Abstract
Mesenchymal stem cells (MSC) have been demonstrated to reside in human adult organs. However, mechanisms of migration of these endogenous MSCs within their tissue of origin are not well understood. Here, we investigate migration of human adult lung-resident mesenchymal progenitor cells. We demonstrate that bioactive lipid lysophosphatidic acid (LPA) plays a principal role in the migration of human lung-resident mesenchymal stem cells (LR-MSCs) through a signaling pathway involving LPA1 induced β-catenin activation. LR-MSCs isolated from human lung allografts and lungs of patients with scleroderma demonstrated a robust migratory response to LPA in vitro. Furthermore, LPA levels correlated with LR-MSC numbers in bronchoalveolar lavage (BAL), providing demonstration of the in vivo activity of LPA in human adult lungs. Migration of LR-MSCs was mediated via LPA1 receptor ligation and LPA1 silencing significantly abrogated the migratory response of LR-MSCs to LPA as well as human BAL. LPA treatment of LR-MSCs induced PKC-mediated glycogen synthase kinase-3β (GSK3β) phosphorylation, with resulting cytoplasmic accumulation and nuclear translocation of β-catenin. TCF/LEF dual luciferase gene reporter assay demonstrated a significant increase in transcriptional activity after LPA treatment. LR-MSC migration and increase in reporter gene activity in the presence of LPA was abolished by transfection with β-catenin siRNA demonstrating that β-catenin is critical in mediating LPA-induced LR-MSC migration. These data delineate a novel signaling pathway through which ligation of a G protein coupled receptor by a biologically relevant lipid mediator induces migration of human tissue-resident mesenchymal progenitors.
Keywords: Mesenchymal stem cell, tissue-resident, Lung, Migration, Lysophosphatidic acid, β-catenin
Introduction
Reparative processes in human solid organs require mobilization of tissue-resident progenitors and migration of cells within an organ plays an essential role in both organized and disorganized repair. Mesenchymal cells migration, considered to be an important first step in wound healing, is critical in the evolution of fibrotic diseases. Infiltration of the functional subunits by mesenchymal cells, resulting in the failure of the organ function, is an integral part of immune-mediated fibrotic diseases such as scleroderma. Similar pathology ensues in the transplanted solid organs with a fibrotic graft response to allo-immune injury presenting as bronchiolitis obliterans syndrome (BOS) in the lungs1, allograft vasculopathy in the heart, and chronic allograft nephropathy in the kidney. While multiple origins of mesenchymal cells have been identified, recent data suggest dominant contribution of locally-resident tissue-specific mesenchymal progenitors in fibrogenesis.2–4 Hence, deciphering mechanism(s) of mobilization of mesenchymal progenitor with an organ is fundamental for understanding disorganized repair and fibrosis in human adult solid organs.
Tissue-specific mesenchymal progenitor cells have been demonstrated to reside in human adult organs such as lung, heart and kidney.4–7 Accumulation of these multipotent mesenchymal stem cells (MSCs) accompanies repair responses following transplantation of the human adult lungs.8 These mesenchymal progenitors recruited in response to injury are locally resident, tissue-specific cells as demonstrated by their donor origin7 and unique expression of embryonic lung-specific mesenchymal factors such as Forkhead box protein F1.4 Lung-resident MSCs (LR-MSCs), recruited in response to injury, have also been shown to be the major contributors to fibrogenesis in the lung allograft. However, paracrine factors, cell surface receptors and intra-cellular signaling pathways mediating migration of endogenous organ-resident mesenchymal progenitors remain to be determined.
Lysophosphatidic acid (LPA) is a bioactive lipid mediator which exerts a wide range of cellular functions has been demonstrated to induce cell migration.9, 10 LPA signals via three closely related G protein coupled receptors (GPCRs), LPA1, LPA2, and LPA3 (formerly EDG2, EDG4, and EDG7).11, 12 The role of LPA in fibrotic diseases is emerging. Studies utilizing specific antagonists or genetic deletion of LPA receptors have demonstrated an important role for LPA in the development of kidney, lung and skin fibrosis in animal models.13–16 However, the biological role of LPA in adult human organs and the signaling mechanisms involved in mediating its fibrotic responses remain to be fully elucidated. β-catenin is an integral cell-cell adhesion adaptor protein as well as transcriptional co-regulator which regulates cell fate decisions during embryogenesis. β-catenin plays an essential role in the induction of mesoderm during embryogenesis17, 18 and forced activation of β-catenin has been demonstrated to increase migration and invasiveness of murine and human mesenchymal cells.19, 20 The best known activator of beta catenin is Wnt-1 which leads to the accumulation of a cytoplasmic free pool of β-catenin, which then translocates into the nucleus and binds to transcription factors of the LEF-1/TCF family to regulate β-catenin/LEF/TCF-dependent gene expression.21 However, recent studies indicate various other ligands can also potentially activate the β-catenin pathway.22
Here, we demonstrate what we believe to be a previously unknown mesenchymal cell signaling pathway triggered by LPA interaction with GPCR LPA1 in human lung-resident mesenchymal stem cells (LR-MSCs). This pathway involves PKC–mediated phosphorylation of GSK3β at Ser-9 leading in turn to cytoplasmic β-catenin accumulation and nuclear translocation. One of the biological effects of this LPA mediated activation of the β-catenin pathway, shown here, is the induction of cell migration.
Material and Methods
Isolation of LR-MSCs from BAL
LR-MSCs were isolated from BAL fluid of lung transplant recipients with no evidence of rejection or infection and scleroderma patients under a protocol approved by the Institutional Review Board of the University of Michigan as previously described.4, 7, 8 Briefly, mesenchymal colony forming units were isolated and expanded from the adherent culture of BAL cells. Cells were maintained in high-glucose DMEM medium supplemented with 10% FBS, 100 U/ml Penicillin/Streptomycin and 0.5% fungizone (Invitrogen, Carlsbad, CA) at 37 °C and 5% CO2/ 95% air. Flowcytometric analysis demonstrated positive expression of cell surface markers CD73, CD105, CD90, CD44 and negative expression of hematopoietic markers CD45. Multi-lineage differentiation potential was confirmed by induction into other mesenchymal lineages such as osteocytes, adipocytes, and chondrocytes as previously demonstrated.4, 7, 8 All cells used for experiments were between passages 3 and 6.
RNA Isolation and Analysis
Total RNA was isolated from LR-MSCs using the RNeasy mini kit (Qiagen, Inc. Valencia, CA) as per manufacturer’s instructions. Real Time RT-PCR assay was performed using a one-step real time RT-PCR kit (Applied Biosystems, Foster City, CA) as per manufacturer’s protocol. TaqMan gene expression assay primers for LPA-1, LPA-2 and LPA-3 were purchased from Applied Biosystems (Hs00173500_m1, Hs00173704_m1 and Hs00173857_m1 respectively).
Protein Isolation and Western Blot Analysis
LR-MSCs whole cell lysates were collected using Cell Lysis Buffer purchase from Cell Signaling Technologies (Danvers, MA). LR-MSCs nuclear and cytoplasmic extracts were collected using NXTRACT kit (Sigma, St. Louis, MO) as per manufacturer’s protocol. Proteins from whole cell or nuclear extracts were separated by electrophoresis in a 10% Bis-Tris polyacrylamide gels and transferred onto PVDF membranes (Invitrogen, Carlsbad, CA). Immunoblotting was done using primary antibodies against LPA-1, β-catenin, p-GSK3β, GAPDH (all from Abcam, Cambridge, MA), SAM68 (Santa Cruz Biotechnology, Santa Cruz, CA) and horseradish peroxidase conjugated secondary antibodies (Sigma, St. Louis, MO). Signal was developed using pico or dura kit from Thermo Scientific (Rockford, IL).
Cell Migration Assay
Cells’ ability to migrate was assessed using Matrigel coated (8μm) transwells chamber set up purchased from BD biosciences (San Jose, CA). LR-MSCs or normal lung fibroblast (CCL-210) (ATCC, Manassas, VA) were seeded in transwells (upper chamber) at 1 × 105 cells per 200μl serum free media and placed into 24-well plates containing 600μl serum free media that were supplemented with or without LPA (Cayman, Ann Arbor, MI), Fetal Bovine Serum (FBS), Platelet derived growth factor (PDGF), Stromal Cell-Derived Factor (CXCL12), Hepatocyte Growth Factor (HGF), C-C Motif Ligand 21 (CCL21), Epidermal Growth Factor (EGF), N-FORMYL-MET-LEU-PHE (FMLP), Lipoteichoic Acid (LTA), C-C Motif Ligand 19 (CCL19) (All from Sigma, St. Louis, MO) or BAL supernatant at the specified concentrations. For migration assays where LPA-1/LPA-3 or cPKC specific antagonists were used, cells were treated with 10μM of either VPC12249 or VPC32183 (Avanti Polar Lipids, Alabaster, AL) or 1μM of GO6906 (Calbiochem, San Diego, CA) respectively for 30 minutes before they were seeded into the upper chamber. After 4 hours, cells that were migrated through transwells and attached on the surface underneath were fixed and stained using Hema 3 staining kit (Fisher Scientific, Kalamazoo, MI) and washed with water. Transwells’ upper surface was cleaned with cotton swabs to remove any remaining cells and transwells were left to dry. Transwells’ surface was removed and mounted on slides and examined under a microscope. Cells were counted (5 high power fields per transwell) and results were presented as average number of cells migrated per high power field per transwell. All experiments conditions were set up in triplicates.
BAL LPA Analysis
Lipids were extracted from BAL fluid using a SEP-PAK technique. Briefly, BAL fluid was applied to pre-conditioned C18 Sep-Pak columns (Waters, Milford, MA). Columns were washed with buffer followed by water. Lipids were eluted with 80% methanol and 20% ddH2O, dried under nitrogen in the evaporator and frozen at −20°C. LPA concentration was measured in eluted samples using LPA ELISA kit from Echelon Biosciences (Salt Lake City, UT) according to manufacturer’s protocol.
Small Interfering RNA
Small interfering RNA (siRNA) targeting β-catenin, LPA-1 receptor and non-targeting scrambled siRNA were purchased from Applied Biosystems (Foster City, CA). LR-MSCs were transiently transfected with siRNA using Oligofectamine (Invitrogen, Carlsbad, CA) for 24 hours and serum starved for another 24 hours before any treatment was added.
TCF/LEF Luciferase Reporter Assay
LR-MSCs were transfected with an inducible dual luciferase reporter plasmid containing TCF/LEF DNA binding sites, negative control (a non-inducible luciferase construct used to eliminate background) or positive control (a GFP expressing construct used for visual confirmation and optimization of transfection) vector using Oligofectamine according to manufacturer’s protocol. To determine β-catenin activity, LR-MSCs were co-transfected with β-catenin or non-targeting siRNA. Cells were serum starved for 24 hours before they were stimulated with LPA (10 μM) for 2 hours. Reporter activity was assayed using the dual luciferase system from Promega (Madison, WI) according to manufacturer’s protocol.
Results
LPA induces migration of human LR-MSCs via LPA1 receptor
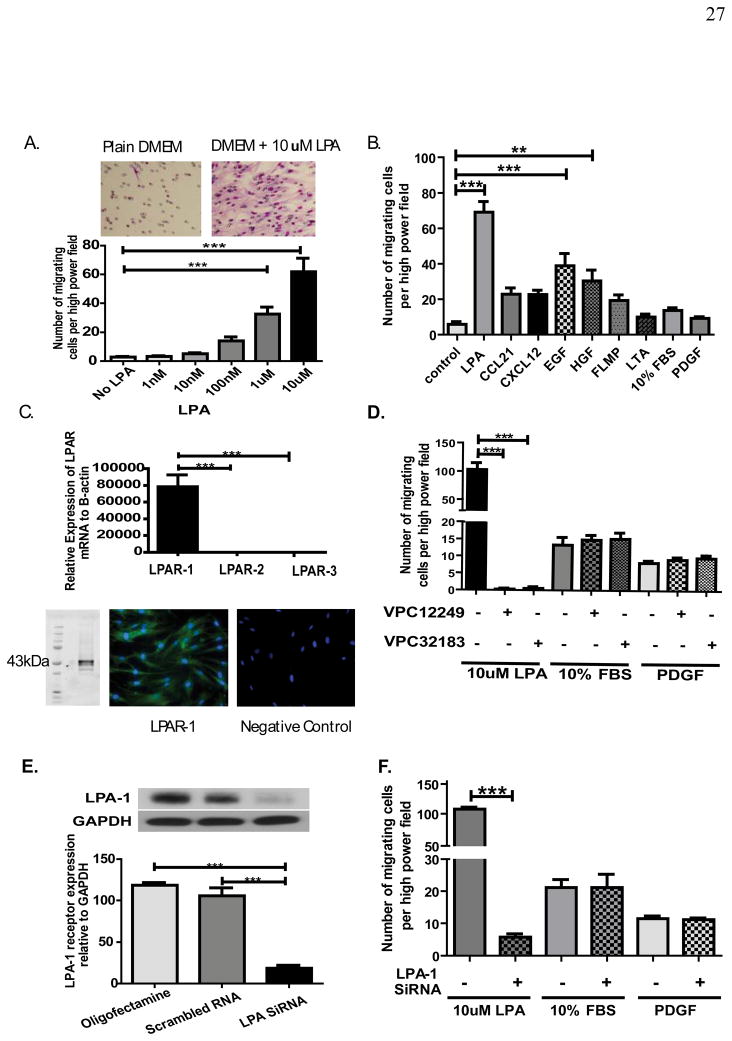
LR-MSCs were isolated from the BAL fluid of human lung allografts as previously described.7 The effect of LPA on LR-MSC migration was first evaluated in vitro using a modified Boyden chamber. Migration was quantified by staining the migratory cells on the lower side of insert membrane. A dose dependent migration of human LR-MSCs was noted in the presence of LPA (Fig. 1A). Effect of LPA on LR-MSC migration was compared to that of other known cellular chemo-attractants (Fig. 1B). A significant migratory response of LR-MSCs was noted in response to LPA, EGF and HGF. However, LPA induced migration was significantly higher than that for either EGF (P< 0.001) or HGF (p<0.001). The migratory response of LR-MSCs to LPA was noted to be significantly higher when compared to normal human lung fibroblasts (CCL-210). A 2.5 fold increase in migration was noted in human lung fibroblast in response to LPA (p=0.014). In comparison, LPA induced a 14 fold increase in migration of human LR-MSCs (P< 0.0001). These data establish LPA as a highly potent inducer of human LR-MSC migration.
Figure 1. LPA induces migration of human lung-resident mesenchymal stem cells via LPA1 receptor.
(A & B) LPA induces LR-MSC migration in a dose dependent manner. Human LR-MSCs were added to the upper chamber of the Transwell and allowed to migrate through the porous membrane into the lower chamber containing medium alone or medium with LPA. Migrated cells on the lower side of the membrane were stained with Hema3 stain and counted with Olympus BX41 light microscope using a ×20 objective. n=3 separate cell lines with 3 replicates of each condition and 5 counted high power fields for each replicate. Representative image from transwell demonstrating robust migration of LR-MSCs in response to LPA are shown above. LPA induced migration was compared to that induced by 10ng/ml CCL21, 30ng/ml CXCL12, 10ng/ml EGF, 20ng/ml HGF, 0.1uM FMLP, 1ug/ml LTA, 10% FBS, and 10nM PDGF, as shown in B. n=4 individual cell lines with 3 replicates of each condition and 5 counted high power fields for each replicate. (C) LPA receptors profile of human LR-MSCs. LPA1, LPA2 and LPA3 mRNA expression by real-time quantitative PCR in human LR-MSCs. n=4 LR-MSC lines. A representative image from western blot analysis and immunofluorescent staining demonstrating LPA1 protein expression in LR-MSCs is shown below. (D) Effect of LPA receptor antagonists on LPA-induced LR-MSC migration. LR-MSCs were pre-treated with competitive antagonists of the LPA1 and LPA3 receptors (1μM VPC12249 or 1μM VPC32183) for 30 min before migration assay. Data represent mean ± SEM from 3 independent experiments. (E & F) Effect of LPA1 siRNA on LPA-induced migration of human LR-MSCs. LR-MSCs were transfected with (20nM) validated LPA1 or scrambled siRNA for 24 h and serum starved for another 24 h. Protein was collected for western blot analysis to test transfection efficiency as shown in E (n=3). Effect of LPA1 siRNA on LR-MSC migration was studied using the migration assay. Data shown in F represent mean ± SEM from 3 independent experiments. *** P < 0.0001.
LPA1 was found to be the predominant receptor expressed on human LR-MSCs with a 4,726 and 9,311 fold higher expression of LPA1 mRNA noted as compared to LPA2 and LPA3 respectively (Fig. 1C). LPA1 receptor expression was also confirmed at protein level by western blot analysis and immunostaining (Fig. 1C). LPA1/LPA3 antagonists (VPC12249 and VPC32183) markedly diminished LPA-induced migration of LR-MSC without effecting PDGF or 10% FBS induced migration (Fig. 1D). As neither of these pharmacological inhibitors is specific for LPA1 receptor only, the results were further confirmed with siRNA directed toward LPA1 receptor. LPA1 siRNA transfection of LR-MSCs was associated with a greater than 80% reduction in LPA1 protein expression (Fig. 1E) and a 95% decrease in LPA-induced migration of LR-MSCs (Fig. 1F), demonstrating that LPA1 is the predominant receptor mediating LPA-induced migration of LR-MSCs. LPA1 siRNA had no effect on PDGF and FBS induced migration (Fig. 1F).
Human BAL LPA levels correlates with LR-MSC numbers and BAL-induced Migration of MSCs is inhibited by LPA1 antagonists
To investigate the in vivo role of LPA in inducing LR-MSC migration in human lungs, correlation of LPA with number of LR-MSCs in BAL was examined. LPA levels were measured in the supernatant from the BAL samples derived from human lung allografts. Mesenchymal colony-forming unit (CFUs) were quantitated in the cell pellet from the same BAL samples as previously described.8 A significantly higher LPA concentration was noted in BAL fluid from lung transplant patients with high CFU count (CFU per 2 × 106 cell ≥ 10) as compared to those with low CFU count (CFU per 2 × 106 cell < 10) (Fig. 2A). This CFU level cutoff was determined based on our previously published data.8 CFU count as a continuous variable also correlated significantly with LPA levels (r =0.63; p=0.007). Next, cell migration assay was performed using BAL fluid as a chemoattractant. Supernatant from BAL with high mesenchymal CFUs but not from those with low mesenchymal CFUs induced significant LR-MSCs migration in vitro (Fig. 2B). This migratory effect of supernatant from BAL samples with high CFUs on LR-MSCs was completely abolished in the presence of LPA1/LPA3 antagonist, VPC12249 (Fig. 2B). These data demonstrate that LPA accounts for the majority of mesenchymal cell migratory activity of BAL from human lung allografts, findings which are compatible with data from patients with idiopathic pulmonary fibrosis.16
Figure 2. Demonstration of the in vivo role of LPA in human lung allografts and the role of β-catenin activation in mediating LPA induced lung-resident mesenchymal stem cell (LR-MSC) migration.
(A) Correlation of LPA concentration with number of lung-resident mesenchymal stem cell in human bronchoalveolar lavage fluid. LR-MSCs were quantitated in BAL fluid from human lung transplant recipients by measuring number of mesenchymal CFUs per 2 ×106 million cells. LPA concentration in supernatant of these BAL samples with high CFUs (CFU ≥ 10, n=10) and low CFUs (CFU< 10, n=10) was determined. **P = 0.004 (B) LPA accounts for chemotactic activity of BAL derived from human lung allografts. Ability of supernatant from samples with high and low CFUs to induce LR-MSC and its modulation by LPA receptor antagonist (VPC12249) was assessed by Transwell assay. Two LR-MSC lines (± VPC12449) were exposed to supernatant from 4 BAL samples with high CFUs (CFUs of 40, 13, 13 and 50) and 4 BAL samples with low CFUs (CFUs of 2, 0, 0, 0). Data is shown as mean ± SEM of migration induced by these BAL samples. Control signifies plain DMEM. *** p < 0.0001. (C) Effect of LPA treatment on β-catenin protein expression in human LR-MSCs. Serum starved LR-MSCs were treated with 10uM LPA for various time points. β-catenin protein expression in the cytoplasmic fraction was quantitated by western blot analysis. Data represent mean ± SEM from 4 individual LR- MSC lines. (D & E) Effect of β-catenin siRNA on LPA-induced LR-MSC migration. LR-MSCs were transfected with β-catenin siRNA (20nM) or scrambled siRNA for 24 hours. β-catenin protein was measured by western blot analysis in aliquots of transfected cells as shown in B. Effect of β-catenin silencing on LR-MSC migration in response to LPA and other control chemoattractants (10% FBS and PDGF) was studied using the Transwell migration assay. Data represent mean ± SEM from 4 individual experiments. ** p< 0.001, *** p < 0.0001.
LPA induced migration of LR-MSCs is dependent on the β-catenin pathway
β-catenin is an important component of the cell cytoskeleton and a key player in the signaling mechanisms that regulates cell migration in response to Wnt pathway ligands.23 Activation of the canonical Wnt signal transduction pathway in MSCs and forced activation of β-catenin signaling in human and murine mesenchymal cells has been shown to impart increased motility and invasiveness.19, 20, 24
Investigation of the intra-cellular signaling pathways activated by LPA receptor ligation demonstrated a robust cytoplasmic β-catenin accumulation with LPA treatment (Fig. 2C). To investigate if β-catenin activation is responsible for LPA induced LR-MSCs migration, cells were transfected with β-catenin siRNA and their migratory response investigated. Measurement of β-catenin protein expression in aliquots of transfected cells confirmed the efficacy of silencing with a significant ( 85%) reduction in protein expression seen in the presence of β-catenin siRNA (Fig 2D). As shown in Fig. 2E, β-catenin silencing significantly abrogated LR-MSCs migration in response to LPA with mean number of migrating LR-MSCs decreasing from 64.89 ± 7.53 to 8.38 ± 7.29 per high power field (p < 0.0001). β-catenin siRNA did not inhibit PDGF or 10% FBS induced migration.
LPA induces GSK3β phosphorylation, β-catenin nuclear accumulation and promotes β-catenin induced transcription in LR-MSCs
β-catenin levels in the cytoplasm are tightly regulated by its phosphorylation, ubiquitination and proteosomal degradation.25 Glycogen synthase kinase (GSK3β) interacts with adenomatous polyposis coli (APC) and axin proteins to form a destruction complex that phosphorylate β-catenin and makes it a target for proteasome-mediated degradation. Phosphorylation of GSK3β at Ser-9 causes inhibition of its activity resulting in cytoplasmic β-catenin accumulation, nuclear translocation and TCF/LEF target genes transcription activation. To investigate whether LPA induced β-catenin up regulation is the result of GSK3β inhibition; we studied the effect of LPA on GSK3β phosphorylation at Ser-9. As shown in Fig. 3A, LPA treatment was associated with a significant increase in GSK3β phosphorylation, thus explaining the increase in cytoplasmic β-catenin expression with LPA treatment. Thirty minutes after LPA stimulation, a significant elevation in the β-catenin protein level in the nuclear fraction was noted (Fig. 3B). Nuclear β-catenin protein levels peaked at 1 hour and were still significantly elevated at 4 hours. TCF/LEF dual luciferase gene reporter assay performed 2 hours after LPA treatment demonstrated a significant increase in transcriptional activity. The increase in reporter gene activity in response to LPA was completely abolished when β-catenin siRNA were used (Fig. 3C). These findings suggest that LPA stimulation of LR-MSCs leads GSK3β phosphorylation which inhibits β-catenin degradation and leads to β-catenin pathway activation.
Figure 3. LPA induces GSK3β phosphorylation and activates β-catenin pathway.
(A) Effect of LPA treatment on GSK3β phosphorylation. Serum starved LR-MSCs were treated with 10μM LPA for 30 or 60 minutes. GSK3β phosphorylation was studied by western blot analysis using phospho-specific antibody (phospho-Ser-9) against GSK3β. n=4. (B) LPA induces nuclear translocation of β-catenin. Nuclear protein fractions of LR-MSCs treated with LPA were used to detect nuclear β-catenin accumulation by western blot analysis. Results were normalized to nuclear SAM 68 as a nuclear loading control. n = 4. (C) TCF/LEF/B-catenin transcription activation by LPA. LR-MSCs were transfected with TCF/LEF luciferase reporter construct (an inducible luciferase reporter), negative control (a non-inducible luciferase reporter used for background elimination) or positive control vector (a GFP expressing luciferase construct used to test transfection efficiency, data not shown) using Oligofectamine. In some conditions, LR-MSCs were also co-transfected with β-catenin siRNA (20nM). Transfected cells were then treated with either control vehicle or 10uM LPA for 2 hours. Reporter activity was assayed using dual luciferase assay system and data are shown as fold induction in relative light units (RLU). Experiments were done in triplicates. n=5 LR-MSC lines. (D& E) LPA1 receptor is the key receptor in LPA activation of β-catenin pathway. LR-MSCs were transiently transfected with (20nM) LPA1 siRNA for 24 hours before treatment with 10μM LPA. Total protein was collected at various time points after LPA treatment and analyzed for GSK3β phosphorylation by western blot analysis. β-catenin protein expression in nuclear fractions 1hr after LPA is shown. n=3.
LPA1 ligation mediated β-catenin activation in LR-MSCs
The predominant expression of LPA1 on LR-MSCs and the role of this receptor in mediating LPA-induced LR-MSC migration led us to evaluate if LPA activation of β-catenin pathway is mediated by LPA1 receptor. To investigate this, GSK3β phosphorylation and nuclear expression of β-catenin was measured in LPA-stimulated LR-MSCs with and without siRNA directed toward LPA1 receptor. LPA1 silencing significantly inhibited LPA induced GSK3β phosphorylation (Fig. 3D) and nuclear β-catenin protein expression (Fig. 3E), suggesting that LPA1 receptor is the key receptor mediating LPA activation of β-catenin pathway in human LR-MSCs.
LPA induced GSK3β phosphorylation, β-catenin nuclear translocation and migration of LR-MSCs is mediated via protein kinase C activation
A primary response of most cell types to LPA is PLC activation, which results in IP3 and diacylglycerol production with consequent intracellular calcium mobilization and protein kinase C (PKC) activation. Conventional PKC (cPKC) specific inhibitor GÖ6976 was found to abolish LPA-induced GSK3β phosphorylation and strongly inhibited nuclear accumulation of β-catenin (Fig. 4A and B). The migratory effect of LPA on LR-MSCs was also abrogated in the presence of GÖ6976 (Fig. 4C). These results suggest that cPKC activation mediates the LPA-induced phosphorylation of GSK3β leading to β-catenin nuclear translocation and subsequent cell migration.
Figure 4. LPA induced cPKC activation is essential for LPA-induced β-catenin pathway activation and migration.
Serum starved LR-MSCs were pretreated with specific cPKC inhibitor (GÖ6976) for 30 min before treatment with 10μM LPA. The effect of cPKC inhibitor on GSK3β phosphorylation (A) and nuclear β-catenin expression (B) was studied by western blot analysis. Cell migration (C) was assayed in the presence of cPKC inhibitor. N=4. ** p< 0.001, *** p < 0.0001.
LPA causes migration of scleroderma LR-MSCs via an LPA1 mediated phosphorylation and inhibition of GSK-3β and subsequent β-catenin translocation
To investigate the robustness of the data and to determine the importance of this signaling pathway in other immune mediated fibrotic diseases, we investigated this mechanism of migration in MSCs isolated from the lungs of patients with scleroderma. LR-MSCs isolated from BAL of patients with scleroderma demonstrated migratory response to stimulation by LPA in a robust and dose dependent manner (Fig. 5A & B). LPA1 was found to be the predominant LPA receptor and the role of LPA1 in mediating migratory response to LPA was demonstrated by utilizing chemical inhibition and siRNA approach (Fig. 5C to F). The role of beta catenin activation in LPA-induced migration of scleroderma LR-MSCs was established by GSK3β phosphorylation and β-catenin nuclear translocation in response to LPA stimulation (Fig. 5G & H) and demonstration of complete abrogation of migration with β-catenin siRNA (Fig. 5I).
Figure 5. LPA causes migration of scleroderma LR-MSCs via an LPA1 mediated phosphorylation and inhibition of GSK-3β and subsequent β-catenin translocation.
(A & B) LPS induces robust migration of scleroderma lung derived MSCs. Migration of LR-MSCs derived from patients with scleroderma in response to varying doses of LPA was assessed in vitro using transwell system. LPA induced migration was compared to that induced by 10% FBS, and 10nM PDGF as shown in B. (C) LPA1 is the predominant receptor on of scleroderma lung derived MSCs. mRNA expression of LPA receptors by real-time quantitative PCR in scleroderma LR-MSCs is shown (D to F) LPA1 receptor mediates LPA induced migration of scleroderma lung-derived MSCs. Effect of LPA receptor antagonists on LPA induced LR-MSC migration was studied by pre-treating cells with competitive antagonists of the LPA1 and LPA3 receptors (1μM VPC12249 or 1μM VPC32183) for 30 min before migration assay. Furthermore, effect of LPA1 siRNA on LPA-induced migration of human LR-MSCs was studied. (G & H) LPA induces GSK3β phosphorylation and nuclear translocation of β-catenin in scleroderma lung derived MSCs. Serum starved LR-MSCs were treated with 10μM LPA for 30 or 60 minutes. GSK3β phosphorylation was studied by western blot analysis using phospho-specific antibody (phospho-Ser-9) against GSK3β. Nuclear protein fractions of LR-MSCs treated with LPA were used to detect nuclear β-catenin accumulation by western blot analysis. Results were normalized to nuclear SAM 68 as a nuclear loading control. (I) LPA induced migration of scleroderma lung-derived MSCs is dependent on the β-catenin pathway. LR-MSCs were transfected with β-catenin siRNA (20nM) or scrambled siRNA for 24 hours. Effect of β-catenin silencing on LR-MSC migration in response to LPA was studied using the Transwell migration assay.
N= 3 scleroderma cell lines. ** p < 0.001, *** p < 0.0001.
Discussion
Delineation of embryonic lung mesenchyme and expression of mesenchyme-specific transcription factors is essential for branching morphogenesis and epithelial cell specification in the fetal lung.26 We have recently demonstrated that lung-specific mesenchymal progenitors reside in the human adult lung.4, 7 These lung-resident mesenchymal stromal cells (LR-MSCs) express embryonic lung-specific mesenchymal factors such as FOXF14 and can modulate lung epithelium via paracrine mechanisms such as secretion of keratinocyte growth factor (KGF) as well as via direct gap junction communications.27 An increase in LR-MSC population is seen in response to injury such as after adult lung transplantation in humans8 and likely plays a role in lung repair. LR-MSCs can also differentiate into myofibroblasts in response to pro-fibrotic factors such as transforming growth factor-β and an altered pro-fibrotic phenotype is noted in LR-MSCs derived from lungs in diseases such as bronchiolitis obliterans.4 Mobilization of the LR-MSC population to lung airspaces precedes development of chronic allograft rejection or bronchiolitis obliterans syndrome.8 Here we investigate the signaling pathways mediating migration of these endogenous organ-resident mesenchymal progenitors. We demonstrate that ligation of G protein coupled receptor LPA1 leads to migration of LR-MSCs via GSK3β phosphorylation and subsequent β-catenin accumulation and nuclear translocation. These data suggest an important role for β-catenin activation in mobilization of tissue-resident mesenchymal progenitors.
Mechanism(s) of migration of MSCs has garnered significant attention secondary to the potential of utilizing exogenously administered or endogenously recruited bone marrow-derived MSCs (BM-MSCs) for tissue repair in solid adult organs.28, 29 These studies have identified several chemokines and growth factors as chemoattractants for BM-MSC in vitro. However, the present study investigates migration of endogenous adult organ-resident MSCs, a cellular population which has been demonstrated to be distinct from BM-MSCs. In this first investigation of migratory function of organ-resident MSCs, MSCs derived from human adult lungs demonstrated a robust migration in response to LPA. Furthermore, correlation of LPA levels with number of LR-MSCs in the human lung lavage samples and the ability of LPA1 receptor antagonism to abolish the migratory response of LR-MSCs to BAL provides demonstration of its in vivo activity in human adult lungs.
A novel aspect of this study is that it identifies LPA1 receptor ligation as an activator of β-catenin pathway in human LR-MSCs. This finding links a key lipid mediator with a key intracellular protein, both of which have been independently demonstrated to be important in both development and disease pathogenesis. β-catenin is essential for development of mesenchyme during embryogenesis.17, 18 In adults, activation of β-catenin pathway has been demonstrated to plays a key role in migration and invasiveness of various human cancer cell lines.30 β-catenin has also been implicated in fibrosis with migration of fibroblasts induced by forced activation of β-catenin.19, 20 Similarly, LPA is linked to invasiveness of cancer cells and data from animal studies demonstrates a key role for LPA in the development of pulmonary, liver and renal fibrosis.13–16 However, the mechanism of how inhibition of LPA1 receptor engagement mediates these effects is fully understood. Our study of human LR-MSCs demonstrates that LPA1 receptor ligation leads to GSKβ phosphorylation and subsequent nuclear translocation of β-catenin. This finding also has important implications in a disease like scleroderma where while β-catenin activation has been described in skin and lung mesenchymal cells,17 the ligand(s) causing that have not been identified.
Activation of β-catenin by non-WNT pathway ligands has garnered significant interest and several physiologically relevant mediators capable of signaling to the canonical β-catenin have been identified in specific cells.22 LPA has been previously reported to induce proliferation of colon cancer cells via activation of β-catenin pathway.31 However, LPA2 was the most abundantly expressed receptor and mediated the effect of LPA on proliferation in colon cancer cells. Furthermore, LPA2 and LPA3, but not LPA1 was shown to activate β-catenin pathway.31 This is in contrast to our observation that LPA1 receptor ligation activates β-catenin pathway and induces cell migration. LR-MSC, unlike colon cancer cells demonstrated predominant expression of LPA1 in comparison to LPA2 or LPA3. Silencing LPA receptor not only abolished LPA induced MSCs migration but also prevented LPA-induced GSK3β phosphorylation and nuclear translocation of β-catenin. Common to both these studies was the finding that PKC activation mediates GSK3β phosphorylation. This is not surprising as both LPA1 and LPA2 have been demonstrated to be potent inducers of PLC activation in embryonic fibroblasts.32 Thus, our study not only demonstrates the role of LPA1 ligation in β-catenin activation for the first time but also establishes the applicability and biological consequences of the phenomenon of β-catenin activation by GPCRs in non-transformed primary human cells. However, while our studies utilizing β-catenin downregulation by siRNA demonstrate that β-catenin mediates the migratory effect of LPA on LR-MSCs, these studies do not elucidate whether this effect is mediated via its effects on structural organization and cadherin/β-catenin interaction or via its activation of nuclear transcription factors of the TCF/LEF family. 23, 33, 34 These studies will be the subject of future investigation.
In summary, our studies have defined what we believe to be a previously unknown mesenchymal signaling pathway triggered by LPA interaction with GPCR LPA1. This pathway involves PKC–mediated phosphorylation of GSK3β at Ser-9 that in turn leads to cytoplasmic β-catenin accumulation and nuclear translocation. Stabilization of β-catenin then activates transcription of Lef/Tcf target genes. One of the biological effects of this LPA signaling cascade is enhancement of cell migration. Collectively, these data offer novel insight into the mechanism of recruitment of endogenous mesenchymal progenitor cells and provide a mechanistic explanation for the important role of LPA in pathogenesis of tissue fibrosis.13–16
Acknowledgments
NIH grants RO1HL094622 (to V. N. Lama), the Scleroderma Research Foundation Award, and the Brian and Mary Campbell and Elizabeth Campbell Carr research gift fund (to V.N.Lama).
Footnotes
Author contributions: L.B.: conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; V.N.L: conception and design, data analysis and interpretation, manuscript writing, financial support and final approval of manuscript.
References
- 1.Belperio JA, Weigt SS, Fishbein MC, et al. Chronic lung allograft rejection: mechanisms and therapy. Proc Am Thorac Soc. 2009;6:108–121. doi: 10.1513/pats.200807-073GO. [DOI] [PubMed] [Google Scholar]
- 2.Hoyles RK, Derrett-Smith EC, Khan K, et al. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor beta receptor. Am J Respir Crit Care Med. 2011;183:249–261. doi: 10.1164/rccm.201002-0279OC. [DOI] [PubMed] [Google Scholar]
- 3.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker N, Badri L, Wettlaufer S, et al. Resident tissue-specific mesenchymal progenitor cells contribute to fibrogenesis in human lung allografts. Am J Pathol. 2011;178:2461–2469. doi: 10.1016/j.ajpath.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno S, Bussolati B, Grange C, et al. Isolation and Characterization of Resident Mesenchymal Stem Cells in Human Glomeruli. Stem cells and development. 2009 doi: 10.1089/scd.2008.0320. [DOI] [PubMed] [Google Scholar]
- 6.Hoogduijn MJ, Crop MJ, Peeters AM, et al. Donor-derived mesenchymal stem cells remain present and functional in the transplanted human heart. Am J Transplant. 2009;9:222–230. doi: 10.1111/j.1600-6143.2008.02450.x. [DOI] [PubMed] [Google Scholar]
- 7.Lama VN, Smith L, Badri L, et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. The Journal of clinical investigation. 2007;117:989–996. doi: 10.1172/JCI29713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badri L, Murray S, Liu LX, et al. Mesenchymal stromal cells in bronchoalveolar lavage as predictors of bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2011;183:1062–1070. doi: 10.1164/rccm.201005-0742OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moolenaar WH. Development of our current understanding of bioactive lysophospholipids. Ann N Y Acad Sci. 2000;905:1–10. doi: 10.1111/j.1749-6632.2000.tb06532.x. [DOI] [PubMed] [Google Scholar]
- 10.Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol. 2008;160:25–46. doi: 10.1007/112_0507. [DOI] [PubMed] [Google Scholar]
- 11.Anliker B, Chun J. Lysophospholipid G protein-coupled receptors. J Biol Chem. 2004;279:20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima N, Ishii I, Contos JJ, et al. Lysophospholipid receptors. Annu Rev Pharmacol Toxicol. 2001;41:507–534. doi: 10.1146/annurev.pharmtox.41.1.507. [DOI] [PubMed] [Google Scholar]
- 13.Castelino FV, Seiders J, Bain G, et al. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 2011;63:1405–1415. doi: 10.1002/art.30262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pradere JP, Gonzalez J, Klein J, et al. Lysophosphatidic acid and renal fibrosis. Biochim Biophys Acta. 2008;1781:582–587. doi: 10.1016/j.bbalip.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradere JP, Klein J, Gres S, et al. LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol. 2007;18:3110–3118. doi: 10.1681/ASN.2007020196. [DOI] [PubMed] [Google Scholar]
- 16.Tager AM, LaCamera P, Shea BS, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 17.Haegel H, Larue L, Ohsugi M, et al. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 18.Huelsken J, Vogel R, Brinkmann V, et al. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheon SS, Cheah AY, Turley S, et al. beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A. 2002;99:6973–6978. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam AP, Flozak AS, Russell S, et al. Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol. 2011;45:915–922. doi: 10.1165/rcmb.2010-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novak A, Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci. 1999;56:523–537. doi: 10.1007/s000180050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shevtsov SP, Haq S, Force T. Activation of beta-catenin signaling pathways by classical G-protein-coupled receptors: mechanisms and consequences in cycling and non-cycling cells. Cell Cycle. 2006;5:2295–2300. doi: 10.4161/cc.5.20.3357. [DOI] [PubMed] [Google Scholar]
- 23.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neth P, Ciccarella M, Egea V, et al. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24:1892–1903. doi: 10.1634/stemcells.2005-0503. [DOI] [PubMed] [Google Scholar]
- 25.Bullions LC, Levine AJ. The role of beta-catenin in cell adhesion, signal transduction, and cancer. Curr Opin Oncol. 1998;10:81–87. doi: 10.1097/00001622-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Maeda Y, Dave V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiological reviews. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 27.Badri L, Walker NM, Ohtsuka T, et al. Epithelial Interactions and Local Engraftment of Lung-resident Mesenchymal Stem Cells. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2010-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maijenburg MW, van der Schoot CE, Voermans C. Mesenchymal Stromal Cell Migration: Possibilities to Improve Cellular Therapy. Stem cells and development. 2011 doi: 10.1089/scd.2011.0270. [DOI] [PubMed] [Google Scholar]
- 29.Tolar J, Le Blanc K, Keating A, et al. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai TY, Su CC, Kuo WW, et al. beta-catenin plays a key role in metastasis of human hepatocellular carcinoma. Oncol Rep. 2011;26:415–422. doi: 10.3892/or.2011.1323. [DOI] [PubMed] [Google Scholar]
- 31.Yang M, Zhong WW, Srivastava N, et al. G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the {beta}-catenin pathway. Proc Natl Acad Sci U S A. 2005;102:6027–6032. doi: 10.1073/pnas.0501535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contos JJ, Ishii I, Fukushima N, et al. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2) Mol Cell Biol. 2002;22:6921–6929. doi: 10.1128/MCB.22.19.6921-6929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bienz M. beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol. 2005;15:R64–67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 34.Harris TJ, Peifer M. Decisions, decisions: beta-catenin chooses between adhesion and transcription. Trends Cell Biol. 2005;15:234–237. doi: 10.1016/j.tcb.2005.03.002. [DOI] [PubMed] [Google Scholar]