Abstract
Borderline Personality Disorder (BPD) is associated with behavioral and emotional dysregulation, particularly in social contexts; however, the underlying pathophysiology at the level of brain function is not well understood. Previous studies found abnormalities in frontal cortical and limbic areas suggestive of poor frontal regulation of downstream brain regions. However, the striatum, which is closely connected with the medial frontal cortices and plays an important role in motivated behaviors and processing of rewarding stimuli, has been understudied in BPD. Here we hypothesized that, in addition to frontal dysfunction, BPD patients may show abnormal striatal function. In this study, 38 BPD patients with intermittent explosive disorder (BPD-IED) and 36 healthy controls (HC) participated in the Point Subtraction Aggression Paradigm (PSAP), a computer game played with a fictitious other player. 18Fluoro-deoxyglucose positron emission tomography (FDG-PET) measured relative glucose metabolism (rGMR) within caudate and putamen in response to aggression-provoking and non-provoking versions of the PSAP. Male BPD-IED patients had significantly lower striatal rGMR than all other groups during both conditions, although male and female BPD-IED patients did not differ in clinical or behavioral measures. These sex differences suggest differential involvement of frontal-striatal circuits in BPD-IED, and are discussed in relation to striatal involvement in affective learning and social decision-making.
Keywords: Borderline personality disorder, intermittent explosive disorder, striatum, aggression, positron emission tomography
Introduction
Borderline Personality Disorder (BPD) is a chronic illness characterized by behavioral disinhibition, including impulsivity, aggression and affective lability (Sanislow et al., 2000; Sanislow et al., 2002). Impulsive aggression and affective dysregulation/instability are core traits of BPD (Siever, Torgersen et al. 2002; Skodol, Siever et al. 2002; McGlashan, Grilo et al. 2005), and contribute substantially to the morbidity and mortality associated with BPD. Impulsive aggression in BPD can manifest in a variety of behaviors, including destruction of property, assault, domestic violence, self-injurious and suicidal behavior, or substance abuse (New, Gelernter et al. 1998).
Although earlier research supported a higher prevalence of BPD among women, as reflected in the 3:1 female to male ratio reported in the most recent edition of the DSM (DSM-IV-TR) (APA 2000), more recent data suggest that there are no sex differences in the prevalence of BPD (Grant, Chou et al. 2008). The available data also suggest that there are no gender differences in BPD with regard to self-harm behaviors such as self-cutting and presenting levels of psychological distress (Sansone and Sansone 2011). However, there appear to be gender differences with regard to personality traits (with men having higher rates of explosive temperaments and high levels of novelty seeking), Axis I (with men having higher rates of substance abuse whereas women are more likely to suffer eating, mood, anxiety, and posttraumatic stress disorders) and Axis II comorbidity (with men more likely than women to have antisocial personality traits), and treatment utilization histories (men are more likely to have had treatment for substance abuse whereas women are likely to have used more pharmacotherapy and psychotherapy) (Sansone and Sansone 2011).
Brain imaging studies in BPD (see New at al. for a review (New et al., 2008)) have shown abnormalities in structure, function and connectivity of medial frontal cortical and limbic regions. These findings have been interpreted as decreased frontal top-down control of limbic areas involved in affective responsiveness and impulsive aggression, resulting in disinhibited behavior and increased impulsive aggression (New et al., 2009). However, the striatum, which is closely connected with the medial frontal cortices and plays an important role in motivated behaviors and processing of rewarding stimuli (Ernst & Fudge, 2009), has been understudied in BPD.
The striatum, which is part of the basal ganglia, is composed of the caudate nucleus and putamen (Ernst & Fudge, 2009). Corticostriatal pathways have been implicated in motivated goal-directed behavior (Ernst & Fudge, 2009; Hollerman et al., 1998; Kawagoe et al., 1998; O’Doherty, 2004; Schultz & Romo, 1988), habit learning, economic and social decision-making (de Quervain et al., 2004; Rilling et al., 2008). The striatum is activated by primary (Gottfried et al., 2003; O’Doherty et al., 2001; Pagnoni et al., 2002) and secondary (Delgado et al., 2000; Kirsch et al., 2003; Knutson et al., 2001) reinforcement, including maternal (Bartels & Zeki, 2004) and romantic love (Aron et al., 2005), suggesting a role in processing socially rewarding cues. Dysregulation of the basal ganglia and corticostriatal networks has been associated with aggressive behavior (Amen et al., 1996; Cummings, 1993; Mendez et al., 1989; Richfield et al., 1987; Soderstrom et al., 2002), schizophrenia (Buchsbaum et al., 1982; Sheppard et al., 1983), unipolar and bipolar depression (Baxter et al., 1985; Buchsbaum et al., 1986), generalized anxiety disorder (Wu et al., 1991), obsessive compulsive disorder (Baxter et al., 1987; Martinot et al., 1990) and alcoholism (Volkow et al., 1994).
However, only four studies have examined striatal activity or structure in BPD. One study using 18Fluoro-deoxyglucose (FDG) positron emission tomography (18FDG-PET) showed hypometabolism throughout thalamo-cortico-basal ganglia circuits in BPD (De La Fuente et al., 1997), although another study found no differences in basal ganglia metabolism with 18FDG-PET during resting state in BPD patients compared to controls (Salavert et al., 2011). Another study showed lower α-[11C]methyl-L-tryptophan (α-[11C]MTrp) trapping in cortico-striatal pathways, suggesting decreased serotonin synthesis capacity, in BPD (Leyton et al., 2001). Finally, significantly increased right and left putamen volumes were observed in male BPD subjects with substance use disorders (Brambilla et al., 2004).
In the present study, we aimed to extend these earlier findings by comparing volume and striatal activity using 18FDG-PET in a group of BPD patients selected for serious impulsive aggression (meeting criteria for intermittent explosive disorder-revised (IED-R)) and healthy controls (HCs) in an aggression provocation behavioral paradigm (New et al., 2009). We aimed to determine whether striatal dysfunction is present in BPD patients during the provocation of aggression and whether it correlates with behavioral and self-reported impulsive aggression. We also aimed to explore any possible gender effects or gender by diagnosis interaction on striatal volume and activity.
We hypothesized that BPD-IED patients and HCs would differ in striatum metabolism during aggression provocation. However, the direction of hypothesized group differences was unclear in light of the ambiguity in the literature.
Method
Participants
Participants and the diagnostic assessments for this study were described in detail previously (New et al., 2009) and are briefly described here. Although we previously published 18FDG-PET data from this sample, the analyses focused on the amygdala and prefrontal cortex, and the striatal data was not examined. We previously published a whole brain statistical probability mapping analysis that specifically focused on differences between the provoked and non-provoked conditions, and did not analyze males and females separately, as no sex differences were discovered in amygdala and prefrontal cortex. Briefly, 38 patients who met DSM-IV criteria for BPD and IED-R (BPD-IED) (22 male, 16 female) and 36 healthy controls (HC; 18 male, 18 female) with no personal or first-degree family history of psychiatric disorders were included. We chose to study a subset of BPD patients meeting criteria for IED-R to find a homogeneous group of subjects with clinically significant impulsive aggression. Groups were sex- and age-matched (mean age: BPD-IED 30.5 years, SD 8.5; HC 28.4 years, SD 7.1; (New et al., 2009)). All participants gave informed consent and the study was approved by the Mount Sinai School of Medicine Institutional Review Board.
All subjects were medically healthy and free of psychiatric medication for at least 2 months and substance abuse or dependence for 6 months. All subjects had negative urine toxicology screens on each testing day (1 HC and 2 BPD-IED excluded for positive test), and females had negative pregnancy tests on each scan day (one female, BPD-IED, was excluded for a positive test). Three subjects (1 HC, 2 BPD-IED) were excluded because they did not believe the task deception.
Axis I diagnoses were made by a psychologist using the Structured Clinical Interview for DSM-IV Disorders (First et al., 1996) and Axis II with the Structured Interview for DSM-IV Personality Disorders (Pfohl B, 1997). Exclusion criteria were schizophrenia, schizophrenia-related psychotic disorders, bipolar I disorder, or current major depressive disorder (MDD). BPD-IED patients with a past history of MDD or posttraumatic stress disorder (PTSD) were not excluded, because of the high comorbidity with these disorders and a goal of selecting a representative sample of patients with BPD (Grilo et al., 2000). Subjects were also assessed with the Module for Intermittent Explosive Disorder-Revised (Coccaro, 1989); see (New et al., 2009).
All subjects completed the Barratt Impulsivity Scale-II (BIS-II)(Barratt, 1965), Affective Lability Scale (ALS) (Harvey et al., 1989), Beck Depression Inventory (BDI) (Beck et al., 1961), Overt Aggression Scale-Modified (OAS-M, including three subscales: aggression, irritability, and suicidality) (Yudofsky et al., 1986), and State-Trait Anger Expression Inventory (STAXI) (Spielberger, 1988). Subjects completed the Buss-Durkee Hostility Inventory (BDHI) (Buss & Durkee, 1957) or the Buss-Perry Aggression Questionnaire (BPAQ—an updated BDHI)(Buss & Perry, 1992); a composite score was calculated, using Z scores (t-BUSS) (See Table 1).
Table 1.
Self-report measures by diagnosis and sex.
| Males (n=40) | Females (n=34) | |||||
|---|---|---|---|---|---|---|
| HC (n=18) | BPD-IED (n=22) | HC (n=18) | BPD-IED (n=16) | |||
| Mean (SD) | Mean (SD) | Statistic | Mean (SD) | Mean (SD) | Statistic | |
| t-BUSS | 41.16 (7.83) | 76.71 (9.28) | t=12.16; df=35; p<0.000001 | 41.38 (6.26) | 76.25 (7.84) | t=12.84; df=25; p<0.000001 |
| STAXI-trait | 45.38 (2.73) | 47.05 (7.02) | t=0.90; df=35; p=0.374 | 43.33 (4.33) | 49.64 (6.84) | t=2.99; df=27; p=0.005 |
| BIS* | 43.49 (33.62–63.64) | 68.38 (61.31–111.65) |
U=63.00 p=0.0008 |
43.13 (34.54–48.18) | 62.32 (57.27–78.48) |
U=31.00 p=0.005 |
| ALS* | 0.33 (0.14–0.77) | 1.82 (1.43–2.00) |
U=3.00 p<0.000001 |
0.08 (0.02–0.61) | 1.83 (1.26–2.19) |
U=9.00 p=0.00001 |
| BDI* | 0.00 (0.00–1.50) | 19.00 (14.00–22.00) |
U=5.00 p=0.000001 |
0.00 (0.00–2.50) | 14.5 (6.00–25.00) |
U=9.5 p=0.00002 |
| OAS-M | ||||||
| Aggression* | 0.00 (0.00–3.00) | 16.50 (5.50–27.50) |
U=30.50 p=0.0001 |
0.00 (0.00–1.00) | 19.00 (9.00–41.00) |
U=1.00 p=0.000004 |
| Irritability* | 1.00 (1.00–2.00) | 6.50 (4.50–7.00) |
U=12.50 p=0.000009 |
1.00 (0.00–2.00) | 7.00 (6.00–9.00) |
U=0.50 p=0.000004 |
| Suicidality* | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | U=112.00 p=0.34 |
0.00 (0.00–0.00) | 0.00 (0.00–1.00) |
U=60.00 p=0.03 |
Median and interquartile range are provided for non-normally distributed variables.
Significant comparisons are in bold.
ALS, Affective Lability Scale; BDI, Beck Depression Inventory; BIS, Barratt Impulsivity Scale; BPD, borderline personality disorder; HC, Healthy Controls; IED, intermittent explosive disorder; IR, Interquartile Range; OAS-M, Overt Aggression Scale-Modified; SD, Standard Deviation; STAXI, State-Trait Anger Expression Inventory; t, Student t test; t-BUSS, Composite of Buss-Durkee Hostility Inventory (BDHI) and Buss-Perry Aggression Questionnaire (BPAQ); U, Mann Whitney U
The Point Subtraction Aggression Paradigm (PSAP)
The PSAP was used to provoke aggressive behavior in study subjects (Cherek et al., 1997). Subjects played a computer game with a fictitious partner with the goal of earning points, which were exchanged for money. Unbeknownst to the participant, there was no live opponent. Subjects were instructed to play by pressing one of three buttons: pressing the A button 100 times earned 1 point; pressing the B button 10 times subtracted a point from the “opponent” (the aggressive response, since no points were earned by the subject for B presses); and pressing the C button would protect the subject for a period from having points subtracted by the “opponent” (no net gain or loss of points).
In the non-provoking condition, the “opponent” at no time subtracted points from the participant. In the provoking condition, the “opponent” subtracted points from the participant approximately every 62.5 sec. All participants were debriefed about the task deception upon study completion.
Image Acquisition and Processing
Each subject underwent one anatomical magnetic resonance imaging (MRI) scan and two positron emission tomography (PET) scans, one under the provoking condition and one non-provoking (counterbalanced order).
T-1 weighted MRI scans (GE Signa 5x system, acquisition parameters: TR=24 ms, TE=5 ms, flip angle=40°, slice thickness=1.2 mm, matrix=256×256, field of view=23 cm) were conducted prior to the PET protocols in order to obtain an anatomical template to interpret PET scan results.
PET scans were conducted 1 to 4 weeks apart. Prior to each PET scan, the subject received 5mCi of 18fluoro-deoxyglucose, and played PSAP in a dimly-lit, sound-attenuated room for the 35-min tracer uptake period. Subjects were positioned in the PET scanner (GE2048 head-dedicated scanner (GE/Scanditronix, Stockholm, Sweden) resolution 4.5 mm in plane, 5.0 mm axially) for 45-min data acquisition following standard protocols (Buchsbaum et al., 2007; Hazlett et al., 2004; Haznedar et al., 1997; New et al., 2007; New et al., 2009).
Positron emission tomography images were obtained in nanocuries/pixel and standardized as relative glucose metabolic rate (rGMR) by dividing each pixel by the mean value for the entire brain, as reported in previous studies (New et al., 2007; Siever et al., 1999; Soloff et al., 2000).
Tracing of the caudate and putamen
For each participant, the caudate nucleus and putamen were outlined precisely in each hemisphere on their MRI scan as in our previous reports (Brickman et al., 2003; Buchsbaum et al., 2003). Five equally-spaced slices were selected for tracing, using a semi-automated boundary-finding method, within this dorsal-ventral distance. All tracing was performed by a single experimenter (E.R.), who was blind to patient diagnosis and session type (provoking/non-provoking), eliminating inter-tracer variability.
In order to measure relative glucose metabolism within the caudate and putamen, the PET and MRI images were co-registered with a surface-fitting method (Woods et al., 1993). Coregistration ensured accurate anatomical specificity for functional data analysis.
All striatal analyses were performed with relative values (relative to whole brain glucose for PET and relative to whole brain size for volume).
Statistical Methods
Striatal metabolism and diagnosis
To examine whether BPD-IED patients and HCs would differ in striatum metabolism during aggression provocation, a Diagnostic group (HC vs. BPD-IED) × Sex (M, F) × Condition (provoked, non-provoked) × Structure (caudate, putamen) × Hemisphere (R, L) × Slice (1, 2, 3, 4, 5; ventral to dorsal) mixed-model multivariate analysis of variance (MANOVA) was performed on rGMR data. Diagnostic group and sex were between-subjects factors and all other factors were repeated measures. Post-hoc tests were carried out with Fisher’s Least Significant Difference (LSD) tests for all significant interactions involving Diagnostic group. We also Bonferroni corrected for multiple comparisons.
Correlations of striatal metabolism with clinical measures and behavior during the task
Pearson (for normally distributed variables) and non-parametric Spearman (for non-normally distributed variables) correlations determined whether striatal metabolism was correlated with clinical measures. Mean striatal rGMR for each structure (caudate and putamen) in each hemisphere (collapsed across slices) was calculated separately for provoking and non-provoking conditions in patients and controls and in males and females. Clinical self-report measures tested for correlation included total BIS-II score, BDI and t-BUSS scores, mean ALS, STAXI trait, and OAS-M aggression, irritability, and suicidality subscales (state measures were excluded because questionnaires were not necessarily given on the same testing day across subjects). Fisher’s R to Z transformation (in Statistica) and two-tailed testing was used to assess the significance of between-group differences in correlation coefficients.
Pearson correlations also tested for relationships between button-pressing behavior and mean striatal rGMR for each structure (caudate and putamen) in each hemisphere (collapsed across slices) in provoking and non-provoking conditions in patients and controls and in males and females.
Volume Analysis
The volume (cc3) of each traced slice was determined automatically as the traced area times slice thickness. Pearson correlations tested for relationships between structure volume and rGMR. To test whether striatal volume differed by diagnosis or sex, a Diagnosis (HC vs. BPD-IED) × Sex (M, F) mixed-model MANOVA was carried out.
Behavioral Differences
A Diagnosis (HC vs. BPD-IED) × Sex (M, F) × Condition (non-provoked, provoked) × Button Press (A, B, C) mixed-model MANOVA tested for differences in button pressing behavior between male and female HCs and BPD-IED patients. Three behavioral measures were tested: number of A, B and C button presses. Post-hoc tests were carried out with Fisher’s LSD test.
Results
Striatal metabolism and diagnosis
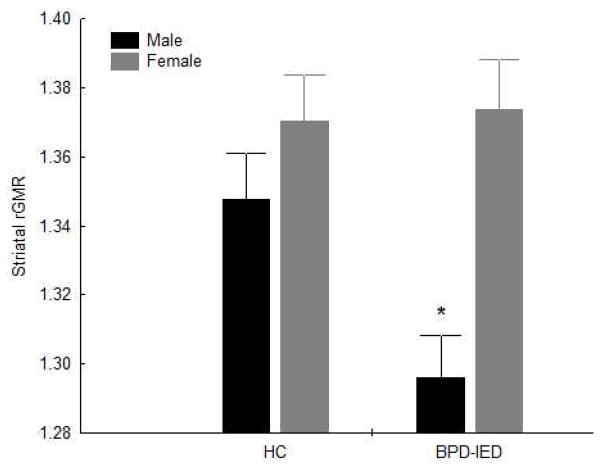
A MANOVA examining Diagnostic group (HC vs. BPD-IED) × Sex (M, F) × Condition (provoked, non-provoked) × Structure (caudate, putamen) × Hemisphere (R, L) × Slice (1, 2, 3, 4, 5; ventral to dorsal) on rGMR showed a non-significant trend for a main effect of Diagnosis (F1,70=3.18, p=0.08), a significant main effect of Sex (F1,70=13.96, p=0.0004), and a Diagnostic group × Sex interaction (F1,70=4.23, p=0.04; Figure 1). Post-hoc tests showed that male BPD-IED patients had significantly lower striatal rGMR than male HC (Fisher’s LSD, p=0.006; Bonferroni, p=0.037), female HC (p=0.0001; Bonferroni, p=0.0007), and female BPD-IED patients (p=0.0001; Bonferroni, p=0.0006). There were no differences, however, between male and female HCs; between female HC and female BPD-IED patients; or between male HC and female BPD-IED patients. Only male BPD-IED patients differed from the other three groups. This difference was not affected by provocation level (Diagnosis × Sex × Condition interaction, F1,70=0.05, p=0.83), and was similar in both striatal structures (Diagnosis × Sex × Structure interaction, F1,70=0.59, p=0.44) and both hemispheres (Diagnosis × Sex × Hemisphere interaction, F1,70=0.002, p=0.97). There was also a significant Diagnosis × Sex × Structure × Slice interaction (GG: ε=0.50, F(adjusted df: 2.0, 140.6)=3.24, p=0.04). Among males, rGMR was lower in BPD-IED patients compared to HCs in both structures (caudate, putamen) and all slices. Among females, rGMR was similar in BPD-IED patients compared to HCs in all slices in the caudate, but in the putamen rGMR was higher among BPD-IED patients in the more dorsal slices, while it was higher among HCs in the more ventral slices.
Figure 1.
Mean striatal rGMR is shown for healthy control subjects (HC) and borderline personality disorder-Intermittent explosive disorder patients (BPD-IED). Males (black bars) with BPD-IED had lower mean striatal rGMR than all other groups. Gray bars represent females. There was a significant Diagnosis × Sex interaction (F1,70=4.23, p=0.04).
* Post-hoc comparisons significant at p<0.01.
To test whether motor activity (total button presses) could be responsible for this finding, we reanalyzed the data covarying for total button presses, and the results remained significant (i.e., male BPD patients have lower striatal rGMR than male HCs (F(1,36)=7.01, p<0.02)). We also reanalyzed the data covarying for proportion of B button presses and the results remained unchanged (data not shown).
Correlations of striatal metabolism with clinical measures and behavior during the task
We assessed whether mean rGMR in the left and right caudate and/or putamen (collapsed across slices) correlated with eight self-report measures of impulsivity, affective lability, depression, trait anger and aggression. Because our analysis demonstrated an effect of sex and diagnosis on striatal rGMR, we assessed males and females and patients and controls separately (results were Bonferroni corrected for multiple comparisons). No correlations reached significance when BPD patients and HCs were analyzed separately, possibly due to the limited sample sizes.
We also tested whether button pressing was associated with striatal rGMR. Since each of two PSAP conditions (provoked and non-provoked) were tested for three possible correlations (A, B, and C button presses), results were Bonferroni corrected for multiple comparisons. In female HCs, less rGMR in the right caudate was associated with more B button presses under the non-provoking condition (r=−0.61, p=0.042, Bonferroni corrected).
Striatal Volume
We assessed whether rGMR varied by structure volume and whether the effects of diagnosis and sex on rGMR could be related to systematic variation in striatal volume across groups.
None of the correlations between volume and corresponding rGMR reached significance (all p>0.12).
MANOVA found no main effect of diagnosis (F1,70=0.08, p=0.88), a trend-level effect of sex, with females having lower volumes than males (F1,70=2.60, p=0.11), and no interaction of Diagnosis × Sex (F1,70=0.05, p=0.19) on striatal volumes (Supplementary Figure S1). Therefore, differences in rGMR cannot be attributed to different striatal sizes in our study.
Clinical/Behavioral Measures and Sex Effects
We found a diagnosis by sex interaction, with healthy females pressing significantly less buttons (averaged across A, B, and C) than any other group (F(1,67)=4.68; p=0.03, all post- hoc tests p<0.02, Supplementary Figure S2A)
We found a Diagnosis by Sex by button type (A, B, C) interaction (GG: ε=0.56, F(adjusted df: 1.2, 75.2)=5.64, p=0.02, Supplementary Figure S2B). This difference was accounted for by A button presses. Healthy females had a significantly lower number of A button presses than BPD females while the opposite pattern was observed for the males, with healthy males making significantly higher number of A button presses than male BPD-IED patients (both post-hoc tests, p<0.005).
We also found a main effect of button type (F(1.2,75.2)=873.7, p<0.0001), with A button being the most often pressed. There was also a condition (provoked vs non-provoked) by button type interaction (F(1.1, 74.4)=13.5, p=0.0003).
We previously reported a main effect of diagnosis for all of the following clinical measures: impulsivity (BIS-II), affective lability (ALS), depression (BDI), State and trait anger, (STAXI), and the recent aggression (OAS-M-covering the prior 2 weeks) and lifetime aggression (t-Buss) (New et al, 2009). In the present analysis, we observed that the effect of diagnosis on clinical measures remained significant after analyzing males and females separately (see Table 1). We tested a Diagnosis by Sex interaction for all these clinical measures in light of our rGMR results, but none were significant.
Discussion
The main finding of this study is that male but not female borderline patients selected for impulsive aggression show reduced rGMR in the striatum compared with healthy controls during a behavioral aggression task, both under provoking and non-provoking conditions. This raises the question as to whether male BDP patients have reduced striatal rGMR at rest. This finding was not accounted for by differences in striatal volumes or total button presses. These data suggest a sex-specific striatal abnormality in BPD-IED patients. These results extend our previous findings that BPD patients responded more aggressively in the PSAP than HCs (New et al., 2009), and of a dysregulation of frontal cortical and amygdala activity during this task.
Striatum and Aggression
Several lines of evidence suggest links between dysfunction in the basal ganglia and aggression (Amen et al., 1996; Cummings, 1993; Mendez et al., 1989; Richfield et al., 1987; Soderstrom et al., 2002). Patients with basal ganglia lesions often exhibit disinhibition, impulsivity and violent outbursts (Bhatia & Marsden, 1994; Mendez et al., 1989; Richfield et al., 1987). Similar symptoms accompany Huntington’s Disease (Folstein & Folstein, 1983) and neuroacanthocytosis (Wyzynski, 1989), a rare neurodegenerative disorder affecting primarily the caudate (Danek et al., 2005). Larger caudate volumes, which may represent disruption of normal frontal-subcortical function, are associated with greater aggression in schizophrenia patients (Hoptman et al., 2006). In contrast, Soderstrom et al. (Soderstrom et al., 2002) found that lower activity in the head of the caudate did not correlate with violence or antisocial behavior in psychopathy, but predicted interpersonal features such as deceitfulness and domination. Moreover, one of the suggested roles of the caudate is to identify and provide the behavioral context necessary for the frontal lobe to select an appropriate action strategy (Houk & Wise, 1995; Seger & Cincotta, 2006). It may be hypothesized that our finding of lower striatum activation in male BPD-IED patients may reflect an inability of their striatum to provide the context (i.e. that this is just a game) to allow the PFC to down regulate aggressive responses.
We had previously shown that PSAP aggressive responding (B button presses) was higher in patients than in controls in both the provoked and non provoked conditions, with a main effect of Diagnosis and a main effect of Condition but no significant interaction (New et al., 2009). It appeared that even in the non-provoked condition, both groups were responding somewhat aggressively, but particularly the BPD-IED patients. This may explain why we found no effect of condition on striatal activation.
The most robust difference in button pressing behavior was found in the A button; male controls pressed the A button more frequently, garnering more points and thereby more money at the end of the task, than did the male BPD-IED patients. The opposite was observed for females; female patients pressed the A button on average more than did female controls. This raises the possibility that in male patients, low activity in the striatum is related to relatively fewer A button presses, although the correlations between button pressing and striatal metabolism were not significant in the patient group. By the nature of the PSAP task, when a participant is pressing the B button they are unable to also be pressing the A button at the same time. Therefore, it might be hypothesized that participants that press B more often (possibly leading to pressing A less often) have lower striatum rGMR because they are drawn away from the motivated goal-directed behaviors and reinforcement portions of the task (i.e. pressing the A button). This would be consistent with our finding that lower rGMR in the right caudate was associated with higher number of B button presses in female HCs. However, we could not find any correlation between A, B or C button presses and striatal rGMR in the BPD patients.
Interestingly, none of our clinical symptom measures revealed a Diagnosis × Sex interaction nor any sex differences, although for most measures there was a main effect of diagnosis (see Table 1).
Striatum in Affective Learning and Social Decision Making
Some have suggested that the Cluster B personality disorders are due to an imbalance of normal responses to reward/punishment (Comings & Blum, 2000; Scerbo et al., 1990; Vollm et al., 2007). The striatum plays a key role in affective learning and decision-making, essential cognitive processes that link one’s own actions to ensuing outcomes. The striatum is involved in reinforcement learning (McClure et al., 2003; O’Doherty et al., 2003; Schonberg et al., 2007; Tobler et al., 2007), as evidenced by impairments in reinforcement learning tasks following striatal lesion in rats (e.g.(Killcross & Coutureau, 2003; Yin & Knowlton, 2004) and neurophysiology in monkeys (Apicella et al., 1991; Ravel et al., 2003). The striatum is activated in affective learning based on primary (Gottfried et al., 2003; O’Doherty et al., 2001; Pagnoni et al., 2002) and secondary (Delgado et al., 2008; Delgado et al., 2000; Kirsch et al., 2003; Knutson et al., 2001) reinforcers.
The striatum has also been implicated in social decision making, which may be related to the PSAP task. Paradigms in which a subject must cooperate with a partner (e.g. the Trust Game, Prisoner’s Dilemma) recruit striatum (e.g. (Delgado et al., 2005a; Delgado et al., 2005b; Rilling et al., 2002; Rilling et al., 2008)). Reciprocated trust activates striatal regions while unreciprocated trust deactivates them (for review see (Rilling et al., 2008)). In the trust game, caudate activity reflects the fairness of a social partner’s actions and the intention to trust that individual (King-Casas et al., 2005). In our PSAP task, point subtraction by the putative opponent was intended to be a negative social maneuver, and could have been interpreted by the subject as unfair or untrustworthy play. Lower striatal activation in male BPD-IED patients could reflect either a more pronounced negative interpretation of point subtraction compared to HCs, or the decision to reciprocate the negative social maneuver.
Another way of construing this is in terms of “punishment”. Caudate activity has also been found to correlate with retaliatory behavior (de Quervain et al., 2004). Caudate activity increases with deserved punishment and is lowered when a deserved punishment is not delivered. Perhaps the decreased striatal activity in male BPD-IED patients reflects the subject’s interpretation of inadequate punishment of the other player for subtracting points, although a limitation of our study is that retaliatory feelings or satisfaction with retaliatory button presses were not specifically assessed. Interestingly, deficits in trust and poor cooperation have been shown in BPD patients during similar experimental conditions (King-Casas et al., 2008; Unoka et al., 2009). This leads to the intriguing hypothesis that striatal dysregulation is linked to higher-order social impairments, particularly in male BPD patients. To elucidate these hypotheses, we would need to do future studies in which appraisal of the “other” player were evaluated.
Sex differences in aggression and BPD-IED
It is interesting to note that in this study, we found a Diagnosis by Sex interaction on striatal rGMR, while in prior work examining other brain regions (e.g., prefrontal cortex, amygdala; New et al., 2009) this was not observed. The present findings suggest that striatal function may play an important role in sex differences observed in disorders involving aggression. We found significantly lower striatal rGMR in male BPD patients than in matched male HCs, but found no difference between groups in females. This is consistent with other studies which have reported sex differences in PET studies of BPD patients (Soloff et al., 2005). There have been very few FDG-PET studies looking at gender differences in striatal metabolism. Most studies have not reported any striatal findings, or have analyzed males and females separately. Early resting FDG-PET studies did not find any sex differences in striatal metabolism in healthy volunteers (Kawachi, Ishii et al. 2002), which is consistent with our results in HCs. However, sex differences in striatal dopaminergic function have been reported (Laakso, Vilkman et al. 2002), and there is some evidence of neurofunctional modulation of the reward system including the striatum by gonadal steroid hormones (Dreher, Schmidt et al. 2007).
Although the effect of sex was much more robust in BPD-IED patients, there was also a trend toward lower striatal rGMR in male than female HCs, suggesting sex differences are also present in the healthy population (Baxter et al., 1987) but exaggerated in BPD-IED patients.
We show no significant effect of sex in self-reported symptom severity. This absence of sex differences in BPD symptoms has been reported previously (Johnson et al., 2003). Indeed, some symptoms that normally have a sex-bias in the general population, such as aggression or detachment (more prevalent in males) and dependency (more prevalent in females) (Clark, 1993) were not different in male and female BPD patients (Johnson et al., 2003). This raises the possibility that BPD abolishes some sex-typical behavior patterns (Johnson et al., 2003). Our finding of a sex specific abnormality in striatal activity during the PSAP suggests that the neural substrate underlying abnormal behaviors in BPD may differ between males and females, but that other factors come into play such that clinical symptoms are quite similar between the sexes.
Conclusions
This study represents the first attempt to understand how the striatum might contribute to social aggression in BPD-IED patients. We showed that male but not female BPD-IED patients have reduced striatal rGMR during a behavioral aggression task and lower A button pressing and point accumulation than did healthy controls.
There are a number of limitations in the current study. Since we did not perform PET studies at rest, it is not clear whether sex differences in striatal metabolism in BPD are present at rest. We did not assess appraisal of putative other during the PSAP. The absence of correlations between striatal activity and behavioral aggression (B-button pressing) in the patient group raises the possibility that there is no direct relationship between striatal activity and aggressive behavior in this task, although we found a correlation between rGMR and B button presses in female HCs. The PSAP task was designed to provoke social aggression; however, it is a complex paradigm that is likely to recruit a wide variety of cognitive processes, any one of which may involve striatal processing.
Another limitation is that, while Axis I and II diagnoses were made by semi-structured interview and consensus diagnosis, severity of symptoms was assessed by self-report scales. The scales we employed have good psychometric properties, but may be less accurate in patients with BPD; BPD patients have higher-than-normal levels of alexithymia (New, in press), with particular difficulty identifying and describing their own emotional responses.
Another limitation is that the caudate and putamen are heterogeneous, and include anatomic and functional subdivisions with different connections to cortical and sub-cortical structures.
Finally, all BPD subjects also carried a diagnosis of IED. We selected patients with both BPD and IED to examine a relatively homogeneous group of BPD patients. However, this limits the degree to which we can generalize our findings to other patients with BPD. Further studies on pure BPD samples would be required to clarify this.
Supplementary Material
Striatal volume is shown for healthy control subjects (HC) and borderline personality disorder-Intermittent explosive disorder patients (BPD-IED). Black bars represent males, and gray bars represent females. MANOVA found no main effect of diagnosis (F1,70=0.08, p=0.88), a trend-level effect of sex (F1,70=2.60, p=0.11) and no interaction of Diagnosis × Sex (F1,70=0.05, p=0.19) on striatal volumes.
Button presses are shown for healthy control subjects (HC, blue bars) and borderline personality disorder-Intermittent explosive disorder patients (BPD-IED, red bars) in males and females.
We found a diagnosis by sex interaction, with healthy females pressing significantly less buttons (averaged across A, B, and C) than any other group (F(1,67)=4.68; p=0.03, all post- hoc tests p<0.02, Supplementary Figure 2A)
We found a Diagnosis by Sex by button type (A, B, C) interaction (GG: ε=.56, F(adjusted df: 1.2, 75.2)=5.64, p=0.02, Supplementary Figure 2B). This difference was accounted for by A button presses. Healthy females had a significantly lower number of A button presses than BPD females while the opposite pattern was observed for the males, with healthy males making significantly higher number of A button presses than male BPD-IED patients (both post-hoc tests, p<0.005).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amen DG, Stubblefield M, Carmicheal B. Brain SPECT findings and aggressiveness. Ann Clin Psychiatry. 1996;3:129–137. doi: 10.3109/10401239609147750. [DOI] [PubMed] [Google Scholar]
- 2.APA. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text rev. [Google Scholar]
- 3.Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res. 1991;85:491–500. doi: 10.1007/BF00231732. [DOI] [PubMed] [Google Scholar]
- 4.Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–37. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- 5.Barratt ES. Factor analysis of some psychometric measures of impulsiveness and anxiety. Psychol Rep. 1965;16:547–554. doi: 10.2466/pr0.1965.16.2.547. [DOI] [PubMed] [Google Scholar]
- 6.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Baxter LR, Jr, Mazziotta JC, Phelps ME, Selin CE, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res. 1987;21:237–45. doi: 10.1016/0165-1781(87)90028-x. [DOI] [PubMed] [Google Scholar]
- 8.Baxter LR, Jr, Phelps ME, Mazziotta JC, Schwartz JM, Gerner RH, Selin CE, Sumida RM. Cerebral metabolic rates for glucose in mood disorders. Studies with positron emission tomography and fluorodeoxyglucose F 18. Arch Gen Psychiatry. 1985;42:441–7. doi: 10.1001/archpsyc.1985.01790280019002. [DOI] [PubMed] [Google Scholar]
- 9.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–76. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- 11.Brambilla P, Soloff PH, Sala M, Nicoletti MA, Keshavan MS, Soares JC. Anatomical MRI study of borderline personality disorder patients. Psychiatry Res. 2004;131:125–33. doi: 10.1016/j.pscychresns.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Brickman AM, Buchsbaum MS, Shihabuddin L, Hazlett EA, Borod JC, Mohs RC. Striatal size, glucose metabolic rate, and verbal learning in normal aging. Brain Res Cogn Brain Res. 2003;17:106–16. doi: 10.1016/s0926-6410(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 13.Buchsbaum M, Wu J, Delisi L, Holcomb H, Kessler R, Johnson J, King A, Hazlett E, Langston K, Post R. Frontal cortex and basal ganglia metabolic rates assessed by positron emisssion tomography with FDG in affective illness. J Affec Dis. 1986;10:137–152. doi: 10.1016/0165-0327(86)90036-4. [DOI] [PubMed] [Google Scholar]
- 14.Buchsbaum MS, Haznedar MM, Aronowitz J, Brickman AM, Newmark RE, Bloom R, Brand J, Goldstein KE, Heath D, Starson M, Hazlett EA. FDG-PET in never-previously medicated psychotic adolescents treated with olanzapine or haloperidol. Schizophr Res. 2007;94:293–305. doi: 10.1016/j.schres.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Buchsbaum MS, Ingvar DH, Kessler R, et al. Cerebral glucography with positron tomography. Arch Gen Psychiatry. 1982;39:251–259. doi: 10.1001/archpsyc.1982.04290030001001. [DOI] [PubMed] [Google Scholar]
- 16.Buchsbaum MS, Shihabuddin L, Brickman AM, Miozzo R, Prikryl R, Shaw R, Davis K. Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophr Res. 2003;64:53–62. doi: 10.1016/s0920-9964(02)00526-1. [DOI] [PubMed] [Google Scholar]
- 17.Buss A, Perry M. The Aggression Questionnaire. J Pers Social Psychology. 1992;63:452–9. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 18.Buss AH, Durkee A. An inventory for assessing different kinds of hostility. J Consult Psychol. 1957;21:343–348. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- 19.Cherek DR, Moeller G, Schnapp W, Dougherty DM. Studies of violent and non-violent male parolees: I. Laboratory and psychometric measurements of aggression. Biol Psychiatry. 1997;41:514–522. doi: 10.1016/s0006-3223(96)00059-5. [DOI] [PubMed] [Google Scholar]
- 20.Clark LM, JL, Collard LM, Hickok LG. Symptoms and traits of personality disorder: Two new methods for their assessment. Psychological Assessment. 1993;5:81–91. [Google Scholar]
- 21.Coccaro E. Central Serotonin and impulsive aggression. British Journal of Psychiatry. 1989;155:52–62. [PubMed] [Google Scholar]
- 22.Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–41. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- 23.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–80. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 24.Danek A, Jung HH, Melone MA, Rampoldi L, Broccoli V, Walker RH. Neuroacanthocytosis: new developments in a neglected group of dementing disorders. J Neurol Sci. 2005;229–230:171–86. doi: 10.1016/j.jns.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 25.De La Fuente JM, Goldman S, Stanus E, Vizuete C, Morlan I, Bobes J, Mendlewicz J. Brain glucose metabolism in borderline personality disorder. J Psychiatr Res. 1997;31:531–41. doi: 10.1016/s0022-3956(97)00001-0. [DOI] [PubMed] [Google Scholar]
- 26.de Quervain DJ, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, Fehr E. The neural basis of altruistic punishment. Science. 2004;305:1254–8. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- 27.Delgado MR, Frank RH, Phelps EA. Perceptions of moral character modulate the neural systems of reward during the trust game. Nat Neurosci. 2005a;8:1611–8. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- 28.Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11:880–1. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005b;24:862–73. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 31.Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33:367–82. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I Disorders- Patient Edition. New York Biometrics Research:New York State Psychiatric Institute; 1996. [Google Scholar]
- 33.Folstein SE, Folstein MF. Psychiatric features of Huntington’s disease: recent approaches and findings. Psychiatr Dev. 1983;1:193–205. [PubMed] [Google Scholar]
- 34.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–7. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 35.Grant BF, Chou SP, Goldstein RB, Huang B, Stinson FS, Saha TD, Smith SM, Dawson DA, Pulay AJ, Pickering RP, Ruan WJ. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008;69:533–545. doi: 10.4088/jcp.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grilo CM, McGlashan TH, Skodol AE. Stability and course of personality disorders: the need to consider comorbidities and continuities between axis I psychiatric disorders and axis II personality disorders. Psychiatr Q. 2000;71:291–307. doi: 10.1023/a:1004680122613. [DOI] [PubMed] [Google Scholar]
- 37.Harvey P, Greenberg B, Serper M. The affective lability scales: development reliability and validity. J Clin Psychol. 1989;45:786–93. doi: 10.1002/1097-4679(198909)45:5<786::aid-jclp2270450515>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 38.Hazlett EA, Buchsbaum MS, Hsieh P, Haznedar MM, Platholi J, LiCalzi EM, Cartwright C, Hollander E. Regional glucose metabolism within cortical Brodmann areas in healthy individuals and autistic patients. Neuropsychobiology. 2004;49:115–25. doi: 10.1159/000076719. [DOI] [PubMed] [Google Scholar]
- 39.Haznedar M, Buchsbaum M, Metzer M, Solimando A, Speigel-Cohen J, Hollander E. Anterior cingulate gyrus volume in glucose metabolism in autistic disorder. Am J Psychiatry. 1997;154:1043–5. [Google Scholar]
- 40.Hollerman JR, Tremblay L, Schultz W. Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol. 1998;80:947–63. doi: 10.1152/jn.1998.80.2.947. [DOI] [PubMed] [Google Scholar]
- 41.Hoptman MJ, Volavka J, Czobor P, Gerig G, Chakos M, Blocher J, Citrome LL, Sheitman B, Lindenmayer JP, Lieberman JA, Bilder RM. Aggression and quantitative MRI measures of caudate in patients with chronic schizophrenia or schizoaffective disorder. J Neuropsychiatry Clin Neurosci. 2006;18:509–15. doi: 10.1176/appi.neuropsych.18.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houk JC, Wise SP. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb Cortex. 1995;5:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- 43.Johnson DM, Shea MT, Yen S, Battle CL, Zlotnick C, Sanislow CA, Grilo CM, Skodol AE, Bender DS, McGlashan TH, Gunderson JG, Zanarini MC. Gender differences in borderline personality disorder: findings from the Collaborative Longitudinal Personality Disorders Study. Compr Psychiatry. 2003;44:284–92. doi: 10.1016/S0010-440X(03)00090-7. [DOI] [PubMed] [Google Scholar]
- 44.Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci. 1998;1:411–6. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- 45.Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb Cortex. 2003;13:400–8. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- 46.King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321:806–10. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308:78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- 48.Kirsch P, Schienle A, Stark R, Sammer G, Blecker C, Walter B, Ott U, Burkart J, Vaitl D. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: an event-related fMRI study. Neuroimage. 2003;20:1086–95. doi: 10.1016/S1053-8119(03)00381-1. [DOI] [PubMed] [Google Scholar]
- 49.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–7. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 50.Leyton M, Okazawa H, Diksic M, Paris J, Rosa P, Mzengeza S, Young SN, Blier P, Benkelfat C. Brain Regional alpha-[11C]methyl-L-tryptophan trapping in impulsive subjects with borderline personality disorder. Am J Psychiatry. 2001;158:775–82. doi: 10.1176/appi.ajp.158.5.775. [DOI] [PubMed] [Google Scholar]
- 51.Martinot JL, Allilaire JF, Mazoyer BM, Hantouche E, Huret JD, Legaut-Demare F, Deslauriers AG, Hardy P, Pappata S, Baron JC, et al. Obsessive-compulsive disorder: a clinical, neuropsychological and positron emission tomography study. Acta Psychiatr Scand. 1990;82:233–42. doi: 10.1111/j.1600-0447.1990.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 52.McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–46. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 53.McGlashan TH, Grilo CM, McGlashan TH, Grilo CM, Sanislow CA, Ralevski E, Morey LC, Gunderson JG, Skodol AE, Shea MT, Zanarini MC, Bender D, Stout RL, Yen S, Pagano M. Two-year prevalence and stability of individual DSM-IV criteria for schizotypal, borderline, avoidant, and obsessive-compulsive personality disorders: toward a hybrid model of axis II disorders. Am J Psychiatry. 2005;162:883–889. doi: 10.1176/appi.ajp.162.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendez MF, Adams NL, Lewandowski KS. Neurobehavioral changes associated with caudate lesions. Neurology. 1989;39:349–54. doi: 10.1212/wnl.39.3.349. [DOI] [PubMed] [Google Scholar]
- 55.New AS, aan het Rot M, Ripoll LH, Perez-Rodriguez MM, Lazarus S, Zipursky E, Weinstein SR, Koenigsberg HW, Hazlett EA, Goodman M, Siever LJ. Empathy and Alexithymia in Borderline Personality Disorder: Clinical and Laboratory Measures. J Pers Disord. doi: 10.1521/pedi.2012.26.5.660. in press. [DOI] [PubMed] [Google Scholar]
- 56.New AS, Goodman M, Triebwasser J, Siever LJ. Recent advances in the biological study of personality disorders. Psychiatr Clin North Am. 2008;31:441–61. vii. doi: 10.1016/j.psc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 57.New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, Trisdorfer R, Haznedar MM, Koenigsberg HW, Flory J, Siever LJ. Amygdala-Prefrontal Disconnection in Borderline Personality Disorder. Neuropsychopharmacology. 2007;32:1629–40. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- 58.New AS, Hazlett EA, Newmark RE, Zhang J, Triebwasser J, Meyerson D, Lazarus S, Trisdorfer R, Goldstein KE, Goodman M, Koenigsberg HW, Flory JD, Siever LJ, Buchsbaum MS. Laboratory Induced Aggression: A Positron Emission Tomography Study of Aggressive Individuals with Borderline Personality Disorder. Biol Psychiatry. 2009;66:1107–14. doi: 10.1016/j.biopsych.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.New AS, Gelernter J, Yovell Y, Trestman RL, Nielsen DA, Silverman J, Mitropoulou V, Siever LJ. Tryptophan hydroxylase genotype is associated with impulsive-aggression measures: a preliminary study. Am J Med Genet. 1998;81:13–17. doi: 10.1002/(sici)1096-8628(19980207)81:1<13::aid-ajmg3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 60.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 61.O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–37. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 62.O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 63.Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nat Neurosci. 2002;5:97–8. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- 64.Pfohl BBN, Zimmerman M. Structured clinical interview for DSM-IV Personality (SIDP-IV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 65.Ravel S, Legallet E, Apicella P. Responses of tonically active neurons in the monkey striatum discriminate between motivationally opposing stimuli. J Neurosci. 2003;23:8489–97. doi: 10.1523/JNEUROSCI.23-24-08489.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richfield EK, Debowey DL, Penney JB, Young AB. Basal ganglia and cerebral cortical distribution of dopamine D1- and D2-receptors in neonatal and adult cat brain. Neurosci Lett. 1987;73:203–8. doi: 10.1016/0304-3940(87)90245-x. [DOI] [PubMed] [Google Scholar]
- 67.Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 68.Rilling JK, King-Casas B, Sanfey AG. The neurobiology of social decision-making. Curr Opin Neurobiol. 2008;18:159–65. doi: 10.1016/j.conb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 69.Salavert J, Gasol M, Vieta E, Cervantes A, Trampal C, Gispert JD. Fronto-limbic dysfunction in borderline personality disorder: A 18F-FDG positron emission tomography study. J Affect Disord. 2011;131:260–7. doi: 10.1016/j.jad.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Sanislow CA, Grilo CM, McGlashan TH. Factor analysis of the DSM-III-R borderline personality disorder criteria in psychiatric inpatients. Am J Psychiatry. 2000;157:1629–33. doi: 10.1176/appi.ajp.157.10.1629. [DOI] [PubMed] [Google Scholar]
- 71.Sanislow CA, Grilo CM, Morey LC, Bender DS, Skodol AE, Gunderson JG, Shea MT, Stout RL, Zanarini MC, McGlashan TH. Confirmatory factor analysis of DSM-IV criteria for borderline personality disorder: findings from the collaborative longitudinal personality disorders study. Am J Psychiatry. 2002;159:284–90. doi: 10.1176/appi.ajp.159.2.284. [DOI] [PubMed] [Google Scholar]
- 72.Sansone RA, Sansone LA. Gender Patterns in Borderline Personality Disorder. Innov Clin Neurosci. 2011;8:16–20. [PMC free article] [PubMed] [Google Scholar]
- 73.Scerbo A, Raine A, O’Brien M, Chan CJ, Rhee C, Smiley N. Reward dominance and passive avoidance learning in adolescent psychopaths. J Abnorm Child Psychol. 1990;18:451–63. doi: 10.1007/BF00917646. [DOI] [PubMed] [Google Scholar]
- 74.Schonberg T, Daw ND, Joel D, O’Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J Neurosci. 2007;27:12860–7. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schultz W, Romo R. Neuronal activity in the monkey striatum during the initiation of movements. Exp Brain Res. 1988;71:431–6. doi: 10.1007/BF00247503. [DOI] [PubMed] [Google Scholar]
- 76.Seger CA, Cincotta CM. Dynamics of frontal, striatal, and hippocampal systems during rule learning. Cereb Cortex. 2006;16:1546–55. doi: 10.1093/cercor/bhj092. [DOI] [PubMed] [Google Scholar]
- 77.Sheppard G, Gruzelier J, Manchanda R, Hirsch SR, Wise R, Frackowiak R, Jones T. 15O positron emission tomographic scanning in predominantly never-treated acute schizophrenic patients. Lancet. 1983;2:1448–52. doi: 10.1016/s0140-6736(83)90798-5. [DOI] [PubMed] [Google Scholar]
- 78.Siever LJ, Buchsbaum MS, New AS, Spiegel-Cohen J, Wei T, Hazlett EA, Sevin E, Nunn M, Mitropoulou V. d,l-fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology. 1999;20:413–23. doi: 10.1016/S0893-133X(98)00111-0. [DOI] [PubMed] [Google Scholar]
- 79.Siever LJ, Torgersen S, Gunderson JG, Livesley WJ, Kendler KS. The borderline diagnosis III: identifying endophenotypes for genetic studies. Biol Psychiatry. 2002;51:964–968. doi: 10.1016/s0006-3223(02)01326-4. [DOI] [PubMed] [Google Scholar]
- 80.Skodol A, Siever LJ, Livesley WJ, Gunderson JG, Pfohl B, Widiger TA. The borderline diagnosis II: biology, genetics, and clinical course. Biol Psychiatry. 2002;51:951–963. doi: 10.1016/s0006-3223(02)01325-2. [DOI] [PubMed] [Google Scholar]
- 81.Soderstrom H, Hultin L, Tullberg M, Wikkelso C, Ekholm S, Forsman A. Reduced frontotemporal perfusion in psychopathic personality. Psychiatry Res. 2002;114:81–94. doi: 10.1016/s0925-4927(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 82.Soloff PH, Meltzer CC, Becker C, Greer PJ, Constantine D. Gender differences in a fenfluramine-activated FDG PET study of borderline personality disorder. Psychiatry Res. 2005;138:183–95. doi: 10.1016/j.pscychresns.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 83.Soloff PH, Meltzer CC, Greer PJ, Constantine D, Kelly TM. A fenfluramine-activated FDG-PET study of borderline personality disorder. Biol Psychiatry. 2000;47:540–547. doi: 10.1016/s0006-3223(99)00202-4. [DOI] [PubMed] [Google Scholar]
- 84.Spielberger C. State-Trait Anger Expression Inventory, Research Edition. Professional ManualOdessa, Florida: Psychological Assessment Resources; 1988. [Google Scholar]
- 85.Tobler PN, O’Doherty JP, Dolan RJ, Schultz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol. 2007;97:1621–32. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Unoka Z, Seres I, Aspan N, Bodi N, Keri S. Trust game reveals restricted interpersonal transactions in patients with borderline personality disorder. J Pers Disord. 2009;23:399–409. doi: 10.1521/pedi.2009.23.4.399. [DOI] [PubMed] [Google Scholar]
- 87.Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Overall JE, Burr G, Wolf AP. Recovery of brain glucose metabolism in detoxified alcoholics. Am J Psychiatry. 1994;151:178–83. doi: 10.1176/ajp.151.2.178. [DOI] [PubMed] [Google Scholar]
- 88.Vollm B, Richardson P, McKie S, Elliott R, Dolan M, Deakin B. Neuronal correlates of reward and loss in Cluster B personality disorders: a functional magnetic resonance imaging study. Psychiatry Res. 2007;156:151–67. doi: 10.1016/j.pscychresns.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 89.Woods R, Mazziota J, Cherry S. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Wu JC, Buchsbaum MS, Hershey TG, Hazlett E, Sicotte N, Johnson JC. PET in generalized anxiety disorder. Biol Psychiatry. 1991;29:1181–99. doi: 10.1016/0006-3223(91)90326-h. [DOI] [PubMed] [Google Scholar]
- 91.Wyzynski B, Merriam A, Medalia A, Lawrence C. Choreoacanthocytosis. Neuropsychiatry Neuropsychol Behav Neurol. 1989;2:137–144. [Google Scholar]
- 92.Yin HH, Knowlton BJ. Contributions of striatal subregions to place and response learning. Learn Mem. 2004;11:459–63. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D. The Overt Aggression Scale for the objective rating of verbal and physical aggression. Am J Psychiatry. 1986;143:35–9. doi: 10.1176/ajp.143.1.35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Striatal volume is shown for healthy control subjects (HC) and borderline personality disorder-Intermittent explosive disorder patients (BPD-IED). Black bars represent males, and gray bars represent females. MANOVA found no main effect of diagnosis (F1,70=0.08, p=0.88), a trend-level effect of sex (F1,70=2.60, p=0.11) and no interaction of Diagnosis × Sex (F1,70=0.05, p=0.19) on striatal volumes.
Button presses are shown for healthy control subjects (HC, blue bars) and borderline personality disorder-Intermittent explosive disorder patients (BPD-IED, red bars) in males and females.
We found a diagnosis by sex interaction, with healthy females pressing significantly less buttons (averaged across A, B, and C) than any other group (F(1,67)=4.68; p=0.03, all post- hoc tests p<0.02, Supplementary Figure 2A)
We found a Diagnosis by Sex by button type (A, B, C) interaction (GG: ε=.56, F(adjusted df: 1.2, 75.2)=5.64, p=0.02, Supplementary Figure 2B). This difference was accounted for by A button presses. Healthy females had a significantly lower number of A button presses than BPD females while the opposite pattern was observed for the males, with healthy males making significantly higher number of A button presses than male BPD-IED patients (both post-hoc tests, p<0.005).