radiation therapy was 0.90.9 Alpha reliability for the SAI with this sample was 0.94.
Symptom Distress Scale (SDS)
The SDS is a general indicator of symptoms experienced by cancer patients.28 It measures the occurrence of specific symptoms and provides an overall score of symptom distress. The SDS is a 13-item Likert-type scale in which patients rate specific symptoms on a scale of 1–5. Total scores range from 13 to 65.28 A correlation of 0.90 between SDS score and scores on the Ware’s health perception questionnaire demonstrates convergent validity.29 The reliability (coefficient alpha) ranged between 0.79 and 0.89 in numerous samples of adult cancer patients.29 Coefficient alpha for the instrument in this study was 0.87.
Open-ended questionnaire
An open-ended questionnaire was used to elicit the participant’s evaluation of the intervention. This questionnaire provided descriptive data regarding the participant’s impression of the intervention and will be used to improve administration of the intervention in future studies.
Procedures
This study was conducted at the outpatient center of a Southeastern comprehensive cancer center. Oncologists, liaison nurses and other healthcare providers identified potential study participants. Prior to the first scheduled chemotherapy treatment, the investigator contacted potential participants via telephone to briefly explain the study and to inform them that they would be asked to participate in the study at their chemotherapy appointment.
At the clinic, potential participants were escorted to a private consultation room. The researcher administered the MMSE as part of the eligibility requirements. The researcher then explained the study, demonstrated the VR equipment, answered questions, and obtained written informed consent. Participants completed the initial set of questionnaires (demographic form, SDS, SAI and PFS). The women were then randomly assigned to receive the VR treatment during either their first chemotherapy treatment or during their next chemotherapy treatment.
For the control condition, when the subjects did not use the VR intervention, normal and customary procedures such as pre-treatment teaching, obtaining venous access, administration of antiemetic medication were performed by the outpatient clinic registered nurse. Immediately following the completion of intravenous chemotherapy, the subject again completed the SDS, SAI, and PFS. Arrangements were made for the researcher to contact the subject via telephone 48–52 h following the completion of chemotherapy to complete a third SDS, SAI, and PFS.
The above procedures were followed for the other chemotherapy treatment except that, during this treatment, the participants received the VR distraction intervention. For this treatment, the researcher demonstrated the virtual equipment and provided a brief explanation of how to use the equipment. The participants were able to choose from three different VR scenarios. The subjects used the VR equipment throughout the intravenous administration of chemotherapy. They were free to change scenarios or remove the headset at any time. As in the control condition, the clinic nurse followed all normal and customary nursing procedures. Following the completion of their chemotherapy, the VR equipment was removed, and a subjective evaluation of the intervention was completed in addition to the standard measures. The researcher recorded the amount of time that the VR was used and asked participants to estimate the amount of time that they used the distraction intervention. Additional data was collected regarding chemotherapy regimen and dosing as well as antiemetic or analgesics given. Participants were given a gift worth approximately $10 in return for their time and effort.
Statistical analysis
Following data collection, data were entered in a SPSS data file for analysis. A frequencies procedure was used to determine distributions and variances, and to identify outliers. No outliers were identified. To ensure that there were no differences in baseline symptoms between the group that received the VR during the first chemotherapy treatment or the group that received the VR during the second chemotherapy treatment, paired t-tests were used to test for group differences related to sequencing effects of the chemotherapy treatments. Since no differences were found, data were combined into a control and VR intervention group for further analysis. Paired t-tests were used to determine the mean difference in SDS, SAI, and PFS values immediately following the chemotherapy treatment and the mean difference SDS, SAI, and PFS values 48 h following the chemotherapy treatment. The level of significance for this pilot study was set at p ≤ 0.10.
RESULTS
Analysis of hypotheses
1. Is virtual reality an effective distraction intervention for reducing chemotherapy-related symptom distress levels in older women with breast cancer?
Results of analysis using paired t-test demonstrated that there was a significant difference in the SAI (p = 0.10, 15 df) scores immediately following the chemotherapy treatment when subjects used the VR intervention (Table 2). Mean SDS and PFS scores were lower following the use of the VR, but no significant differences were found. Cohen’s effect size for the intervention was calculated to be 0.44 for the SAI. The hypothesis that VR could mitigate chemotherapy-related symptom distress as a distraction intervention was partially supported.
Table 2.
Data Analysis: Research Question 1
| Instrument | t | p-value | Effect size |
|---|---|---|---|
| Symptom Distress Scale | −0.49 | 0.63 | 0.12 |
| Piper Fatigue | −0.12 | 0.91 | 0.03 |
| State Anxiety | −1.76 | 0.10a | 0.44 |
p ≤ 0.10, df = 15.
2. Does virtual reality have a lasting effect?
Again, paired t-tests were used to answer the hypothesis. While there were no significant changes in any of the measures of symptom distress, fatigue, or anxiety 2 days later, there was a trend toward lower scores with the VR condition (Table 3).
Table 3.
Data Analysis: Research Question 2
| Instrument | t | p-value |
|---|---|---|
| Symptom Distress Scale | −1.32 | 0.21 |
| Piper Fatigue | −1.13 | 0.28 |
| State Anxiety | −0.40 | 0.70 |
p ≤ 0.10, df = 15.
Additional findings
During the experimental condition, the participants used the VR equipment throughout the intravenous administration of chemotherapy. Table 4 shows the various scenarios and corresponding amount of time each was used. Women were free to change scenarios or remove the headset if they wished to discontinue the intervention. While several women changed scenarios, no one stopped using the VR. The researcher recorded the amount of time in minutes that the VR was used and also asked participants to estimate the amount of time that they had used the distraction intervention. The average amount of time women thought that they used VR was 43 min, significantly less (p < 0.001) than the actual mean recorded time of 78 minutes (Fig. 2).
Table 4.
Time Spent Using Virtual Reality Scenarios
| Virtual reality scenario | No. of subjects | Range (min) | Mean (min) |
|---|---|---|---|
| Titanic® | 7 | 35–100 | 78 |
| World of Art® | 8 | 10–65 | 39 |
| Oceans Below® | 7 | 25–105 | 54 |
| All scenarios | 16 | 55–115 | 78 |
FIG. 2.
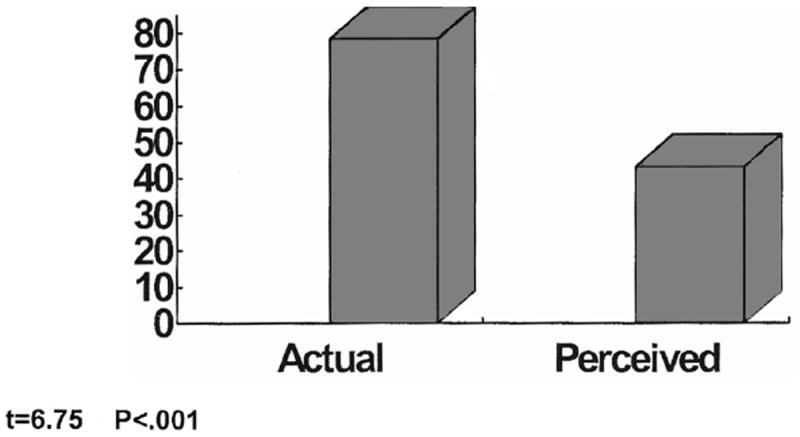
Perception of time in minutes.
Responses to the open-ended questionnaire provided descriptive data regarding the subjects’ evaluation of the intervention. Some key findings included that the women thought the head set was easy to use, they reported experiencing no unusual sensations or cybersickness, all subjects preferred the chemotherapy treatment with VR treatment, and 100% indicated that they would be willing to use the intervention again.
DISCUSSION
Findings from this study demonstrate that it is feasible to use VR distraction intervention in a population of older women and that the intervention reduced treatment-related anxiety in this small sample. Subjective comments regarding use of VR equipment have been positive. 94% of older women were able to use the headset without difficulty. Concerns about the headsets included weight and quality of visual image. Comments from nurses in the clinic indicate that patients are tolerating the treatments better when they use VR. One nurse was able to infuse the chemotherapy faster because the patient did not complain of “coldness” during the infusion when she was using VR. Clinic nurses mentioned that they have not received any symptom management calls from patients following chemotherapy treatments when women used the VR. All eligible individuals have been able to receive VR intervention during their chemotherapy treatment without delay or interruption due to technical problems.
The findings of this pilot study differ from previous studies in that there was a significant decrease in State Anxiety scores and no significant differences in Symptom Distress Scale or Piper Fatigue Scale values. Possible explanations for this are the novelty of the intervention and the lower baseline of symptom distress. For this population, rather than the computer-based intervention inducing anxiety, the distracting qualities of the VR appeared to decrease situational anxiety related to the chemotherapy treatment. In addition, consistent with the literature that older women experience less symptom distress than younger women following chemotherapy,30 it is possible that, given their life experiences, this sample of older women had already established effective coping strategies for managing symptom distress. The findings from this study partially support the theoretical premise that distraction enhances adaptation to chemotherapy-related symptoms.
The limitations of this pilot study are the small sample size and single study site. To date all of the studies exploring the effectiveness of VR have employed small sample sizes. Few controlled studies have been conducted using VR as a distraction technique. The findings of this study offer direction for future research: (1) the study should be repeated using an experimental design and multiple study sites, (2) future studies could compare VR to other distractors, (3) the VR intervention could be tested with different samples and different outcome variables, and (4) research should explore how coping style and immersive tendency affect the use of distraction interventions. Ongoing study of this distraction technique will determine if VR is an effective intervention. In summary, the VR distraction decreased chemotherapy-related anxiety in a sample of older women with breast cancer. The intervention was easy to implement and positively received by the study participants.
Acknowledgments
This study was funded by a grant from the National Institutes of Nursing Research (1 P20 NR07795–01, Clipp PI). We wish to acknowledge the support of the nurses and physicians at the Morris Outpatient Treatment facility at Duke University Medical Center Comprehensive Cancer Center. Thanks also to the study participants for sharing their time and insights.
References
- 1.Jemal A, Murray T, Samuels A, et al. Cancer statistics. CA: A Cancer Journal for Clinicians. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. 2000 On-line: www.cancer.org.
- 3.Goldhirsch A, Glick JH, Gelber RD, et al. Meeting highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Journal of the National Cancer Institute. 1998;90:1601–1608. doi: 10.1093/jnci/90.21.1601. [DOI] [PubMed] [Google Scholar]
- 4.Castiglione M, Gelber RD, Goldhirsch A. Adjuvant systemic therapy for breast cancer in the elderly: competing causes of mortality. International Breast Cancer Study Group Journal of Clinical Oncology. 1990;8:519–526. doi: 10.1200/JCO.1990.8.3.519. [DOI] [PubMed] [Google Scholar]
- 5.Christman K, Muss HB, Case LD, et al. Chemotherapy of metastatic breast cancer in the elderly. The Piedmont Oncology Association experience. JAMA. 1992;268:57–62. [PubMed] [Google Scholar]
- 6.Lyman GH, Lyman S, Bakducci L, et al. Age and the risk of breast cancer recurrence. Cancer Control. 1996;3:421–427. [PubMed] [Google Scholar]
- 7.Hockenberry MJ, Bologna-Vaughan S. Preparation for intrusive procedures using noninvasive techniques in children with cancer: state of the art vs. new trends. Cancer Nursing. 1985;8:97–102. [PubMed] [Google Scholar]
- 8.Vasterling J, Jenkins RA, Tope DM, et al. Cognitive distraction and relaxation training for the control of side effects due to cancer chemotherapy. Journal of Behavioral Medicine. 1993;16:65–80. doi: 10.1007/BF00844755. [DOI] [PubMed] [Google Scholar]
- 9.Kolcaba K, Fox C. The effects of guided imagery on comfort in women with early stage breast cancer undergoing radiation therapy. Oncology Nursing Forum. 1999;26:67–72. [PubMed] [Google Scholar]
- 10.Ezzone S, Baker C, Rosselet R, et al. Music as an adjunct to antiemetic therapy. Oncology Nursing Forum. 1998;25:1551–1556. [PubMed] [Google Scholar]
- 11.Arthur C. Did reality move for you? New Scientist. 1992;134:22–27. [Google Scholar]
- 12.Pratt DR, Zyda M, Kelleher K. Virtual reality: in the mind of the beholder. Computer. 1995;7:17–19. [Google Scholar]
- 13.Schneider SM, Workman ML. Effects of virtual reality on symptom distress in children receiving cancer chemotherapy. CyberPsychology & Behavior. 1999;2:125–134. doi: 10.1089/cpb.1999.2.125. [DOI] [PubMed] [Google Scholar]
- 14.Schneider SM, Prince-Paul M, Allen M, et al. Virtual reality as a distraction intervention for women receiving chemotherapy. Oncology Nursing Forum. doi: 10.1188/04.ONF.81-88. in press. [DOI] [PubMed] [Google Scholar]
- 15.Wint SS, Eshelman D, Steele J, et al. Effects of distraction using virtual reality glasses during lumbar punctures in adolescents with cancer. Oncology Nursing Forum. 2002;29:31. doi: 10.1188/02.ONF.E8-E15. Online: www.ons.org/xp6/ONS/Library.xml/ONS_Publications.xml/ONF.xml/ONF2002.xml/Jan_Feb_2002.xml. [DOI] [PubMed] [Google Scholar]
- 16.Kozarek RA, Raltz SL, Neal L, et al. Prospective trial using virtual vision as a distraction technique in patients undergoing gastric laboratory procedures. Gastroenterology Nursing. 1997;20:12–14. doi: 10.1097/00001610-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Schneider SM, Workman ML. Virtual reality as a distraction intervention for children receiving chemotherapy. Pediatric Nursing. 2000;26:593–597. [PubMed] [Google Scholar]
- 18.Schneider SM. Effect of virtual reality on symptom distress in breast cancer patients. Presented at the Virtual Reality and Mental Health Symposium, Medicine Meets Virtual Reality Conference; January 24–27; Newport Beach, CA. 2001. [Google Scholar]
- 19.Oshuga M, Tatsuno Y, Shimono F, et al. CyberPsychology & Behavior. 1998;1:10–112. [Google Scholar]
- 20.Hoffman HG, Patterson DR, Carrougher GJ. Use of virtual reality for adjunctive treatment of adult burn pain during physical therapy: a controlled study. Clinical Journal of Pain. 2000;16:244–250. doi: 10.1097/00002508-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman HG, Patterson DR, Carrougher GJ, et al. Effectiveness of virtual reality-based pain control with multiple treatments. Clinical Journal of Pain. 2001;17:229–235. doi: 10.1097/00002508-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Lazarus RS, Folkman S. Stress, appraisal and coping. New York: Springer; 1984. [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Piper BF, Dibble SL, Dodd MJ, et al. The revised Piper fatigue scale: psychometric evaluation in women with breast cancer. Oncology Nursing Forum. 1998;25:35–44. [PubMed] [Google Scholar]
- 25.McNair DM, Lorr M, Doppleman LF. POMS: manual for Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 26.Yoshitake H. Three characteristic patterns of subjective fatigue symptoms. Ergonomics. 1978;21:213–233. doi: 10.1080/00140137808931718. [DOI] [PubMed] [Google Scholar]
- 27.Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- 28.McCorkle R, Young K. Development of a symptom distress scale. Cancer Nursing. 1978;1:373–378. [PubMed] [Google Scholar]
- 29.McCorkle R. The measurement of symptom distress. Seminars in Oncology Nursing. 1987;3:248–256. doi: 10.1016/s0749-2081(87)80015-3. [DOI] [PubMed] [Google Scholar]
- 30.Given CW, Given BA, Stommel M. The impact of age, treatment, and symptoms on the physical and mental health of cancer patients. A longitudinal perspective. Cancer. 1994;74(7 Suppl):2128–2138. doi: 10.1002/1097-0142(19941001)74:7+<2128::aid-cncr2820741721>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]


