Abstract
The breast cancer resistance protein (Bcrp) is an efflux transporter that participates in the biliary and renal excretion of drugs and environmental chemicals. Recent evidence suggests that pharmacological activation of the peroxisome proliferator activated receptor alpha (PPARα) can up-regulate the hepatic expression of Bcrp. The current study investigated the regulation of hepatic and renal Bcrp mRNA and protein in mice treated with the PPARα agonist perfluorooctanoic acid (PFOA) and the ability of PFOA to alter human BCRP function in vitro. Bcrp mRNA and protein expression were quantified in the livers and kidneys of male C57BL/6 mice treated with vehicle or PFOA (1 or 3 mg/kg/day oral gavage) for 7 days. PFOA treatment increased liver weights as well as the hepatic mRNA and protein expression of the PPARα target gene, cytochrome P450 4a14. Compared to vehicle-treated control mice, PFOA increased hepatic Bcrp mRNA and protein between 1.5- and 3-fold. Immunofluorescent staining confirmed enhanced canalicular Bcrp staining in liver sections from PFOA-treated mice. The kidney expression of cytochrome P450 4a14 mRNA, but not Bcrp, was increased in mice treated with PFOA. Micromolar concentrations of PFOA decreased human BCRP ATPase activity and inhibited BCRP-mediated transport in inverted membrane vesicles. Together, these studies demonstrate that PFOA induces hepatic Bcrp expression in mice and may inhibit human BCRP transporter function at concentrations that exceed levels observed in humans.
Keywords: ABCG2, BCRP, PFOA, PPARα, transporter
1. Introduction
During the last century, perfluorooctanoic acid (PFOA) became a frequent intermediate in the production of non-stick cookware, lubricants, cosmetics, fire fighting foam, hydraulic fluid, carpets, upholstery, and other commercial products. PFOA is a synthetic fluorinated compound with an eight carbon backbone. Because carbon-fluorine bonds are stable, PFOA resists degradation and metabolism, which has led to its bioaccumulation and persistence in the environment. Moreover, PFOA is negligibly cleared in humans, which leads to a long serum half-life of 2.3 to 3.5 years (Olsen et al., 2007; Bartell et al., 2010). A number of adverse health effects including hyperuricemia (Steenland et al., 2010) and hypercholesterolemia (Costa et al., 2009; Steenland et al., 2009; Frisbee et al., 2010) have been reported in workers occupationally exposed to PFOA as well as individuals who lived or worked in districts with water contaminated by PFOA from a chemical plant. Because of the environmental persistence, limited excretion in humans, and the potential adverse effects of PFOA, the U.S. Environmental Protection Agency has called for a voluntary phase-out by eight major manufacturers (reviewed in Post et al., 2012). Despite this effort, further human exposure is expected due to the use of existing PFOA-containing products and the potential for other domestic and international companies to continue production (reviewed in Post et al., 2012).
PFOA is an agonist of the rodent and human nuclear receptor peroxisome proliferator activated receptor alpha (PPARα) (Maloney and Waxman, 1999). Activation of PPARα in rodents is associated with increased liver weight, proliferation of peroxisomes, tumorigenesis, and enhanced catalase activity (Reddy and Qureshi, 1979; Ikeda et al., 1985; Issemann and Green, 1990; Uy-Yu et al., 1990). Hepatic activation of PPARα signaling by PFOA is often monitored by up-regulation of cytochrome (Cyp) P450 4a genes in rodents (Sohlenius et al., 1992; Diaz et al., 1994). While humans do not exhibit hepatic peroxisome proliferation nor tumorigenesis following PPARα activation (reviewed in Reddy and Lalwai, 1983; Klaunig et al., 2003), mouse and human PPARα do share a number of target genes involved in metabolism and transport following activation by PFOA (Nakamura et al., 2009; Bjork et al., 2011). More recent work has also demonstrated that PFOA and related compounds such as perfluorodecanoic acid can activate other nuclear receptors including the constitutive androstane receptor and the pregnane x receptor (Cheng and Klaassen, 2008; Rosen et al., 2008a; Bjork et al., 2011).
Breast cancer resistance protein (Bcrp) is an efflux transporter encoded by the ATP binding cassette G subfamily, isoform 2 (Abcg2) gene. The rodent transporter is denoted by lowercase letters (Bcrp) and the human isoform is written in capitalized letters (BCRP). Bcrp/BCRP is widely expressed on the apical epithelial cell surface of excretory tissues such as the liver, kidney, and intestines as well as in sensitive organs such as the placenta, testes, and brain (reviewed in Klaassen and Aleksunes, 2010). BCRP transports a wide array of endogenous substrates, including urate and folate, as well as anticancer drugs, sulfasalazine, and glyburide (reviewed in Klaassen and Aleksunes, 2010). In the liver and kidneys, Bcrp secretes chemicals into the bile and urine, respectively. PFOA is readily excreted in the urine of rats (Vanden Heuvel et al., 1991; 1992). Prior studies point to a role for organic anion transporters (OATs) in the renal secretion and reabsorption of PFOA (Weaver et al., 2010; Yang et al., 2010; Han et al., 2012). However, it is unknown whether efflux transporters such as Bcrp can contribute to its renal secretion.
Recent evidence suggests that xenobiotic activation of PPARα can up-regulate the hepatic and brain expression of Bcrp (Moffit et al., 2006; Hirai et al., 2007; Hoque et al., 2012). However, little is known about the ability of the PPARα agonist PFOA to regulate Bcrp expression and function. Therefore, the purpose of the present study was to 1) determine whether PFOA treatment alters the mRNA and protein expression of Bcrp in the liver and kidneys of mice and 2) assess the ability of PFOA to activate and inhibit human BCRP ATPase activity and transport in vitro.
2. Materials and Methods
2.1. Chemicals
Unless otherwise specified, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
2.2. Animal Treatment
Adult male C57BL/6 mice (027 strain) were purchased from Charles River Laboratories (Wilmington, MA). Mice (n=6) were dosed daily with vehicle (deionized water, 10 ml/kg), 1, or 3 mg/kg/day of perfluorooctanoic acid ammonium salt (77262, Sigma-Aldrich, St. Louis, MO) for seven days by oral gavage. PFOA doses and dosing regimen were previously used to investigate the regulation of hepatic nuclear receptor pathways in mice (Rosen et al., 2008b). Terminal weights were recorded and mice were sacrificed by decapitation 24 hours following the final dose. Organs and tissues were collected and weighed, snap frozen in liquid nitrogen, and stored at −80°C until use for RNA and protein analyses. The Rutgers University Institutional Animal Care and Use Committee approved these studies.
2.3. RNA Isolation
Total RNA was isolated using RNABee (Tel-Test, Friendswood, TX) and the RNeasy Midi Kit according to the manufacturers’ recommendations (Qiagen, Valencia, CA). RNA integrity was confirmed using an Amersco Formaldehyde-Free RNA Gel Kit (ISC Bioexpress, Kaysville, UT). Total RNA concentrations were quantified at 260 nm using a NanoDrop Spectrophotometer (Thermo Scientific, Rockford, IL).
2.4. Branched DNA (bDNA) Analysis
The mRNA expression of mouse Cyp4a14 and Bcrp were quantified using the bDNA signal amplification assay (Panomics QuantiGene, High Volume bDNA Signal Amplification Kit 1.0; Affymetrix, Santa Clara, CA). Multiple oligonucleotide probe sets (containing capture, label, and blocker probes) specific to mouse mRNA transcripts were designed previously using ProbeDesigner software (version 1.0; Bayer Corp., Diagnostics Div., Tarrytown, NY). Probe sequences for Bcrp and Cyp4a14 were published previously (Cheng et al., 2005; Tanaka et al., 2005). Luminescence was read on a Spectramax luminometer (Molecular Devices, Sunnyville, CA).
2.5. Western Blot Analysis
Livers and kidneys were homogenized in sucrose-Tris buffer (10 mM tris base, 250 mM sucrose) containing 1% protease inhibitor cocktail (Sigma P8340). Protein concentrations were measured using a bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL). Magic Mark XP molecular weight markers (Life Technologies, Carlsbad, CA) or fifty μg of protein homogenate were loaded per well onto an 8% SDS-PAGE resolving gel (XCell SureLock Midi Gel System; Life Technologies) and run at 100 volts at room temperature for 3 hours. Proteins were transblotted onto a nitrocellulose membrane in a 7-minute transfer (iBlot, Life Technologies). Membranes were then blocked with 5% non-fat dry milk in 0.5% PBS/Tween-20 overnight at 4°C. Primary antibodies were hybridized overnight at 4°C. The following primary antibodies were used: Cyp4a14 (sc-46087 1:1000 dilution, Santa Cruz Biotechnology, Santa Cruz, CA), Bcrp (Bxp-53 1:5000 dilution, Enzo Life Sciences, Farmingdale, NY) and beta actin (ab8227 1:2000 dilution, Abcam, Cambridge, MA). Following incubation with species-appropriate secondary antibodies, membranes were washed and subsequently incubated with 2 ml of SuperSignal West Dura Extended Duration Substrate for 2 minutes (Thermo Scientific, Rockford, IL). Protein band intensity was semi-quantified using a FluorChem Imager (ProteinSimple, Santa Clara, CA).
2.6. Indirect Immunofluorescence
Cryosections (5 μm) were allowed to warm to room temperature. Slides were fixed for 5 minutes in a coplin jar with 4% paraformaldehyde and then rinsed with phosphate-buffered saline (PBS) for 5 minutes. Goat serum (5%) in 0.1% triton X in PBS was used to block slides for 60 minutes. Primary antibody (Bxp-53, 1:100 in 5% goat serum/PBS-Triton) was added and incubated overnight at 4°C. Slides were rinsed with PBS and then incubated in secondary antibody (Antirat AlexiFluor 488 IgG, 1:100 in 5% goat serum/PBS-Triton) for 60 minutes (Life Technologies). Sections were air dried and mounted in Prolong Gold with 4′,6-diamidino-2-phenylindole (Life Technologies). Images were acquired using a Zeiss Observer D1 microscope and an x-cite series 120Q fluorescent illuminator (Zeiss Inc., Thornwood, NY) and a Jenoptik camera with ProgRes CapturePro 2.8 software (Jenoptik, Easthampton, MA). All sections were both stained and imaged under the same conditions for each tissue. Negative controls did not contain primary antibody and were included to ensure minimal non-specific staining (data not shown).
2.7. Human BCRP ATPase Assay
BCRP-expressing membranes were purchased from Xenotech (Lenexa, KS). The ATPase assay was used to quantify the activation and inhibition of transporter-dependent ATPase activity by PFOA according to the manufacturer’s protocol. Activation. To determine activation, plasma membranes from Spodoptera frugiperda (Sf9) cells transfected with human BCRP were incubated at 37°C in 96-well plates with assay medium, 2 mM ATP, and PFOA (0.03–100 μM) in the presence and absence of 1.2 mM sodium orthovanadate for 30 minutes. Sodium orthovanadate inhibits the ATPase function of ABC transporters and was used to calculate vanadate-sensitive activity by subtraction from the total ATPase activity. Inhibition. Inhibition was analyzed in a similar manner, with the addition of a specific BCRP activator (sulfasalazine, 10 μM) to the assay medium. Hoechst 33342 (0.1 mM) was used as a positive control for inhibitory activity. Following incubation of membranes at 37°C with a colorimetric reagent, liberation of inorganic phosphate was observed and quantified by absorption at 610 nm.
2.8. Human BCRP Membrane Vesicles
Control and human BCRP membrane vesicles (5 mg/ml, 500 μl) were purchased from Cellz Direct (Durham, NC). Vesicles were generated from the plasma membranes of Sf9 insect cells infected with a baculovirus expressing the human BCRP transproter. ATP-dependent transport of Lucifer yellow (50 μM) over a 10 minute period at 37°C was used to quantify BCRP activity in membrane vesicles (20 μg) according to the manufacturer’s recommendation. Control vesicles and no MgATP incubations were used as negative controls to account for background diffusion of Lucifer yellow into vesicles. Reactions were terminated with ice-cold stopping buffer (40 mM MOPS-Tris, 70 mM KCl, 7.5 mM MgCl2) and vesicles were washed in a 96-well filter plate using vacuum filtration (Millipore, Billerica, MA). Vesicles were solubilized by addition of 50% methanol for 15 minutes at room temperature and vesicle contents were filtered through to a new 96-well plate. Lucifer yellow fluorescence was read at an excitation wavelength of 430 nm and emission wavelength of 538 nm.
2.9. Statistical Analysis
Data are presented as mean ± standard error (SE). GraphPad Prism® version 5 software (GraphPad Software, La Jolla, CA) was used for statistical analysis. Differences among groups were determined by a one-way analysis of variance with a Newman-Keuls posthoc test for multiple comparisons. Differences were considered statistically significant at p<0.05.
3. Results
3.1. Hepatic and kidney weights in PFOA-treated mice
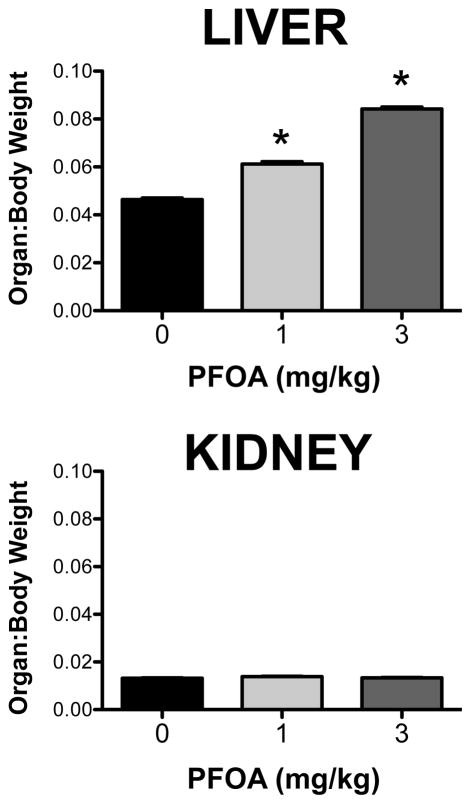
Treatment of mice with 1 and 3 mg/kg PFOA daily for 7 days increased liver weights by 38% and 91%, respectively (data not shown). Likewise, the liver-to-body weight ratios increased in a dose-dependent manner in PFOA-treated mice (Figure 1). Conversely, PFOA did not alter the absolute nor the relative kidney weights compared to vehicle-treated mice.
Figure 1. Liver and kidney weights in PFOA-treated mice.
Adult male C57BL/6 mice were treated with vehicle or PFOA daily for 7 days by oral gavage and tissues were collected 24 hours after the final dose. Terminal body and organ weights were recorded during necropsy. Organ weights are presented as ratios relative to body weight (n=6). Data are shown as mean ± SE. Asterisks (*) represent statistically significant differences (p < 0.05) compared to 0 mg/kg control mice.
3.2. Hepatic and renal expression of Cyp4a14 and Bcrp mRNA in PFOA-treated mice
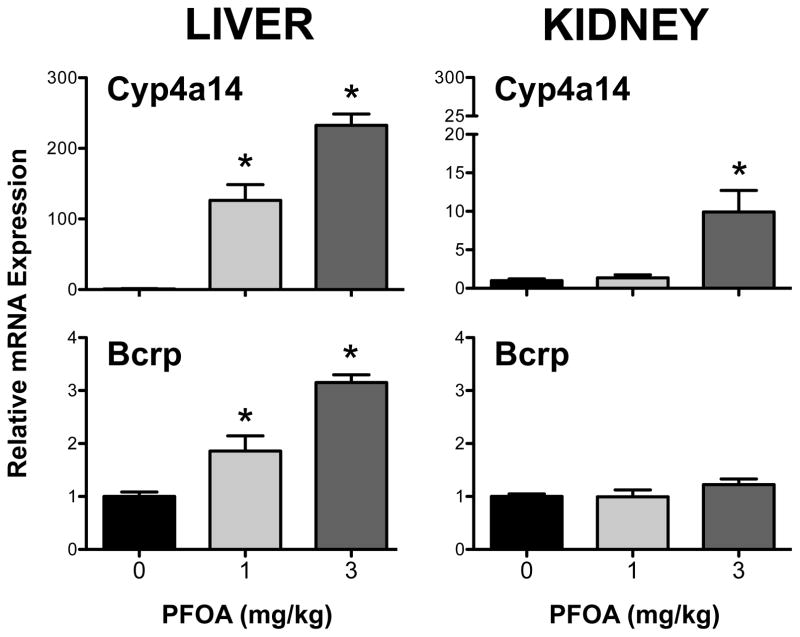
Compared to vehicle-treated mice, Cyp4a14 mRNA was induced more than 100-fold in the liver at both PFOA doses. A 10-fold up-regulation was observed only in kidneys of mice treated with 3 mg/kg PFOA (Figure 2). Bcrp mRNA was increased 1.9- and 3.2-fold in the livers of mice treated with 1 mg/kg and 3 mg/kg PFOA, respectively. No change in kidney Bcrp mRNA was observed in PFOA-treated mice.
Figure 2. Messenger RNA expression of Cyp4a14 and Bcrp in livers and kidneys of PFOA-treated mice.
Quantification of mRNA in livers and kidneys was determined after 7-day exposure to PFOA using the bDNA assay. Induction of Cyp4a14 mRNA was used as positive control for PPARα activation. Data are presented as mean ± SE (n=5). Asterisks (*) represent statistically significant differences (p < 0.05) compared to 0 mg/kg control mice.
3.3. Hepatic and renal expression of Cyp4a14 and Bcrp protein in PFOA-treated mice
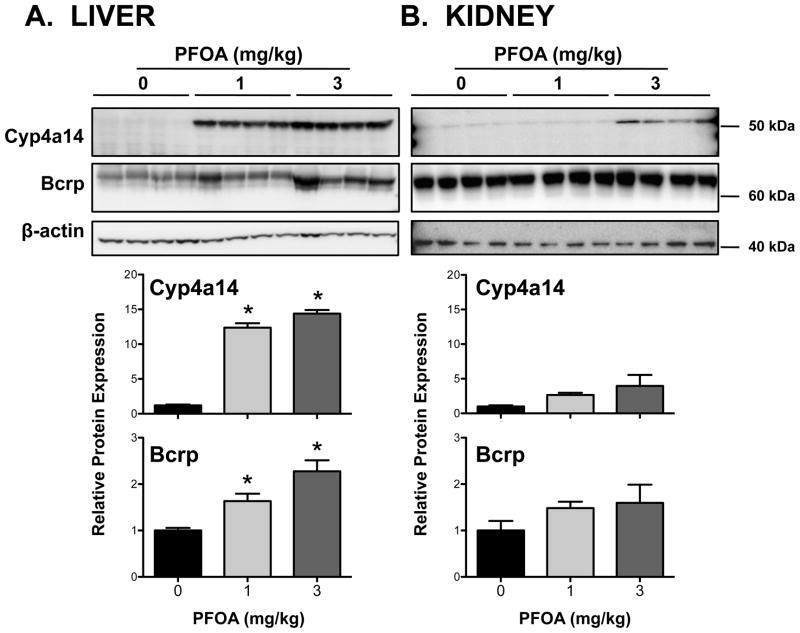
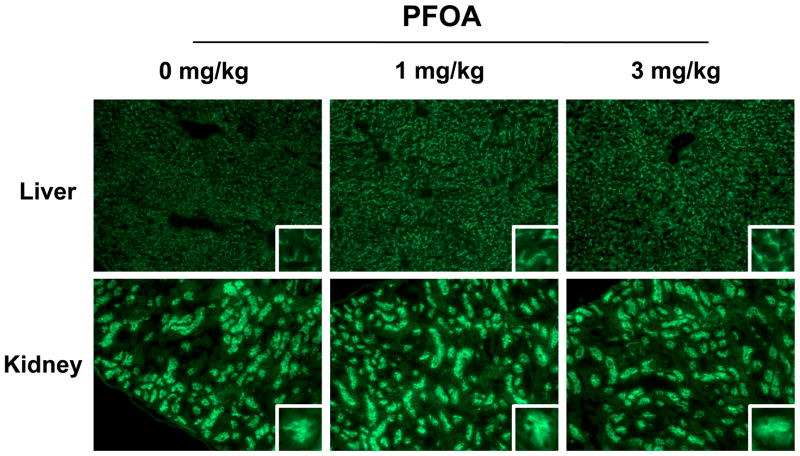
Similar to mRNA expression, levels of Cyp4a14 protein were increased up to 15-fold in livers of PFOA-treated mice (Figure 3A). Likewise, Cyp4a14 protein was elevated up to 4-fold in the kidneys, however, it was not statistically significant (Figure 3B). Bcrp protein was detected in the liver and kidneys with a molecular weight estimated to be 72–74 kDa (Figures 3A and 3B), which is consistent with the published size in the kidneys and livers of wild-type, but not Bcrp-null mice (Jonker et al., 2002). In the kidneys, an additional faint band was also observed at 33–34 kDa (data not shown). Consistent with Cyp4a14, Bcrp protein expression in livers was increased up to 2.3-fold in response to PFOA with no change observed in the kidneys. Immunofluorescent staining revealed apical localization of BCRP on hepatocytes and proximal tubule epithelial cells (Figure 4). PFOA treatment increased Bcrp staining intensity throughout the livers of mice; no change was observed in the kidneys. It is presumed that the staining in the kidneys is primarily due to the ~72–74kDa protein detected on western blot. In the liver, the extent of Bcrp staining was similar between centrilobular and periportal regions suggesting that the induction following PFOA treatment was not limited to discrete regions of the liver.
Figure 3. Protein expression of Cyp4a14 and Bcrp in livers and kidneys of PFOA-treated mice.
Semi-quantification of protein expression was determined after 7-day exposure to PFOA using western blot analysis. Induction of Cyp4a14 protein was used as positive control for PPARα activation in PFOA-treated mice. Molecular weight markers are designated on the right side of the blot images. β-actin was used as a loading control. The western blot data are presented as individual blots and mean relative protein expression (normalized to β-actin). Data are presented as mean ± SE (n=4). Asterisks (*) represent statistically significant differences (p < 0.05) compared to 0 mg/kg control mice.
Figure 4. Immunofluorescent staining of Bcrp in livers and kidneys of PFOA-treated mice.
Indirect immunofluorescence staining of the apical Bcrp transporter (green) was conducted on liver and kidney cryosections after 7-day exposure to PFOA. Representative regions are shown. Magnification, x100. Images were cropped, enlarged, and provided as insets.
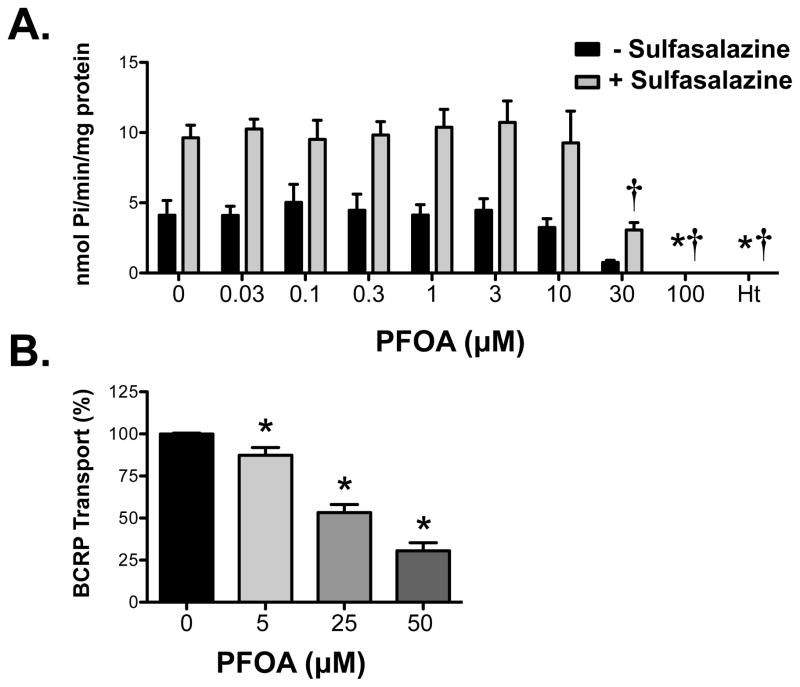
3.4. PFOA inhibition of human BCRP ATPase activity and transport in inverted membrane vesicles
Plasma membranes expressing human BCRP were used to indirectly test whether PFOA is a substrate by quantifying the liberation of inorganic phosphate following ATP hydrolysis. Increasing concentrations of PFOA did not alter the rate of ATP hydrolysis in BCRP-expressing membranes (Figure 5A). At high concentrations of PFOA (30 and 100 μM), the constitutive activity of BCRP was reduced below baseline. Additional experiments aimed to determine whether PFOA can inhibit BCRP activity. For this purpose, BCRP-expressing membranes were activated with the substrate sulfasalazine (Figure 5A). High concentrations of PFOA (30 and 100 μM) decreased BCRP hydrolysis of ATP in a manner similar to the inhibitor Hoechst 33342. In addition, PFOA inhibited BCRP-mediated transport of Lucifer yellow in inverted vesicles from BCRP-expressing cells at concentrations ranging between 5 and 50 μM (Figure 5B).
Figure 5. Interaction of PFOA with human BCRP transporter.
(A) BCRP membranes were incubated with ATP and varying concentrations of PFOA in the presence and absence of sulfasalazine (10 μM) for 30 minutes. Additional reactions included sodium orthovanadate in order to determine the ATPase activity attributed to transport. The amount of inorganic phosphate released was determined by spectrophotometry following addition of a colorimetric reagent. Hoechst 33342 (Ht) was used as a positive control inhibitor of BCRP activity. Data are presented as vanadate-sensitive ATPase activity from 3 separate experiments expressed as mean ± SE (n=3). Asterisks (*) represent statistically significant differences (p < 0.05) compared to 0 μM PFOA in the absence of sulfasalazine. Daggers (†) represent statistically significant differences (p < 0.05) compared to 0 μM PFOA in the presence of sulfasalazine. (B) Inverted BCRP-expressing vesicles (20 μg) were incubated with Lucifer yellow (50 μM) for 10 minutes in the presence and absence of ATP and increasing concentrations of PFOA. Data are presented as mean ± SE (n=5) normalized to maximal ATP-dependent BCRP activity (no PFOA) from 2 separate experiments. Asterisks (*) represent statistically significant differences (p < 0.05) compared to 0 μM PFOA.
4. Discussion
The present study demonstrated that daily treatment of mice with PFOA for 7 days increased Bcrp mRNA and protein in liver, but not in kidneys. Elevated hepatic Bcrp expression was associated with markers of peroxisome proliferation including up-regulation of Cyp4a14 mRNA and protein as well as hepatomegaly; this suggests that PPARα-mediated signaling may regulate Bcrp in livers of PFOA-treated mice. Little activation of PPARα was observed in kidneys with an increase in Cyp4a14 mRNA seen only at the highest PFOA dose. Using in vitro models of human BCRP transport, it was also demonstrated that PFOA can inhibit BCRP-mediated ATP hydrolysis and efflux at high micromolar concentrations. These values can be compared to the circulating levels found in individuals thought to be highly exposed to PFOA. The mean serum concentration of PFOA in Ohio and West Virginia residents has been reported as 69.2 – 80.3 ng/ml (~0.2 μM) following high exposure to PFOA (Steenland et al., 2009; Frisbee et al., 2010). As a result, it is unlikely that BCRP-mediated transport can be inhibited at concentrations of PFOA achieved in humans exposed occupationally or in contaminated locations.
Evidence suggests that PPARα plays a role in mediating Bcrp basal and inducible expression in the livers of mice (Moffit et al., 2006). Exposure of CD-1 mice to clofibrate, a PPARα agonist, significantly up-regulates hepatic Bcrp mRNA and protein. Additionally, mice lacking PPARα exhibited decreased constitutive Bcrp protein and impaired induction of Bcrp mRNA in response to clofibrate treatment (Moffit et al., 2006). Similarly, Bcrp mRNA is induced by other PPARα agonists (Wy 1463 and GW7647) in the livers of wild-type mice, but not PPARα-null mice (Hirai et al., 2007). However, no change in Bcrp expression was observed in the intestines of either genotype. This suggests tissue-specific PPARα-mediated Bcrp regulation. Similarly, the current study demonstrated Bcrp up-regulation in the liver, but not the kidneys, of PFOA-treated mice. More recently, the ability of human PPARα to regulate BCRP mRNA and protein expression was demonstrated in hCMEC/D3 endothelial cells, which serve as a model of the human blood-brain barrier (Hoque et al., 2012). Down-regulation of PPARα using small interfering RNAs reduced BCRP expression and function. It was also observed that PPARα binding to the BCRP/ABCG2 promoter region increased when hCMEC/D3 cells were exposed to clofibrate (Hoque et al., 2012). Results from this study provided direct evidence of PPARα regulation of BCRP in human brain microvessel endothelial cells. In addition to PPARα, recent evidence suggests that ligand-activated PPARγ can also up-regulate BCRP mRNA in dendritic cells by directly binding to a conserved enhancer region in the promoter of the human BCRP/ABCG2 gene (Szatmari et al., 2006). Based on the ability of PPARα and PPARγ to transactivate the human BCRP/ABCG2 gene, future studies should be aimed at determining whether PFOA can upregulate BCRP in human hepatocytes at environmentally-relevant concentrations. Furthermore, PFOA can activate signaling through the constitutive androstane receptor and the pregnane x receptor (Cheng and Klaassen, 2008; Rosen et al., 2008a; Bjork et al., 2011). Additional studies are needed to determine whether these transcription factors can up-regulate the expression of Bcrp mRNA in the liver.
Using in vitro expression systems, it has been demonstrated that OATs and organic anion transporting polypeptides (OATPs) can transport PFOA and other perfluorinated carboxylates of various carbon backbone lengths (Weaver et al., 2010). The basolateral Oat1/OAT1, Oat3/OAT3, and Oatp1a1 and apical Oat2 and urate (URAT) transporters increase the in vitro uptake of PFOA (Nakagawa et al., 2008; Weaver et al., 2010). Similarly, human OAT4 and URAT proteins can influx PFOA into cells which may enhance renal PFOA reabsorption and contribute to its long half-life in humans (Yang et al., 2010). It is currently unknown whether apical efflux transporters participate in the renal disposition of PFOA. Based on initial data obtained from the ATPase assay (Figure 5A), PFOA does not appear to be a substrate of BCRP however direct transport measures are needed to confirm these findings. Likewise, the multidrug resistance-associated protein 2 is not thought to secrete PFOA into urine (Katakura et al., 2007). Thus, further investigation is needed to identify the efflux transporter(s) involved in the renal secretion of PFOA.
In conclusion, PFOA up-regulates Bcrp mRNA and protein only in the livers of mice with no change observed in the kidneys. While it is unknown whether the doses of PFOA used can similarly up-regulate BCRP following environmental or occupational exposure, these findings add to our mechanistic understanding of PFOA target genes. Functional in vitro studies suggest that PFOA may inhibit BCRP transport at concentrations that exceed levels observed in humans.
Highlights.
PFOA increased liver weight and Cyp4a14 mRNA and protein expression in mice.
PFOA increased kidney Cyp4a14 mRNA in mice.
PFOA increased Bcrp mRNA and protein in livers, but not kidneys, of mice.
PFOA inhibited activation of human BCRP ATPase activity in vitro.
PFOA inhibited human BCRP transport in inverted membrane vesicles.
Acknowledgments
The authors thank Mrs. Myrna Trumbauer for assistance with dosing mice and tissue collections. This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK080774] and the National Institutes of Health National Institute of Environmental Health Sciences [Grants ES020522, ES005022, ES020721, ES007148]. Michael Little was supported by an institutional summer research training grant from the American Society for Pharmacology and Experimental Therapeutics. Kristin Bircsak was supported by a Predoctoral Fellowship from the American Foundation for Pharmaceutical Education.
Abbreviations
- Abc
ATP-binding cassette
- Bcrp
breast cancer resistance protein
- bDNA
branched DNA
- Cyp4a14
cytochrome P450 4a14
- Oat
organic anion transporter
- Oatp
organic anion transporting polypeptide
- PPARα
peroxisome proliferator-activated receptor alpha
- PFOA
perfluorooctanoic acid
- SE
standard error
- Sf9
Spodoptera frugiperda 9
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lobna M. Eldasher, Email: lmeldasher@gmail.com.
Xia Wen, Email: wen@eohsi.rutgers.edu.
Michael S. Little, Email: littlem4@mail.montclair.edu.
Kristin M. Bircsak, Email: kbircsak@eden.rutgers.edu.
Lindsay L. Yacovino, Email: lindsay.yacovino@gmail.com.
Lauren M. Aleksunes, Email: aleksunes@eohsi.rutgers.edu.
References
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect. 2010;118:222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JA, Butenhoff JL, Wallace KB. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology. 2011;288:8–17. doi: 10.1016/j.tox.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Cheng X, Klaassen CD. Perfluorocarboxylic acids induce cytochrome P450 enzymes in mouse liver through activation of PPAR-alpha and CAR transcription factors. Toxicol Sci. 2008;106:29–36. doi: 10.1093/toxsci/kfn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Maher J, Dieter MZ, Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos. 2005;33:1276–1282. doi: 10.1124/dmd.105.003988. [DOI] [PubMed] [Google Scholar]
- Costa G, Sartori S, Consonni D. Thirty years of medical surveillance in perfluooctanoic acid production workers. J Occup Environ Med. 2009;51:364–372. doi: 10.1097/JOM.0b013e3181965d80. [DOI] [PubMed] [Google Scholar]
- Diaz MJ, Chinje E, Kentish P, Jarnot B, George M, Gibson G. Induction of cytochrome P4504A by the peroxisome proliferator perfluoro-n-octanoic acid. Toxicology. 1994;86:109–122. doi: 10.1016/0300-483x(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, Ducatman AM. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Arch Pediatr Adolesc Med. 2010;164:860–869. doi: 10.1001/archpediatrics.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW. Renal elimination of perfluorocarboxylates (PFCAs) Chem Res Toxicol. 2012;25:35–46. doi: 10.1021/tx200363w. [DOI] [PubMed] [Google Scholar]
- Hirai T, Fukui Y, Motojima K. PPARalpha agonists positively and negatively regulate the expression of several nutrient/drug transporters in mouse small intestine. Biol Pharm Bull. 2007;30:2185–2190. doi: 10.1248/bpb.30.2185. [DOI] [PubMed] [Google Scholar]
- Hoque MT, Robillard KR, Bendayan R. Regulation of breast cancer resistant protein by peroxisome proliferator-activated receptor alpha in human brain microvessel endothelial cells. Mol Pharmacol. 2012;81:598–609. doi: 10.1124/mol.111.076745. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Aiba K, Fukuda K, Tanaka M. The induction of peroxisome proliferation in rat liver by perfluorinated fatty acids, metabolically inert derivatives of fatty acids. J Biochem. 1985;98:475–482. doi: 10.1093/oxfordjournals.jbchem.a135302. [DOI] [PubMed] [Google Scholar]
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Buitelaar M, Wagenaar E, Van Der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RP, Rosing H, Beijnen JH, Schinkel AH. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakura M, Kudo N, Tsuda T, Hibino Y, Mitsumoto A, Kawashima Y. Rat organic anion transporter 3 and organic anion transporting polypeptide 1 mediate perfluorooctanoic acid transport. J Health Sci. 2007;53:77–83. [Google Scholar]
- Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62:1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Babich MA, Baetcke KP, Cook JC, Corton JC, David RM, DeLuca JG, Lai DY, McKee RH, Peters JM, Roberts RA, Fenner-Crisp PA. PPARalpha agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol. 2003;33:655–780. doi: 10.1080/713608372. [DOI] [PubMed] [Google Scholar]
- Maloney EK, Waxman DJ. trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol Appl Pharmacol. 1999;161:209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- Moffit JS, Aleksunes LM, Maher JM, Scheffer GL, Klaassen CD, Manautou JE. Induction of hepatic transporters multidrug resistance-associated proteins (Mrp) 3 and 4 by clofibrate is regulated by peroxisome proliferator-activated receptor alpha. J Pharmacol Exp Ther. 2006;317:537–545. doi: 10.1124/jpet.105.093765. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Hirata T, Terada T, Jutabha P, Miura D, Harada KH, Inoue K, Anzai N, Endou H, Inui K, Kanai Y, Koizumi A. Roles of organic anion transporters in the renal excretion of perfluorooctanoic acid. Basic Clin Pharmacol Toxicol. 2008;103:1–8. doi: 10.1111/j.1742-7843.2007.00155.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ito Y, Yanagiba Y, Ramdhan DH, Kono Y, Naito H, Hayashi Y, Li Y, Aoyama T, Gonzalez FJ, Nakajima T. Microgram-order ammonium perfluorooctanoate may activate mouse peroxisome proliferator-activated receptor alpha, but not human PPARalpha. Toxicology. 2009;265:27–33. doi: 10.1016/j.tox.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post GB, Cohn PD, Cooper KR. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ Res. 2012;116:93–117. doi: 10.1016/j.envres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Lalwai ND. Carcinogenesis by hepatic peroxisome proliferators: evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. Crit Rev Toxicol. 1983;12:1–58. doi: 10.3109/10408448309029317. [DOI] [PubMed] [Google Scholar]
- Reddy JK, Qureshi SA. Tumorigenicity of the hypolipidaemic peroxisome proliferator ethyl-alpha-p-chlorophenoxyisobutyrate (clofibrate) in rats. Br J Cancer. 1979;40:476–482. doi: 10.1038/bjc.1979.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, Abbott BD, Lau C, Corton JC. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol Sci. 2008a;103:46–56. doi: 10.1093/toxsci/kfn025. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, Abbott BD, Lau C, Corton JC. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol Sci. 2008b;103:46–56. doi: 10.1093/toxsci/kfn025. [DOI] [PubMed] [Google Scholar]
- Sohlenius AK, Lundgren B, DePierre JW. Perfluorooctanoic acid has persistent effects on peroxisome proliferation and related parameters in mouse liver. J Biochem Toxicol. 1992;7:205–212. doi: 10.1002/jbt.2570070403. [DOI] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Frisbee S, Ducatman A, Vaccarino V. Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am J Epidemiol. 2009;170:1268–1278. doi: 10.1093/aje/kwp279. [DOI] [PubMed] [Google Scholar]
- Steenland K, Tinker S, Shankar A, Ducatman A. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ Health Perspect. 2010;118:229–233. doi: 10.1289/ehp.0900940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari I, Vamosi G, Brazda P, Balint BL, Benko S, Szeles L, Jeney V, Ozvegy-Laczka C, Szanto A, Barta E, Balla J, Sarkadi B, Nagy L. Peroxisome proliferator-activated receptor gamma-regulated ABCG2 expression confers cytoprotection to human dendritic cells. J Biol Chem. 2006;281:23812–23823. doi: 10.1074/jbc.M604890200. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2005;326:181–187. doi: 10.1016/j.bbrc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Uy-Yu N, Kawashima Y, Horii S, Kozuka H. Effects of chronic administration of perfluorooctanoic acid on fatty acid metabolism in rat liver: relationship among stearoyl-coenzyme A desaturase, 1-acylglycerophosphocholine acyltransferase, and acyl composition of microsomal phosphatidylcholine. J Pharmacobiodyn. 1990;13:581–590. doi: 10.1248/bpb1978.13.581. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Davis JW, 2nd, Sommers R, Peterson RE. Renal excretion of perfluorooctanoic acid in male rats: inhibitory effect of testosterone. J Biochem Toxicol. 1992;7:31–36. doi: 10.1002/jbt.2570070107. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, Peterson RE. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J Biochem Toxicol. 1991;6:83–92. doi: 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- Weaver YM, Ehresman DJ, Butenhoff JL, Hagenbuch B. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicol Sci. 2010;113:305–314. doi: 10.1093/toxsci/kfp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Glover KP, Han X. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol Sci. 2010;117:294–302. doi: 10.1093/toxsci/kfq219. [DOI] [PubMed] [Google Scholar]