Abstract
Recent progress in tissue-resident adult stem/progenitor cell research has inspired great interest because these immature cells from your own body can act as potential, easily accessible cell sources for cell transplantation in regenerative medicine and cancer therapies. The use of adult stem/progenitor cells endowed with a high self-renewal ability and multilineage differentiation potential, which are able to regenerate all the mature cells in the tissues from their origin, offers great promise in replacing non-functioning or lost cells and regenerating diseased and damaged tissues. The presence of a small subpopulation of adult stem/progenitor cells in most tissues and organs provides the possibility of stimulating their in vivo differentiation, or of using their ex vivo expanded progenies for cell-replacement and gene therapies with multiple applications in humans without a high-risk of graft rejection and major side effects. Among the diseases that could be treated by adult stem cell-based therapies are hematopoietic and immune disorders, multiple degenerative disorders such as Parkinson's and Alzheimer's diseases, types 1 and 2 diabetes mellitus as well as skin, eye, liver, lung, tooth and cardiovascular disorders. In addition, a combination of the current cancer treatments with an adjuvant treatment consisting of an autologous or allogeneic adult stem/progenitor cell transplantation also represents a promising strategy for treating and even curing diverse aggressive, metastatic, recurrent and lethal cancers. In this chapter, we reviewed the most recent advancements on the characterization of phenotypic and functional properties of adult stem/progenitor cell types found in bone marrow, heart, brain and other tissues and discussed their therapeutic implications in the stem cell-based transplantation therapy.
INTRODUCTION
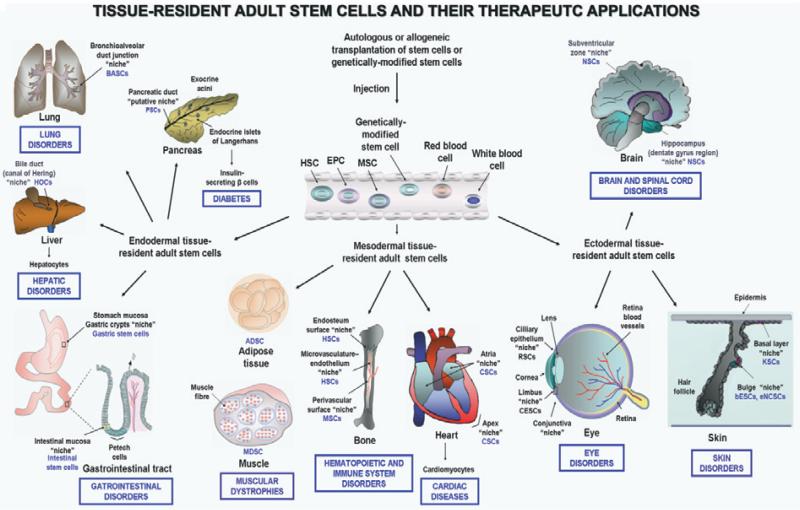
Recent advances in the field of the stem cell biology have led to the characterization of different tissue-resident adult stem/progenitor cells in most mammalian tissues and organs that constitute potential and easily accessible sources of immature cells with multiple promising therapeutic applications in stem cell-based transplantation therapies. Among the tissues harboring a small subpopulation of adult stem/progenitor cells, there are bone marrow (BM), vascular walls, heart, brain, tooth, skeletal muscles, adipose tissues as well as the epithelium of the skin, eye, lung, liver, digestive tract, pancreas, breast, ovary, uterus, prostate and testis (Fig. 1).1-14 Numerous studies have allowed researchers to define the unique features of each tissue-resident adult stem/progenitor cell type and their specialized local microenvironment designated as a niche (Fig. 1).1-4,6-15 The tissue-resident adult stem/progenitor cells and their early progenies endowed with a high self-renewal and multilineage differentiation potential generally provide critical physiological functions in the regenerative process for tissue homeostatic maintenance, and repair after intense injuries, such as chronic inflammatory atrophies and fibrosis.1-4,6-14 Multipotent adult stem/progenitor cells are able to give rise to different differentiated cell lineages in tissues from which they originate in physiological conditions, and thereby regenerate the tissues and organs throughout the lifespan of an individual. Importantly, it has also been shown that certain adult stem/progenitor cells, including BM-derived stem/progenitor cells, may be attracted at distant extramedullary peripheral sites after intense injuries, and thereby participate in the tissue repair through remodeling and regeneration of damaged areas.1,2,9-11,14-17
Figure 1.
Scheme showing the anatomic localizations of tissue-resident adult stem/progenitor cells and their niches and adult stem cell-based transplantation therapies for treating diverse human disorders. The clinical treatments consisting of an injection of autologous or allogeneic adult stem/progenitor cell transplant, including bone marrow (BM)-derived stem cells (HSC, EPC, MSC), peripheral blood (PB) or genetically-modified adult stem/progenitor cells into peripheral circulation or diseased areas in the same patient or a host patient is illustrated. The tissue-specific degenerating disorders and diseases which might be treated by the autologous or allogeneic transplantation of adult stem/progenitor cells are also indicated.
Of clinical interest, it has been shown that the small pools of endogenous adult stem/progenitor cells can be successfully used for cell replacement-based therapies in regenerative medicine and cancer therapy in humans.3,9-11,14,16-36 The use of autologous adult stem/progenitor cell transplant may reduce the high-risk of graft rejection and severe secondary effects observed with allogenic transplant or embryonic stem cell (ESC)-based transplantation therapies. Particularly, the in vivo stimulation of endogenous tissue-resident adult stem/progenitor cells or the replacement of nonfunctioning or lost adult stem/progenitor cells by new ex vivo expanded immature cells or their differentiated progenies have been recognized as promising therapeutic strategies.3,9-11,14,16-21,23-36 Among the human diseases that could be treated by stem cell-based transplantation therapies, there are hematopoietic and immune disorders, type 1 or 2 diabetes mellitus, cardiovascular, neurodegenerative and musculoskeletal diseases and skin, eye, tooth, liver, lung, and gastrointestinal disorders and aggressive and recurrent cancers (Figs. 1 and 2).3,9-11,14,16-21,23-36 In regard with this, we discussed the most recent progress in basic and clinical research in the adult stem/progenitor cell field in terms of their implications in the development of novel stem cell-based transplantation therapies. The emphasis is on the phenotypic and functional properties of adult stem/progenitor cells found in BM, heart and brain and their potential therapeutic applications to treat diverse severe disorders and aggressive cancers.
Figure 2.
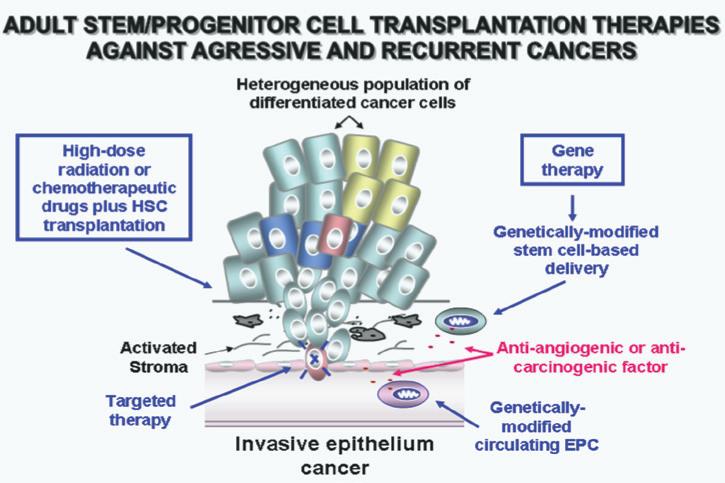
Scheme showing potential combination therapies against locally invasive and metastatic epithelial cancers. The therapeutic strategies consisting of targeted therapy of tumor-initiating cells and their local microenvironment, including stromal components, high-dose radiation or chemotherapy plus adult stem/progenitor cell transplantation and selective delivery of anti-tumoral drugs by using genetically-modified stem cells, are also illustrated.
BONE MARROW-DERIVED STEM/PROGENITOR CELLS AND THEIR THERAPEUTIC APPLICATIONS IN TRANSPLANTATION THERAPIES
Hematopoietic Stem/Progenitor Cells and their Clinical Applications
The BM-resident hematopoietic stem cells (HSCs) provide critical functions for the maintenance of hematopoiesis and the immune system by generating all of the mature myeloid and lymphoid cell lineages in the peripheral circulation along the lifespan of an individual.1,9-11 The most immature and quiescent HSCs are colocalized with the osteoblasts in a specialized niche within a BM region designated as endosteum (Fig. 1).1,2,9,11,12 These multipotent HSCs are characterized by the expression of specific biomarkers including telomerase, high levels of aldehyde dehydrogenase and CD34- or CD34+/CD38-/low/Thy1+/CD90+/C-kit-/lo/Lin-/CD133+/vascular endothelial growth factor receptor 2 (VEGFR2+)/ABCG2 multidrug transporter. Moreover, another HSC subpopulation found in a BM microvasculature-sinusoidal endothelium niche also can contribute to rapidly supplying new mature blood cell lineages, which have a short life in the peripheral circulation (Fig. 1).1,11 In regard with this, the results from a recent study have also revealed the presence of postnatal CD34+/Lin-/CD10+/CD24- hematopoietic progenitor cells co-expressing recombination activating gene 1, terminal deoxynucleotide transferase, paired box protein 5 (PAX5), interleukin 7 receptor-α and CD3ε in the BM and peripheral blood.37 These hematopoietic progenitor cells, which exhibited a very low potential along myeloid commitment, were able to migrate from BM to thymus and generate B-, T-, and natural killer (NK)-lymphocytes.37 Additionally, the primitive KIT+ hematopoietic progenitor cells endowed with a long-term self-renewal capacity have also been identified in the adult spleen in humans.38 The activation of HSCs and their early progenitors implicates the interplay of a complex signaling network mediated by different growth factors including hedgehog, Wnt/β-catenin, Notch, fibroblast growth factor (FGF) and Polycom group proteins, such as BMI-1 and interactions with the niche components that control their self-renewal ability versus differentiation capacity.1,2,9,11,12,39
In clinical practice, autologous or allogeneic HSC transplantation is currently used to treat the patients with diverse hematopoietic disorders to reconstitute the hematopoietic cell lineages and immune system defense.9-11,18,19 BM-derived HSCs may be collected from BM aspirate or by aphaeresis after their mobilization in peripheral blood (PB) by using diverse mobilizing agents such as granulocyte-stimulating factor (G-CSF), granulocyte colony-stimulating factor (GM-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF) and/or synthetic chemical compounds-like bicyclam derivative, AMD3100 (Plerixafor).9,11,40 Hence, BM or mobilized PB HSC-containing samples or isolated HSC preparations may be retransplanted into the same patients (autografts) or different patients (allografts) by injection into the bloodstream (Fig. 1). Transplanted HSCs spontaneously migrate and engraft at the BM compartment where they establish their novel homing, and thereby contribute to replenish all the mature blood cell types and restore immune system functions. Moreover, a treatment of patients with a myeloablative conditioning regimen consisting of high-dose chemotherapy or radiotherapy is generally made prior to allogeneic HSC transplantation to reduce the immune response and risk of graft rejection and improve the anti-tumoral efficacy. The HSC transplants, alone or in combination therapies, may be used to treat HSC aging related-intrinsic functional defects, inherited immunodeficient and autoimmune diseases such as multiple sclerosis, refractory and severe aplastic anemias, congenital thrombocytopenia, osteoporosis, cardiovascular disorders, chronic inflammatory Bowel disorders (IBDs) including Crohn's disease and ulcerative colitis, and diabetes mellitus.9-11,20,21 Particularly, HSC transplant may improve the immune response of patients, and thereby help to repair damaged tissues at distant sites in diverse pathological conditions and prevent infectious diseases after the transplantation of tissue or organ grafts.9,11,18 Moreover, high-dose or intermittent systemic chemotherapy or ionizing radiation therapy plus HSC transplantation represents a potential therapeutic option to treat and even cure the high risk patients with advanced and/or relapsed cancers (Fig. 2). Among them, there are leukemias, multiple myeloma, Hodgkin's and non- Hodgkin's lymphomas, melanoma and aggressive and metastatic solid tumors such as sarcomas, retinoblastoma, kidney, brain, lung, pancreatic, prostate, breast and ovarian cancers.9-11,19,41,42 In fact, HSC transplant may restore the hematopoietic and immune systems after myeloablative effects induced by high-dose irradiation or chemotherapy following a treatment of cancer patients (Fig. 2).19
Although there have been important advances in HSC transplantation procedures, the graft-versus-host diseases (GVHDs), toxicity of cytoreductive conditioning regimens, the presence of residual malignant cells in allograft as well as the lack of appropriate donors for some patients, represent the major limiting factors for their clinical applications in safe conditions.43 Particularly, several secondary effects may be manifested after HSC transplantation in certain patients and contribute to a poor quality of life.44 For example, acute or GVHD is a common late complication of allogeneic transplantation characterized by specific clinical and pathologic signs related with the fact that immunocompetent donor cells may attack to fast proliferating recipient tissues such as the skin, liver and gastrointestinal tract.45 GVHD may be associated with the occurrence of severe vascular and fibrotic lesions. In order to reduce the toxicity of myeloablative regimens, non-myeloablative or reduced-intensity myeloablative conditioning regimens may be used in certain cases, and more particularly, for old patients or patients with comorbidities that are unable to tolerate this immunosuppressor treatment.11,14 Moreover, an autograft after purging of malignant cells may constitute another alternative treatment in patients with high-risk leukemic relapse when no stem cell donor is available. For instance, the autologous transplantation of CD133+ selected HSCs may be used for pediatric patients with relapsed CD34+/CD133- leukemia.42 The results from a recent investigation have also revealed that the homing and engraftment of BCR-ABL+ leukemic stem cells (LSCs) in the BM of patients with chronic myelogenous leukemias (CMLs) is highly dependent on CD44 adhesion molecule expression in respect to normal HSCs.46 Therefore, the targeting of CD44 LSC using an anti-CD44 antibody also may constitute an alternative approach for improving the efficacy of HSC transplantation in CML patients.46 In addition, bank stored-umbilical cord (UC) cells, including umbilical cord blood (UCB), placenta cells and fetal tissue-derived HSC transplants, which generally induce a less intense detrimental alloreactive response, may also constitute other HSC sources for autograft or allograft in certain clinical or experimental settings.9-11
Mesenchymal Stem Cells and Endothelial Progenitor Cells and Their Therapeutic Applications
The BM stroma, PB, skin dermis and placenta as well as the walls of large and small blood vessels in most tissues and organs, including the brain, spleen, liver, kidney, lung, muscle, adipose tissues, thymus, uterus and pancreas, also contain the multipotent mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs).9-11,47,48 Much of the work conducted on adult stem/progenitor cells has focused on MSCs found within the BM stroma. More particularly, the MSCs expressing CD49a and CD133 markers are localized in a perivascular niche in BM, and may give rise to the osteoblasts that are colocalized with HSCs, and which may support the hematopoiesis by producing the growth factors and cytokines that promote the expansion and/or differentiation of HSCs (Fig. 1).1,2,5,9-11,49 It has also been reported that the lung-resident mice CD45 side subpopulation containing MSCs and expressing a high telomerase level and mesenchymal markers (CD44, CD90, CD105, CD106, CD73, Sca-I) can differentiate into chondrocytes, adipocytes and osteocytes.50 The results from differentiation studies have also indicated that CD146+ Notch3+ cells sorted from cultured BM-derived MSCs were capable of adipogenic and osteogenic differentiation, while ITGA11+ cells mainly displayed an osteogenic differentiation profile with limited adipogenic fate.15 The BM-derived or tissue-resident MSCs can generate diverse mesodermal cell lineages involved in osteogenesis, adipogenesis, cartilage and muscle formation, including the osteoblasts, osteocytes, adipocytes, chondrocytes, myoblasts and myocytes under appropriate culturing conditions ex vivo and in vivo.5,9-11 Moreover, MSCs may also be induced to differentiate into fibroblasts, neuronal cells, pulmonary cells, pancreatic islet β cells, corneal epithelial cells and cardiomyocytes ex vivo and/or in vivo using specific growth factors and cytokines.5,9,10
In the case of EPCs, which are derived-like HSCs from the embryonic hemangioblasts, they may be distinguished by the expression of different biomarkers, including CD34+ or CD34-, CD133, VEGFR2+ also designated as Flk-1 (fetal liver kinase-1), KIT and CXC chemokine receptor 4 (CXCR4).14 EPCs may contribute in a significant manner to give rise to mature endothelial cells that form new vascular walls of vessels after intense injury and vascular diseases as well as the new vessel formation in tumors.9-11 The critical role of circulating EPCs in endothelial cell maintenance after tissue injury is notably supported by the observation that their number and function is inversely associated with the progression of atherosclerosis and an enhanced risk of cardiovascular diseases. For instance, it has been proposed that the number of circulating EPCs may be increased by down-regulating EPC senescence through plasma high-density lipoprotein (HDL)-induced nitride oxide (NO) production and telomerase activity via the phosphatidylinositol 3′ kinase (PI3K)/Akt signaling pathway.22 These molecular events may promote the angiogenic process, and thereby decrease the incidence of atherosclerosis-related ischemic diseases.22 Moreover, the CXCR4 gene transfer in EPCs has been observed to promote their migration and adhesion to the endothelial cell layer and vascular re-endothelization in nude mice subjected to carotid artery injury as compared to non-transduced EPCs, while a treatment with neutralizing antibodies against CXCR4 or/and JAK-2 inhibitor AG490 attenuated these beneficial effects.17 In the same way, the transplantation of CXCR4-expressing EPCs also resulted in their migration to ischemic brain regions and promoted neurovascular repair, and improved long-term neurobehavioral outcomes in an animal model.23 It has been noticed that an up-regulated expression of stromal-derived factor-1 (SDF-1) level in ischemic heart or brain can contribute in part to the recruitment of CXCR4-expressing EPC cells at injured tissues.16,23
All of the aforementioned functional properties of MSCs and EPCs made them the promising sources of immature cells for treating numerous degenerative and vascular disorders in human. The autologous or allogeneic transplantation of a BM or PB sample can lead to the homing and engraftment of functional HSCs, MSCs and EPCs and/or their differentiated progenies at the BM and distant damaged tissues. Thus, this supports the feasibility of this strategy for improving the tissue remodeling and healing processes after severe injury as well as in the treatment of diverse human disorders, including osteogenesis imperfecta, atherosclerotic lesions, visual loss associated with choroidal neovascularization, ischemic cardiovascular and muscular diseases.9-11,24,25 It has been reported that MSCs, EPCs and their progenies can contribute to the vasculogenesis and regenerative process of several tissues, including bone, cartilage, tendon, muscle, adipose, brain, heart, lung, skin, pancreas, kidney and eye.10,24,25 Importantly, adult BM-derived and tissue-resident MSCs are a little immunogenic and display immunomodulatory and anti-inflammatory effects in host in vivo.19 In this regard, the results from a clinical trial consisting of a transplantation of autologous BM-derived MSCs to 41 patients between January 1998 and November 2008 have revealed that neither tumors nor infections were observed between 5 and 137 months of follow-up after transplantation.25 Therefore, these therapeutic properties of MSCs also support their potential clinical applications to prevent the tissue or organ allograft rejection and severe acute and chronic GVHDs as well as to treat the autoimmune disorders such as inflammatory bowel diseases and inflammation of the heart muscle walls associated with autoimmune myocarditis, in which immunomodulation and tissue repair are required.19 Indeed, MSCs can prolong skin allograft survival and reverse severe acute GVHDs in vivo supporting their use in treating skin diseases as well as in the maxillofacial surgery.51,52 In counterpart, the migration and proliferation of vascular smooth muscle cells (SMCs) derived from BM cells, including HSCs and MSCs, in the vascular injured area leading to an excessive cell accumulation, may contribute to the development of vascular pathologies such as intimal hyperplasia and atherosclerotic lesions.10,53 Therefore, future investigations are necessary to optimize the BM-derived cell transplantation strategies and establish the specific mechanism(s) of action and physiological effects of HSCs, MSCs and EPCs in the long term. This should allow for improvement of their therapeutic and curative benefits and prevent their detrimental clinical effects in treated patients.
CARDIAC STEM/PROGENITOR CELLS AND THEIR THERAPEUTIC APPLICATIONS
The myocardial regeneration and cardiac function recovery in physiological and pathological conditions may occur via the activation of a small pool of interstitial cardiac stem/progenitor cells (CSCs or CPCs) found within the specialized niches localized at the apex and atria of the heart (Fig. 1).3,4,9-11 Mammalian heart-resident adult CSCs or their early progenies expressing different stem cell-like markers, including telomerase, nestin, KIT (also designated CD117), multidrug resistance-1 (MDR-1) and ABCG2 multidrug transporters, Islet1 transcription factor and/or Sca-1 (in mice), are endowed with a high self-renewal capacity and multilineage differentiation potential.3,4,9-11 These immature cells are able to give rise to three major cell types constituting the myocardium, including cardiomyocytes, smooth muscles and vascular endothelial cells in homeostatic conditions and after myocardial injuries.3,4,9-11 Therefore, the in vivo stimulation of CSCs and early progenies by administration of diverse exogenous growth factors or the intravascular, intramyocardial or catheter-based delivery of ex vivo expanded CSCs or their differentiated progenies may represent the potential therapeutic strategies to treat and even cure diverse heart diseases (Fig. 1).3,9,10 Moreover, the transplantation of genetically-modified adult stem/progenitor cells also offers great promise by permitting delivery of a specific therapeutic gene product such as an angiogenic agent or cardioprotective factor in the ischemic or non-ischemic heart disease areas (Fig. 1).10,26 These treatment types, alone or in combination with the conventional clinical therapies by using pharmacological agents such as angiotensin-converting enzyme (ACE) inhibitors, β-adrenergic blockers, and nitroglycerin, represent promising strategies to improve the long-term survival of patients diagnosed with heart failures resulting from ischemic heart disease, hypertension and myocardial infarction.3,9-11,26,54
In addition, the use of other stem/progenitor cell types such as ESCs, UCB-derived stem cells (CD133+ cells, HSCs or MSCs), alveolar epithelial stem cells (AECs), BM-derived stem cells (CD133+ cells, HSCs, MSCs or EPCs), adipose-derived stem cells (ADSCs), muscle-derived stem cells (MDSCs), pancreatic stem cells (PSCs) and adult testicular stem cells or their progenies, which can differentiate into functional and contractile cardiomyocytes and/or vascular endothelial cells in vitro and/or in vivo also represent potential therapeutic stem/progenitor cell sources.3,10,16,17,26,27,55 Consistent with this, the results from numerous investigations carried out on animal injury models in vivo have revealed the potential benefit of using these stem/progenitor cell types or their differentiated progenies with the cardiomyogenic properties to repair the damaged myocardium and improving the coronary revascularisation and cardiac function.10,11,21,26,27,55 For instance, the data from small clinical trials consisting of the transplantation of human BM-derived stem cells, mobilized PB cells or purified CD133+ BM-derived stem cells into patients with advanced ischemic heart diseases have also indicated that this treatment generally improves the vascularization process and/or myocardial function.56-58 Importantly, the results from a recent study have indicated that transendocardial injections of BM-derived MSCs also can improve the cardiac function by stimulating host heart-resident KIT+ CSCs proliferation in an animal model of myocardial infraction.27
An optimization of cell delivery methods and cell-replacement therapies are required to establish the beneficial effects of these treatments on the ischemic and non-ischemic cardiac disorders versus their potential risk before they deemed safe to use in the clinical setting.
NEURAL STEM/PROGENITOR CELLS AND THEIR THERAPEUTIC APPLICATIONS
Phenotypic and Functional Properties of Neural Stem/Progenitor Cells
Adult neurogenesis and tissue repair in central and peripheral nervous tissues may occur through the activation of adult neural stem and progenitor cells (NSCs and NPCs).10,59 The neural stem/progenitor cells have been identified within two specific germinal regions of the brain, the subventricular zone bordering lateral ventricle in the forebrain and dentate gyrus in hippocampus (Fig. 1).6-11 Multipotent NSCs localized in the germinal subraventricular zone, which express different stem cell-like markers, such as CD133 and nestin and possess a high self-renewal potential, can give rise to three principal cell lineages, including mature neurons and glial cells, astrocytes and oligodentrocytes.6-11 NSCs can generate the progenitor cells that migrate along the blood vessels at distant damaged areas of the brain and participate to regenerate and repair the injured tissues by generating further differentiated and functional progenies.60 Moreover, NPCs found in the subgranular cell layer of hippocampus, which are also designated neural precursor cells, can generate the granule cell projection neurons that integrate into existing neuronal circuitry.6-9,11 In addition, multipotent adult stem/progenitor cells expressing the glial markers that are able to give rise to the dopaminergic glomus cells have also been identified in the peripheral nervous system within a germinal center termed carotid body.61 Importantly, recent accumulating lines of experimental evidence have also indicated the possibility of using these different immature neural stem/progenitor cells in transplantation therapies for treating diverse neurological disorders.
Potential Stem Cell-Based Therapies for Neurological Disorders
The discovery that the adult neural stem/progenitor cells can actively contribute to neurogenesis, astrogliogenesis and tissue repair in central and peripheral nervous systems in the postnatal developing brain and throughout adult life has given new avenues to develop novel stem cell-based therapies for treating diverse neurodegenerative disorders, cerebrovascular dysfunctions and primary brain tumors.10,59,62 More specifically, the transplantation of neural stem/progenitor cells and/or oligodentrocyte precursor cells (OPCs) or other adult stem/progenitor cell types may constitute a potential cell-replacement strategy for treating diverse severe brain injuries and devastating neurodegenerative diseases that are associated with a partial or substantial loss of functional neurons and/or glial cells. These neurological disorders include traumatic brain injury, Parkinson's and Alzheimer's, Lou Gehrig's and Huntington's diseases, temporal lobe epilepsy, stroke/cerebral ischemia, cerebellar ataxia, multiple sclerosis and amyotrophic lateral sclerosis.7,9-11,59,62-69 It has been shown that ex vivo expanded neural stem/progenitor cells may be transplanted in specific brain regions where they can proliferate, survive, migrate at damaged areas and differentiate into functional neuronal and glial cells in vivo.7,9-11,59,62-69 For instance, the intracerebral transplantation of neural stem/progenitor cells has been observed to improve neuronal survival under conditions of focal cerebral ischemia via the hypoxia-inducible factor-1α (HIF-1α)-induced VEGF expression.69 Interestingly, it has also been observed that the neural progenitor cells from the olfactory organ of patients with Parkinson's disease were able to generate dopaminergic cells in vitro and reduce the behavioral asymmetry resulting from the dopaminergic neuron loss in the rat model of Parkinson's disease.67 In regard with this, the intrastriatal transplantation of CB-stem/progenitor cells or their progenies also might constitute a potential cell source for anti-Parkinsonian therapy.61 In the same way, the intravenous injection of human NSCs also resulted in their migration to the striatal lesion, where they attenuated the striatal atrophy and induced a long-term functional improvement in an animal model mimicking the striatal degeneration observed in Huntington's disease.63
In addition, ESCs, fetal stem/progenitor cells, umbilical cord-derived stem cells and other adult stem cell types, such as AECs, BM-derived MSCs, ADSCs and skin-, tooth- and endometrium-derived stem cells, may also be induced to differentiate or trans-differentiate into functional neurons, astrocytes or oligodendrocytes in vitro and/or in vivo.10,28-30 For example, it has been observed that the transplantation of embryonic medial ganglionic eminence (MGE) cells into the striatum ameliorated motor symptoms in a rodent model of Parkinson's disease.28 Moreover, the data from a small clinical trial with seven patients with Parkinson's disease treated with a single-dose of unilateral autologous BM-MSCs transplanted into the sublateral ventricular zone have indicated that three patients exhibited a steady improvement of the symptoms.10,29 Importantly, it has also been reported that the hippocampal NSC transplantation rescued the cognitive decline in the learning and memory observed in aged animal model of Alzheimer disease by increasing hippocampal synaptic density induced by brain-derived neurothrophic factor.30 In this matter, recent studies have also underlined the importance of considering the intrinsic and extrinsic factors that modulate neural stem/progenitor cell behavior as well as the influence of their local microenvironment to design more effective stem cell-based transplantation therapies for treating the brain disorders.
Stem Cell Transplant Plus Modulators of Neural Stem/Progenitor Cell Behavior
The ex vivo treatment or in vivo administration of trophic factors that can modulate the functional integration, proliferation, long-term survival, fate specification and/or migration of neural stem/progenitor cells and/or their local microenvironment, alone or in combination with stem cell transplant, might represent potential therapeutic strategies for treating specific neurological diseases.10,31-36 In this regard, it has been reported that the stimulation of sonic hedgehog (SHH) cascade using exogenous SHH ligand or chemically-synthesized agonist (SAG) of smoothened (SMO) hedgehog coreceptor induced the proliferation of neuronal and glial precursors in vitro.34 Moreover, the intracerebroventricular administration of SHH or SAG to adult rats promoted the survival of newly generated neural cells in adult rat models.34 Importantly, it has also been reported that the transplantation of neural stem/progenitor cells, chondroitinas ABC and growth factors, EGF, basic FGF and platelet-derived growth factor-AA (PDGF-AA) principally resulted in their differentiation in oligodentrocytes and synergistically promoted the functional repair of chronically injured spinal cord in an animal model.35 In the same way, the MSCs transplanted into the neurogenic areas of the hippocampus of the young rodent brain also were able to survive, migrate at distinct sites of graft and generate new mature neurons synthesizing neurotransmitters.36 In contrast, the implantation of MSCs into non-neurogenic areas of striatum was associated with a massive cell degeneration and no migration of cells was seen under these conditions.36 These results suggest then that the MSC behavior may be influenced by the local microenvironment prevalent within the site of implantation. Consistently, the induction of neuronal differentiation of MSCs by increasing cyclic adenosine monophosphate in the culture medium before cell implantation has been observed to promote their differentiation in vivo.36 In addition, another promising strategy to treat neurological disorders and primary brain cancers also includes the transplantation of genetically-engineered adult stem/progenitor cells.
Genetically-Engineered Stem Cell-Based Transplantation Therapies
The transplantation of genetically-engineered ex vivo expanded NSCs or other adult stem/progenitor cell types, including BM-derived stem cells, alone or in combination therapies, has been shown to effectively restore diverse neurological deregulations or suppress the brain tumor growth.70-77 In fact, NSCs and adult progenitor stem cells such as MSCs possess an inherent tropic property and typically migrate throughout the brain to reach the injured areas or primary brain tumor sites, and more specifically in the tumor hypoxia region. Hence, human NSCs may be used as a delivery vehicle for a specific release of therapeutic gene products at damaged brain regions or primary brain tumors, including medulloblastomas and GBMs. For instance, it has been shown that the transplantation of NSCs expressing HIF-1α, which can act, in part, by promoting angiogenesis, improved behavioral recovery in a rat stroke model.75 The transplantation of fetal-derived NSCs engineered to express interleukin-12 or tumor necrosis factor-α (TNF-α) related apoptosis inducing ligand (TRAIL), has also been observed to result in their specific recruitment within intracranial glioma, and the release of therapeutic gene product concomitant with an inhibition of tumor growth.78,79 Similarly, the co-injection of neural stem/progenitor cells expressing cyclophosphamide (CPA)-activating enzyme cytochrome p450 2B6 (CYP2B6), which catalyzes CPA prodrug transformation into membrane diffusible DNA-alkylating metabolites with GMB cells followed by a CPA administration, markedly impaired brain tumor growth.80 It has also been noted that the intracerebally transplantation of CPA gene-engineered neural stem/progenitor cells resulted in their migration through the brain parenchyma to the neoplastic site concomitant with a tumor growth inhibition through the activation of CPA.80
Overall, these observations support the therapeutic interest of using adult stem/progenitor cells, which are able to integrate and migrate through the brain and reach the damaged brain regions, alone or in combination with other therapeutic agents, for developing effective transplantation therapies to rescue damaged areas of the brain or specifically target the brain tumor cells.
OTHER TISSUE-RESIDENT ADULT STEM/PROGENITOR CELL TYPES AND THEIR THERAPEUTIC IMPLICATIONS
Among the other tissues and organs harboring an adult stem/progenitor cell subpopulation, there are skin, liver, intestinal crypts and gastric glands, adipose tissues, muscles, eye and pancreas (Fig. 1).9,10,14 It has been shown that the in vivo stimulation of these adult stem/progenitor cells and/or the replacement of their dysfunctional counterparts and/or their further differentiated progenies by functional cells, also may constitute potential therapeutic strategies for the treatment of numerous pathological disorders in humans.9,10,14 Particularly, the adult stem/progenitor cell-based transplantation therapies could result in the restoration of the regeneration program in these tissues, and thereby prevent the progressive loss of functions of these adult stem/progenitor cells with aging and lead to the treatment of diverse human disorders. Among the disorders that could be treated by adult stem/progenitor cell transplantations, there are skin disorders (chronic nonhealing wounds and ulcers, ectodermal dysplasia congenital disorders); lung disorders (interstitial lung diseases, cystic fibrosis, asthma, chronic bronchitis and emphysema); chronic liver injuries (hepatitis and liver cirrhosis); gastrointestinal disorders (chronic inflammatory bowel diseases and ulcers); bone and cartilage disorders (osteoporosis, osteogenesis imperfecta); musculoskeletal disorders (Duchenne and Becker dystrophies and amyotrophic lateral sclerosis); eye diseases (partial or total limbal and/or conjunctival stem cell deficiency, bullous keratopathy, glaucoma and retinal damages) and pancreatic disorders (types 1 and 2 diabetes mellitus) (Fig. 1).9,10 More particularly, the stimulation of PSCs in vivo or transplantation of ex vivo expanded pancreatic β cells in the host disease recipient may constitute a therapeutic strategy for restoring the β cell mass lost over time in diabetic patients.10,13,14 Consistently, it has also been reported that the transplantation of purified pancreatic duct cells from islet-depleted human pancreatic tissue plus stromal cell preparation generated the insulin-producing cells in normoglycemic non-obese diabetes/severe combined immunodeficient (NOD/SCID) mice model in vivo.81 Importantly, the gene therapy using insulin-producing cells, such as human adipose tissue-derived MSCs with unfractionated cultured BM, has also been observed to be effective for treating insulinopenic patients with type 1 diabetes mellitus.82
In addition, it has been reported that the human endometrial gland-derived mesenchymal cells (EMCs) and menstrual blood-derived mesenchymal cells (MMCs) expressing CD29 and CD105 were more proliferative than MSCs from umbilical cord.83,84 These pluripotent immature cells were able to differentiate into cardiomyocytic, respiratory epithelial, neurocytic, myocytic, endothelial, pancreatic, hepatic, adipocytic, and osteogenic cells in vitro as well as trans-differentiate into cardiac tissue-layer in vivo.83,84 This suggests then that EMCs and MMCs may constitute new potential pluripotent stem cell sources, easily accessible for cell replacement-based therapy.83
On the other hand, the adult stem/progenitor cells engineered to express cytotoxic agents or antibodies targeting their malignant counterparts, cancer stem/progenitor cells and their local microenvironment also offer great promises for the development of new therapeutic approaches for treating aggressive, metastatic and recurrent cancers derived from these different tissue-resident adult stem/progenitor cells (Fig. 2).10,14,85,86 As a matter of fact, it has been shown that the use of BM-derived EPCs engineered to express a soluble truncated form of VEGFR-2 impaired tumor growth in vivo.87 MSCs engineered to produce and deliver tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) into tumor sites also caused the apoptotic death of lung (A549), breast (MDAMB231), squamous (H357) and cervical (Hela) cancer cells in coculture experiments in vitro as well as a significant reduction of xenografted tumor growth and lung metastatic tumor burden in vivo.85
CONCLUSION AND PERSPECTIVES
Together, these recent advancements in the field of the tissue-resident adult stem cell biology have led to the development of potential transplantation therapies for treating patients with diverse devastating diseases, including hematopoietic, cardiovascular and neurodegenerative diseases and aggressive and recurrent cancers.
Although important progress has been made, future studies are necessary to optimize the transplantation procedures in order to promote the functional integration, proliferation, differentiation and migration of transplanted adult stem stem/progenitor cells to damaged tissues and their long-term survival after implantation. Especially, the optimization of administration modes of tissue-resident adult stem/progenitor cell transplants and the identification of the specific intrinsic and extrinsic factors that regulate their behavior in physiological and pathological conditions is necessary for the design of new therapeutic strategies for improving the cell recovery and delivery in the specific damaged tissue areas after transplantation. These future studies should lead to more effective and safe transplantation therapies that could be translated in clinical settings for treating and even curing diverse human diseases which remain incurable in the clinics with the current conventional therapies.
ACKNOWLEDGEMENTS
The authors on this work are supported by the grants from the U.S. Department of Defense [PC04502 and PC074289] and the National Institutes of Health [Grants CA78590, CA111294, CA133774, CA131944 and CA138791].
REFERENCES
- 1.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 2.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 3.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 4.Bollini S, Smart N, Riley PR. Resident cardiac progenitor cells: at the heart of regeneration. J Mol Cell Cardiol. 2010;50:296–303. doi: 10.1016/j.yjmcc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Bernardo ME, Emons JA, Karperien M, et al. Human mesenchymal stem cells derived from bone marrow display a better chondrogenic differentiation compared with other sources. Connect Tissue Res. 2007;48:132–140. doi: 10.1080/03008200701228464. [DOI] [PubMed] [Google Scholar]
- 6.Watts C, McConkey H, Anderson L, Caldwell M. Anatomical perspectives on adult neural stem cells. J Anat. 2005;207:197–208. doi: 10.1111/j.1469-7580.2005.00448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10:S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 8.Lim DA, Huang YC, Alvarez-Buylla A. The adult neural stem cell niche: lessons for future neural cell replacement strategies. Neurosurg Clin N Am. 2007;18:81–92. doi: 10.1016/j.nec.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Mimeault M, Batra SK. Recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24:2319–2345. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- 10.Mimeault M, Batra SK. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev. 2008;4:27–49. doi: 10.1007/s12015-008-9008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mimeault M, Hauke R, Batra SK. Stem cells—A revolution in therapeutics—Recent advances on the stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82:252–264. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed F, Arseni N, Glimm H, et al. Constitutive expression of the ATP-binding cassette transporter ABCG2 enhances the growth potential of early human hematopoietic progenitors. Stem Cells. 2008;26:810–818. doi: 10.1634/stemcells.2007-0527. [DOI] [PubMed] [Google Scholar]
- 13.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 14.Mimeault M, Batra SK. Recent progress on normal and malignant pancreatic stem/progenitor cell research: therapeutic implications for the treatment of type 1 or 2 diabetes mellitus and aggressive pancreatic cancer. Gut. 2008;57:1456–1468. doi: 10.1136/gut.2008.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaltz N, Ringe J, Holzwarth C, et al. Novel markers of mesenchymal stem cells defined by genome-wide gene expression analysis of stromal cells from different sources. Exp Cell Res. 2010;316:2609–2617. doi: 10.1016/j.yexcr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Castellani C, Padalino M, China P, et al. Bone-marrow-derived CXCR4-positive tissue-committed stem cell recruitment in human right ventricular remodeling. Hum Pathol. 2010;41:1566–1576. doi: 10.1016/j.humpath.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Wu F, Xia WH, et al. CXCR4 gene transfer contributes to in vivo reendothelialization capacity of endothelial progenitor cells. Cardiovasc Res. 2010;88:462–470. doi: 10.1093/cvr/cvq207. [DOI] [PubMed] [Google Scholar]
- 18.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol. 2006;169:338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ringden O. Immunotherapy by allogeneic stem cell transplantation. Adv Cancer Res. 2007;97C:25–60. doi: 10.1016/S0065-230X(06)97002-X. [DOI] [PubMed] [Google Scholar]
- 20.Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446–4455. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon J, Choi SC, Park CY, et al. Bone marrow-derived side population cells are capable of functional cardiomyogenic differentiation. Mol Cells. 2008;25:216–223. [PubMed] [Google Scholar]
- 22.Pu DR, Liu L. HDL slowing down endothelial progenitor cells senescence: a novel anti-atherogenic property of HDL. Med Hypotheses. 2008;70:338–342. doi: 10.1016/j.mehy.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou HY, Liang HL, Wang YS, et al. A therapeutic strategy for choroidal neovascularization based on recruitment of mesenchymal stem cells to the sites of lesions. Mol Ther. 2010;45:1–4. doi: 10.1038/mt.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakitani S, Okabe T, Horibe S, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5:146–150. doi: 10.1002/term.299. [DOI] [PubMed] [Google Scholar]
- 26.Povsic TJ, O'Connor CM. Cell therapy for heart failure: the need for a new therapeutic strategy. Expert Rev Cardiovasc Ther. 2010;8:1107–1126. doi: 10.1586/erc.10.99. [DOI] [PubMed] [Google Scholar]
- 27.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez-Cerdeno V, Noctor SC, Espinosa A, et al. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell. 2010;6:238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkataramana NK, Kumar SK, Balaraju S, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson's disease. Transl Res. 2010;155:62–70. doi: 10.1016/j.trsl.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 30.Blurton-Jones M, Kitazawa M, Martinez-Coria H, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamane J, Nakamura M, Iwanami A, et al. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. J Neurosci Res. 2010;88:1394–1405. doi: 10.1002/jnr.22322. [DOI] [PubMed] [Google Scholar]
- 32.Massouh M, Saghatelyan A. De-routing neuronal precursors in the adult brain to sites of injury: role of the vasculature. Neuropharmacology. 2010;58:877–883. doi: 10.1016/j.neuropharm.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 33.Sugaya K, Kwak YD, Ohmitsu O, et al. Practical issues in stem cell therapy for Alzheimer's disease. Curr Alzheimer Res. 2007;4:370–377. doi: 10.2174/156720507781788936. [DOI] [PubMed] [Google Scholar]
- 34.Bragina O, Sergejeva S, Serg M, et al. Smoothened agonist augments proliferation and survival of neural cells. Neurosci Lett. 2010;482:81–85. doi: 10.1016/j.neulet.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 35.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, et al. Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci. 2010;30:1657–1676. doi: 10.1523/JNEUROSCI.3111-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lepski G, Jannes CE, Strauss B, et al. Survival and neuronal differentiation of mesenchymal stem cells transplanted into the rodent brain are dependent upon microenvironment. Tissue Eng Part A. 2010 doi: 10.1089/ten.TEA.2009.0686. [DOI] [PubMed] [Google Scholar]
- 37.Six EM, Bonhomme D, Monteiro M, et al. A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J Exp Med. 2007;204:3085–3093. doi: 10.1084/jem.20071003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dor FJ, Ramirez ML, Parmar K, et al. Primitive hematopoietic cell populations reside in the spleen: studies in the pig, baboon, and human. Exp Hematol. 2006;34:1573–1582. doi: 10.1016/j.exphem.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Konuma T, Oguro H, Iwama A. Role of the polycomb group proteins in hematopoietic stem cells. Dev Growth Differ. 2010;52:505–516. doi: 10.1111/j.1440-169X.2010.01191.x. [DOI] [PubMed] [Google Scholar]
- 40.Brave M, Farrell A, Ching LS, et al. FDA review summary: mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology. 2010;78:282–288. doi: 10.1159/000315736. [DOI] [PubMed] [Google Scholar]
- 41.Rossi DJ, Bryder D, Weissman IL. Hematopoietic stem cell aging: mechanism and consequence. Exp Gerontol. 2007;42:385–390. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barfield RC, Hale GA, Burnette K, et al. Autologous transplantation of CD133 selected hematopoietic progenitor cells for treatment of relapsed acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;48:349–353. doi: 10.1002/pbc.20687. [DOI] [PubMed] [Google Scholar]
- 43.Vogel W, Kopp HG, Kanz L, et al. Myeloma cell contamination of peripheral blood stem-cell grafts can predict the outcome in multiple myeloma patients after high-dose chemotherapy and autologous stem-cell transplantation. J Cancer Res Clin Oncol. 2005;131:214–218. doi: 10.1007/s00432-004-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen SL. Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. Dan Med Bull. 2007;54:112–139. [PubMed] [Google Scholar]
- 45.Hausermann P, Walter RB, Halter J, et al. Cutaneous graft-versus-host disease: a guide for the dermatologist. Dermatology. 2008;216:287–304. doi: 10.1159/000113941. [DOI] [PubMed] [Google Scholar]
- 46.Krause DS, Lazarides K, von Andrian UH, et al. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 47.Sellheyer K, Krahl D. Skin mesenchymal stem cells: Prospects for clinical dermatology. J Am Acad Dermatol. 2010;63:859–865. doi: 10.1016/j.jaad.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 48.Tu TC, Kimura K, Nagano M, et al. Identification of human placenta-derived mesenchymal stem cells involved in re-endothelialization. J Cell Physiol. 2010;226:224–235. doi: 10.1002/jcp.22329. [DOI] [PubMed] [Google Scholar]
- 49.Gindraux F, Selmani Z, Obert L, et al. Human and rodent bone marrow mesenchymal stem cells that express primitive stem cell markers can be directly enriched by using the CD49a molecule. Cell Tissue Res. 2007;327:471–483. doi: 10.1007/s00441-006-0292-3. [DOI] [PubMed] [Google Scholar]
- 50.Martin J, Helm K, Ruegg P, et al. Adult lung side population cells have mesenchymal stem cell potential. Cytotherapy. 2008;10:140–151. doi: 10.1080/14653240801895296. [DOI] [PubMed] [Google Scholar]
- 51.Shanti RM, Li WJ, Nesti LJ, et al. Adult mesenchymal stem cells: biological properties, characteristics, and applications in maxillofacial surgery. J Oral Maxillofac Surg. 2007;65:1640–1647. doi: 10.1016/j.joms.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Yoshikawa T, Mitsuno H, Nonaka I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860–877. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- 53.Wang CH, Cherng WJ, Yang NI, et al. Late-outgrowth endothelial cells attenuate ntimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arterioscler Thromb Vasc Biol. 2007;28:54–60. doi: 10.1161/ATVBAHA.107.147256. [DOI] [PubMed] [Google Scholar]
- 54.Haider HK, Elmadbouh I, Jean-Baptiste M, et al. Non-viral vector gene modification of stem cells for myocardial repair. Mol Med. 2008;14:79–86. doi: 10.2119/2007-00092.Haider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang J, Wang J, Zheng F, et al. Combination of chemokine and angiogenic factor genes and mesenchymal stem cells could enhance angiogenesis and improve cardiac function after acute myocardial infarction in rats. Mol Cell Biochem. 2010;339:107–118. doi: 10.1007/s11010-009-0374-0. [DOI] [PubMed] [Google Scholar]
- 56.McMullen NM, Pasumarthi KB. Donor cell transplantation for myocardial disease: does it complement current pharmacological therapies? Can J Physiol Pharmacol. 2007;85:1–15. doi: 10.1139/Y06-105. [DOI] [PubMed] [Google Scholar]
- 57.Sohn RL, Jain M, Liao R. Adult stem cells and heart regeneration. Expert Rev Cardiovasc Ther. 2007;5:507–517. doi: 10.1586/14779072.5.3.507. [DOI] [PubMed] [Google Scholar]
- 58.Ahmadi H, Baharvand H, Ashtiani SK, et al. Safety analysis and improved cardiac function following local autologous transplantation of CD133(+) enriched bone marrow cells after myocardial infarction. Curr Neurovasc Res. 2007;4:153–160. doi: 10.2174/156720207781387141. [DOI] [PubMed] [Google Scholar]
- 59.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 60.Kandasamy M, Couillard-Despres S, Raber KA, et al. Stem cell quiescence in the hippocampal neurogenic niche is associated with elevated transforming growth factor-beta signaling in an animal model of Huntington disease. J Neuropathol Exp Neurol. 2010;69:717–728. doi: 10.1097/NEN.0b013e3181e4f733. [DOI] [PubMed] [Google Scholar]
- 61.Pardal R, Ortega-Saenz P, Duran R, et al. Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell. 2007;131:364–377. doi: 10.1016/j.cell.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 62.Delcroix GJ, Schiller PC, Benoit JP, et al. Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials. 2010;31:2105–2120. doi: 10.1016/j.biomaterials.2009.11.084. [DOI] [PubMed] [Google Scholar]
- 63.Lee ST, Chu K, Park JE, et al. Intravenous administration of human neural stem cells induces functional recovery in Huntington's disease rat model. Neurosci Res. 2005;52:243–249. doi: 10.1016/j.neures.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 64.Keene CD, Chang RC, Lopez-Yglesias AH, et al. Suppressed accumulation of cerebral amyloid {beta} peptides in aged transgenic alzheimer's disease mice by transplantation with wild-type or prostaglandin E2 receptor subtype 2-null bone marrow. Am J Pathol. 2010;177:346–354. doi: 10.2353/ajpath.2010.090840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee HJ, Lee JK, Lee H, et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer's disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.03.024. in press. [DOI] [PubMed] [Google Scholar]
- 66.Freedman MS, Bar-Or A, Atkins HL, et al. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler. 2010;16:503–510. doi: 10.1177/1352458509359727. [DOI] [PubMed] [Google Scholar]
- 67.Murrell W, Wetzig A, Donnellan M, et al. Olfactory mucosa is a potential source for autologous stem cell therapy for Parkinson's disease. Stem Cells. 2008;26:2183–2192. doi: 10.1634/stemcells.2008-0074. [DOI] [PubMed] [Google Scholar]
- 68.Jones J, Jaramillo-Merchan J, Bueno C, et al. Mesenchymal stem cells rescue Purkinje cells and improve motor functions in a mouse model of cerebellar ataxia. Neurobiol Dis. 2010;40:415–423. doi: 10.1016/j.nbd.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Harms KM, Li L, Cunningham LA. Murine neural stem/progenitor cells protect neurons against ischemia by HIF-1alpha-regulated VEGF signaling. PLoS ONE. 2010;5:e9767. doi: 10.1371/journal.pone.0009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou Z, Jiang X, Zhang W, et al. Efficacy of Tyrosine Hydroxylase gene modified neural stem cells derived from bone marrow on Parkinson's disease—a rat model study. Brain Res. 2010;1346:279–286. doi: 10.1016/j.brainres.2010.05.071. [DOI] [PubMed] [Google Scholar]
- 71.Oh JS, Ha Y, An SS, et al. Hypoxia-preconditioned adipose tissue-derived mesenchymal stem cell increase the survival and gene expression of engineered neural stem cells in a spinal cord injury model. Neurosci Lett. 2010;472:215–219. doi: 10.1016/j.neulet.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Somoza R, Juri C, Baes M, et al. Intranigral transplantation of epigenetically-induced BDNF-secreting human mesenchymal stem cells: implications for cell-based therapies in Parkinson s disease. Biol Blood Marrow Transplant. 2010;16:1530–1540. doi: 10.1016/j.bbmt.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 73.Chang DY, Yoo SW, Hong Y, et al. The growth of brain tumors can be suppressed by multiple transplantation of mesenchymal stem cells expressing cytosine deaminase. Int J Cancer. 2010;127:1975–1983. doi: 10.1002/ijc.25383. [DOI] [PubMed] [Google Scholar]
- 74.Kim SU. Neural Stem Cell-based Gene Therapy for Brain Tumors. Stem Cell Rev. 2011;7:130–140. doi: 10.1007/s12015-010-9154-1. [DOI] [PubMed] [Google Scholar]
- 75.Wu W, Chen X, Hu C, et al. Transplantation of neural stem cells expressing hypoxia-inducible factor-1alpha (HIF-1alpha) improves behavioral recovery in a rat stroke model. J Clin Neurosci. 2010;17:92–95. doi: 10.1016/j.jocn.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 76.Mercapide J, Rappa G, Anzanello F, et al. Primary gene-engineered neural stem/progenitor cells demonstrate tumor-selective migration and antitumor effects in glioma. Int J Cancer. 2010;126:1206–1215. doi: 10.1002/ijc.24809. [DOI] [PubMed] [Google Scholar]
- 77.Rattigan Y, Hsu JM, Mishra PJ, et al. Interleukin 6 mediated recruitment of mesenchymal stem cells to the hypoxic tumor milieu. Exp Cell Res. 2010;316:3417–3424. doi: 10.1016/j.yexcr.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Ehtesham M, Kabos P, Kabosova A, et al. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–5663. [PubMed] [Google Scholar]
- 79.Ehtesham M, Kabos P, Gutierrez MA, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–7174. [PubMed] [Google Scholar]
- 80.Mercapide J, Rappa G, Anzanello F, et al. Primary gene-engineered neural stem/progenitor cells demonstrate tumor-selective migration and antitumor effects in glioma. Int J Cancer. 2010;126:1206–1215. doi: 10.1002/ijc.24809. [DOI] [PubMed] [Google Scholar]
- 81.Wu DC, Byod AS, Wood KJ. Embryonic stem cell transplantation: potential applicability in cell replacement therapy and regenerative medicine. Front Biosci. 2007;12:4525–4535. doi: 10.2741/2407. [DOI] [PubMed] [Google Scholar]
- 82.Trivedi HL, Vanikar AV, Thakker U, et al. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplant Proc. 2008;40:1135–1139. doi: 10.1016/j.transproceed.2008.03.113. [DOI] [PubMed] [Google Scholar]
- 83.Hida N, Nishiyama N, Miyoshi S, et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26:1695–1704. doi: 10.1634/stemcells.2007-0826. [DOI] [PubMed] [Google Scholar]
- 84.Meng X, Ichim TE, Zhong J, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57. doi: 10.1186/1479-5876-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loebinger MR, Eddaoudi A, Davies D, et al. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zischek C, Niess H, Ischenko I, et al. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann Surg. 2009;250:747–753. doi: 10.1097/SLA.0b013e3181bd62d0. [DOI] [PubMed] [Google Scholar]
- 87.Davidoff AM, Ng CY, Brown P, et al. Bone marrow-derived cells contribute to tumor neovasculature and, when modified to express an angiogenesis inhibitor, can restrict tumor growth in mice. Clin Cancer Res. 2001;7:2870–2879. [PubMed] [Google Scholar]