Abstract
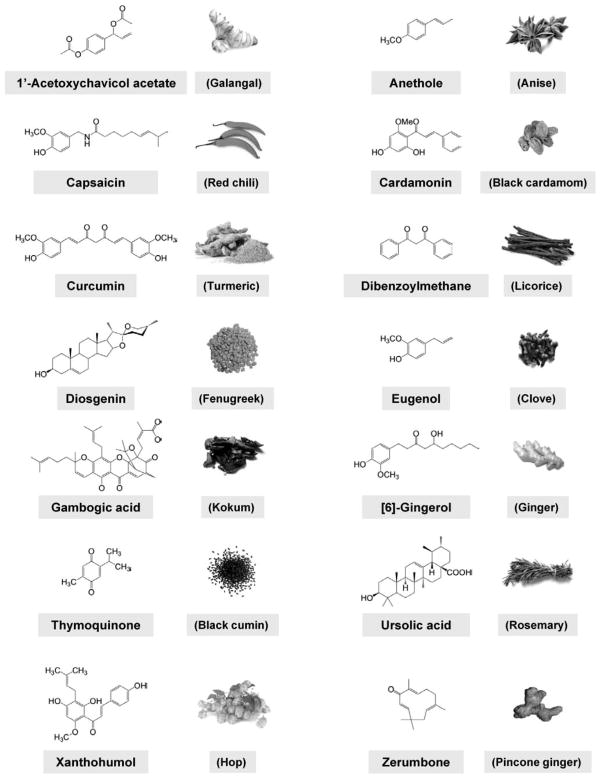
Extensive research within the last half a century has revealed that cancer is caused by dysregulation of as many as 500 different gene products. Most natural products target multiple gene products and thus are ideally suited for prevention and treatment of various chronic diseases, including cancer. Dietary agents such as spices have been used extensively in the Eastern world for a variety of ailments for millennia, and five centuries ago they took a golden journey to the Western world. Various spice-derived nutraceuticals, including 1′-acetoxychavicol acetate, anethole, capsaicin, car-damonin, curcumin, dibenzoylmethane, diosgenin, eugenol, gambogic acid, gingerol, thymoquinone, ursolic acid, xanthohumol, and zerumbone derived from galangal, anise, red chili, black cardamom, turmeric, licorice, fenugreek, clove, kokum, ginger, black cumin, rosemary, hop, and pinecone ginger, respectively, are the focus of this review. The modulation of various transcription factors, growth factors, protein kinases, and inflammatory mediators by these spice-derived nutraceuticals are described. The anticancer potential through the modulation of various targets is also the subject of this review. Although they have always been used to improve taste and color and as a preservative, they are now also used for prevention and treatment of a wide variety of chronic inflammatory diseases, including cancer.
INTRODUCTION
Four decades after U.S. President Nixon officially declared the “War on Cancer,” the overall rates of cancer have not substantially changed. Despite significant progress in the treatment of certain forms of cancer (such as childhood leukemia), cancer in general remains a major cause of death. Why are we losing the war against cancer? Is cancer a more complex and challenging disease than expected (1)? In any case, what is the future of cancer research? We argue that the primary cause is a too narrow focus in the effort to develop cancer drugs for a single target, usually a single gene, gene product, or signaling pathway that has been identified on the basis of genetic analysis or biological observations (2). Theoretically, targeting therapy should be sufficient to achieve a significant therapeutic effect; in reality, however, such therapies have had very little therapeutic impact (3–5). In fact, they have generally been highly ineffective against complex diseases (e.g., cancer) or diseases affecting multiple tissues or cell types (e.g., diabetes and immunoinflammatory disorders).
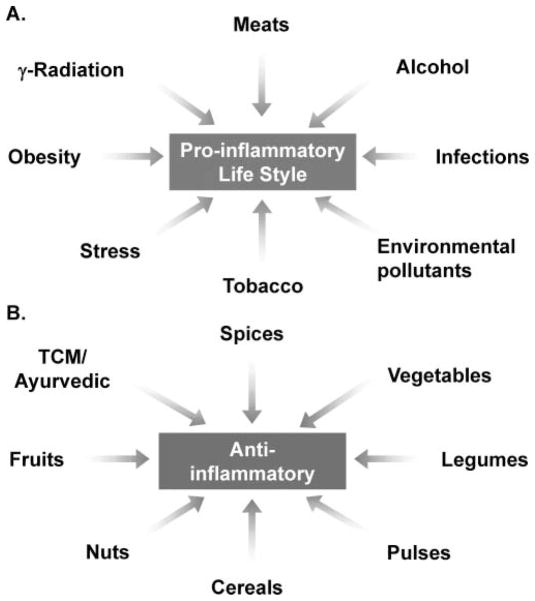
Only 5% to 10% of all cancers are caused by inheritance of mutated genes and somatic mutations, whereas the remaining 90–95% has been linked to lifestyle factors and the environment (6). Almost 30% of all cancers have been attributed to tobacco smoke, 35% to diet, 14–20% to obesity, 18% to infections, and 7% to radiation and environmental pollutants. The underlying mechanisms by which these risk factors induce cancer are becoming increasingly evident. One process that seems to be common to all these risk factors is inflammation (6–9). Therefore, most risk factors for cancer, including tobacco, obesity, alcohol, infections, stress, food carcinogens (e.g., grilled meat), and environmental pollutants, have been shown to be components of a proinflammatory lifestyle, one leading to tumorigenesis (Fig. 1A).
FIG. 1.
Various life factors induced proinflammatory lifestyle related to tumorigenesis and chemopreventive agents, including spices, suppress cancer.
The World Cancer Research Foundation 2007 report (10) estimates that 35% of the cancer incidence worldwide could be attributable to lifestyle factors such as food, nutrition, and physical activity. Increasing evidence has indicated that a diet protective against cancer would include fruits, vegetables, spices, cereals, pulses, and nuts (Fig. 1B). The specific substances in these dietary foods that are responsible for preventing cancer and the mechanisms by which they achieve this have also been examined extensively.
According to the U.S. Food and Drug Administration, spice is an “aromatic vegetable substance in the whole, broken, or ground form, the significant function of which in food is seasoning rather than nutrition” and from which “no portion of any volatile oil or other flavoring principle has been removed.” Although spices have been used for thousands of years and are known for their flavor, taste, and color in the food, they are not usually recognized for their medicinal value. The results from Italy with gastric cancer patients and healthy people indicate that individuals who consume more fresh fruit, raw vegetables, and spices were associated with lower incidence of cancer (11). In addition, in a comparison of the incidence of the various types of cancer between the United States and India, the United States was found to have much higher rates of colorectal cancer. In 2000, the United States had 356 colon cancer cases reported and 139 deaths per 1 million people. In contrast, India only had 40 reported cases of colon cancer and 26 deaths per 1 million people. Why cancer incidence is so much lower in India than in most Western countries is not fully understood, but the high spice consumption could be one of the contributing factors (12).
In this review, we will focus on the selected nutraceuticals derived from spices (Fig. 2) that target multiple cellular signaling pathways in tumorigenesis. The spicy nutraceuticals, described here, do indeed show great potential for modulating multiple targets such as transcription factors (e.g., NF-κB, STAT3, activator protein (AP-1), NRF-2, peroxisome proliferator-activated receptor (PPARγ), and HIF-1α), growth factor receptors [e.g., vascular endothelial growth factor receptor (VEGFR), epidermal growth factor receptor (EGFR), HER2 (EGFR2), and insulin-like growth factor-1 receptor (IGF-1R), kinases (e.g., phosphoinositide 3-kinase (PI3K), AMP-activated protein kinase (AMPK), Bcr-abl, and Raf/Ras], inflammatory mediators, and other targets involved in tumor progression.
FIG. 2.
Chemical structures of selected spice-derived nutraceuticals.
MOLECULAR TARGETS OF SPICE-DERIVED NUTRACEUTICALS
As described earlier, cancer is not a simple disease but a complex interaction between multiple signaling pathways with various target molecules. In this review, we will focus on some selected spice-derived nutraceuticals (see Fig. 2 and Table 1). This first section will focus on some of the most commonly known targets that cause undesired effects in tumor development (Fig. 3). How spice-derived nutraceuticals modulate these particular targets will then be discussed.
TABLE 1.
A list of spice-derived nutraceuticals and natural sources
| Nutraceuticals | Sources | Botanical name |
|---|---|---|
| [6]-Gingerol | Ginger, marjoram | Zingiber officinale Roscoe; Origanum majorana |
| Anethol and analogues* | Anise, fennel, cloves, licorice, star anise, tarragon | Pimpinella anisum; Foeniculum vulgare; Syzygium anisatum; Glycyrrhiza glabra; Illicum verum; Artemisia dracunculus L. |
| Capsaicinoids | Pepper, red chili, paprika | Capsaicum spp.; Euphorbia spp.; C. annum; C. frutens |
| Curcumin | Turmeric | Curcuma longa |
| Dibenzoylmethane | Licorice | Glycyrrhiza sp. |
| Diosgenin | Fenugreek, crape ginger | Trigonella foenum graecum; Costus speciosus |
| Eugenol | Clove, nutmeg, cinnamon, basil, bay leaf, allspice, coriander | S. aromaticum; Myristica fragrans; genus Cinnamomum; Ocimum basilicum; Laurus nobilis; Pimenta dioica; Coriandrum sativum |
| Gambogic acid | Gamboge | Garcinia sp. |
| Thymoquinone | Blackseed | Nigella sativa |
| Ursolic acid | Basil, salvia, rosemary, berries | Rosmarinus officinalis; O. sanctum; Aronia melanocarpa; Oxycoccus quadripetalus; Origanum majorana; Diospyros melanoxylon; Salvia przewalskii Maxim |
| Xanthohumol | Hop | Humulus lupulus L. |
| Zerumbone | Pinecone ginger | Zingiber zerumbet; Curcuma ochrorhiza; C. heyneana |
FIG. 3.
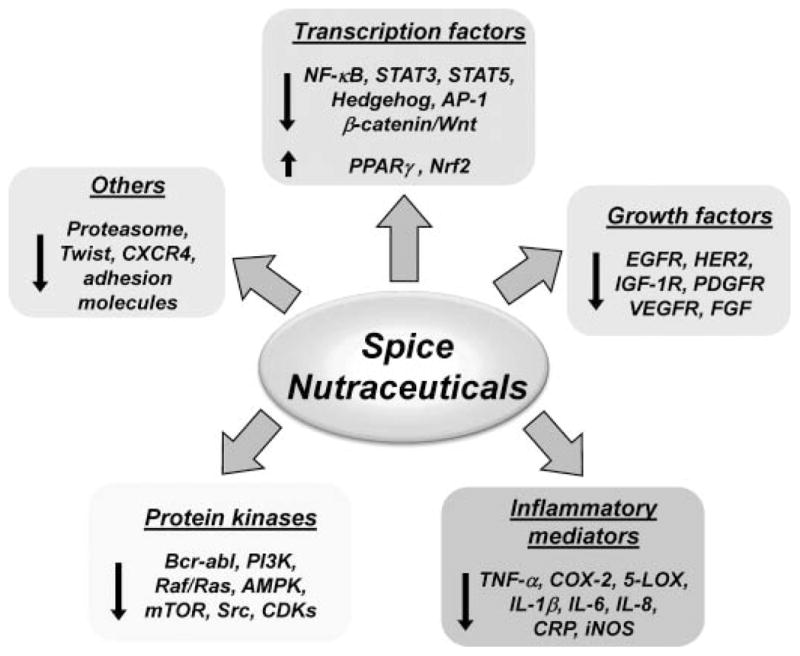
Molecular targets of spice-derived nutraceuticals in cancer. The nutraceuticals from spices modulates multiple targets including transcription factors, growth factors, and kinases involved in cancer cell growth, proliferation, survival, and metastasis. The single-head arrow indicates activation or positive regulation, whereas the blunt-end arrow indicates inhibition or negative regulation. 5-LOX indicates 5-lipoxygenase; AMPK, 5′ adenosine monophosphate (AMP)-activated protein kinase; AP-1, activator protein 1; CDK, cyclin-dependent kinase; COX-2, cyclooxygenase-2; CRP, C-reactive protein; CXCR4, C-X-C chemokine receptor type 4; EGFR, epidermal growth factor receptor; FGF, fibroblast growth factor; IGF-1R, insulin-like growth factor-1 receptor; IL-1β, interleukin 1beta; iNOS, inducible nitric oxide synthase; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-kappa B; NRF-2, NF-E2-related factor 2; PDGFR, platelet-derived growth factor receptor; PI3K, phosphoinositide 3-kinase; PPARγ, peroxisome proliferator-activated receptor gamma; STAT3, signal transducer activator of transcription 3; TNF-α, tumor necrosis factor alpha; VEGFR, vascular endothelial growth factor receptor.
Transcription Factors
Transcription factors regulate the expression of genes within a cell and ultimately control cell behavior. Thousands of these factors have been identified. They are frequently locked in an “on” position in cancer cells; while the transcription factors are shut off, a cancer cell will generally stop growing or begin to die. A number of oncogenic transcription factors such as AP-1, NF-κB, STAT3, and others are overactivated in human cancer and thus may present promising targets for treatment and prevention of cancer (13–16). Although the effect of various nutraceuticals on the transcription factors is discussed below individually, there is extensive cross-talk between these factors as recently described from our laboratory (13). Although the modulation of some these targets in some cases by spice-derived nutraceuticals may occur directly, in other cases the effects may be indirect.
Nuclear Factor-Kappa B (NF-κB)
We know that a number of genes involved in regulation and control of cancer growth and its’ metastasis are controlled by certain transcription factors. Among these, NF-κB plays a major role in development and progression of cancer because it regulates more than 500 genes, ones involved in inflammation, cell survival, cell proliferation, invasion, angiogenesis, and metastasis. In 1986, Sen and Baltimore discovered NF-κB as a nuclear factor that binds to the enhancer region of the κB chain of immunoglobulin in B cells (17). Upon activation, it is translocated to the nucleus, where it induces the expression of target genes. Many of the target genes are critical to the establishment of the early and late stages of aggressive cancers, including expression of cyclin D1, apoptosis suppressor proteins such as Bcl-2 and Bcl-xL, and those required for metastasis and angiogenesis, such as matrix metalloproteases (MMP) and VEGF. NF-κB is constitutively expressed in almost all cancer types and suppresses apoptosis in a wide variety of tumors. Its constitutive expression has been reported in human cancer cell lines in culture, carcinogen-induced mouse mammary tumors, and biopsies from cancer patients (13, 18).
A number of studies from our laboratory have shown that spice-derived nutraceuticals exert their anticancer effects through the suppression of NF-κB. Curcumin as well as various other curcuminoids from turmeric mediate their therapeutic effects by regulating NF-κB and the NF-κB-regulated gene products cyclooxygenase-2 (COX-2), cyclin D1, adhesion molecules, MMPs, inducible nitric oxide synthase, Bcl-2, Bcl-xL, and tumor necrosis factor (TNF) (19,20). The fennel-derived nutraceutical anethole blocks both early and late cellular responses transduced by tumor necrosis factor through suppression of NF-κB activation. Thus, its analogues eugenol and isoeugenol also inhibit TNF-induced NF-κB activation (21). Numerous spice-derived phytochemicals, such as cap-saicin (22), cardamonin (23), dibenzoylmethane (DBM) (24), diosgenin (25), gambogic acid (26), [6]-gingerol (27), thymoquinone (28), xanthohumol (29), ursolic acid (30), and zerum-bone (31) may also suppress NF-κB activation and antiapoptotic gene products and induce apoptosis in cancer cells.
Signal Transducer and Activator of Transcription 3
STAT3 is a transcription factor, first identified in 1994 as a DNA-binding factor that selectively binds to the interleukin (IL)-6-responsive element in the promoter of acute-phase genes from IL-6-stimulated hepatocytes (32). STAT3 was also independently identified as a DNA-binding protein in response to epidermal growth factor (EGF) (33). It is normally present in the cytoplasm of most cells. In response to certain inflammatory stimuli (e.g., IL-6) and growth factors (e.g., EGF), STAT3 undergoes sequential tyrosine phosphorylation, homodimerization, nuclear translocation, DNA binding, and gene transcription. Several protein kinases that cause specific phosphorylation of STAT3 have been identified, including Janus-activated kinase 1, 2, and 3. Protein phosphatases that dephosphorylate STAT3 also have been identified. The molecule is associated with in-flammation, cellular transformation, survival, proliferation, invasion, angiogenesis, and metastasis of cancer. Gene products linked with survival (e.g., Bcl-xL), proliferation (e.g., cyclin D1), and angiogenesis (e.g., VEGF) are regulated by STAT3 activation (16). STAT3 is constitutively active in most tumor cells but not in normal cells. Its activation has also been associated with chemoresistance and radioresistance (34).
One nutraceutical with potential to target STAT3 pathways is curcumin, a potent anticancer agent that induces apoptosis by inhibiting the STAT3 pathway. As first reported by Bharti et al. (35), curcumin has the potential to suppress STAT3 activation in human multiple myeloma (MM) cells. The same research group also reported that STAT3 is constitutively active in CD138+ cells derived from MM patients, and curcumin can inhibit STAT3 activation (36). The suppression of STAT3 by curcumin also occurs in a variety of other human cancer cells including glioma (37), cutaneous T-cell lymphoma (38), Hodgkin’s lymphoma (39), T-cell leukemia (40), ovarian cancer (41), endometrial cancer (41), and head and neck cancer (42). Bhutani et al. (43) found that capsaicin suppressed the STAT3 signaling pathway in human MM cells and inhibited the growth of human MM xenograft tumors in male athymic nu/nu mice. They showed that capsaicin inhibited the activation of janus-activated kinase-1 and c-Src, which are both implicated in STAT3 activation. In glial tumors, capsaicin was reported to downregulate the IL-6/STAT3 pathway by depleting intracellular gp130 pools through the endoplasmic reticulum (44). Li et al. (45) found that the spice-derived steroidal saponin, diosgenin, inhibited the STAT3 signaling pathway, leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cells. Thymoquinone is also known to inhibit the activation of STAT3 and potentiate the apoptotic effects of thalidomide and bortezomib in MM cells (46). Pathak et al. (47) found that ursolic acid in basil inhibited both inducible and constitutive activation of STAT3. Ursolic acid downregulated STAT3-regualted antiapoptotic genes such as Bcl-2, Bcl-xL, survivin, and Mcl-1 and inhibited proliferation in human MM cells.
Signal Transducer and Activator of Transcription 5
Stat5A was discovered as a transcription factor regulating milk protein expression. It was originally identified as a mammary gland factor (48) but renamed Stat5 according to homology within the Stat family (49). Further studies demonstrated that Stat5 has 2 different isoforms A and B. Stat5B is a crucial signaling protein mediating the biological effects of growth hormone, whereas the key function of Stat5A is to transduce the signals initiated by prolactin receptors (50). In addition, Stat5A/B can be activated by several other ligands including IL-2, IL-3, IL-5, IL-7, granulocyte-macrophage colony-stimulating factor, insulin, erythropoietin, and thrombopoietin (51).
Stat5 is persistently activated in certain human cancer cell lines and tumor tissues (52,53). The activation of Stat5 signaling has been shown to promote tumorigenesis in a number of cases, such as chronic myelogenous leukemia (CML) and myeloproliferative disease that is induced by TEL–JAK2 (50). In CML, the oncogenic BCR-ABL chromosomal translocation also known to cause persistent activation of Stat5 (54). Stat5 is also constitutively activated by the FMS-like tyrosine kinase 3 receptor tyrosine kinase (FLT3) in AML and thus an inhibitor for FLT3 blocks Stat5 signaling in these cells (55). In solid tumors instance prostate and breast, have also been shown that activation of Stat5A/B is significantly (56,57). Above information indicate that Stat5 inhibitors might be potent as anticancer therapies.
Among spice-derived compounds, curcumin is known to inhibit Stat5 signaling pathway. Rajasingh et al. (40) showed that curcumin induced a dose-dependent decrease in Stat5 phosphorylation resulting in the induction of growth-arrest and apoptosis in T-cell leukemia lines, MT-2, HuT-102, and SLB-1. Results from other group indicated that curcumin inhibited INFγ-induced nuclear translocation of Stat5 without affecting its phosphorylation of Stat5 in human CML cells (58).
AP-1
AP-1 acts as a dimer consisting mainly of the Jun (c-Jun, JunB, JunD) and Fos (c-Fos, FosB, Fra-1, Fra-2) subfamilies that harbor a basic leucine zipper (bZIP) domain and can form duplexes between themselves and with other bZIP proteins. Bernstein and Colburn (59) elucidated the role of AP-1 in tumorigenesis. They reported a lack of responsiveness to AP-1 activation in transformation-resistant JB6 cells, whereas AP-1 was functional in transformation-sensitive JB6 cells. Later the requirement for AP-1 activity during tumor promotion in JB6 cells was demonstrated by expression of a transactivation-minus mutant of c-Jun (TAM67) (60,61). Numerous data suggest that the activation of AP-1 plays a major role in proliferation and metastasis of tumor cells (62,63). AP-1 regulates the expression of genes that mediate proliferation and angiogenesis such as c-myc and fos, and genes for COX-2, urokinase-type plasminogen activator, MMP-9, cyclin D1, and VEGF (62,64). This transcription factor also represses tumor-suppressor genes such as p53, p21cip1/waf-1, and p16 (65). Curcumin has been shown to suppress the activation of 12-O-tetradecanoylphorbol-13 acetate (TPA)-induced AP-1 in HL-60 and Raji cells (66,67). Curcumin treatment also suppresses constitutive AP-1 activity in prostate cancer cell lines (68). Inhibition of AP-1 transcriptional activity by curcumin correlates with inhibition of Lewis lung carcinoma invasion in an orthotopic implantation model (69). In HT1080 human fibrosarcoma cells, ursolic acid represses the expression of MMP-9 by stimulating the nuclear translocation of glucocorticoid receptor and the translocated glucocorticoid receptor, probably by downmodulating the transactivating function of AP-1 to MMP-9 promoter region (70).
3.1.5. Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2)
Nrf2 is a transcription factor that plays a crucial role in protecting cells against inflammation, as well as oxidative and electrophilic stresses. Under stress conditions such as oxidative or electrophilic stress, Nrf2 translocates into the nucleus, binds to antioxidant response elements (ARE), and transactivates phase II detoxifying and antioxidant genes. Among the antioxidant genes that are regulated by Nrf2 are NAD(P)H:quinone oxidoreductase (NQO1), heme oxygenase-1 (HO-1), thiore-doxin reductase 1, glutamate-cysteine ligase modifier subunit, and glutamate-cysteine ligase catalytic (GCLC) subunit (71). Several lines of evidence have indicated the roles of Nrf2 in susceptibility to carcinogenesis. The colorectal tumor incidence, multiplicity, size, and stage of progression are higher in Nrf2-deficient mice exposed to azoxymethane-dextran sodium sulfate (AOM/DSS) (72). Beside colorectal carcinogenesis, Nrf2-deficient mice are also more susceptible to skin tumorigenesis (73), lung cancer (74), gastric neoplasia (75), urinary bladder carcinoma (76), and hepatocarcinogenesis (77) compared to their wild-type counterparts. A recent review by Hayes and McMahon (78) indicated frequent mutation of KEAP1, an inhibitor of Nrf2, and NRF2 in human cancers. KEAP1 mutation (C23Y) found in tumors from breast cancer patients has been associated with impaired ubiquitination of Nrf2 (79), and recurrent KEAP1 gene alterations were observed in gallbladder cancer with a frequency of 30% (80). Furthermore, it has been noted that patients with lung tumors containing mutant KEAP1 or NRF2 showed a poorer prognosis than patients with nonmutant tumors (81).
Administration of curcumin induced the expression and nuclear translocation of Nrf2 in the liver and lung of mice treated with benzo[a]pyrene (B[a]P) (82). Moreover, curcumin increased ARE-binding of Nrf2 and induced the activity as well as expression of GST and NQO1 and their mRNA transcripts, and the liver and lung of mice treated with dietary curcumin had reduced oxidative stress and inflammation (83). Dibenzoylmethane (DBM), a constituent of licorice, induced the ARE-luciferase reporter activity and attenuated B[a]P-induced DNA adduct formation in the lung of A/J mice. These findings were in agreement with increased mRNA expression of NQO1, GSTA2, and GCLC in mouse hepatoma cells, which was negated by dominant-negative mutation of Nrf2 (84). Recently, a study conducted with DBM on AOM/DSS-induced colon cancer model showed that DBM increased induction of Nrf2 transcription factor and phase II detoxifying enzymes (85). Lee et al. (86) demonstrated that a chalcone, xanthohumol, exerts antiinflammatory activity through Nrf2-ARE signaling and upregulation of downstream HO-1 in mouse microglial BV2 cells. Interestingly, this chalcone alkylated 27 cysteine sulfhydryl groups of Keap1, which led to Nrf2 nuclear accumulation, upregulation of cytoprotective gene expression by the binding of Nrf2 to ARE, and prevention of degenerative diseases, such as cancer (87). Capsaicin, a major pungent ingredient of red pepper, is also reported to have chemoprotective effects through activation of Nrf2 and upregulate the expression of HO-1 (88).
PPAR-γ
PPAR-α, -β (or δ), and -γ are 3 of 100 nuclear receptors in the orphan receptor class. PPARγ (PPARγ) is the most extensively studied subtype of the PPARs. It is mainly expressed in adipose tissue and in colonic epithelium. Lower levels are expressed in beta cells of the pancreas, vascular endothelium, macrophages, and many other tissues. Over the last decade, research on PPARγ unveiled its role in important biological processes, including lipid biosynthesis, glucose metabolism, anti-inflammatory response, and atherosclerosis (89), and in regulating tumor suppression and promotion (90–92). Earlier research suggested a relationship between PPARγ activation and cellular differentiation accompanied by cell cycle arrest (93). Later research demonstrated PPARγ expression in multiple tumor types, leading to further insights into the role of PPARγ in cell cycle arrest and growth inhibition of human and rodent tumor cells (94–96). The role of PPARs in carcinogenesis is not fully understood and remains controversial. However, numerous lines of evidences indicate the dysregulation of PPARγ correlates with carcinogenesis in head and neck cancer (97), thyroid follicular carcinomas (98), colon cancer (99), and bladder cancer (96).
PPARγ agonists in the drug development pipeline are undergoing clinical trials in patients with advanced metastatic cancer, anaplastic thyroid cancer, or leukoplakia (100,101). Curcumin has been reported to significantly induce the expression of PPAR and inhibit cell proliferation, induce apoptosis, and suppress extracellular matrix gene expression. Blocking the transactivity by PPARγ antagonist significantly decreased the effects of curcumin on the inhibition of cell proliferation. Recent studies reported that the activation of PPARγ by curcumin in Moser cells inhibited the growth and mediated the suppression of gene expression of cyclin D1 and EGFR (102). Kim et al. (103) reported that capsaicin had a role as PPARγ agonist and induced apoptosis in HT-29 human colon cancer cells. This capsaicin-induced cell death was completely blocked by bisphenol A diglycidyl ether, a specific PPARγ antagonist.
β-catenin/Wnt
Two genes, one from wingless fruit flies (Drosophila melanogaster) and one for a protooncogene causing mammary tumors (wingless and int-1, respectively) were found to encode identical proteins, and so the new name Wnt was generated (104). Wnt family proteins are secreted lipid-modified glyco-proteins with highly conserved cysteine residues (105). The extracellular Wnt glycoproteins bind to cell surface receptors to stimulate intracellular events. The best characterized Wnt signaling pathway is the canonical Wnt/β-catenin signaling pathway. There are also one or more “non-canonical” or β-catenin-independent Wnt signaling pathways that are less well understood, and that act in a β-catenin-independent manner, leading to changes to cytoskeletal dynamics, adhesion, and motility (106). The highly conserved canonical Wnt/β-catenin signaling pathway is activated by the binding of Wnt ligand to the receptors frizzled (FZD) and low-density lipoprotein receptor-related protein 5/6 (LRP5/6), triggering a series of downstream events that culminate in the cytosolic accumulation and nuclear translocation of the multifunctional protein β-catenin. In the absence of active Wnt ligands, β-catenin is bound to the scaffold proteins axis inhibition protein and adenomatosis polyposis coli (APC) and is constitutively phosphorylated at 4 N-terminal residues via interaction with glycogen synthase kinase (GSK)-3β. Accumulated β-catenin then translocates to the nucleus, where it interacts with transcription factor T-cell factor (TCF) and/or lymphoid enhancer factor (LEF) and regulates the expression of target genes that mediate the ultimate effects of this pathway on cellular processes including cell fate, proliferation, and migration. Wnt pathways have been intimately linked to cancer.
Numerous reports indicate that curcumin downregulates the Wnt/β-catenin signaling pathway. Jaiswal et al. (107) observed that curcumin induced caspase-3-mediated cleavage of β-catenin, E-cadherin, and APC; decreased transactivation of β-catenin/TCF/LEF; decreased promoter DNA-binding activity of the β-catenin/TCF/LEF complex; and decreased levels of c-myc protein in human colon cancer cells. Ryu et al. (108) reported that curcumin derivatives inhibit the Wnt/β-catenin pathway by decreasing the amount of the transcriptional coactivator p300. The inhibition of Wnt/β-catenin by curcumin was also found in estrogen receptor (ER)-positive (MCF-7) and ER-negative (MDA-MB-231) breast cancer cells (109). Interestingly, it was found that curcumin could inhibit mammosphere formation and could also decrease the amount of aldehyde dehydrogenase-positive cells in normal and malignant breast cells through the inhibition of Wnt signaling, suggesting the inhibitory effects of curcumin on breast cancer stem cells (110). Other than curcumin, the spice-derived nutraceuticals ursolic acid (111) and xanthohumol (112) also inhibit β-catenin and thus have anti-cancer properties.
Sonic Hedgehog
Hedgehog (Hh) was first discovered by Christiane Nusslein-Volhard and Eric Wieschaus nearly in 1980 as a “segment-polarity” gene that controls Drosophila embryonic cuticle pattern (113). Hh signaling is important not only in fruit flies, where it serves to pattern their embryonic cuticles and adult appendages, but also in humans, where it helps to determine cell fate and numbers in brains and spinal cords, to pattern limbs and internal organs, and even to regulate body height (114). However, in the past few years, it has become clear that aberrant activation of the Hh signaling pathway can lead to cancer (115,116). Emerging evidence implicates the activation of Hh signaling in the development of a variety of cancers including basal cell carcinomas, medulloblastomas, leukemia, glioma, and cancers of the gastrointestinal, lung, ovary, breast, prostate, and colon (117). The activation of Hh signaling is driven by endogenous expression of Hh ligands such as Sonic and Indian Hh. Key regulatory components of the Hh pathway signaling include Smoothened (SMO), a 7-transmembrane domain cell surface protein essential to pathway activation, and Patched homologue 1 (PTCH1), a cell surface receptor protein that serves as a primary repressor of SMO. Binding of any of 3 Hh ligands to PTCH1 relieves PTCH1 repression of SMO, leading to downstream pathway activation including modification of the 3 GLI family transcription factors (GLI1–GLI3), which in turn promote transcription of genes regulating cell growth and differentiation (117). Activation of the Hh pathway is also associated with poorly differentiated and more aggressive tumors (118, 119). These observations have sparked vigorous interest in the development of novel inhibitors of the Hh pathway.
Recently, Elamin and colleagues (120) reported that curcumin inhibited the Shh-GLI1 signaling pathway by downregulating the Sonic hedgehog (Shh) protein and its most important downstream targets GLI1 and PTCH1 in human medulloblastomas cells. Zerumbone was shown to exert cytotoxic activity in pancreatic cancer cells. This sesquiterpene suppressed GLI-mediated transactivation and led to downmodulation of Hh-related gene expression in PANC1 pancreatic cancer cells, which express Hh/GLI components (121). These results indicate that a spice nutraceutical may represent great promise as Shh-targeted therapy for cancer treatment.
Growth Factors
Most growth factors work through their specific receptors to mediate signals. Receptor tyrosine kinases (RTKs) are the high-affinity cell surface receptors for many polypeptide growth factors. Of the 90 unique tyrosine kinase genes identified in the human genome, 58 encode RTK proteins. Some protein tyrosine kinases are considered attractive targets for the therapy of malignant disease. In selected cancers, activating mutations in a tyrosine kinase appear to be etiologic, initiating the transformation from a benign to a malignant state. However, the drugs targeting RTK produced adverse effects and development of secondary resistance so new inhibitors of these factors are required.
EGFR
Aberrant EGFR signaling is a major characteristic of many human malignancies including breast cancer. Since the discovery of EGF in the 1960’s and its receptor in the 1980s (122,123), our understanding of the EGF/EGFR pathway has been significantly advanced. EGFR is now considered a major oncogenic factor and an attractive therapeutic target (124). A transmembrane RTK, it plays a central role in regulating cell division and death. EGFR belongs to the HER family of receptors, which is composed of 4 related proteins (EGFR [HER1/ErbB1], ERBB2 [HER2], ERBB3 [HER3], and ERBB4 [HER4]). The HER receptors are known to be activated by binding to different ligands, including EGF, transforming growth factor-α, heparin-binding EGF-like growth factor, amphiregulin, betacellulin, and epiregulin. It plays a role in protein phosphorylation and in malignant transformation (125).
So far, 3 anti-EGFR agents have been approved for clinical use: gefitinib (Iressa) for non-small-cell lung cancer, the monoclonal EGFR antibody cetuximab (Erbitux) for metastatic colorectal cancer, and most recently, erlotinib (Tarceva) for metastatic non-small cell lung cancer. These remain in clinical trial and their efficacy is uncertain. In any case, additional drugs that inhibit EGFR are urgently needed, and nutraceuticals are among the candidates. Curcumin, for example, inhibits the ligand-stimulated activation of EGFR, indicating that it has the potential to break the autocrine loops that are established in several advanced cancers (126). Curcumin inhibits EGFR in numerous cancer cells including breast (127), colon (102), prostate (128), lung (129), and head and neck (130) cancer. Ursolic acid suppresses the phosphorylation of EGFR, in direct relation to its cell growth inhibitory effect and also suppresses EGF-stimulated cell proliferation in human colorectal cancer cells (131). Thoennissen et al. (132) demonstrated that capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells in vitro by modulating the EGFR/HER-2 pathway. Capsiate, a capsaicin analog with an ester bond instead of the amide bond between the vanillyl moiety and the fatty acid chain, inhibits UVB-induced EGFR activation, which reduces the expression of inflammatory mediators, such as cytokines and COX-2 and angiogenic factors in vitro and decreases UVB-induced skin damage in vivo (133).
HER2
Growth of human breast cells is closely regulated by steroid hormone as well as peptide hormone receptors. Members of both receptor classes are important prognostic factors in human breast cancer. Clinical data indicate that overexpression of the HER-2 gene is associated with an ER-negative phenotype. Different ligand binding to HER-2 receptors results in dimerization and activation of their intrinsic kinase activity followed by phosphorylation of specific tyrosine residues in the receptor cytoplasmic tails. These phosphorylated tyrosines, in turn, provide recognition sites for intracellular signaling intermediates, which link RTKs to downstream transduction cascades (134). The selection and combination of pathways activated ultimately result in changes in gene expression, thereby triggering the appropriate biological response to the extracellular cues received. Driven by the binding specificities of the bivalent, EGF-related peptide ligands and the complement of receptors available on the cell, HER-2 receptors form different homodimeric and heterodimeric complexes (135). Herceptin, an antibody against the HER-2 for breast cancer patients, binds to the extracellular domain of receptors in the same way, but because its target c-erbB2 has no known directing ligand, it presumably acts by other mechanisms (136). Although these approaches look very promising, confounding issues remain, the most important being side effects and drug resistance.
A nutraceutical alternative, curcumin, has been shown to not only inhibit the tyrosine kinase activity of this receptor but also to deplete the protein itself by interfering with the function of the ATP-dependent GRP94 chaperone protein, which is involved in the maintenance of the properly folded state of the receptor (137). Jung et al. (138) found that curcumin increased the association between CHIP, a chaperone-dependent ubiquitin ligase, and erbB2 (also called HER2), and thus induced ubiquitination and degradation of this receptor. Moreover, they found that curcumin’s Michael reaction acceptor functionality appeared to be the pharmacophore responsible for its ability to promote erbB2 degradation. Curcumin also inhibits cell proliferation and invasion through modulation of HER2 in gastric cancer cells (139).
VEGFR
VEGF is a signal protein produced by cells that stimulates the growth of new blood vessels. Its receptor VEGFR is an important signaling protein involved in both vasculogenesis (the formation of the circulatory system) and angiogenesis (the growth of blood vessels from preexisting vasculature). En-dothelial cells express 3 different VEGFR: VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1), and VEGFR-3 (Flt-4). These all belong to the family of RTKs (140). Structurally, all VEGF receptors contain 7 immunoglobulin-like extracellular domains, 1 transmembrane region, and an intracellular split tyrosine kinase domain (141). VEGF signaling is activated by binding of the growth factor to the receptor, which leads to dimerization. Dimerization, in turn, triggers kinase activation (142). The angiogenic response of VEGF varies between different organs and is dependent on the genetic background of the animal. However, mitogenic activity in endothelial cells is mainly mediated by VEGFR-2, leading to their survival, proliferation, migration, and differentiation.
Several spice-derived nutraceuticals have been shown to downregulate VEGF signaling in vitro and others have been shown to prevent new blood vessel formation in vivo. [6]-Gingerol blocks capillary-like tube formation in endothelial cells, and inhibited sprouting of endothelial cells in rat aorta secretion in human endothelial cells in response to VEGF in vitro (143). Gambogic acid inhibits VEGFR2 signaling, thus inhibiting angiogenesis and prostate tumor growth (144).
Insulin-Like Growth Factor (IGF) 1-Receptor
IGFs exert multiple effects on glucose, fat, and protein metabolism. IGFs also play important roles in regulating cell proliferation, differentiation, apoptosis, and transformation (145). The IGF family consists of 2 ligands (IGF-I and IGF-II), 2 receptors (IGF-IR and IGF-IIR), 6 high-affinity IGF-binding proteins (IGFBP1–6), and other low-affinity IGFBP-related proteins. The interaction between ligand-receptor induces a conformational change in receptor subunits, resulting in activation of the tyrosine kinase of the cytoplasmic domain of IGF-IR. Phosphorylation of adaptor proteins, such as insulin receptor substrate-1 or -2, Src- and collagen-homology, and growth factor receptor-binding protein 2, leads to binding of additional proteins, allowing for signal transduction along several specific pathways. Some of the key pathways and their endpoints include phosphorylation of mitogen-activated protein kinase (MAPK) and a subsequent increase in proliferation, activation of PI3K, leading to decreased apoptosis, and modulation of mammalian target of rapamycin (mTOR), resulting in translational adaptation (146). The IGF system has been implicated in several human malignancies, including various epithelial cancers, sarcomas, multiple myeloma, melanoma, and childhood cancers (147). More recent studies have suggested that high circulating IGF-I levels and/or low IGFBP3 levels are associated with increased risk of several cancers including breast (148), prostate (149), lung (150), colorectal (151), and bladder (152). The negative correlation between IGFBP3 levels and cancer risk is consistent with a protective role of IGFBP3.
It is worth mentioning a chemoprevention approach to therapeutics, given that many agents have the potential of upregulating the IGFBPs. A study conducted by Xia et al. (153) demonstrated that curcumin decreased the secretion of IGF-1 with a concomitant increase of IGFBP-3 in a dose-dependent manner. Thus, the IGF-1-stimulated IGF-1R tyrosine kinase activation was also abrogated by curcumin in human breast cancer cells. Thus, in colorectal cancer cell lines, curcumin enhanced the effect of FOLFOX (5-fluorouracil [5-FU] or 5-FU plus ox-aliplatin) on cell proliferation suppression and apoptosis. These changes were associated with decreased expression and activation (tyrosine phosphorylation) of several receptors, including IGF-1R, and upregulation of IGFBP-3.
Platelet-Derived Growth Factor (PDGF) Receptor
PDGF is the principal mitogen in serum for mesenchymal cells and consists of a family of A, B, C, and D polypeptides that promote cell migration, proliferation, and survival by binding to their cognate homo- or heterodimeric tyrosine kinase receptors, PDGFRα and PDGFRβ (154,155). Enhanced signaling of PDGF has been implicated in the pathogenesis of atherosclerosis, balloon injury induced restenosis, pulmonary fibrosis, angiogenesis, and tumorigenesis (156). In malignant cells, autocrine function of PDGF plays a role on tumor growth by overexpression or hyperactivation of PDGFRs, or by PDGF stimulation of angiogenesis within the tumor. We see only constitutive activation of PDGFRα or PDGFRβ in myeloid cells, and activating mutations of PDGFRα are seen in gastrointestinal tumors. Active PDGFRα was also found in non-small-cell lung cancer (157).
Two main approaches have been taken toward the inhibition of cancer growth when PDGF-PDGFR signaling is activated: 1) direct targeting of tumor cells in which PDGF signaling is activated and 2) indirect inhibition of tumors by targeting pericytes to block tumor angiogenesis independently of PDGF activity. Some natural compounds, such as curcumin and gambogic acid, have been identified as inhibitors of the activation of PDGFR and as blockades of the PDGF-mediated pathway. Yang et al. (158) showed that curcumin-inhibited cell migration, proliferation, and collagen synthesis by attenuating PDGF signal transduction, and inhibited the binding of PDGF to its receptors in vitro and neointima formation after carotid artery injury in rats. Another study, conducted by Lin and Chen (159), showed that the interruption of the PDGF and EGF signaling pathways by curcumin stimulates expression of PPARγ in activated rat hepatic stellate cells in vitro. Recently, Zhao and Huang (160) demonstrated that curcumin blocks the expression of PDGF-BB, PDGFRβ, and ERK1, which might explain curcumin’s antifibrosis activity. Beside curcumin, dehydrozingerone, derived from ginger, and its structural analog isoeugenol also elicited a concentration-dependent inhibition of PDGF-stimulated VSMC migration, proliferation, collagen synthesis, and PDGF/H2O2-stimulated phosphorylation of PDGFRβ and downstream Akt in VSMC cells (161). A study conducted by Liu et al. (162) showed that gambogic acid-induced G0/G1 cell cycle arrest and cell migration inhibition by suppressing PDGFRβ tyrosine phosphorylation and Rac1 activity in rat aortic smooth muscle cells.
Protein Kinases
Of the 25,000 genes in the human genome, there are more than 500 protein kinases that might make targets for drug discovery in cancer. A large number of inhibitors of these protein kinases have been designed and have undergone clinical trials; however, the efficacies are not proved.
PI3K
PI3K signaling plays a pivotal role in translating the detection of extracellular cues into alterations in a variety of cellular functions. It belongs to a large family of PI3K-related kinases. A key downstream effector of PI3K is the serine-threonine kinase Akt, which in response to PI3K activation, phosphorylates and regulates the activity of a number of targets including kinases, transcription factors, and other regulatory molecules (163). The PI3K/Akt signal transduction cascade has been investigated extensively for its roles in oncogenic transformation through its involvement in cell cycle progression, apoptosis, and neoplastic transformation. The amplification of the genomic region containing PIK3CA, the gene coding for the p110 alpha subunit of PI3K, has been identified in 40% of cases of ovarian cancer (164). Activating mutations may occur in as many as 35% of the cases of breast cancer and is associated with a poor prognosis (165). Modifications of PIK3CA have also been identified in colon, brain, and lung cancers (166). PI3K also has a role in the metastatic phenotype (167).
A few nutraceuticals have the demonstrated capability to inhibit PI3K. Ursolic acid treatment moderately decreased PI3K levels in 2 endometrial cancer cell lines, SNG-II and the poorly differentiated HEC108 cell line, and thus induced apoptosis (168). Recently, Tang and colleagues (169) showed that the proapoptotic effects of ursolic acid were mediated by activation of caspase-3 and downregulation of survivin and were highly correlated with inactivation of PI3K/Akt/survivin pathway in human HepG2 cells. Lee et al. (170) reported that diosgenin inhibits melanogenesis by activating the PI3K pathway and also suggested that diosgenin may be an effective inhibitor of hyper-pigmentation. Curcumin-mediated apoptotic effects were observed in T-cell acute lymphoblastic leukemia malignant cells: curcumin suppressed constitutively activated targets of PI3K-kinase (AKT, FOXO, and GSK3), leading to the inhibition of proliferation and induction of caspase-dependent apoptosis (171). A recent study conducted by Chen et al. (172) showed that the level of PI3K in melanoma tumor tissue was lower in a curcumin-treated group (once a day at a dose of 100 mg/kg for 18 days) than the untreated control group.
AMPK
The AMPK is a Ser/Thr protein kinase that was first identified by its activation by AMP and its ability to phosphorylate and inactivate enzymes involved in lipid and cholesterol synthesis (173). At the cellular level, AMPK is activated by metabolic stressors that deplete ATP and increase AMP (e.g., exercise, hypoxia, glucose deprivation) (174). AMPK activation enhances insulin sensitivity, inhibits hepatic glucose production, stimulates glucose uptake in muscle, inhibits fatty acid synthesis and esterification, and diminishes proinflammatory changes (175). It has been shown that AMPK phosphorylates tuberous sclerosis complex-2 (a bona fide tumor suppressor) to inhibit mTOR signals (176). This observation reveals a direct connection of AMPK with cancer. Recently, great attention has been given to linkage between AMPK and cancer. AMPK, by regulating several downstream targets, such as mTORC1, p53, FOXO, and fatty acid synthase, and associated metabolic processes, controls intracellular energy levels in order to maintain the cell growth rate at an appropriate level. Likewise, AMPK activation under metabolic stress or by pharmacological activators can regulate various processes, including cell cycle checkpoint, cell polarity, senescence, autophagy, and apoptosis (177,178).
As has been the case for many other targets in the cancer cell signaling pathways, curcumin strongly activates AMPK, in this case in a p38-dependent manner in CaOV3 ovarian cancer cells, thus inducing cell death (179). Stimulation of AMPK by curcumin downregulates PPARγ in 3T3-L1 adipocytes and decreases COX-2 expression in MCF-7 cells, which in turn affects the proliferation rate (180). Another study, conducted by Lee et al. (181), showed that curcumin exerted antitumorigenic effects through modulation of the AMPK-COX-2 cascade. Curcumin exhibited a potent apoptotic effect on HT-29 colon cancer cells at concentrations of 50 micromol/L and above. These apoptotic effects were correlated with the decrease in phospho-Akt and COX-2, as well as the increase in phospho-AMPK. Another nutraceutical, capsaicin, has been reported to activate AMPK and increase apoptosis in HT-29 colon cancer cells (182).
Bcr-abl
The Bcr-abl oncoproteins are translocation-specific gene products of the Philadelphia chromosome that are detectable in most CML. Bcr-abl regulates proliferation, survival, differentiation, and trafficking of hematopoietic cells by transcriptional and posttranscriptional mechanisms that require tyrosine kinase activity and formation of multiprotein complexes whereby signaling molecules are assembled and activated in the cytoplasm and in the nucleus (183). The expression of Bcr-abl induces resistance of CML to apoptosis induced by chemotherapeutic drugs (184). Overexpression of Bcr-abl also prevent apoptotic cell death by inducing a Bcl-2 expression pathway in leukemia cells (185). Additionally, Bcr-abl has been shown to regulate c-jun gene expression, activation of c-Jun N-terminal kinase, and the ras pathway, which may also contribute to suppression of apoptosis, transformation, and tumorigenesis (186). Downstream mediators of Bcr-abl are known to regulate by the proteasome degradation.
Several proteasome inhibitors such as bortezomib could suppress Bcr-abl signaling (187). Curcumin inhibits the proliferation of K562 cells and the effect is correlated with down-regulation of p210bcr/abl (188). The underlying mechanism of curcumin in downregulating p210bcr/abl was identified later: It dissociates the binding of p210bcr/abl with Hsp90/p23 complex (189). A study conducted by William (190) showed that cur-cumin inhibits proliferation and induces apoptosis of leukemic cells expressing wild-type or T315I-BCR-ABL and prolongs survival of mice with acute lymphoblastic leukemia. Xhantho-humol was also reported to suppress Bcr-abl signaling. Mon-teghirofo et al. (191) showed that xanthohumol strongly inhibited Bcr-abl expression at both mRNA and protein levels. Thus, xanthohumol could induce apoptosis in all of Bcr-abl+ cells, CML cells, and clinical samples and retain its cytotoxicity in imatinib mesylate-resistant K562 cells (191).
Raf/Ras
Raf is a member of a serine/threonine specific protein kinase family and is an immediate downstream target of Ras, which is implicated in the transduction of signals from the cell surface to the nucleus (192). In the resting cell, Ras is tightly bound to GDP. It is activated by binding of extracellular stimuli such as growth factors, RTKs, T-cell receptors, and phorbol-12 myristate-13 acetate (PMA) to cell membrane receptors. Activated Ras interacts specifically with effector proteins, thereby initiating cascades of protein–protein interactions that may finally lead to regulation of cell proliferation, apoptosis, migration, fate specification, and differentiation (193). Ras also can activate a number of signaling pathways, such as Raf/MEK/ERK (extracellular signal-regulated kinases) pathway, the MEKK/SEK/JNK pathway, a PI3K/Akt/NF-κB pathway, a p120-GAP/p190-B/Rac/NF-κB pathway, and a Raf/MEKK1/inhibitor-κB kinase (IKK)/NF-κB pathway (194).
Among the spicy nutraceuticals, curcumin showed strong inhibition on Ras and Ras-related pathways. Curcumin modulates the Ras signal transduction pathway and inhibits the proliferation of K562 cells (188). Limtrakul et al. (195) showed that orally consumed curcumin (0.2% or 1% in diet) significantly inhibited 7,12-dimethylbenz(a)anthracene- and TPA-induced skin tumor formation and ras and fos gene expression in mouse skin.
mTOR
mTOR is a key regulator of various cellular processes needed for growth, cell-cycle progression, and cell metabolism. It belongs to the family of PI3K-related kinases, along with ATM, ATR, DNA-PK, and hSMG1, and it is considered one of the most commonly activated signaling pathways in human cancer (196). Rapamycin, first isolated from the soil of Rapa Nui (Easter Island), was the first identified potent inhibitor of the PI3K/AKT/mTOR pathway. Rapamycin specifically inhibits the function of mTOR, a protein thus named when its rapamycin-inhibiting properties were discovered. The mTOR pathway plays a critical role in cell growth, proliferation, motility, survival, apoptosis, autophagy, and angiogenesis (197). This pathway is activated by a variety of signals, including nutrients such as amino acids, glucose, and oxygen and/or mitogens.
The effects of curcumin on mTOR pathway have been demonstrated in a variety of cancer cell lines. Curcumin exhibits anticancer activity by inhibition of mTOR pathway in adenoid cystic carcinoma (198) intestinal microvascular endothelial cells (199), head and neck squamous cell carcinoma (200), glioblastoma (201), glioma (202,203), colorectal carcinoma (204), and prostate cancer (205,206). Interestingly, curcumin can disrupt the mTOR-raptor complex (207). Curcumin inhibited mTORC1 signaling not by inhibition of the upstream kinases, such as IGF-IR and PDK1, and altered the TSC1/2 interaction. Curcumin was able to inhibit phosphorylation of S6K1 and 4E-BP1 and to dissociate raptor from mTOR, leading to inhibition of mTORC1 activity. These data suggest that curcumin may represent a new class of mTOR inhibitor (207). A study conducted by Deeb et al. (208) showed that the apoptosis induced by xanthohumol was associated with the inhibition of prosurvival Akt, NF-κB, and mTOR signaling proteins and NF-κB-regulated antiapoptotic Bcl-2 and survivin in hormone-sensitive and hormone-refractory human prostate cancer cells line. Diosgenin was reported to suppress fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt, mTOR, and JNK phosphorylation (209). De Angel et al. (210) showed that ursolic acid had potential to disrupt cell cycle progression and induce necrosis in a clonal MMTV-Wnt-1 mammary tumor cell by modulating Akt/mTOR signaling.
Proinflammatory Mediators
The mixture of cytokines and proinflammatory mediators that is produced in the tumor microenvironment has an important role in tumor development and progression. In other words, cancer cells can respond to proinflammatory mediators that promote growth, attenuate apoptosis, and facilitate invasion and metastasis. Therapeutic manipulations that are aimed at inhibiting or attenuating the production of proinflammatory mediators might prevent and treatment cancer.
Proinflammatory Cytokines
Tumor necrosis factor (TNF)
α. Although TNF-α was first isolated as an anticancer cytokine, the major role of this cytokine has been as the key player to an onset of a wide variety of chronic diseases, including cancer (211). TNF-α has itself been shown to be one of the key molecules of inflammation due to its ability to activate NF-κB (212). Almost all cell types, when exposed to TNF, activate NF-κB, leading to the expression of inflammatory genes. These include COX-2, lipoxygenase (LOX)-2, cell-adhesion molecules, inflammatory cytokines, chemokines, and inducible nitric oxide synthase. TNF-α has been found to be a growth factor for most tumor cells (213). TNF-α is produced by cancer cells and can act as an endogenous tumor promoter. The role of TNF-α has been linked to all steps involved in tumorigenesis, including cellular transformation, promotion, survival, proliferation, invasion, angiogenesis, and metastasis. Curcumin has been known to inhibit the expression of both TNF-α mRNA and TNF-α protein in mantle cell lymphoma cell lines. Suppression of TNF-α by curcumin led to inhibition of NF-κB and cell proliferation, as was the case when TNF-α secretion was neutralized using anti-TNF-α antibody (214). [6]-Gingerol, vanillyl ketone from ginger, has been reported to exhibit a strong antiinflammatory activity and suppress TNF-α production in TPA-treated female ICR mice (215). The chalcone, cardamonin, has been identified as inhibitors of lipopolysaccharide (LPS)-and interferon-gamma-induced TNF-α production in monocytes (216). Similarly, eugenol (217,218), gambogic acid (219), thymoquinone (220,221), zerumbone (222), and xanthohumol (223) also exert inhibitory effects on the expression of TNF-α.
Interleukins
Several inflammatory interleukins including IL-1β and IL-6 have been linked with tumorigenesis, which suggests that inflammation is associated with cancer development. Interleukins are involved in different steps in tumorigenesis. IL-1β promotes growth and confers chemoresistance in pancreatic carcinoma cell lines (224). High levels of IL-1β have a key role in chemoresistant in pancreatic cell lines. IL-1β also affects the production of several angiogenic factors from tumor and stromal cells that promotes tumor growth through hyperneovascularization in lung carcinoma growth in vivo (225). Lewis et al. (226) reported that the elevation of IL-1 was found in human breast, colon, lung, head and neck cancers, and melanomas and correlated to bad prognoses in patients with cancer.
IL-6 has a direct growth stimulatory effect on many tumor cells through the activation of several signaling pathways. By activating Ras/Raf pathway, IL-6 induces proliferation of tumor cells (227,228). IL-6 upregulates the expression of cyclins D1, D2, and B1, and myc by activating STAT3 and thus promotes the cell cycle arrest (229–231). IL-6 also increases the expression of antiapoptotic gene products, including Bcl-2, Bcl-xL, Mcl-1, survivin, and XIAP, and contributes chemoresistance and constitutive activation of STAT-3 that are resistant to chemotherapeutic agents (34). IL-6 also plays an important role in promoting metastasis to organs other than the bone. By activating STAT3, IL-6 promotes the growth of metastatic tumors in the brain by upregulating basic fibroblast growth factor, MMP-2, and VEGF, which contribute to invasion and angiogenesis (16).
A number of studies have shown that the spices-derived nutraceuticals possess anticancer potential through the inhibition of interleukins. Curcumin has been found to suppress the expression of several inflammatory cytokines including IL-1 (232,233). Chan et al. (233) showed that curcumin, at 5 μM, inhibited LPS-induced production of TNF-α and IL-1 in human macrophage cell line Mono Mac 6. Although the effect of cap-saicin on cytokine production is controversial, Kang et al. (234) showed that capsaicin suppressed the mRNA and protein expression of IL-6 from the adipose tissues and adipocytes of obese mice, whereas it enhanced the expression of the adiponectin gene and protein. This inhibitory effect of capsaicin is associated with suppression of NF-κB and/or activation of PPARγ. These results suggest that capsaicin may be a useful phytochemical for attenuating obesity-induced inflammatory responses. Recently, Jung et al. (235) examined the effects of diosgenin on the production of inflammatory mediators in macrophage stimulated by LPS/IFN-γ. The pretreatment with diosgenin resulted in the inhibition of the production of IL-1 and IL-6 but not that of TNF-α. Indeed, the inhibition of these inflammatory mediators appears to be at the transcriptional level, since diosgenin decreased LPS/IFN-γ-induced NF-κB and AP-1 activity. These results indicate that diosgenin may exert its immunosuppressive effects by inhibiting proinflammatory cytokine expression. Murakami et al. (222) found that oral feeding of zerumbone significantly lowered the levels of IL-1β [inhibitory rate (IR) = 34%], TNF-α (IR = 29%), and prostaglandin (PG) E2 (IR = 73%) and suppressed DSS-induced colitis mice model. Overall, the intervention of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6, by using spicy nutraceuticals may be attractable therapeutic strategy to prevent tumor progression and treat cancer.
Proinflammatory Enzymes
Cyclooxygenase (COX)-2
COX-2 is one of the key enzymes implicated in the modulation of inflammation and acts by catalyzing the rate-limiting step that leads to the formation of prostaglandins from arachidonic acid. It is also known to be regulated by NF-κB, which mediates tumorigenesis. COX-2 has been implicated in the growth and progression of a variety of human cancers. Indeed, overexpression of COX-2 has been found in a number of cancers (236). Results from clinical studies have shown that dysregulation of COX-2 is correlated with a poor prognosis (236,237). Enhanced COX-2 expression has been found in colon cancer tissues from subjects with clinically diagnosed colorectal cancer (238). Cyclooxygenase regulates colon carcinoma-induced angiogenesis by modulating the production of angiogenic factors by colon cancer cells (239,240). Indeed, the dysregulation of COX-2 is found in invasive breast cancers, lung adenocarcinoma, and head and neck cancer cells (241–244). So far, the clinical strategy to target COX-2 has been via inhibition of its activity. Non-steroidal inflammatory drugs (NSAIDs) are the first class of inhibitors of COXs available on the market for a variety of diseases. However, intake of NSAIDs for a long period caused severe side effects, such as induced gastrointestinal problems or increased incidence of cardiovascular disease (245,246).
Among the spice-derived nutraceuticals, curcumin has been reported to suppress PG production; it has become clear that this compound plays multiple roles toward COX-2 regulation and directly prevents COX-2 gene expression (247). Ammon et al. (248) showed that curcumin exerts antiinflammatory properties in vivo animal models through inhibition of 5-LOX and 12-LOX activity in rat peritoneal neutrophils and COX activity in human platelets. Curcumin also revealed that this phenolic compound could inhibit chenodeoxycholate- or PMA-induced expression of COX-2 in several gastrointestinal cell lines (249). Treatment with chenodeoxycholate or PMA increased binding of AP-1 to DNA. This effect was also blocked by curcumin, leading to downregulation of COX-2. In addition to the above effects on gene expression, Zhang et al. found that curcumin directly inhibit the activity of COX-2 (249). Capsaicin suppresses the expression of both COX-1 and COX-2 by redox status-dependent regulation, leading to apoptosis in human SK-N-SH human neuroblastoma cells (250). [6]-Gingerol and structurally related pungent principles of ginger exert inhibitory effects on biosynthesis of PGs and leukotrienes through suppression of prostaglandin synthase or 5-LOX (251,252). It has been reported that eugenol is able to modulate COX-2 expression by inhibiting NF-κB pathway in human osteoblast (253). Indeed, eugenol exhibited a significant inhibition of PGE2 production (IC50 = 0.37 microM) and suppression of COX-2 expression in LPS-stimulated mouse macrophage cells (254). Eugenol inhibited the proliferation of HT-29 cells and the mRNA expression of COX-2 but not COX-1. This result suggests that eugenol might be a plausible lead candidate for further developing the COX-2 inhibitor as an antiinflammatory or cancer chemopreventive agent. Other than above compounds, cardamonin (216), DBM (255), gambogic acid (26), thymoquinone (256,257), and zerumbone (222) are known to suppress COX-2 expression or activity, thus have the potential to perturb tumorigenesis.
5-LOX
5-LOX is a key enzyme in the metabolism of arachidonic acid to leukotrienes. Several studies suggest that there is a link between 5-LOX and carcinogenesis in humans and animals. In addition to the important role of leukotrienes as mediators in allergy and inflammation, these intermediates are also linked to pathophysiological events in the brain, including cerebral ischemia, brain edema, and increased permeability of the blood-brain barrier in brain tumors (258). The dysregulation of 5-LOX are also found in process of colonic adenoma formation promoted by cigarette smoke (259). The expression of 5-LOX is also regulated by NF-κB, and it has been linked with the progression and development of cancer of the kidney, breast, and pancreas (260–262). Several phytochemicals known to suppress 5-LOX are curcumin (255) and diosgenin (263). Hong and colleagues (255) showed that curcumin potently inhibited the activity of human recombinant 5-LOX, showing estimated IC50 values of 0.7 μM, respectively. The results suggest that curcumin affects arachidonic acid metabolism, inhibiting the catalytic activities of 5-LOX, and this activity may contribute to the antiinflammatory and anticarcinogenic actions of curcumin and its analogs.
Other Important Targets
Proteasome
The synthesis and degradation of protein is a tightly regulated process that is essential for cellular homeostasis. The degradation of as much as 80% of cellular proteins is regulated by the proteasomes. The latter compose a multicatalytic enzyme complex containing 1 catalytic core, the 20S proteasome, and 2 19S regulatory complexes. The proteolytic activity of the proteasome resides in the 20S proteasomal subunits, β1, β2, and β5, which are responsible for caspase-, trypsin-, and chymotrypsin-like activities, respectively (264). Numerous proteins such as cyclins (265), p53 (266), Bax (267), p27 (268), and the inhibitor of NF-κB (IκBα) (269), which are, involved in carcinogenesis and cancer survival, are known as targets for proteasome. Thus, the inhibition of proteasome leads to accumulation of proapoptotic proteins and induces cell death in cancer cells (270,271).
Cancer cells are also known to be more sensitive to proteasome inhibition than normal cells, indicating the potential role of proteasome inhibitors as anticancer drugs (272). Indeed, a proteasome inhibitor, bortezomib (PS-341, Velcade) was approved by the Food and Drug Administration for the treatment of MM (272). Likewise, curcumin possesses inhibitory effects against proteasome, with its greatest potency being chymotrypsin-like activity (273). Inhibition of the proteasome activity by curcumin was associated with colorectal cancer cell apoptosis in vitro and regression of tumor growth in nude mice (273). Mori et al. (274) reported that capsaicin inhibited TNF-α-stimulated NF-κB activation through suppression of degradation of IκBα by inhibition of proteasome activity in human prostate cancer PC-3 cells. Recently, capsaicin was also reported to cause increased accumulation of ubiquitinated proteins as wells as various target substrates, such as p53, Bax, and p27, thereby inducing cell death in mouse neuro 2a cells (275). Thymoquinone has been shown to possess 20S and 26S proteasome inhibition activity and induce the accumulation of p53 and bax, leading to apoptosis in cancer cells (276). The spice-derived chalcone, xantho-humol, also induced a proapoptotic pathway by its proteasome inhibitory properties and was able to induce endoplasmic reticulum stress in human chronic lymphocytic leukemia cell lines (277).
Epigenetic Changes
The term epigenetic (literally “over” or “upon” genetics) was coined by Conrad Waddington in 1942 and was used to explain why genetic variations sometimes did not lead to phenotypic variations and how genes might interact with their environment to yield a phenotype (278). But the word currently refers specifically to the study of mitotically and/or meiotically heritable changes in gene expression that are not attributable to a change in the DNA sequence. Epigenetic regulation includes DNA methylation, posttranslational histone modifications, and noncoding RNA-mediated silencing pathways. The disruption of such changes underlies a wide variety of pathologies, including cancer (279). Therefore, cancer is a multistep process derived from combinational crosstalk between genetic alterations and epigenetic influences through various environmental factors (280). Epigenetic mechanisms controlling gene transcription are often involved in cell proliferation, differentiation, and survival and are casually linked with tumor development. Alterations in epigenetic processes, including chromatin modifications such as DNA methylation and histone acetylation, are common targets studied in cancer epigenomics (281). DNA methylation usually takes place at the 5′ position of the cytosine ring within CpG dinucleotides, and its consequence is the silencing of genes and noncoding genomic regions. DNA methylation is mediated by a family of DNA methyltransferases (DNMT1–3) and can inhibit gene expression either by promoting the recruitment of methyl-binding domains, which in turn recruit histone-modifying and chromatin-remodeling proteins to the methylation sites, or by directly disrupting the recruitment of DNA-binding transcription factors. The methylation of DNA is generally associated with gene silencing (282). In contrast to DNA methylation, histone modifications are highly complex in terms of both the number of sites that can be modified and in the variety of possible modifications. The enzymes that add and remove such modifications are, respectively, histone acetyltransferases (HATs) and deacetylases (HDACs and sirtuins), methyltransferases and demethylases, kinases and phosphatases, ubiquitin ligases and deubiquitinases, SUMO ligases and proteases, and so on. Finally, these modifications recruit additional transcriptional regulators (283).
Among all the spice-derived nutraceuticals, curcumin has been examined maximally for epigenetic changes (284). Recent evidence has shown that curcumin inhibits DNMT activities and histone modification such as HDAC inhibition in tumorigenesis. Molecular docking of the interaction between curcumin and DNMT1 suggested that curcumin covalently blocks the catalytic thiolate of C1226 of DNMT1 to exert its inhibitory effect. Further, curcumin treatment with extracted genomic DNA from a leukemia cell line induced global hypomethylation (285).
Curcumin has been identified as a strong inhibitor for HATs in both in vitro and in vivo cancer models. Balasubramanyam et al. (286) showed that curcumin is a specific inhibitor of p300/CREB-binding protein (CBP) HAT activity, but not of p300/CBP-associated factor, in vitro and in vivo. Filter binding and gel HAT assays showed that acetylation of histones H3 and H4 by p300/CBP was strongly inhibited covalently by curcumin. Another study demonstrated that curcumin restored ultraviolet radiation-induced hyperacetylation in the promoter region of inflammatory-related genes ATF3, COX2, and MKP1 that are involved in inflammation (287).
Besides curcumin, Chen et al. (288) showed that ursolic acid increased histone H3 acetylation in HL60 cells. These results demonstrated that ursolic acid induces cell death partially through increasing acetylation of histone H3 and inhibition of HDAC activity.
CLINICAL TRIALS
Several clinical trials have been conducted with spice-derived nutraceuticals for prevention and treatment for cancer in human (Table 2).
TABLE 2.
A list of clinical trials with spice-derived nutraceuticals in patients with different cancer
| Cancers | Dose/frequency | Patients | End point modulation | References |
|---|---|---|---|---|
| Curcumin | ||||
| Colorectal cancer | 36–180 mg/day × 120 days | 15 | Decreased lymphocytic GST | (289) |
| Colorectal cancer | 450–3,600 mg/day × 120 days | 15 | Lowered inducible serum PGE2 levels | (290) |
| Colorectal cancer | 450–3,600 mg/day × 7 days | 12 | Decreased M1G DNA adducts | (291) |
| External cancer lesions | 62 | Reduction in lesion size and pain | (292) | |
| FAP | 480 mg × 3/day × 180 days | 5 | Decrease in the number of polyps was 60.4% | (293) |
| Metastatic breast cancer | 500–8,000 mg/day × ≥7 days | 14 | Reached to RECIST criteria (57%) with 5 PR and 3 SD | (294) |
| Multiple myeloma | 2–12 g/day | 24 | Inhibits NF-κ B, COX-2 and pSTAT3 | (295) |
| Pancreatic cancer | 8 g/day | 25 | 73% reduction in tumor in one patient | (296) |
| Pancreatic cancer | 8 g/day | 21 | 8 g/day of curcumin is safe and feasible | (297) |
| Pancreatic cancer | 8 g/day ×3 × 4 wk | 17 | One of 11 patients had PR, 4 had SD, and 6 had tumor progression | (298) |
| Precancerous lesions | 8 g/day × 90 days | 19 | Well tolerated up to 3 mo | (299) |
| Precancerous lesions* | 1 g/day × 7 days | 25 | Increased serum and salivary vitamins C and E | (300) |
| Prostate cancer or PIN** | 100 mg/day × 180 days | 100 | Decreased PSA level | (301) |
| Ginger extracts | ||||
| Bone sarcoma | 57 | Moderate to severe vomiting | (302) | |
| Various primary sites | 1~2 g/day | 162 | No differences in the prevalence of nausea, vomiting, CINV, or severity | (303) |
| Various primary sites | 250 mg × 2/day × 3 days | 28 | Reduced the delayed nausea of chemotherapy, reduced use of antiemetic medications | (304) |
| Capsaicin | ||||
| Pancreatic cancer | 0.075% cream × 56 days | 49 | Reduced pain | (305) |
| Various primary sites | 0.02–0.048 g × 2–4 days | 11 | Reduced pain temporary | (306) |
| Anethole | ||||
| Lung cancer*** | 75 mg/day × 6 mo | 112 | Lowered progression rate | (307) |
Patients with oral leukoplakia, oral submucous fibrosis, or lichen planus.
Combination of curcumin (100 mg) + isoflavone (40 mg).
nethole dithiolethione.
FAP indicates familial adenomatous polyposis; CINV, chemotherapy-induced nausea and vomiting; PR, partial response; SD, stable disease; RECIST, response evaluation criteria in solid tumors.
Clinical Trials With Curcumin
Clinical trials with curcumin have been reported in a several cancers such as oral, vulva, breast, skin, liver, colorectal, pancreas, bladder, and cervical cancer (308).
Colorectal Cancer
Sharma and colleagues (289) studied the pharmacodynamic and pharmacokinetic effect of oral Curcuma extract in patients with advanced colorectal cancer. Fifteen patients with advanced colorectal cancer refractory to standard chemotherapies received Curcuma extract daily for up to 4 mo. The extract was well tolerated, and dose-limiting toxicity was not observed. Neither curcumin nor its metabolites were detected in blood or urine, but curcumin was recovered from feces. Ingestion of 440 mg of Cur-cuma extract for 29 days was accompanied by a 59% decrease in lymphocytic glutathione-S-transferase activity. At higher dose levels, this effect was not observed. Leukocytic M(1)G levels were constant within each patient and unaffected by treatment. Radiologically, stable disease was demonstrated in 5 patients for 2 to 4 mo of treatment. Another Phase I study conducted by the same group (290) showed that a daily dose of 3.6 g curcumin engendered 62% and 57% decreases in inducible PGE2 production in blood samples taken 1 h after dose on days 1 and 29, respectively, in advanced colorectal cancer patients. Garcea et al. (309) conducted a pilot trial with 12 patients having hepatic metastasis from colorectal cancer who received 450–3,600 mg of curcumin daily, for 1 wk prior to surgery, to investigate whether oral administration of curcumin results in concentrations of the agent in normal and malignant human liver tissue sufficient to elicit pharmacological activity. They concluded that doses of curcumin required to furnish hepatic levels sufficient to exert pharmacological activity are probably not feasible in humans. Another dose-escalation pilot study, this one conducted by Plummer et al. (310), showed that a standardized formulation of Curcuma extract in 15 patients with advanced colorectal cancer revealed a dose-dependent inhibition of COX-2 activity, measured as basal and LPS-mediated PGE2 production, in blood revealing the efficacy of curcumin in colorectal cancer.
Familial Adenomatous Polyposis
The clinical trial conducted by Cruz-Correa et al. (293) in patients with familial adenomatous polyposis (FAP) showed that curcumin could reduce adenomas in patient with FAP. Five FAP patients received curcumin (480 mg) and quercetin (20 mg) orally 3 times a day for 6 mo prior to colectomy. The number and size of polyps were assessed at baseline and after therapy. All 5 patients had a decreased polyp number (60.4%) and size (50.9%) from baseline with minimal adverse side effects and no laboratory abnormalities after a mean of 6 mo of treatment with curcumin and quercetin.
Various External and Internal Cancerous Lesions in Different Cancers
An early clinical trial with 62 cancer patients having external cancerous lesions of various sites (breast–37, vulva–4, oral–7, skin–7, and others–11) reported reduction in smell (in 90% patients), reduction in itching (in almost all patients), reduction in lesion size and pain (in 10% patients), and reduction in exudates (in 70% patients) after topical application of an ointment containing curcumin. In this study, an adverse reaction in terms of increased local itching was noticed in only 1 scalp melanoma patient out of the 62 patients evaluated (292).
In a Phase I clinical trial, a daily curcumin dose of 8,000 mg taken orally for 3 mo resulted in histological improvement of precancerous lesions in patients having uterine cervical intraepithelial neoplasm (in 1 out of 4 patients), intestinal metaplasia (in 1 out of 9 patients), bladder cancer (in 1 out of 2 patients), and oral leucoplakia (in 2 out of 7 patients) (299).
Metastatic Breast Cancer
An open-label phase I trial with metastatic breast cancer was conducted to investigate the feasibility and tolerability of the combination of docetaxel and curcumin (294). Fourteen patients were accrued in this open-label phase I trial. Curcumin was well tolerated at maximal tolerated dose, 8 g by mouth daily. Eight patients out of 14 had measurable lesions according to RECIST criteria, with 5 partial responses and 3 stable diseases. Some improvements as biological (decrease in carci-noembryonic antigen tumor marker across the treatment) and clinical responses (regression of nonmeasurable lesions) were observed in most patients. Further investigation is ongoing in a Phase II randomized clinical trial to confirm the efficacy of such a combination in advanced and metastatic breast cancer patients.
Multiple Myeloma
Studies conducted by our group showed that curcumin suppressed constitutive activation of NF-κB, STAT3, and expression of COX-2 in the peripheral blood mononuclear cell (PBMC) from multiple myeloma patients. Curcumin was given at 2, 4, 8, and 12 g/day orally, daily, which was well tolerated with no adverse events. Out of 29 patients, 12 patients continued treatment for 12 wk and 5 completed 1 full yr of treatment with stable disease (295).
Advanced Pancreatic Cancer
The results of phase II clinical trial from our group (296) showed that curcumin inhibited pancreatic cancer in patients. Twenty-five patients were enrolled in this study. Patients received 8 g of curcumin orally daily until disease progression, with restaging every 2 mo. Serum cytokine levels for IL-6, IL-8, IL-10, and IL-1 receptor antagonists and PBMC expression of NF-κ B and COX-2 were monitored. Out of 25 patients, 21 were evaluable for response. Two patients showed clinical biologic activity. One had ongoing stable disease for more than 18 mo; interestingly, 1 additional patient had a brief, but marked, tumor regression of 73%, accompanied by significant increases (4- to 35-fold) in serum cytokine levels. No toxicities were observed. Curcumin suppressed expression of NF-κ B, COX-2, and phosphorylated STAT3 in PBMC from patients (most of whom had baseline levels considerably higher than those found in healthy volunteers). This result suggested oral curcumin was well tolerated and, despite its limited absorption, has biological activity in some patients with pancreatic cancer.
Recently, another clinical trial was conducted to evaluate the safety and feasibility of combination therapy using curcumin with gemcitabine-based chemotherapy (297). Gemcitabine-resistant patients (n = 21) with pancreatic cancer received 8 g oral curcumin daily in combination with gemcitabine-based chemotherapy. No dose-limiting toxicities were observed in the phase I study, and oral curcumin 8 g/day was selected as the recommended dose for the phase II study. Median survival time after initiation of curcumin was 161 days (95% confidence interval 109–223 days) and 1-yr survival rate was 19% (4.4–41.4%). This result indicated that combination therapy using 8 g oral curcumin daily with gemcitabine-based chemotherapy was safe and feasible in patients with pancreatic cancer and its efficacy warranted further investigation.
Prostatic Intraepithelial Neoplasia
Rafailov et al. (311) conducted phase I trial to investigate the effect of Zyflamend, a herbal preparation containing curcumin, against prostatic intraepithelial neoplasia (PIN). After 6 mo the biopsy revealed benign prostatic hyperplasia alone, and at the end of 18 mo biopsy was negative for cancer and PIN, indicating that the patient was free of cancer and PIN.
Clinical Trials With Ginger
Ginger (Zingiber officinale), an age-old spice, is best known for its role as a flavor for Asian and Indian kitchens. For the last 4 centuries, the powdered rhizome of ginger has been used in Indian (Ayurvedic) and traditional Chinese medicine to treat gastrointestinal complaints such as nausea and excessive flatulence. Recently, it has been shown to be effective in reducing experimental induced motion sickness, seasickness, postoperative nausea, and vomiting, and nausea-vomiting of pregnancy (312). Pillai et al. (302) conducted a clinical trial to investigate the effect of ginger against acute chemotherapy-induced nausea and vomiting (CINV) in cancer patients. Acute CINV was defined as nausea and vomiting occurring within 24 h of start of chemotherapy (days 1–4) and delayed CINV as that occurring after 24 h of completion of chemotherapy (days 5–10). They found that ginger root powder was effective in reducing the severity of acute and delayed CINV as additional therapy to ondansetron and dexamethasone in patients receiving high emetogenic chemotherapy. Another study conducted by Livine et al. (304) demonstrated that ginger with high-protein meals reduced the delayed nausea of chemotherapy and reduced the need for antiemetic medications. However, in a phase II, randomized, placebo-controlled clinical trial, the efficacy of ginger for chemotherapy-associated nausea in patients with cancer was evaluated: The addition of ginger to currently recommended antiemetic regimens did not improve the CINV (303,312,313). These results suggest that the use of ginger for reduced acute CINV require additional study before further clinical trials are conducted.
Clinical Trials With Capsaicin
Capsaicin (trans-8-N-vanillyl-6-nonenamide) is the primary irritant in Capsicum fruits, both green and red peppers, that are widely used as spices. Recently, the structural analogues of capsaicin, such as capsiate and dihydrocapsiate, the nonirritating capsinoids from CH-19 sweet pepper, possess the anticancer as well as chemopreventive activity to capsaicin but without the pungent property (314). These analogues might be considered replacements for capsaicin with potential in the prevention of cancer.
There has been a turning point on the question of using capsaicin as a pain relief. One pilot study examined the ability of oral capsaicin to provide temporary relief of oral mucositis pain in patients with cancer. Oral capsaicin in a candy (taffy) vehicle that produced substantial pain reduction in 11 patients with oral mucositis pain from cancer therapy. However, this pain relief was not complete for most patients and was only temporary. Results from this study suggested that an additional research would be required to fully utilize the properties of capsaicin desensitization and thus optimize analgesia (306).
Ellison and colleagues (305) performed a clinical trial to examine the efficacy of capsaicin cream (0.075%) for management of surgical neuropathic pain in cancer patients. Ninety-nine assessable cancer patients participated in this placebo-controlled trial. After stratification, patients received treatment with 0.075% capsaicin cream for 8 wk followed by 8 wk of an identical-appearing placebo cream or vice versa. A capsaicin/placebo cream was to be applied to the site with pain, 4 times daily. The results showed that the capsaicin cream arm had substantially more pain relief (P = 0.01) after the first 8 wk, with an average pain reduction of 53% vs. 17%. After completion of the 16-wk study, patients were asked which treatment period was most beneficial. The 60% of the responding patients chose the capsaicin arm, 18% chose the placebo arm, and the rest chose neither. The results indicated that topical capsaicin cream reduced postsurgical neuropathic pain and, despite some toxicity, was preferred by patients over a placebo. Taken together the proof of a practically safe dose of capsaicin should be established before further clinical trials.
Clinical Trials With Anethole
A trial reported by Lam et al. (307) investigated the effects of anethole dithiolethione (ADT) in smokers with bronchial dysplasia. ADT belongs to the dithiolethione class of organosulfur compounds, which have antioxidant, chemotherapeutic, radioprotective, and chemopreventive properties. In the lesion-specific analysis, the progression rate of preexisting dysplastic lesions by 2 or more grades and/or the appearance of new lesions was statistically significantly lower in the ADT group (8%) than in the placebo group (17%) (P < 0.001). This study suggests that ADT may exhibit chemopreventive potential for lung cancer control.
LIMITATIONS AND ADVERSE EFFECTS OF SPICE
The majority of the literature from in vitro and in vivo studies suggest that spice possesses potent chemopreventive activities. However, there are reports that some spices or their components exhibit harmful effects in some people. For instance, turmeric-induced skin allergies have been reported among workers in turmeric factories. Cases of allergic contact dermatitis and contact urticaria from curcumin mediated by 2 different mechanisms have been reported (315). There are also similar reports with ginger, nutmeg, and oregano (316).
Although the anticancer potential of capsaicin was well accepted, its beneficial effect in cancer patients is controversial. An early Italian case-control study revealed that chili consumption was protective against stomach cancer (11), whereas epidemiologic study from Mexico City showed that chili consumers were at greater risk of developing stomach cancer than nonconsumers (317). Indeed, there is evidence that capsaicin or chili extracts may also act as cocarcinogen or tumor promoter (318). There is not enough evidence documenting whether or not smaller amounts of a greater variety of spices, known to have similar health effects, would result in comparable health benefits. However, it is an area requiring further study.
CONCLUSIONS
Although the earliest evidence of the use of spice by humans was around 5,000 B.C., their biological activities have been investigated last several decades. Today, considerable data regarding the beneficial effect of spice nutraceuticals on prevention and treatment of cancer in preclinical settings are available. However, the translation of the in vitro efficacy of nutraceuticals in the prevention of cancer to clinical use requires attention to establishing physiologically relevant concentrations and chronic exposures to imitate in vivo conditions. For optimizing the desired physiological response, further consideration should be given to the significant intake of nutraceuticals, their duration, and their validation in suitable animal models. A huge amount of data from in vivo settings using experimental rodents supports the anticancer potential of spice-derived nutraceuticals. However, clinical trials or further studies with these nutraceuticals must be done in order to elucidate and understand their efficacy in the treatment and prevention of human cancer. There is also a potential that these spices, when consumed in combination, exhibit synergistic potential. This is clearly the case when turmeric is combined with black pepper. This needs to be further explored. Spices should be viewed as a part of an everyday lifestyle rather than as pharmaceuticals. In addition, although over 100 pilot clinical trials have been done with spices and their components in human subjects, more evidence is still needed to demonstrate their potential in prevention and treatment of chronic inflammatory disease, including cancer. The possibility for spice-derived nutraceuticals to exhibit multiple effects and for ability to counteract treatment regimens exists, just as the potential of a once-unknown world was first shown by the explorers Christopher Columbus and Vasco da Gama in their search for valuable spices several centuries before.
Acknowledgments
We would like to thank Walter Pagel for carefully proofreading the manuscript and providing valuable comments. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was partly supported by a grant from a core grant from the National Institutes of Health (CA-16672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and a grant from the Center for Targeted Therapy of MD Anderson Cancer Center.
References
- 1.Kamb A, Wee S, Lengauer C. Why is cancer drug discovery so difficult? Nat Rev Drug Discov. 2007;6:115–120. doi: 10.1038/nrd2155. [DOI] [PubMed] [Google Scholar]
- 2.Knowles J, Gromo G. A guide to drug discovery: target selection in drug discovery. Nat Rev Drug Discov. 2003;2:63–69. doi: 10.1038/nrd986. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S. Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem. 2007;102:580–592. doi: 10.1002/jcb.21500. [DOI] [PubMed] [Google Scholar]
- 4.Fojo T, Parkinson DR. Biologically targeted cancer therapy and marginal benefits: are we making too much of too little or are we achieving too little by giving too much? Clin Cancer Res. 2010;16:5972–5980. doi: 10.1158/1078-0432.CCR-10-1277. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal BB, Danda D, Gupta S, Gehlot P. Models for prevention and treatment of cancer: problems vs. promises. Biochem Pharmacol. 2009;78:1083–1094. doi: 10.1016/j.bcp.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand P, Kunnumakkara AB, Sundaram C, Harikumar KB, Tharakan ST, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James MA, Lee JH, Klingelhutz AJ. Human papillomavirus type 16 E6 activates NF-kappaB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J Virol. 2006;80:5301–5307. doi: 10.1128/JVI.01942-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLuca C, Roulston A, Koromilas A, Wainberg MA, Hiscott J. Chronic human immunodeficiency virus type 1 infection of myeloid cells disrupts the autoregulatory control of the NF-kappaB/Rel pathway via enhanced IkappaBalpha degradation. J Virol. 1996;70:5183–5193. doi: 10.1128/jvi.70.8.5183-5193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 10.Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. American Institute for Cancer Research; Washington, DC: 2007. [Google Scholar]
- 11.Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, et al. A case-control study of gastric cancer and diet in Italy. Int J Cancer. 1989;44:611–616. doi: 10.1002/ijc.2910440409. [DOI] [PubMed] [Google Scholar]
- 12.Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. 2008;19:347–361. doi: 10.1016/j.jnutbio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-kappaB addiction and its role in cancer: “one size does not fit all. Oncogene. 2011;30:1615–1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal BB, Van Kuiken ME, Iyer LH, Harikumar KB, Sung B. Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp Biol Med (Maywood) 2009;234:825–849. doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews CP, Colburn NH, Young MR. AP-1 a target for cancer prevention. Curr Cancer Drug Targets. 2007;7:317–324. doi: 10.2174/156800907780809723. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, et al. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann NY Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 17.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47:921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 18.Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: from bench to bedside. Exp Biol Med (Maywood) 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 19.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, et al. Curcumin (diferuloylmethane) downregulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 21.Chainy GB, Manna SK, Chaturvedi MM, Aggarwal BB. Anethole blocks both early and late cellular responses transduced by tumor necrosis factor: effect on NF-kappaB, AP-1, JNK, MAPKK and apoptosis. Oncogene. 2000;19:2943–2950. doi: 10.1038/sj.onc.1203614. [DOI] [PubMed] [Google Scholar]
- 22.Singh S, Natarajan K, Aggarwal BB. Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is a potent inhibitor of nuclear transcription factor-kappa B activation by diverse agents. J Immunol. 1996;157:4412–4420. [PubMed] [Google Scholar]
- 23.Israf DA, Khaizurin TA, Syahida A, Lajis NH, Khozirah S. Car-damonin inhibits COX and iNOS expression via inhibition of p65NF-kappaB nuclear translocation and Ikappa-B phosphorylation in RAW 264.7 macrophage cells. Mol Immunol. 2007;44:673–679. doi: 10.1016/j.molimm.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Murakami R, Uchida M, Hori O, Matsuura N, Choshi T, et al. Efficacy of dibenzoylmethane derivatives in protecting against endoplasmic reticulum stress and inhibiting nuclear factor kappa b on dextran sulfate sodium induced colitis in mice. Biol Pharm Bull. 2010;33:2029–2032. doi: 10.1248/bpb.33.2029. [DOI] [PubMed] [Google Scholar]
- 25.Shishodia S, Aggarwal BB. Diosgenin inhibits osteoclastogenesis, invasion, and proliferation through the downregulation of Akt, I kappa B kinase activation and NF-kappa B-regulated gene expression. Oncogene. 2006;25:1463–1473. doi: 10.1038/sj.onc.1209194. [DOI] [PubMed] [Google Scholar]
- 26.Pandey MK, Sung B, Ahn KS, Kunnumakkara AB, Chaturvedi MM, et al. Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway. Blood. 2007;110:3517–3525. doi: 10.1182/blood-2007-03-079616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SO, Chun KS, Kundu JK, Surh YJ. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kappaB and p38 MAPK in mouse skin. Biofactors. 2004;21:27–31. doi: 10.1002/biof.552210107. [DOI] [PubMed] [Google Scholar]
- 28.Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-kappa B activation pathway by thymoquinone: role in suppression of antiapop-totic gene products and enhancement of apoptosis. Mol Cancer Res. 2008;6:1059–1070. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- 29.Harikumar KB, Kunnumakkara AB, Ahn KS, Anand P, Krishnan S, et al. Modification of the cysteine residues in IkappaBalpha kinase and NF-kappaB (p65) by xanthohumol leads to suppression of NF-kappaB-regulated gene products and potentiation of apoptosis in leukemia cells. Blood. 2009;113:2003–2013. doi: 10.1182/blood-2008-04-151944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with downregulation of cyclooxygenase 2, matrix metallopro-teinase 9, and cyclin D1. Cancer Res. 2003;63:4375–4383. [PubMed] [Google Scholar]
- 31.Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24:6957–6969. doi: 10.1038/sj.onc.1208845. [DOI] [PubMed] [Google Scholar]
- 32.Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, et al. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 33.Zhong Z, Wen Z, Darnell JE., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, et al. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann NY Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 36.Bharti AC, Shishodia S, Reuben JM, Weber D, Alexanian R, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–3184. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 37.Weissenberger J, Priester M, Bernreuther C, Rakel S, Glatzel M, et al. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res. 2010;16:5781–5795. doi: 10.1158/1078-0432.CCR-10-0446. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C, Li B, Zhang X, Hazarika P, Aggarwal BB, et al. Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients’ PBMCs: potential role for STAT-3 and NF-kappaB signaling. J Invest Dermatol. 2010;130:2110–2119. doi: 10.1038/jid.2010.86. [DOI] [PubMed] [Google Scholar]
- 39.Mackenzie GG, Queisser N, Wolfson ML, Fraga CG, Adamo AM, et al. Curcumin induces cell-arrest and apoptosis in association with the inhibition of constitutively active NF-kappaB and STAT3 pathways in Hodgkin’s lymphoma cells. Int J Cancer. 2008;123:56–65. doi: 10.1002/ijc.23477. [DOI] [PubMed] [Google Scholar]
- 40.Rajasingh J, Raikwar HP, Muthian G, Johnson C, Bright JJ. Curcumin induces growth-arrest and apoptosis in association with the inhibition of constitutively active JAK-STAT pathway in T cell leukemia. Biochem Biophys Res Comm. 2006;340:359–368. doi: 10.1016/j.bbrc.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Saydmohammed M, Joseph D, Syed V. Curcumin suppresses constitutive activation of STAT-3 by upregulating protein inhibitor of activated STAT-3 (PIAS-3) in ovarian and endometrial cancer cells. J Cell Biochem. 2010;110:447–456. doi: 10.1002/jcb.22558. [DOI] [PubMed] [Google Scholar]
- 42.Chakravarti N, Myers JN, Aggarwal BB. Targeting constitutive and interleukin-6-inducible signal transducers and activators of transcription 3 pathway in head and neck squamous cell carcinoma cells by curcumin (diferuloylmethane) Int J Cancer. 2006;119:1268–1275. doi: 10.1002/ijc.21967. [DOI] [PubMed] [Google Scholar]
- 43.Bhutani M, Pathak AK, Nair AS, Kunnumakkara AB, Guha S, et al. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res. 2007;13:3024–3032. doi: 10.1158/1078-0432.CCR-06-2575. [DOI] [PubMed] [Google Scholar]
- 44.Lee HK, Seo IA, Shin YK, Park JW, Suh DJ, et al. Capsaicin inhibits the IL-6/STAT3 pathway by depleting intracellular gp130 pools through endoplasmic reticulum stress. Biochem Biophys Res Comm. 2009;382:445–450. doi: 10.1016/j.bbrc.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Fernandez PP, Rajendran P, Hui KM, Sethi G. Diosgenin, a steroidal saponin, inhibits STAT3 signaling pathway leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cells. Cancer Lett. 2010;292:197–207. doi: 10.1016/j.canlet.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Li F, Rajendran P, Sethi G. Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br J Pharmacol. 2010;161:541–554. doi: 10.1111/j.1476-5381.2010.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pathak AK, Bhutani M, Nair AS, Ahn KS, Chakraborty A, et al. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5:943–955. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 48.Schmitt-Ney M, Happ B, Ball RK, Groner B. Developmental and environmental regulation of a mammary gland-specific nuclear factor essential for transcription of the gene encoding beta-casein. Proc Natl Acad Sci USA. 1992;89:3130–3134. doi: 10.1073/pnas.89.7.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakao H, Gouilleux F, Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994;13:2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin JX, Leonard WJ. The role of Stat5a and Stat5b in signaling by IL-2 family cytokines. Oncogene. 2000;19:2566–2576. doi: 10.1038/sj.onc.1203523. [DOI] [PubMed] [Google Scholar]
- 51.Liao Z, Lutz J, Nevalainen MT. Transcription factor Stat5a/b as a therapeutic target protein for prostate cancer. Int J Biochem Cell Biol. 2010;42:186–192. doi: 10.1016/j.biocel.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 53.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 54.Huang M, Dorsey JF, Epling-Burnette PK, Nimmanapalli R, Landowski TH, et al. Inhibition of Bcr-Abl kinase activity by PD180970 blocks constitutive activation of Stat5 and growth of CML cells. Oncogene. 2002;21:8804–8816. doi: 10.1038/sj.onc.1206028. [DOI] [PubMed] [Google Scholar]
- 55.Levis M, Allebach J, Tse KF, Zheng R, Baldwin BR, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99:3885–3891. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 56.Li H, Ahonen TJ, Alanen K, Xie J, LeBaron MJ, et al. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 2004;64:4774–4782. doi: 10.1158/0008-5472.CAN-03-3499. [DOI] [PubMed] [Google Scholar]
- 57.Vafaizadeh V, Klemmt P, Brendel C, Weber K, Doebele C, et al. Mammary epithelial reconstitution with gene-modified stem cells assigns roles to Stat5 in luminal alveolar cell fate decisions, differentiation, involution, and mammary tumor formation. Stem Cells. 2010;28:928–938. doi: 10.1002/stem.407. [DOI] [PubMed] [Google Scholar]
- 58.Blasius R, Reuter S, Henry E, Dicato M, Diederich M. Curcumin regulates signal transducer and activator of transcription (STAT) expression in K562 cells. Biochem Pharmacol. 2006;72:1547–1554. doi: 10.1016/j.bcp.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 59.Bernstein LR, Colburn NH. AP1/jun function is differentially induced in promotion-sensitive and resistant JB6 cells. Science. 1989;244:566–569. doi: 10.1126/science.2541502. [DOI] [PubMed] [Google Scholar]
- 60.Brown PH, Alani R, Preis LH, Szabo E, Birrer MJ. Suppression of oncogene-induced transformation by a deletion mutant of c-jun. Oncogene. 1993;8:877–886. [PubMed] [Google Scholar]
- 61.Dong Z, Birrer MJ, Watts RG, Matrisian LM, Colburn NH. Blocking of tumor promoter-induced AP-1 activity inhibits induced transformation in JB6 mouse epidermal cells. Proc Natl Acad Sci USA. 1994;91:609–613. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 63.Young MR, Yang HS, Colburn NH. Promising molecular targets for cancer prevention: AP-1, NF-kappa B, and Pdcd4. Trends Mol Med. 2003;9:36–41. doi: 10.1016/s1471-4914(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 64.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 65.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 66.Han SS, Keum YS, Seo HJ, Surh YJ. Curcumin suppresses activation of NF-kappaB and AP-1 induced by phorbol ester in cultured human promyelocytic leukemia cells. J Biochem Mol Biol. 2002;35:337–342. doi: 10.5483/bmbrep.2002.35.3.337. [DOI] [PubMed] [Google Scholar]
- 67.Hergenhahn M, Soto U, Weninger A, Polack A, Hsu CH, et al. The chemopreventive compound curcumin is an efficient inhibitor of Epstein-Barr virus BZLF1 transcription in Raji DR-LUC cells. Mol Carcinog. 2002;33:137–145. doi: 10.1002/mc.10029. [DOI] [PubMed] [Google Scholar]
- 68.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–7609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 69.Ichiki K, Mitani N, Doki Y, Hara H, Misaki T, et al. Regulation of activator protein-1 activity in the mediastinal lymph node metastasis of lung cancer. Clin Exp Metastasis. 2000;18:539–545. doi: 10.1023/a:1011980313237. [DOI] [PubMed] [Google Scholar]
- 70.Cha HJ, Park MT, Chung HY, Kim ND, Sato H, et al. Ursolic acid-induced down-regulation of MMP-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cells. Oncogene. 1998;16:771–778. doi: 10.1038/sj.onc.1201587. [DOI] [PubMed] [Google Scholar]
- 71.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, et al. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 72.Khor TO, Huang MT, Prawan A, Liu Y, Hao X, et al. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev Res (Phila) 2008;1:187–191. doi: 10.1158/1940-6207.CAPR-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu C, Huang MT, Shen G, Yuan X, Lin W, et al. Inhibition of 7,12-dimethylbenz(a)anthracene-induced skin tumorigenesis in C57BL/6 mice by sulforaphane is mediated by nuclear factor E2-related factor 2. Cancer Res. 2006;66:8293–8296. doi: 10.1158/0008-5472.CAN-06-0300. [DOI] [PubMed] [Google Scholar]
- 74.Aoki Y, Sato H, Nishimura N, Takahashi S, Itoh K, et al. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl Pharmacol. 2001;173:154–160. doi: 10.1006/taap.2001.9176. [DOI] [PubMed] [Google Scholar]
- 75.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA. 2001;98:3410–3415. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, et al. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- 77.Kitamura Y, Umemura T, Kanki K, Kodama Y, Kitamoto S, et al. Increased susceptibility to hepatocarcinogenicity of Nrf2-deficient mice exposed to 2-amino-3-methylimidazo[4,5-f]quinoline. Cancer Sci. 2007;98:19–24. doi: 10.1111/j.1349-7006.2006.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 79.Nioi P, Hayes JD. Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix-loop-helix transcription factors. Mutat Res. 2004;555:149–171. doi: 10.1016/j.mrfmmm.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 80.Shibata T, Kokubu A, Gotoh M, Ojima H, Ohta T, et al. Genetic alteration of Keap1 confers constitutive Nrf2 activation and resistance to chemotherapy in gallbladder cancer. Gastroenterology. 2008;135:1358–1368. e1351–e1354. doi: 10.1053/j.gastro.2008.06.082. [DOI] [PubMed] [Google Scholar]
- 81.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, et al. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci USA. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 83.Garg R, Maru G. Dietary curcumin enhances benzo(a)pyrene-induced apoptosis resulting in a decrease in BPDE-DNA adducts in mice. J Environ Pathol Toxicol Oncol. 2009;28:121–131. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i2.40. [DOI] [PubMed] [Google Scholar]
- 84.Thimmulappa RK, Rangasamy T, Alam J, Biswal S. Dibenzoyl-methane activates Nrf2-dependent detoxification pathway and inhibits benzo(a)pyrene induced DNA adducts in lungs. Med Chem. 2008;4:473–481. doi: 10.2174/157340608785700199. [DOI] [PubMed] [Google Scholar]
- 85.Cheung KL, Khor TO, Huang MT, Kong AN. Differential in vivo mechanism of chemoprevention of tumor formation in azoxymethane/dextran sodium sulfate mice by PEITC and DBM. Carcinogenesis. 2010;31:880–885. doi: 10.1093/carcin/bgp285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee IS, Lim J, Gal J, Kang JC, Kim HJ, et al. Antiinflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem Int. 2011;58:153–160. doi: 10.1016/j.neuint.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 87.Luo Y, Eggler AL, Liu D, Liu G, Mesecar AD, et al. Sites of alkylation of human Keap1 by natural chemoprevention agents. J Am Soc Mass Spectrom. 2007;18:2226–2232. doi: 10.1016/j.jasms.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joung EJ, Li MH, Lee HG, Somparn N, Jung YS, et al. Capsaicin induces heme oxygenase-1 expression in HepG2 cells via activation of PI3K-Nrf2 signaling: NAD(P)H:quinone oxidoreductase as a potential target. Antioxid Redox Signal. 2007;9:2087–2098. doi: 10.1089/ars.2007.1827. [DOI] [PubMed] [Google Scholar]
- 89.Evans RM. The nuclear receptor superfamily: a Rosetta stone for physiology. Mol Endocrinol. 2005;19:1429–1438. doi: 10.1210/me.2005-0046. [DOI] [PubMed] [Google Scholar]
- 90.Grommes C, Landreth GE, Heneka MT. Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. Lancet Oncol. 2004;5:419–429. doi: 10.1016/S1470-2045(04)01509-8. [DOI] [PubMed] [Google Scholar]
- 91.Nahle Z. PPAR trilogy from metabolism to cancer. Curr Opin Clin Nutr Metab Care. 2004;7:397–402. doi: 10.1097/01.mco.0000134360.30911.bb. [DOI] [PubMed] [Google Scholar]
- 92.Panigrahy D, Huang S, Kieran MW, Kaipainen A. PPARgamma as a therapeutic target for tumor angiogenesis and metastasis. Cancer Biol Ther. 2005;4:687–693. doi: 10.4161/cbt.4.7.2014. [DOI] [PubMed] [Google Scholar]
- 93.Altiok S, Xu M, Spiegelman BM. PPARgamma induces cell cycle withdrawal: inhibition of E2F/DP DNA-binding activity via downregulation of PP2A. Genes Dev. 1997;11:1987–1998. doi: 10.1101/gad.11.15.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qin C, Morrow D, Stewart J, Spencer K, Porter W, et al. A new class of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists that inhibit growth of breast cancer cells: 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes. Mol Cancer Ther. 2004;3:247–260. [PubMed] [Google Scholar]
- 95.Sato H, Ishihara S, Kawashima K, Moriyama N, Suetsugu H, et al. Expression of peroxisome proliferator-activated receptor (PPAR)gamma in gastric cancer and inhibitory effects of PPARgamma agonists. Br J Cancer. 2000;83:1394–1400. doi: 10.1054/bjoc.2000.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshimura R, Matsuyama M, Segawa Y, Hase T, Mitsuhashi M, et al. Expression of peroxisome proliferator-activated receptors (PPARs) in human urinary bladder carcinoma and growth inhibition by its agonists. Int J Cancer. 2003;104:597–602. doi: 10.1002/ijc.10980. [DOI] [PubMed] [Google Scholar]
- 97.Hamakawa H, Nakashiro K, Sumida T, Shintani S, Myers JN, et al. Basic evidence of molecular targeted therapy for oral cancer and salivary gland cancer. Head Neck. 2008;30:800–809. doi: 10.1002/hed.20830. [DOI] [PubMed] [Google Scholar]
- 98.Kroll TG, Sarraf P, Pecciarini L, Chen CJ, Mueller E, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected] Science. 2000;289:1357–1360. doi: 10.1126/science.289.5483.1357. [DOI] [PubMed] [Google Scholar]
- 99.Sarraf P, Mueller E, Smith WM, Wright HM, Kum JB, et al. Loss-of-function mutations in PPAR gamma associated with human colon cancer. Mol Cell. 1999;3:799–804. doi: 10.1016/s1097-2765(01)80012-5. [DOI] [PubMed] [Google Scholar]
- 100.Khuri FR, Lee JJ, Lippman SM, Kim ES, Cooper JS, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 101.O’Shaughnessy JA, Kelloff GJ, Gordon GB, Dannenberg AJ, Hong WK, et al. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8:314–346. [PubMed] [Google Scholar]
- 102.Chen A, Xu J. Activation of PPAR{gamma} by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am J Physiol Gastrointest Liver Physiol. 2005;288:G447–456. doi: 10.1152/ajpgi.00209.2004. [DOI] [PubMed] [Google Scholar]
- 103.Kim CS, Park WH, Park JY, Kang JH, Kim MO, et al. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor gamma in HT-29 human colon cancer cells. J Med Food. 2004;7:267–273. doi: 10.1089/jmf.2004.7.267. [DOI] [PubMed] [Google Scholar]
- 104.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 105.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 106.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol. 2009;129:1614–1627. doi: 10.1038/jid.2008.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 108.Ryu MJ, Cho M, Song JY, Yun YS, Choi IW, et al. Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem Biophys Res Comm. 2008;377:1304–1308. doi: 10.1016/j.bbrc.2008.10.171. [DOI] [PubMed] [Google Scholar]
- 109.Prasad CP, Rath G, Mathur S, Bhatnagar D, Ralhan R. Potent growth suppressive activity of curcumin in human breast cancer cells: modulation of Wnt/beta-catenin signaling. Chem Biol Interact. 2009;181:263–271. doi: 10.1016/j.cbi.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 110.Kakarala M, Brenner DE, Korkaya H, Cheng C, Tazi K, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res Treat. 2010;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Choi YH, Baek JH, Yoo MA, Chung HY, Kim ND, et al. Induction of apoptosis by ursolic acid through activation of caspases and down-regulation of c-IAPs in human prostate epithelial cells. Int J Oncol. 2000;17:565–571. [PubMed] [Google Scholar]
- 112.Vanhoecke B, Derycke L, Van Marck V, Depypere H, De Keukeleire D, et al. Antiinvasive effect of xanthohumol, a prenylated chalcone present in hops (Humulus lupulus L.) and beer. Int J Cancer. 2005;117:889–895. doi: 10.1002/ijc.21249. [DOI] [PubMed] [Google Scholar]
- 113.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 114.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 115.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 116.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 118.Wang LH, Choi YL, Hua XY, Shin YK, Song YJ, et al. Increased expression of sonic hedgehog and altered methylation of its promoter region in gastric cancer and its related lesions. Mod Pathol. 2006;19:675–683. doi: 10.1038/modpathol.3800573. [DOI] [PubMed] [Google Scholar]
- 119.Fukaya M, Isohata N, Ohta H, Aoyagi K, Ochiya T, et al. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. Gastroenterology. 2006;131:14–29. doi: 10.1053/j.gastro.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 120.Elamin MH, Shinwari Z, Hendrayani SF, Al-Hindi H, Al-Shail E, et al. Curcumin inhibits the sonic hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol Carcinog. 2010;49:302–314. doi: 10.1002/mc.20604. [DOI] [PubMed] [Google Scholar]
- 121.Hosoya T, Arai MA, Koyano T, Kowithayakorn T, Ishibashi M. Naturally occurring small-molecule inhibitors of hedgehog/GLI-mediated transcription. Chembiochem. 2008;9:1082–1092. doi: 10.1002/cbic.200700511. [DOI] [PubMed] [Google Scholar]
- 122.Gregory H. Isolation and structure of urogastrone and its relationship to epidermal growth factor. Nature. 1975;257:325–327. doi: 10.1038/257325a0. [DOI] [PubMed] [Google Scholar]
- 123.Carpenter G, Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- 124.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184–188. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sporn MB, Todaro GJ. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980;303:878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- 126.Korutla L, Cheung JY, Mendelsohn J, Kumar R. Inhibition of ligand-induced activation of epidermal growth factor receptor tyrosine phosphorylation by curcumin. Carcinogenesis. 1995;16:1741–1745. doi: 10.1093/carcin/16.8.1741. [DOI] [PubMed] [Google Scholar]
- 127.Squires MS, Hudson EA, Howells L, Sale S, Houghton CE, et al. Relevance of mitogen activated protein kinase (MAPK) and phosphotidylinositol-3-kinase/protein kinase B (PI3K/PKB) pathways to induction of apoptosis by curcumin in breast cells. Biochem Pharmacol. 2003;65:361–376. doi: 10.1016/s0006-2952(02)01517-4. [DOI] [PubMed] [Google Scholar]
- 128.Kim JH, Xu C, Keum YS, Reddy B, Conney A, et al. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with beta-phenylethyl isothiocyanate and curcumin. Carcinogenesis. 2006;27:475–482. doi: 10.1093/carcin/bgi272. [DOI] [PubMed] [Google Scholar]
- 129.Lev-Ari S, Starr A, Vexler A, Karaush V, Loew V, et al. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, downregulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006;26:4423–4430. [PubMed] [Google Scholar]
- 130.Khafif A, Lev-Ari S, Vexler A, Barnea I, Starr A, et al. Curcumin: a potential radio-enhancer in head and neck cancer. Laryngoscope. 2009;119:2019–2026. doi: 10.1002/lary.20582. [DOI] [PubMed] [Google Scholar]
- 131.Shan JZ, Xuan YY, Zheng S, Dong Q, Zhang SZ. Ursolic acid inhibits proliferation and induces apoptosis of HT-29 colon cancer cells by inhibiting the EGFR/MAPK pathway. J Zhejiang Univ Sci B. 2009;10:668–674. doi: 10.1631/jzus.B0920149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thoennissen NH, O’Kelly J, Lu D, Iwanski GB, La DT, et al. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene. 2010;29:285–296. doi: 10.1038/onc.2009.335. [DOI] [PubMed] [Google Scholar]
- 133.Lee EJ, Jeon MS, Kim BD, Kim JH, Kwon YG, et al. Capsiate inhibits ultraviolet B-induced skin inflammation by inhibiting Src family kinases and epidermal growth factor receptor signaling. Free Radic Biol Med. 2010;48:1133–1143. doi: 10.1016/j.freeradbiomed.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 134.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 135.Gullick WJ. Update on HER-2 as a target for cancer therapy: alternative strategies for targeting the epidermal growth factor system in cancer. Breast Cancer Res. 2001;3:390–394. doi: 10.1186/bcr328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, et al. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- 137.Hong RL, Spohn WH, Hung MC. Curcumin inhibits tyrosine kinase activity of p185neu and also depletes p185neu. Clin Cancer Res. 1999;5:1884–1891. [PubMed] [Google Scholar]
- 138.Jung Y, Xu W, Kim H, Ha N, Neckers L. Curcumin-induced degradation of ErbB2: A role for the E3 ubiquitin ligase CHIP and the Michael reaction acceptor activity of curcumin. Biochim Biophys Acta. 2007;1773:383–390. doi: 10.1016/j.bbamcr.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 139.Cai XZ, Wang J, Li XD, Wang GL, Liu FN, et al. Curcumin suppresses proliferation and invasion in human gastric cancer cells by downregulation of PAK1 activity and cyclin D1 expression. Cancer Biol Ther. 2009;8:1360–1368. doi: 10.4161/cbt.8.14.8720. [DOI] [PubMed] [Google Scholar]
- 140.Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Comm. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 141.Pajusola K, Aprelikova O, Korhonen J, Kaipainen A, Pertovaara L, et al. FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res. 1992;52:5738–5743. [PubMed] [Google Scholar]
- 142.Barleon B, Siemeister G, Martiny-Baron G, Weindel K, Herzog C, et al. Vascular endothelial growth factor upregulates its receptor fms-like tyrosine kinase 1 (FLT-1) and a soluble variant of FLT-1 in human vascular endothelial cells. Cancer Res. 1997;57:5421–5425. [PubMed] [Google Scholar]
- 143.Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, et al. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem Biophys Res Comm. 2005;335:300–308. doi: 10.1016/j.bbrc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- 144.Yi T, Yi Z, Cho SG, Luo J, Pandey MK, et al. Gambogic acid inhibits angiogenesis and prostate tumor growth by suppressing vascular endothelial growth factor receptor 2 signaling. Cancer Res. 2008;68:1843–1850. doi: 10.1158/0008-5472.CAN-07-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Casa AJ, Dearth RK, Litzenburger BC, Lee AV, Cui X. The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front Biosci. 2008;13:3273–3287. doi: 10.2741/2925. [DOI] [PubMed] [Google Scholar]
- 146.Kim SY, Toretsky JA, Scher D, Helman LJ. The role of IGF-1R in pediatric malignancies. Oncologist. 2009;14:83–91. doi: 10.1634/theoncologist.2008-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 148.Allen NE, Roddam AW, Allen DS, Fentiman IS, Dos Santos Silva I, et al. A prospective study of serum insulin-like growth factor-I (IGF-I), IGF-II, IGF-binding protein-3 and breast cancer risk. Br J Cancer. 2005;92:1283–1287. doi: 10.1038/sj.bjc.6602471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stattin P, Bylund A, Rinaldi S, Biessy C, Dechaud H, et al. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst. 2000;92:1910–1917. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- 150.Yu H, Spitz MR, Mistry J, Gu J, Hong WK, et al. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst. 1999;91:151–156. doi: 10.1093/jnci/91.2.151. [DOI] [PubMed] [Google Scholar]
- 151.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 152.Zhao H, Grossman HB, Spitz MR, Lerner SP, Zhang K, et al. Plasma levels of insulin-like growth factor-1 and binding protein-3, and their association with bladder cancer risk. J Urol. 2003;169:714–717. doi: 10.1097/01.ju.0000036380.10325.2a. [DOI] [PubMed] [Google Scholar]
- 153.Xia Y, Jin L, Zhang B, Xue H, Li Q, et al. The potentiation of curcumin on insulin-like growth factor-1 action in MCF-7 human breast carcinoma cells. Life Sci. 2007;80:2161–2169. doi: 10.1016/j.lfs.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 154.Heldin CH, Johnsson A, Wennergren S, Wernstedt C, Betsholtz C, et al. A human osteosarcoma cell line secretes a growth factor structurally related to a homodimer of PDGF A-chains. Nature. 1986;319:511–514. doi: 10.1038/319511a0. [DOI] [PubMed] [Google Scholar]
- 155.Li X, Ponten A, Aase K, Karlsson L, Abramsson A, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 156.Levitzki A. PDGF receptor kinase inhibitors for the treatment of PDGF driven diseases. Cytokine Growth Factor Rev. 2004;15:229–235. doi: 10.1016/j.cytogfr.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 157.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 158.Yang X, Thomas DP, Zhang X, Culver BW, Alexander BM, et al. Curcumin inhibits platelet-derived growth factor-stimulated vascular smooth muscle cell function and injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2006;26:85–90. doi: 10.1161/01.ATV.0000191635.00744.b6. [DOI] [PubMed] [Google Scholar]
- 159.Lin J, Chen A. Activation of peroxisome proliferator-activated receptor-gamma by curcumin blocks the signaling pathways for PDGF and EGF in hepatic stellate cells. Lab Invest. 2008;88:529–540. doi: 10.1038/labinvest.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhao ZD, Huang ZS. [Study on effects of curcumin on expressions of PDGF-BB, PDGFRbeta and ERK1 of HSC] Zhong Yao Cai. 2009;32:732–735. [PubMed] [Google Scholar]
- 161.Liu Y, Dolence J, Ren J, Rao M, Sreejayan N. Inhibitory effect of dehydrozingerone on vascular smooth muscle cell function. J Cardiovasc Pharmacol. 2008;52:422–429. doi: 10.1097/FJC.0b013e31818aed93. [DOI] [PubMed] [Google Scholar]
- 162.Liu Y, Li W, Ye C, Lin Y, Cheang TY, et al. Gambogic acid induces G0/G1 cell cycle arrest and cell migration inhibition via suppressing PDGF receptor beta tyrosine phosphorylation and Rac1 activity in rat aortic smooth muscle cells. J Atheroscler Thromb. 2010;17:901–913. doi: 10.5551/jat.3491. [DOI] [PubMed] [Google Scholar]
- 163.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 164.Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 165.Li SY, Rong M, Grieu F, Iacopetta B. PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat. 2006;96:91–95. doi: 10.1007/s10549-005-9048-0. [DOI] [PubMed] [Google Scholar]
- 166.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 167.Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 168.Achiwa Y, Hasegawa K, Udagawa Y. Regulation of the phosphatidylinositol 3-kinase-Akt and the mitogen-activated protein kinase pathways by ursolic acid in human endometrial cancer cells. Biosci Biotechnol Biochem. 2007;71:31–37. doi: 10.1271/bbb.60288. [DOI] [PubMed] [Google Scholar]
- 169.Tang C, Lu YH, Xie JH, Wang F, Zou JN, et al. Downregulation of survivin and activation of caspase-3 through the PI3K/Akt pathway in ursolic acid-induced HepG2 cell apoptosis. Anticancer Drugs. 2009;20:249–258. doi: 10.1097/CAD.0b013e328327d476. [DOI] [PubMed] [Google Scholar]
- 170.Lee J, Jung K, Kim YS, Park D. Diosgenin inhibits melanogenesis through the activation of phosphatidylinositol-3-kinase pathway (PI3K) signaling. Life Sci. 2007;81:249–254. doi: 10.1016/j.lfs.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 171.Hussain AR, Al-Rasheed M, Manogaran PS, Al-Hussein KA, Platanias LC, et al. Curcumin induces apoptosis via inhibition of PI3′-kinase/AKT pathway in acute T cell leukemias. Apoptosis. 2006;11:245–254. doi: 10.1007/s10495-006-3392-3. [DOI] [PubMed] [Google Scholar]
- 172.Chen LX, He YJ, Zhao SZ, Wu JG, Wang JT, et al. Inhibition of tumor growth and vasculogenic mimicry by cucumin through downregulation of the EphA2/PI3K/MMP pathway in a murine choroidal melanoma model. Cancer Biol Ther. 2011;11 doi: 10.4161/cbt.11.2.13842. [DOI] [PubMed] [Google Scholar]
- 173.Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- 174.Hardie DG. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 175.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 176.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 177.Fay JR, Steele V, Crowell JA. Energy homeostasis and cancer prevention: the AMP-activated protein kinase. Cancer Prev Res (Phila) 2009;2:301–309. doi: 10.1158/1940-6207.CAPR-08-0166. [DOI] [PubMed] [Google Scholar]
- 178.Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6:457–470. doi: 10.2217/fon.09.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pan W, Yang H, Cao C, Song X, Wallin B, et al. AMPK mediates curcumin-induced cell death in CaOV3 ovarian cancer cells. Oncol Rep. 2008;20:1553–1559. [PubMed] [Google Scholar]
- 180.Lee YK, Lee WS, Hwang JT, Kwon DY, Surh YJ, et al. Curcumin exerts antidifferentiation effect through AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and antiproliferatory effect through AMPKalpha-COX-2 in cancer cells. J Agric Food Chem. 2009;57:305–310. doi: 10.1021/jf802737z. [DOI] [PubMed] [Google Scholar]
- 181.Lee YK, Park SY, Kim YM, Park OJ. Regulatory effect of the AMPK-COX-2 signaling pathway in curcumin-induced apoptosis in HT-29 colon cancer cells. Ann NY Acad Sci. 2009;1171:489–494. doi: 10.1111/j.1749-6632.2009.04699.x. [DOI] [PubMed] [Google Scholar]
- 182.Kim YM, Hwang JT, Kwak DW, Lee YK, Park OJ. Involvement of AMPK signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cells. Ann NY Acad Sci. 2007;1095:496–503. doi: 10.1196/annals.1397.053. [DOI] [PubMed] [Google Scholar]
- 183.Zou X, Calame K. Signaling pathways activated by oncogenic forms of Abl tyrosine kinase. J Biol Chem. 1999;274:18141–18144. doi: 10.1074/jbc.274.26.18141. [DOI] [PubMed] [Google Scholar]
- 184.Bedi A, Barber JP, Bedi GC, el-Deiry WS, Sidransky D, et al. BCR-ABL-mediated inhibition of apoptosis with delay of G2/M transition after DNA damage: a mechanism of resistance to multiple anticancer agents. Blood. 1995;86:1148–1158. [PubMed] [Google Scholar]
- 185.Sanchez-Garcia I, Grutz G. Tumorigenic activity of the BCR-ABL oncogenes is mediated by BCL2. Proc Natl Acad Sci USA. 1995;92:5287–5291. doi: 10.1073/pnas.92.12.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Burgess GS, Williamson EA, Cripe LD, Litz-Jackson S, Bhatt JA, et al. Regulation of the c-jun gene in p210 BCR-ABL transformed cells corresponds with activity of JNK, the c-jun N-terminal kinase. Blood. 1998;92:2450–2460. [PubMed] [Google Scholar]
- 187.Jagani Z, Song K, Kutok JL, Dewar MR, Melet A, et al. Proteasome inhibition causes regression of leukemia and abrogates BCR-ABL-induced evasion of apoptosis in part through regulation of forkhead tumor suppressors. Cancer Res. 2009;69:6546–6555. doi: 10.1158/0008-5472.CAN-09-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wu LX, Xu JH, Wu GH, Chen YZ. Inhibitory effect of curcumin on proliferation of K562 cells involves down-regulation of p210(bcr/abl) initiated Ras signal transduction pathway. Acta Pharmacol Sin. 2003;24:1155–1160. [PubMed] [Google Scholar]
- 189.Wu LX, Xu JH, Huang XW, Zhang KZ, Wen CX, et al. Downregulation of p210(bcr/abl) by curcumin involves disrupting molecular chaperone functions of Hsp90. Acta Pharmacol Sin. 2006;27:694–699. doi: 10.1111/j.1745-7254.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 190.William BM, Goodrich A, Peng C, Li S. Curcumin inhibits proliferation and induces apoptosis of leukemic cells expressing wild-type or T315I-BCR-ABL and prolongs survival of mice with acute lymphoblastic leukemia. Hematology. 2008;13:333–343. doi: 10.1179/102453308X343437. [DOI] [PubMed] [Google Scholar]
- 191.Monteghirfo S, Tosetti F, Ambrosini C, Stigliani S, Pozzi S, et al. Antileukemia effects of xanthohumol in Bcr/Abl-transformed cells involve nuclear factor-kappaB and p53 modulation. Mol Cancer Ther. 2008;7:2692–2702. doi: 10.1158/1535-7163.MCT-08-0132. [DOI] [PubMed] [Google Scholar]
- 192.Daum G, Eisenmann-Tappe I, Fries HW, Troppmair J, Rapp UR. The ins and outs of Raf kinases. Trends Biochem Sci. 1994;19:474–480. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 193.Wiesmuller L, Wittinghofer F. Signal transduction pathways involving Ras. Mini review. Cell Signal. 1994;6:247–267. doi: 10.1016/0898-6568(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 194.Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 195.Limtrakul P, Anuchapreeda S, Lipigorngoson S, Dunn FW. Inhibition of carcinogen induced c-Ha-ras and c-fos proto-oncogenes expression by dietary curcumin. BMC Cancer. 2001;1:1. doi: 10.1186/1471-2407-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 197.Ciuffreda L, Di Sanza C, Incani UC, Milella M. The mTOR pathway: a new target in cancer therapy. Curr Cancer Drug Targets. 2010;10:484–495. doi: 10.2174/156800910791517172. [DOI] [PubMed] [Google Scholar]
- 198.Sun ZJ, Chen G, Zhang W, Hu X, Liu Y, et al. Curcumin dually inhibits both mTOR and NF-{kappa}B pathways through a crossed PI3K/Akt/IKK signaling axis in adenoid cystic carcinoma. Mol Pharmacol. 2011;79:106–118. doi: 10.1124/mol.110.066910. [DOI] [PubMed] [Google Scholar]
- 199.Rafiee P, Binion DG, Wellner M, Behmaram B, Floer M, et al. Modulatory effect of curcumin on survival of irradiated human intestinal microvascular endothelial cells: role of Akt/mTOR and NF-{kappa}B. Am J Physiol Gastrointest Liver Physiol. 2010;298:G865–877. doi: 10.1152/ajpgi.00339.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Clark CA, McEachern MD, Shah SH, Rong Y, Rong X, et al. Curcumin inhibits carcinogen and nicotine-induced mammalian target of rapamycin pathway activation in head and neck squamous cell carcinoma. Cancer Prev Res (Phila) 2010 doi: 10.1158/1940-6207.CAPR-09-0244. [DOI] [PubMed] [Google Scholar]
- 201.Su CC, Wang MJ, Chiu TL. The anti-cancer efficacy of curcumin scrutinized through core signaling pathways in glioblastoma. Int J Mol Med. 2010;26:217–224. doi: 10.3892/ijmm_00000455. [DOI] [PubMed] [Google Scholar]
- 202.Shinojima N, Yokoyama T, Kondo Y, Kondo S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy. 2007;3:635–637. doi: 10.4161/auto.4916. [DOI] [PubMed] [Google Scholar]
- 203.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, et al. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 204.Johnson SM, Gulhati P, Arrieta I, Wang X, Uchida T, et al. Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer Res. 2009;29:3185–3190. [PMC free article] [PubMed] [Google Scholar]
- 205.Li M, Zhang Z, Hill DL, Wang H, Zhang R. Curcumin, a dietary component, has anticancer, chemosensitization, and radiosensitization effects by down-regulating the MDM2 oncogene through the PI3K/mTOR/ETS2 pathway. Cancer Res. 2007;67:1988–1996. doi: 10.1158/0008-5472.CAN-06-3066. [DOI] [PubMed] [Google Scholar]
- 206.Yu S, Shen G, Khor TO, Kim JH, Kong AN. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol Cancer Ther. 2008;7:2609–2620. doi: 10.1158/1535-7163.MCT-07-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Beevers CS, Chen L, Liu L, Luo Y, Webster NJ, et al. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res. 2009;69:1000–1008. doi: 10.1158/0008-5472.CAN-08-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Deeb D, Gao X, Jiang H, Arbab AS, Dulchavsky SA, et al. Growth inhibitory and apoptosis-inducing effects of xanthohumol, a prenylated chalone present in hops, in human prostate cancer cells. Anticancer Res. 2010;30:3333–3339. [PMC free article] [PubMed] [Google Scholar]
- 209.Chiang CT, Way TD, Tsai SJ, Lin JK. Diosgenin, a naturally occurring steroid, suppresses fatty acid synthase expression in HER2-overexpressing breast cancer cells through modulating Akt, mTOR and JNK phosphorylation. FEBS Lett. 2007;581:5735–5742. doi: 10.1016/j.febslet.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 210.De Angel RE, Smith SM, Glickman RD, Perkins SN, Hursting SD. Antitumor effects of ursolic acid in a mouse model of postmenopausal breast cancer. Nutr Cancer. 2010;62:1074–1086. doi: 10.1080/01635581.2010.492092. [DOI] [PubMed] [Google Scholar]
- 211.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 212.Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002;13:135–141. doi: 10.1016/s1359-6101(01)00020-x. [DOI] [PubMed] [Google Scholar]
- 213.Sugarman BJ, Aggarwal BB, Hass PE, Figari IS, Palladino MA, Jr, et al. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985;230:943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- 214.Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70:700–713. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 215.Surh YJ, Park KK, Chun KS, Lee LJ, Lee E, et al. Antitumor-promoting activities of selected pungent phenolic substances present in ginger. J Environ Pathol Toxicol Oncol. 1999;18:131–139. [PubMed] [Google Scholar]
- 216.Ahmad S, Israf DA, Lajis NH, Shaari K, Mohamed H, et al. Cardamonin, inhibits proinflammatory mediators in activated RAW 264.7 cells and whole blood. Eur J Pharmacol. 2006;538:188–194. doi: 10.1016/j.ejphar.2006.03.070. [DOI] [PubMed] [Google Scholar]
- 217.Lee YY, Hung SL, Pai SF, Lee YH, Yang SF. Eugenol suppressed the expression of lipopolysaccharide-induced proinflammatory mediators in human macrophages. J Endod. 2007;33:698–702. doi: 10.1016/j.joen.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 218.Kaur G, Athar M, Alam MS. Eugenol precludes cutaneous chemical carcinogenesis in mouse by preventing oxidative stress and inflammation and by inducing apoptosis. Mol Carcinog. 2010;49:290–301. doi: 10.1002/mc.20601. [DOI] [PubMed] [Google Scholar]
- 219.Zhang L, Yi Y, Chen J, Sun Y, Guo Q, et al. Gambogic acid inhibits Hsp90 and deregulates TNF-alpha/NF-kappaB in HeLa cells. Biochem Biophys Res Commun. 2010;403:282–287. doi: 10.1016/j.bbrc.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 220.El-Mahmoudy A, Shimizu Y, Shiina T, Matsuyama H, Nikami H, et al. Macrophage-derived cytokine and nitric oxide profiles in type I and type II diabetes mellitus: effect of thymoquinone. Acta Diabetol. 2005;42:23–30. doi: 10.1007/s00592-005-0170-6. [DOI] [PubMed] [Google Scholar]
- 221.Tekeoglu I, Dogan A, Demiralp L. Effects of thymoquinone (volatile oil of black cumin) on rheumatoid arthritis in rat models. Phytother Res. 2006;20:869–871. doi: 10.1002/ptr.1964. [DOI] [PubMed] [Google Scholar]
- 222.Murakami A, Hayashi R, Tanaka T, Kwon KH, Ohigashi H, et al. Suppression of dextran sodium sulfate-induced colitis in mice by zerumbone, a subtropical ginger sesquiterpene, and nimesulide: separately and in combination. Biochem Pharmacol. 2003;66:1253–1261. doi: 10.1016/s0006-2952(03)00446-5. [DOI] [PubMed] [Google Scholar]
- 223.Lupinacci E, Meijerink J, Vincken JP, Gabriele B, Gruppen H, et al. Xanthohumol from hop (Humulus lupulus L.) is an efficient inhibitor of monocyte chemoattractant protein-1 and tumor necrosis factor-alpha release in LPS-stimulated RAW 264.7 mouse macrophages and U937 human monocytes. J Agric Food Chem. 2009;57:7274–7281. doi: 10.1021/jf901244k. [DOI] [PubMed] [Google Scholar]
- 224.Arlt A, Vorndamm J, Muerkoster S, Yu H, Schmidt WE, et al. Autocrine production of interleukin 1beta confers constitutive nuclear factor kappaB activity and chemoresistance in pancreatic carcinoma cell lines. Cancer Res. 2002;62:910–916. [PubMed] [Google Scholar]
- 225.Saijo Y, Tanaka M, Miki M, Usui K, Suzuki T, et al. Proinflammatory cytokine IL-1 beta promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. J Immunol. 2002;169:469–475. doi: 10.4049/jimmunol.169.1.469. [DOI] [PubMed] [Google Scholar]
- 226.Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med. 2006;4:48. doi: 10.1186/1479-5876-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ogata A, Chauhan D, Teoh G, Treon SP, Urashima M, et al. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–2221. [PubMed] [Google Scholar]
- 228.Ara T, Song L, Shimada H, Keshelava N, Russell HV, et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 2009;69:329–337. doi: 10.1158/0008-5472.CAN-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 230.Quintanilla-Martinez L, Kremer M, Specht K, Calzada-Wack J, Nathrath M, et al. Analysis of signal transducer and activator of transcription 3 (Stat 3) pathway in multiple myeloma: Stat 3 activation and cyclin D1 dysregulation are mutually exclusive events. Am J Pathol. 2003;162:1449–1461. doi: 10.1016/S0002-9440(10)64278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Niu G, Bowman T, Huang M, Shivers S, Reintgen D, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–7010. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 232.Xu YX, Pindolia KR, Janakiraman N, Noth CJ, Chapman RA, et al. Curcumin, a compound with antiinflammatory and antioxidant properties, downregulates chemokine expression in bone marrow stromal cells. Exp Hematol. 1997;25:413–422. [PubMed] [Google Scholar]
- 233.Chan MM. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem Pharmacol. 1995;49:1551–1556. doi: 10.1016/0006-2952(95)00171-u. [DOI] [PubMed] [Google Scholar]
- 234.Kang JH, Kim CS, Han IS, Kawada T, Yu R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007;581:4389–4396. doi: 10.1016/j.febslet.2007.07.082. [DOI] [PubMed] [Google Scholar]
- 235.Jung DH, Park HJ, Byun HE, Park YM, Kim TW, et al. Diosgenin inhibits macrophage-derived inflammatory mediators through downregulation of CK2, JNK, NF-kappaB, and AP-1 activation. Int Immunopharmacol. 2010;10:1047–1054. doi: 10.1016/j.intimp.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 236.Cao Y, Prescott SM. Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol. 2002;190:279–286. doi: 10.1002/jcp.10068. [DOI] [PubMed] [Google Scholar]
- 237.Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010;2010:215158. doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.DuBois RN, Shao J, Tsujii M, Sheng H, Beauchamp RD. G1 delay in cells overexpressing prostaglandin endoperoxide synthase-2. Cancer Res. 1996;56:733–737. [PubMed] [Google Scholar]
- 239.Takeda H, Sonoshita M, Oshima H, Sugihara K, Chulada PC, et al. Cooperation of cyclooxygenase 1 and cyclooxygenase 2 in intestinal polyposis. Cancer Res. 2003;63:4872–4877. [PubMed] [Google Scholar]
- 240.Einspahr JG, Krouse RS, Yochim JM, Danenberg PV, Danenberg KD, et al. Association between cyclooxygenase expression and colorectal adenoma characteristics. Cancer Res. 2003;63:3891–3893. [PubMed] [Google Scholar]
- 241.Half E, Tang XM, Gwyn K, Sahin A, Wathen K, et al. Cyclooxygenase-2 expression in human breast cancers and adjacent ductal carcinoma in situ. Cancer Res. 2002;62:1676–1681. [PubMed] [Google Scholar]
- 242.Kundu N, Fulton AM. Selective cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic disease in a murine model of breast cancer. Cancer Res. 2002;62:2343–2346. [PubMed] [Google Scholar]
- 243.Wolff H, Saukkonen K, Anttila S, Karjalainen A, Vainio H, et al. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- 244.Pold M, Zhu LX, Sharma S, Burdick MD, Lin Y, et al. Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC Ligand (CXCL) 5 and interleukin-8/CXCL8 in human non-small cell lung cancer. Cancer Res. 2004;64:1853–1860. doi: 10.1158/0008-5472.can-03-3262. [DOI] [PubMed] [Google Scholar]
- 245.Davenport HW. Damage to the gastric mucosa: effects of salicylates and stimulation. Gastroenterology. 1965;49:189–196. [PubMed] [Google Scholar]
- 246.Gottlieb S. COX 2 inhibitors may increase risk of heart attack. BMJ. 2001;323:471. [PMC free article] [PubMed] [Google Scholar]
- 247.Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, et al. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813–819. [PubMed] [Google Scholar]
- 248.Ammon HP, Safayhi H, Mack T, Sabieraj J. Mechanism of antiinflammatory actions of curcumine and boswellic acids. J Ethnopharmacol. 1993;38:113–119. doi: 10.1016/0378-8741(93)90005-p. [DOI] [PubMed] [Google Scholar]
- 249.Zhang F, Altorki NK, Mestre JR, Subbaramaiah K, Dannenberg AJ. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis. 1999;20:445–451. doi: 10.1093/carcin/20.3.445. [DOI] [PubMed] [Google Scholar]
- 250.Lee YS, Kwon EJ, Jin DQ, Park SH, Kang YS, et al. Redox status-dependent regulation of cyclooxygenases mediates the capsaicin-induced apoptosis in human neuroblastoma cells. J Environ Pathol Toxicol Oncol. 2002;21:113–120. [PubMed] [Google Scholar]
- 251.Kiuchi F, Iwakami S, Shibuya M, Hanaoka F, Sankawa U. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chem Pharm Bull (Tokyo) 1992;40:387–391. doi: 10.1248/cpb.40.387. [DOI] [PubMed] [Google Scholar]
- 252.Flynn DL, Rafferty MF, Boctor AM. Inhibition of 5-hydroxy-eicosatetraenoic acid (5-HETE) formation in intact human neutrophils by naturally occurring diarylheptanoids: inhibitory activities of curcuminoids and yakuchinones. Prostaglandins Leukot Med. 1986;22:357–360. doi: 10.1016/0262-1746(86)90146-0. [DOI] [PubMed] [Google Scholar]
- 253.Lee YY, Yang SF, Ho WH, Lee YH, Hung SL. Eugenol modulates cyclooxygenase-2 expression through the activation of nuclear factor kappa B in human osteoblasts. J Endod. 2007;33:1177–1182. doi: 10.1016/j.joen.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 254.Kim SS, Oh OJ, Min HY, Park EJ, Kim Y, et al. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003;73:337–348. doi: 10.1016/s0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]
- 255.Hong J, Bose M, Ju J, Ryu JH, Chen X, et al. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: effects on cytosolic phospholipase A(2), cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25:1671–1679. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- 256.Marsik P, Kokoska L, Landa P, Nepovim A, Soudek P, et al. In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1- and -2-catalyzed prostaglandin E2 biosyntheses. Planta Med. 2005;71:739–742. doi: 10.1055/s-2005-871288. [DOI] [PubMed] [Google Scholar]
- 257.Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA. Antiinflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (Oxford) 2009;11:373–381. doi: 10.1111/j.1477-2574.2009.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Boado RJ, Pardridge WM, Vinters HV, Black KL. Differential expression of arachidonate 5-lipoxygenase transcripts in human brain tumors: evidence for the expression of a multitranscript family. Proc Natl Acad Sci USA. 1992;89:9044–9048. doi: 10.1073/pnas.89.19.9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ye YN, Wu WK, Shin VY, Bruce IC, Wong BC, et al. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis. 2005;26:827–834. doi: 10.1093/carcin/bgi012. [DOI] [PubMed] [Google Scholar]
- 260.Faronato M, Muzzonigro G, Milanese G, Menna C, Bonfigli AR, et al. Increased expression of 5-lipoxygenase is common in clear cell renal cell carcinoma. Histol Histopathol. 2007;22:1109–1118. doi: 10.14670/HH-22.1109. [DOI] [PubMed] [Google Scholar]
- 261.Jiang WG, Douglas-Jones AG, Mansel RE. Aberrant expression of 5-lipoxygenase-activating protein (5-LOXAP) has prognostic and survival significance in patients with breast cancer. Prostaglandins Leukot Essent Fatty Acids. 2006;74:125–134. doi: 10.1016/j.plefa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 262.Hennig R, Grippo P, Ding XZ, Rao SM, Buchler MW, et al. 5-Lipoxygenase, a marker for early pancreatic intraepithelial neoplastic lesions. Cancer Res. 2005;65:6011–6016. doi: 10.1158/0008-5472.CAN-04-4090. [DOI] [PubMed] [Google Scholar]
- 263.Lepage C, Liagre B, Cook-Moreau J, Pinon A, Beneytout JL. Cyclooxygenase-2 and 5-lipoxygenase pathways in diosgenin-induced apoptosis in HT-29 and HCT-116 colon cancer cells. Int J Oncol. 2010;36:1183–1191. doi: 10.3892/ijo_00000601. [DOI] [PubMed] [Google Scholar]
- 264.Adams J. The proteasome: structure, function, and role in the cell. Cancer Treat Rev. 2003;29(Suppl 1):3–9. doi: 10.1016/s0305-7372(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 265.Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- 266.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 267.Li B, Dou QP. Bax degradation by the ubiquitin/proteasome-dependent pathway: involvement in tumor survival and progression. Proc Natl Acad Sci USA. 2000;97:3850–3855. doi: 10.1073/pnas.070047997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 269.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, et al. Signal-induced site-specific phosphorylation targets I kappa B alpha to the ubiquitin-proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 270.Drexler HC. Activation of the cell death program by inhibition of proteasome function. Proc Natl Acad Sci USA. 1997;94:855–860. doi: 10.1073/pnas.94.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Orlowski RZ. The role of the ubiquitin-proteasome pathway in apoptosis. Cell Death Diff. 1999;6:303–313. doi: 10.1038/sj.cdd.4400505. [DOI] [PubMed] [Google Scholar]
- 272.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 273.Milacic V, Banerjee S, Landis-Piwowar KR, Sarkar FH, Majumdar AP, et al. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res. 2008;68:7283–7292. doi: 10.1158/0008-5472.CAN-07-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mori A, Lehmann S, O’Kelly J, Kumagai T, Desmond JC, et al. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006;66:3222–3229. doi: 10.1158/0008-5472.CAN-05-0087. [DOI] [PubMed] [Google Scholar]
- 275.Maity R, Sharma J, Jana NR. Capsaicin induces apoptosis through ubiquitin-proteasome system dysfunction. J Cell Biochem. 2010;109:933–942. doi: 10.1002/jcb.22469. [DOI] [PubMed] [Google Scholar]
- 276.Cecarini V, Quassinti L, Di Blasio A, Bonfili L, Bramucci M, et al. Effects of thymoquinone on isolated and cellular proteasomes. FEBS J. 2010;277:2128–2141. doi: 10.1111/j.1742-4658.2010.07629.x. [DOI] [PubMed] [Google Scholar]
- 277.Lust S, Vanhoecke B, Vang M, Boelens J, Vanm H, et al. Xanthohumol activates the proapoptotic arm of the unfolded protein response in chronic lymphocytic leukemia. Anticancer Res. 2009;29:3797–3805. [PMC free article] [PubMed] [Google Scholar]
- 278.Waddington CH. The epigenotype. Endeavour. 1942;1:18–20. [Google Scholar]
- 279.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther. 2009;8:1409–1420. doi: 10.1158/1535-7163.MCT-08-0860. [DOI] [PubMed] [Google Scholar]
- 281.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8:286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 282.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 283.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 284.Reuter S, Gupta SC, Park B, Goel A, Aggarwal BB. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011 doi: 10.1007/s12263-011-0222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Liu S, Shen T, Huynh L, Klisovic MI, Rush LJ, et al. Interplay of RUNX1/MTG8 and DNA methyltransferase 1 in acute myeloid leukemia. Cancer Res. 2005;65:1277–1284. doi: 10.1158/0008-5472.CAN-04-4532. [DOI] [PubMed] [Google Scholar]
- 286.Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, et al. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279:51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- 287.Pollack BP, Sapkota B, Boss JM. Ultraviolet radiation-induced transcription is associated with gene-specific histone acetylation. Photochem Photobiol. 2009;85:652–662. doi: 10.1111/j.1751-1097.2008.00485.x. [DOI] [PubMed] [Google Scholar]
- 288.Chen IH, Lu MC, Du YC, Yen MH, Wu CC, et al. Cytotoxic triterpenoids from the stems of Microtropis japonica. J Nat Prod. 2009;72:1231–1236. doi: 10.1021/np800694b. [DOI] [PubMed] [Google Scholar]
- 289.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 290.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 291.Garcea G, Berry DP, Jones DJ, Singh R, Dennison AR, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev. 2005;14:120–125. [PubMed] [Google Scholar]
- 292.Kuttan R, Sudheeran PC, Josph CD. Turmeric and curcumin as topical agents in cancer therapy. Tumori. 1987;73:29–31. doi: 10.1177/030089168707300105. [DOI] [PubMed] [Google Scholar]
- 293.Cruz-Correa M, Shoskes DA, Sanchez P, Zhao R, Hylind LM, et al. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2006;4:1035–1038. doi: 10.1016/j.cgh.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 294.Bayet-Robert M, Kwiatkowski F, Leheurteur M, Gachon F, Planchat E, et al. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol Ther. 2010;9:8–14. doi: 10.4161/cbt.9.1.10392. [DOI] [PubMed] [Google Scholar]
- 295.Vadhan VS, Weber D, Giralt S, Alexanian R, Thomas S, et al. Curcumin downregulates NF-kappaB and related genes in patients with multiple myeloma: results of a phase1/2 study. American Society of Hematology; 2007. [Google Scholar]
- 296.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 297.Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68:157–164. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 298.Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62:1137–1141. doi: 10.1080/01635581.2010.513802. [DOI] [PubMed] [Google Scholar]
- 299.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or premalignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 300.Rai B, Kaur J, Jacobs R, Singh J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J Oral Sci. 2010;52:251–256. doi: 10.2334/josnusd.52.251. [DOI] [PubMed] [Google Scholar]
- 301.Ide H, Tokiwa S, Sakamaki K, Nishio K, Isotani S, et al. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate. 2010;70:1127–1133. doi: 10.1002/pros.21147. [DOI] [PubMed] [Google Scholar]
- 302.Pillai AK, Sharma KK, Gupta YK, Bakhshi S. Anti-emetic effect of ginger powder versus placebo as an add-on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatr Blood Cancer. 2011;56:234–238. doi: 10.1002/pbc.22778. [DOI] [PubMed] [Google Scholar]
- 303.Zick SM, Ruffin MT, Lee J, Normolle DP, Siden R, et al. Phase II trial of encapsulated ginger as a treatment for chemotherapy-induced nausea and vomiting. Support Care Cancer. 2009;17:563–572. doi: 10.1007/s00520-008-0528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Levine ME, Gillis MG, Koch SY, Voss AC, Stern RM, et al. Protein and ginger for the treatment of chemotherapy-induced delayed nausea. J Altern Complement Med. 2008;14:545–551. doi: 10.1089/acm.2007.0817. [DOI] [PubMed] [Google Scholar]
- 305.Ellison N, Loprinzi CL, Kugler J, Hatfield AK, Miser A, et al. Phase III placebo-controlled trial of capsaicin cream in the management of surgical neuropathic pain in cancer patients. J Clin Oncol. 1997;15:2974–2980. doi: 10.1200/JCO.1997.15.8.2974. [DOI] [PubMed] [Google Scholar]
- 306.Berger A, Henderson M, Nadoolman W, Duffy V, Cooper D, et al. Oral capsaicin provides temporary relief for oral mucositis pain secondary to chemotherapy/radiation therapy. J Pain Symptom Manage. 1995;10:243–248. doi: 10.1016/0885-3924(94)00130-D. [DOI] [PubMed] [Google Scholar]
- 307.Lam S, MacAulay C, Le Riche JC, Dyachkova Y, Coldman A, et al. A randomized phase IIb trial of anethole dithiolethione in smokers with bronchial dysplasia. J Natl Cancer Inst. 2002;94:1001–1009. doi: 10.1093/jnci/94.13.1001. [DOI] [PubMed] [Google Scholar]
- 308.Kunnumakkara AB, Anand P, Aggarwal BB. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 309.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- 311.Rafailov S, Cammack S, Stone BA, Katz AE. The role of Zyflamend, an herbal anti-inflammatory, as a potential chemopreventive agent against prostate cancer: a case report. Integr Cancer Ther. 2007;6:74–76. doi: 10.1177/1534735406298843. [DOI] [PubMed] [Google Scholar]
- 312.Hickok JT, Roscoe JA, Morrow GR, Ryan JL. A Phase II/III Randomized, placebo-controlled, double-blind clinical trial of ginger (zingiber of-ficinale) for nausea caused by chemotherapy for cancer: a currently accruing URCC CCOP cancer control study. Support Cancer Ther. 2007;4:247–250. doi: 10.3816/SCT.2007.n.022. [DOI] [PubMed] [Google Scholar]
- 313.Manusirivithaya S, Sripramote M, Tangjitgamol S, Sheanakul C, Lee-lahakorn S, et al. Antiemetic effect of ginger in gynecologic oncology patients receiving cisplatin. Int J Gynecol Cancer. 2004;14:1063–1069. doi: 10.1111/j.1048-891X.2004.14603.x. [DOI] [PubMed] [Google Scholar]
- 314.Pyun BJ, Choi S, Lee Y, Kim TW, Min JK, et al. Capsiate, a nonpungent capsaicin-like compound, inhibits angiogenesis and vascular permeability via a direct inhibition of Src kinase activity. Cancer Res. 2008;68:227–235. doi: 10.1158/0008-5472.CAN-07-2799. [DOI] [PubMed] [Google Scholar]
- 315.Liddle M, Hull C, Liu C, Powell D. Contact urticaria from curcumin. Dermatitis. 2006;17:196–197. doi: 10.2310/6620.2006.06004. [DOI] [PubMed] [Google Scholar]
- 316.Futrell JM, Rietschel RL. Spice allergy evaluated by results of patch tests. Cutis. 1993;52:288–290. [PubMed] [Google Scholar]
- 317.Lopez-Carrillo L, Hernandez Avila M, Dubrow R. Chili pepper consumption and gastric cancer in Mexico: a case-control study. Am J Epidemiol. 1994;139:263–271. doi: 10.1093/oxfordjournals.aje.a116993. [DOI] [PubMed] [Google Scholar]
- 318.Surh YJ, Lee SS. Capsaicin in hot chili pepper: carcinogen, co-carcinogen or anticarcinogen? Food Chem Toxicol. 1996;34:313–316. doi: 10.1016/0278-6915(95)00108-5. [DOI] [PubMed] [Google Scholar]