Abstract
The dominant view of cerebellar function has been that it is exclusively concerned with motor control and coordination. Recent results from neuroanatomical, behavioral and imaging studies have profoundly changed this view. Neuroanatomical studies using virus transneuronal tracers have demonstrated that the output from the cerebellum reaches vast areas of the neocortex, including regions of prefrontal and posterior parietal cortex. Furthermore, it has recently become clear that the cerebellum is reciprocally connected with the basal ganglia, indicating that the two subcortical structures are part of a densely interconnected network. Altogether, these results provide the neuroanatomical substrate for cerebellar involvement in non-motor functions mediated by the prefrontal and posterior parietal cortex, as well as in processes traditionally associated with the basal ganglia.
Keywords: cerebellum, cerebral cortex, basal ganglia, non-motor function, transneuronal tracers
Changing views of cerebellar connections and function
The classical view of cerebro-cerebellar interconnections is that the cerebellum receives information from widespread neocortical areas, including portions of the frontal, parietal, temporal, and occipital lobes (Figure 1) [1, 2]. The cerebellum was thought to funnel this information back through the ventrolateral nucleus of the thalamus to gain access to a single area of the cerebrum, the primary motor cortex (M1) (e.g., [3]). Thus, cerebellar connections with the cerebral cortex were viewed as means of collecting information from broad regions of the cerebral cortex to influence the generation and control of movement at the level of M1. According to this view, cerebellar output was entirely within the domain of motor control and abnormal activity in this circuit would lead to purely motor deficits.
Figure 1. Origin of projections from the cerebral cortex to the cerebellum and the cortical targets of cerebellar output.
Top: The relative density of cerebro-pontine neurons is indicated by the gray dots on the lateral and medial views of the monkey brain. Red labels indicate areas of the cerebral cortex that are the target of cerebellar output. Blue labels indicate areas that are not the target of cerebellar output. The numbers refer to cytoarchitectonic areas. Bottom: Histogram of relative density of cerebro-pontine cells in the different cytoarchitectonic areas of the monkey. AIP, anterior intraparietal area; AS, arcuate sulcus; CgS, cingulate sulcus; FEF, frontal eye field; IP, intraparietal sulcus; LS, lateral sulcus; Lu, lunate sulcus; M1, face, arm, and leg areas of the primary motor cortex; PMd arm, arm area of the dorsal premotor area; PMv arm, arm area of the ventral premotor area; PrePMd, predorsal premotor area; PreSMA, presupplementary motor area; PS, principal sulcus; SMA arm, arm area of the supplementary motor area; ST, superior temporal sulcus; TE, area of inferotemporal cortex. Adapted from [17].
More recent analyses of cerebellar output have resulted in a dramatic shift in this view (e.g., [4–12]). It is now clear that efferents from the cerebellar nuclei project to multiple subdivisions of the thalamus (for a classic review, see [13]), which, in turn, project to a myriad of neocortical areas, including premotor, prefrontal and posterior parietal areas of the cerebral cortex. Moreover, recent findings have shown that the cerebellum and basal ganglia are densely interconnected [14, 15]. Taken together, these neuroanatomical findings, along with results from behavioral and imaging studies, provide a new framework for understanding cerebellar involvement in motor, as well as non-motor function. Specifically, it is now clear that the cerebellum can influence the generation and control of movement not only at the level of M1, but also through interactions with premotor cortical areas and sensorimotor regions of the basal ganglia. Furthermore, the cerebellum can no longer be considered an exclusively motor structure, and likely contributes to non-motor processes mediated by the prefrontal and parietal cortex, such as cognition and visuospatial reasoning, as well as non-motor operations of the basal ganglia, such as reward-related learning.
We begin our review by presenting the evidence that cerebellar output reaches not only M1, but also premotor, prefrontal, and posterior parietal areas of the cerebral cortex. These findings are important because they provide the anatomical substrate for the output of the cerebellum to influence non-motor behavior. We then present the data for segregated motor and non-motor domains in a major output nucleus of the cerebellum, the dentate, and in the cerebellar cortex. Additionally, we consider the evidence that the fundamental unit of cerebro-cerebellar operations is a closed-loop circuit. Finally, we describe the new findings for interconnections between the cerebellum and basal ganglia. Throughout, we discuss how these new anatomical results provide the neural substrate for cerebellar contributions to a wide range of behaviors.
Cerebellar output
The use of neurotropic viruses as transneuronal tracers (Box 1) has been essential for the identification of the areas of the cerebral cortex that are the targets of cerebellar output [4–12, 16, 17]. These studies have shown that cerebellar projections to M1 originate largely from neurons in the dentate nucleus. Furthermore, there is a rostral to caudal sequence of dentate outputs to the leg, arm, and face representations in M1 (Figure 2). This arrangement corresponds well with the somatotopic organization of the dentate previously proposed on the basis of physiological studies (e.g., [18–20]).
Box 1: Virus tracing.
Prior neuroanatomical approaches for examining cerebro-cerebellar and cerebello-basal ganglia circuits have been hindered by a number of technical limitations. Chief among these limitations is the multisynaptic nature of these pathways and the inability of conventional tracers to label more than the direct inputs or outputs of an area. To overcome these and other problems, neurotropic viruses have been used as transneuronal tracers in the central nervous system of primates (for references and a review, see [10, 138]. Selected strains of virus move transneuronally in either the retrograde or anterograde direction [10]. Thus, one can examine either the inputs to or the outputs from a site. The viruses used as tracers move from neuron to neuron exclusively at synapses, and the transneuronal transport occurs in a time dependent fashion. Careful adjustment of the survival time after a virus injection allows for the study of neural circuits composed of two (2nd order), three (3rd order), or four (4th order) synaptically connected neurons [139].
In an initial series of studies, the retrograde transneuronal transport of the McIntyre-B strain of herpes simplex virus type 1 (H129) was used to identify cerebellar outputs to selected neocortical areas [11]. In subsequent studies, the retrograde transneuronal transport of the CVS-11 (challenge virus strain 11) and N2c strains of rabies virus (RV) were used to identify cerebellar outputs to selected neocortical areas and to the basal ganglia [4, 9, 10, 15, 22]. In other studies, anterograde transneuronal transport of the H129 strain of HSV1 was used to identify neocortical inputs to the cerebellar cortex [10]. In the figure, we show a schematic of the retrograde transneuronal transport of RV (red) and anterograde transneuronal transport of H129 (blue) following neocortical injections in separate animals. DN: deep cerebellar nuclei; GC: granule cells; PC: Purkinje cells; PN: pontine nuclei.
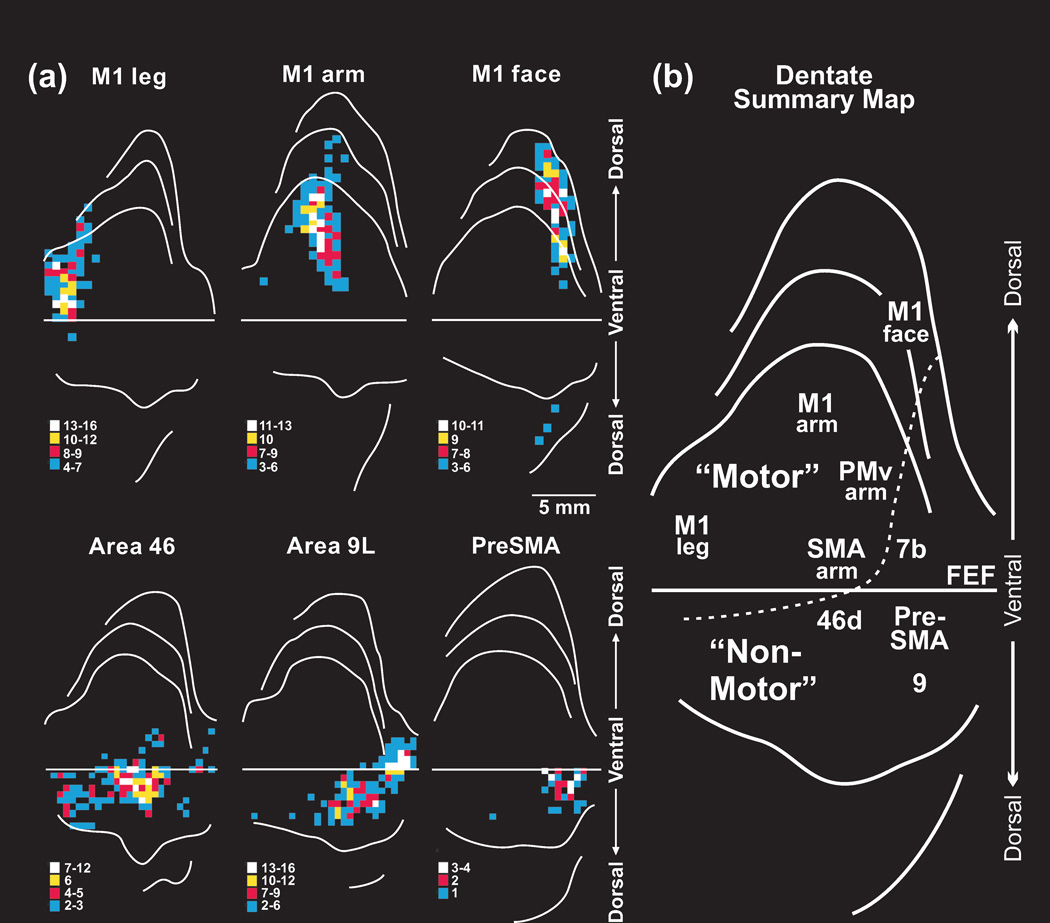
Figure 2. Output channels in the dentate.
(a) Top: Dorsal location of output channels to primary motor cortex (M1). Note the somatotopic organization of output channels to leg, arm and face M1. Bottom: Ventral location of output channels to prefrontal cortex. The key below each diagram indicates density of neurons in bins through the nucleus. (b) Summary map of dentate topography. The lettering on the unfolded map indicates the neocortical target of different output channels. The location of different output channels divides the dentate into motor and non-motor domains. Staining for monoclonal antibody 8B3 is most intense in the non-motor domain. The dashed line marks the limits of intense staining for this antibody. The numbers refer to cytoarchitectonic areas. FEF, frontal eye field; M1, face, arm, and leg areas of the primary motor cortex; PMv arm, arm area of the ventral premotor area; PreSMA, presupplementary motor area; SMA arm, arm area of the supplementary motor area. Adapted from [17].
The region of the dentate that contains neurons that project to M1 occupies only 30% of the nucleus [6, 21]. This implies that a substantial portion of the dentate projects to neocortical targets other than M1. A series of experiments were aimed to test this proposal and to define the neocortical targets of the unlabeled regions of the dentate. Briefly, these experiments demonstrated that the arm representations in multiple premotor areas, including the ventral premotor area (PMv), dorsal premotor cortex (PMd), and supplementary motor area (SMA), are targets of cerebellar output [4, 22, 23]. Cerebellar outputs to these premotor areas originate from the same dorsal region of the dentate that provides output to arm M1 (Figure 2). The clustering of output channels to M1 and the premotor areas in the dorsal region of the dentate creates a motor domain within the nucleus [21]. The outputs to the arm representations of M1, PMv, PMd and SMA appear to be in register within the dorsal region of the dentate. This raises the possibility that a single, integrated map of the body is represented within the motor domain of the dentate (Box 2).
Box 2: Questions for Future Research.
Is there an integrated map of the body within the motor domain of the dentate?
What are all the neocortical targets of the dentate, interpositus and fastigial nuclei?
What is the functional organization of the non-motor domain in the cerebellar cortex?
What is the nature of the information exchanged between the cerebellum and the basal ganglia?
How does cerebellar input improve basal ganglia function?
How does basal ganglia input improve cerebellar function?
How do the cerebellum and basal ganglia and interact during different forms of learning?
How do the cerebellum and basal ganglia interact and contribute to disorders of movement, cognition and affect?
Remarkably, even after establishing the origin of cerebellar output to multiple motor areas of the cerebral cortex, the cortical targets of a large portion of the dentate remained unidentified. Therefore, several experiments were performed to determine whether regions of prefrontal and posterior parietal cortex are targets of cerebellar output (Figure 2) [8, 9, 12, 16, 24]. When the results of all of these experiments are considered together, it is clear that the dentate contains distinct output channels that project to areas 9m, 9l, and 46d, 7b, the anterior intraparietal area (AIP), medial intraparietal area (MIP), and ventral lateral intraparietal area (LIPv), but not to areas 12, 46v, 7a or inferotemporal cortex (TE) (Figure 1 and 2). Importantly, the extent of the dentate that is occupied by an output channel to a specific area of prefrontal or posterior parietal cortex is comparable to that occupied by an output channel to a cerebral motor area (Figure 2). Thus, it is likely that the signal from the dentate to prefrontal and posterior parietal areas of cortex is as important to their function as the signal the nucleus sends to motor areas of the cerebral cortex.
The output channels to prefrontal cortex are clustered together in a ventral region of the nucleus that is entirely outside the more dorsally located motor domain. The output channels to prefrontal cortex are also rostral to the output channel that targets the frontal eye field [11]. Interestingly, the topographic arrangement of output channels in the dentate does not mirror the arrangement of their targets in the cerebral cortex. For example, the presupplementary motor area (PreSMA) is adjacent to the SMA on the medial surface of the hemisphere (Figure 1), but the output channels to these neocortical areas are spatially separate in the dentate (Figure 2b). Although PreSMA has traditionally been included with the motor areas of the frontal lobe, evidence indicates that it should be considered a region of prefrontal cortex (for review, see [25]). The location of the output channel to the PreSMA in the non-motor domain of the dentate supports this proposal. Overall, these results support the notion that the topographic arrangement of output channels in the dentate nucleus reflects their connectional and functional relationship, rather than spatial relationships of the neocortical areas they innervate.
The division of the dentate into separate motor and non-motor domains is reinforced by underlying molecular gradients within the nucleus of cebus monkeys and macaques [4, 5, 26]. Immunoreactivity for a monoclonal antibody (8B3) is most intense in ventral regions of the dentate that project to prefrontal and posterior parietal areas of cortex. Thus, 8B3 may “recognize” a significant portion of the non-motor domain within the dentate. If this is the case, measurements indicate that the non-motor domain occupies approximately 40% of the dentate nucleus.
Anatomical and imaging studies provide evidence for a similar organization of the human dentate. For example, it has long been recognized that the human dentate is composed of a dorsal, microgyric portion and a ventral, macrogyric portion (for references and illustration, see [27]). Comparative studies suggest that the dentate has expanded in great apes and humans relative to the other cerebellar nuclei [28]. In fact, most of this increase appears to be due to an expansion in the relative size of the ventral dentate [29]. If the human ventral dentate has cortical outputs like that of the monkey, then the non-motor portion of the dentate grows in size and importance in great apes and humans.
There have been relatively few imaging studies of the human deep cerebellar nuclei, due to the challenges that their small size and high iron content pose to magnetic resonance imaging (MRI) [30, 31]. The available experiments, using resting state imaging to establish functional connectivity (fcMRI), report that the human dentate is connected with both motor and non-motor networks of the cerebral cortex [32, 33]. In addition, the few studies that examined functional activation of the dentate during task performance (fMRI) reported activation of the dentate during both motor and non-motor tasks (see [34] for a review). Furthermore, the results of a recent fMRI study provided evidence for segregated motor activation in dorsal and rostral regions of the dentate nucleus, whereas cognitive tasks led to activation in caudal aspects of the dentate nucleus [35]. Taken together, the results in human studies are remarkably consistent with the neuroanatomical findings in monkeys. They support the conclusion that the dentate has separate motor and non-motor domains that influence motor and non-motor areas of the cerebral cortex.
Cerebro-cerebellar loops
A number of general principles have emerged from a comparison of the areas of the cerebral cortex that project to the cerebellum, and the areas of the cerebral cortex that are the target of cerebellar output (Figure 1 and 2). To date, all of the areas in the cerebral cortex that are targets of cerebellar output (e.g., M1, premotor areas in the frontal lobe, and selected regions of prefrontal and posterior parietal cortex) also have prominent projections to the cerebellum (Figure 1). On the other hand, several areas of the cerebral cortex that lack substantial projections to the cerebellum (e.g., areas 46v, 12 and TE) do not appear to be major targets of cerebellar output (Figure 1). If these principles apply to all cerebro-cerebellar networks, then all of the areas of cerebral cortex that project to the cerebellum may be the targets of cerebellar output. This would include such diverse areas as regions of extrastriate cortex, cingulate cortex, and the parahippocampal gyrus (Figure 1) [1, 36–41]. Portions of the dentate with currently unidentified cortical targets (see Figure 2) may project to these regions. In addition, the interpositus and fastigial nuclei send efferents to the thalamus [e.g., 19, 42–44]. The neocortical targets of these deep nuclei remain to be fully determined, but are likely to include both motor and non-motor areas. For example, there is evidence for projections from the interpositus nucleus to primary motor cortex [6] and neocortical areas in the intraparietal sulcus [24]. Additionally, classic electrophysiological evidence suggests that cerebellar stimulation, especially in portions of the fastigial nucleus and associated regions of vermal cortex, evokes responses at limbic sites, including regions of cingulate cortex and the amygdala [45, 46]. Therefore, the fastigial nucleus may be a source of input to a number of sites within the limbic system. This possibility needs to be more fully explored (Box 2). Even so, it should be clear from current evidence that the output of the cerebellum, and its impact on non-motor function, is more extensive than previously suspected.
Another important implication of the general patterns of their connectivity is that multiple “closed-loops” may characterize the input-output organization of cerebro-cerebellar networks. This possibility has been tested for two cortical areas: M1 and area 46 [10]. In essence, anterograde transneuronal transport of the H129 strain of herpes simplex virus type 1 was used to determine the regions of the cerebellar cortex that receive input from M1 and the regions that receive input from area 46. Then, retrograde transneuronal transport of rabies virus was used to define the regions of the cerebellar cortex that project to M1 and the regions that project to area 46. This approach demonstrated that lobules IV-V, HVIIB and HVIII receive input from M1 and project to M1 (Figure 3). Similarly, lobule VII (largely hemispheric Crus II, but also vermis) receives input from area 46 and projects to area 46 (Figure 3). These results suggest that the fundamental macro-architectural unit of cerebro-cerebellar circuits is a closed-loop circuit.
Figure 3. Input-output organization of cerebellar loops with M1 and with area 46.
Top: Organization of cerebellar loops with M1. Left, the distribution of Purkinje cells (small dots) that project to the arm area of M1. These neurons were labeled after retrograde transneuronal transport of rabies from injections into the arm area of M1. Right, the distribution of granule cells (fine lines) that receive input from the arm area of M1. These neurons were labeled after anterograde transneuronal transport of the H129 strain of HSV1 from injections into the arm area of M1. Bottom: Organization of cerebellar loops with area 46. Left, the distribution of Purkinje cells (small dots) that project to area 46. These neurons were labeled after retrograde transneuronal transport of rabies virus from injections into area 46. Right, the distribution of granule cells (fine lines) that receive input from the area 46. These neurons were labeled after anterograde transneuronal transport of the H129 strain of HSV1 from injections into the area 46. Adapted from [10].
The obvious spatial separation of the cerebellar regions that are interconnected with M1 and area 46 indicate that the distinct motor and non-motor domains observed in the dentate nucleus (Figure 2b) have their counterparts in cerebellar cortex. Specifically, the motor domain includes two regions: one largely in the anterior lobe (lobules III-VI) and another largely in the paramedial lobule and adjacent posterior lobe (HVIIB and HVIII). The non-motor domain involves cerebellar territory between these areas of motor representation, including portions of the vermis and hemisphere.
Considerable support for the separation of cerebellar cortex into motor and non-motor domains comes from considering both classical and recent electrophysiological and imaging studies. Early electrophysiological studies in cats and monkeys demonstrated the presence of two somatotopic representations of the body in the cerebellar cortex (for review, see [47]). These maps are located in regions that are directly interconnected with the primary motor cortex (Figure 3) and reciprocally linked with the medial and dorsal accessory nuclei of the inferior olivary complex, which in turn receive afferents from the spinal cord [48]. Imaging studies have confirmed these representations in humans using fcMRI and fMRI (Figure 4) [49–51]. The orientation of the body map is inverted in the anterior lobe and upright in lobule VIII of the posterior lobe (Figure 4).
Figure 4. Somatomotor topography in the cerebellar cortex.
(a) The cerebral (right hemisphere) and cerebellar (left hemisphere) locations of the foot (green), hand (red), and face (blue) somatomotor representations in the monkey, from physiological responses evoked by stimulation. (b) Somatomotor topography in the cerebral cortex related to the foot (green), hand (red), and tongue (blue). Left hemisphere shows sites of fMRI activation during motor tasks. Right hemisphere shows seed regions for studies of functional connectivity MRI (fcMRI). (c) Cerebellar somatomotor topography as measured by task fMRI (right hemisphere) and fcMRI (left hemisphere, the seed regions are shown on b, right hemisphere). (d) Three views of somatomotor representation within the cerebellar cortex. Displayed coordinates represent the plane in MNI atlas space. Adapted from [50], with permission.
The functional organization of the extensive non-motor domain in cerebellar cortex remains under active investigation. As noted above, a portion of this domain in the monkey is interconnected with the prefrontal cortex (Figure 3). There is also evidence, in the monkey, that this domain has interconnections with selected regions of posterior parietal cortex [24]. In the human, experiments using fcMRI have provided complementary evidence that the non-motor domain in cerebellar cortex is functionally coupled to association areas of the cerebral cortex (Figure 5a). Across various studies, activity in large portions of the cerebellar cortex, including hemispheric lobule VII, is correlated with activity in fronto-parietal association areas, including the cognitive control and default networks [33, 50, 52, 53]. The extensive functional coupling of the cerebellar cortex with association neocortical areas likely reflects an expanded role for the human cerebellum in non-motor function. This notion is supported by the finding that the non-motor domain in the cerebellar cortex of great apes and humans has expanded to mirror the enlargement of the prefrontal cortex [54]. Difussion tractography MRI results also provide evidence for corresponding increases in prefrontal contributions to the cortico-ponto-cerebellar system in humans [55]. Interestingly, some association cortical regions that have limited or no known projections to the cerebellum in the monkey (such as ventral area 46 and regions in orbitofrontal cortex, see [1] and Figure 1) show functional coupling to cerebellar cortex in the human. Thus, an expanded array of neocortical areas may interact with the cerebellum in humans.
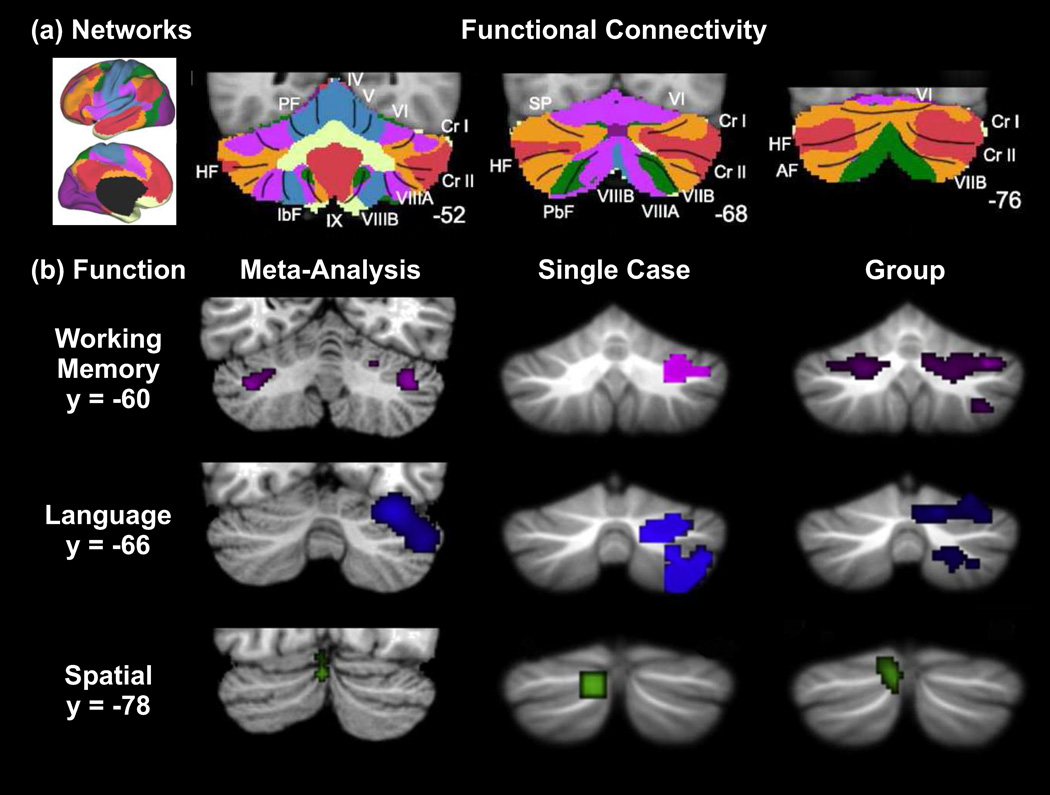
Figure 5. Topography in the non-motor regions of the cerebellar cortex.
(a) Maps of human cerebellum (panels on the right) based on functional connectivity with 7 major networks in the cerebrum (leftmost panel, based on [135]). Every voxel within the cerebellum is colored based on its maximal functional correlation with a cerebral network in a sample of 1000 subjects. The coordinates at the bottom right of the cerebellar panels represent the section level in the MNI atlas space. AF: ansoparamedian fissure; HF: horizontal fissure; IbF: intrabiventer fissure; IcF: intraculminate fissure; PbF: prepyramidal/prebiventer fissure; PF: primary fissure; PLF: posterolateral fissure; PrcF: preculminate fissure; SF: secondary fissure; SPF: superior posterior fissure. Adapted from [50], with permission. (b) Cerebellar non-motor topography from a meta-analysis of published imaging data, a single case study and a group study. Consistently active clusters during working memory (purple), language (blue), and spatial (green) paradigms are shown on coronal cerebellar slices. Y-Coordinates represent the section level in MNI atlas space. Adapted from [136], with permission.
There is abundant evidence for activation of cerebellar cortex in a wide variety of non-motor processes, including executive function, working memory, language, timing, music and emotion (Figure 5b). Since several recent reviews discuss results from functional imaging of the human cerebellum and provide considerations on the functional topography in cerebellar cortex (e.g., [17, 56–60]), this subject will not be presented in detail here. However, we do want to point out that the results of these studies are fully consistent with the idea that cerebellar cortex has distinct motor and non-motor domains. For example, cognitive and emotion tasks consistently activate regions in lobule VI, lateral lobule VII (Crus I, Crus II, VIIB) and medial lobule VII, regions that are functionally coupled with associative regions of the cerebral cortex (Figure 5a). Although future research is needed to reveal the detailed topography of the cerebellar cortex (Box 2), it is clear that the sites of activation in cognitive and emotion tasks are quite separate from those observed in motor tasks (Figure 4).
The concept that the cerebellar cortex and dentate nucleus contain distinct motor and non-motor domains provides an anatomical explanation for the observation that global dysfunction of the cerebellar cortex has wide-ranging effects on behavior (e.g., [61]), whereas localized dysfunction of a portion of the cerebellar cortex leads to more limited motor or non-motor deficits (e.g., [62–65]). The non-motor disorders produced by cerebellar dysfunction, by their very nature, may be less obvious that the disorders of movement, but they are no less real.
The results we have summarized concerning the functional organization of the cerebellum highlight the inadequacy of the term “cerebellar patient.” For comparison, no neuroscientist would ever characterize a subject as a “cerebral cortex patient.” Everyone recognizes that the site of a lesion in the cerebral cortex is of paramount importance in determining the nature of the ensuing deficits. The cerebellar cortex, like the neocortex, is not functionally homogenous. Therefore, the precise localization of damage or activation is just as important for studies of the cerebellum as it is for the cerebral cortex.
The cerebellum is interconnected with the basal ganglia
The loops that link the cerebellum with the cerebral cortex have traditionally been considered to be anatomically and functionally distinct from those that link the basal ganglia with the cerebral cortex [66, 67]. The outputs from the cerebellum and basal ganglia to the cerebral cortex are relayed through distinct thalamic nuclei [13, 68]. Any interactions between cerebro-cerebellar and cerebro-basal ganglia loops were thought to occur primarily at the neocortical level. Results from recent anatomical experiments challenge this perspective and provide evidence for disynaptic pathways that directly link the cerebellum with the basal ganglia. These experiments used retrograde transneuronal transport of rabies virus from injections into nuclei of the basal ganglia and cerebellar cortex (Figure 6).
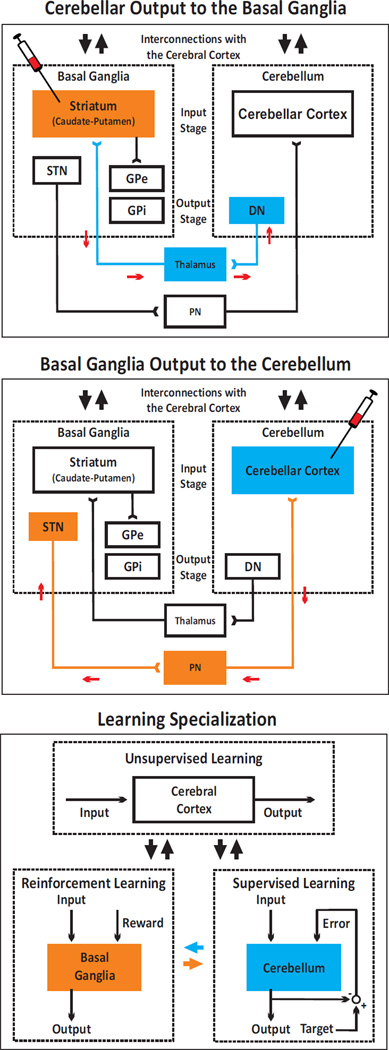
Figure 6. Experimental paradigms and circuits interconnecting the cerebellum and the basal ganglia.
The top panel depicts the experimental paradigm and results from [15] describing cerebellar (blue) output to the basal ganglia (orange). Rabies virus was injected into the striatum. The virus went through two stages of transport: retrograde transport to first-order neurons in the thalamus that innervate the injection site and then, retrograde transneuronal transport to second-order neurons in the dentate nucleus (DN) that innervate the first-order neurons. The middle panel of the figure depicts the experimental paradigm and results from [14], describing basal ganglia (orange) output to the cerebellum (blue). Rabies virus was injected into the cerebellar cortex. The virus went through two stages of transport: retrograde transport to first-order neurons in the pontine nuclei (PN) that innervate the injection site and then, retrograde transneuronal transport to second-order neurons in the subthalamic nucleus (STN) that innervate the first-order neurons. These interconnections enable two-way communication between the basal ganglia and the cerebellum. The small red arrows in the two top panels indicate the direction of virus transport. The bottom panel shows the theoretical specialization of the cerebellum, the basal ganglia, and the cerebral cortex for different types of learning, according to [66]. The cerebellum is specialized for supervised learning, guided by the error signal from the inferior olive. The basal ganglia are specialized for reinforcement learning, guided by the reward signal encoded in the dopaminergic input from the substantia nigra pars compacta. The cerebral cortex is specialized for unsupervised learning, guided by statistical properties of the input and neuromodulatory inputs. Interconnections between different structures are shown by large arrows (black arrows for interconnections with the cerebral cortex, orange arrow for the connection from the basal ganglia to the cerebellum, blue arrow for the connection from the cerebellum to the basal ganglia). DN: dentate nucleus; GPe: external segment of the globus pallidus; GPi: internal segment of the globus pallidus; PN: pontine nuclei; STN: subthalamic nucleus. Adapted from [137] (top panels) and [66] (bottom panel), with permission.
Virus transport demonstrated that the dentate nucleus projects disynaptically to the striatum (caudate and putamen) (Figure 6) [15]. Projections to the striatum originate from motor and non-motor domains in the dentate. Furthermore, the projections terminate in regions of putamen and caudate known to be within the “sensorimotor” and “associative territories” of these nuclei (for an overview of the organization of the striatum, see [69]). These findings indicate that the disynaptic pathway from the dentate to the striatum enables an output from the cerebellum to influence non-motor, as well as motor, function within the basal ganglia.
In a comparable set of experiments, virus transport demonstrated that the subthalamic nucleus (STN) projects disynaptically to cerebellar cortex (Figure 6) [14]. Projections to the cerebellar cortex originate from motor and non-motor domains within the STN (for an overview of the organization of the STN, see [70]). Furthermore, the projections terminate in motor and non-motor regions of the cerebellar cortex. These findings indicate that the disynaptic pathway from the STN to the cerebellar cortex enables an output from the basal ganglia to influence non-motor, as well as motor function within the cerebellum.
Taken together, these studies indicate that the cerebellum and basal ganglia are components of a densely interconnected network concerned with motor and non-motor aspects of behavior. As a consequence, these major subcortical systems are likely to interact as part of their normal function. Such interactions imply that abnormal activity in one system would have important effects on the other. Several observations support these predictions (see Figure 8). Below, we provide evidence that implicates both the cerebellum and the basal ganglia in associative reward-related learning as well as in the manifestations of neuropsychiatric and motor disorders.
Figure 8. Cerebellar activation associated with learning paradigms and neuropsychiatric conditions.
(a) fMRI study of appetitive conditioning with a pleasant taste reward. Activation (white) in the basal ganglia and the cerebellum correlates with temporal difference prediction error. Adapted from [75], with permission. (b) fMRI study of higher-order aversive conditioning. Activation (yellow/orange colors) in the basal ganglia and cerebellum correlates with temporal difference prediction error. Adapted from [80], with permission. (c) Cerebellar involvement in addiction. Summary results of cerebellar activation associated with cue-induced craving. Different shapes indicate results from different studies. Adapted from [95], with permission. (d) Tics in Tourette syndrome (tics minus sleep contrast) activate both the cerebellum and basal ganglia. Adapted from [134], with permission. In all panels, blue arrows point to sites of cerebellar activation, orange arrows point to sites of basal ganglia activation.
The cerebellum and basal ganglia are typically viewed as segregated modules that participate in different aspects of learning. The cerebellum is thought to be involved in adaptive modification of behavior and error-based learning, whereas the basal ganglia is thought to be involved in reward prediction and reward-based learning (see [66, 71], Figure 6c). Future research is needed to determine the computational benefits of interconnecting a reinforcement learning module with a supervised learning module in order to better understand how the basal ganglia and cerebellum may interact (Box 2). So far, accounts of reward-related learning have strongly emphasized the role of the basal ganglia because of the hypothesis that dopamine neurons reflect reward-prediction error and facilitate reinforcement learning in striatal target neurons [72]. Indeed, lesions and inactivations of the ventral striatum significantly impair previously acquired conditioned responses to food [73, 74]. Human fMRI studies have also shown that activity in the striatum is correlated with reward prediction error in Pavlovian reward association tasks [75, 76]. Strikingly, reward prediction error in these imaging studies is also strongly correlated with cerebellar signals (Figure 8a) [75]. In light of the findings that the cerebellum is closely interconnected with the basal ganglia, such a result need not be surprising. There is substantial evidence for cerebellar contributions to associative learning (for reviews, see [77, 78]). Early studies indicate that the cerebellum is both necessary and sufficient for the establishment of classical conditioning with aversive stimuli [79]. The cerebellum is activated in neuroimaging studies of aversive conditioning in humans, along with regions in the striatum (Figure 8b) [80, 81]. Co-activations of the cerebellum and the basal ganglia (Figure 8a and b) [75, 80–82] suggest that they may interact in support of processes involving reward-related learning. Although there is convincing evidence that both the cerebellum and the basal ganglia contribute to reward-related learning (for reviews, see [78, 83]), further work is needed to determine precisely how these systems interact during this and other processes (Box 2).
Cerebellar and basal ganglia interactions in reward-related learning may explain, in part, why lesions in both regions impair reward-based reversal learning [84, 85] and may help interpret findings that implicate the cerebellum in addiction. Although dopaminergic function and reinforcement learning implemented in the basal ganglia are considered key elements in the process of addiction (for reviews, see [86, 87]), the cerebellum may also play an important role in this disorder (for a review, see [88]). Neuroimaging studies in addicted individuals provide compelling evidence for this perspective (see Figure 8c). For example, neuroimaging studies reported that cognitive deficits in addicted individuals were associated with abnormal cerebellar activity [89]. Furthermore, imaging studies consistently reveal that the cerebellum is active when addicts interact with conditioned drug cues that increase craving (Figure 8c). Such activations have been observed across addiction studies irrespective of the drug of abuse and include responses to smoking cues [90], alcohol cues [91], heroin cues [92] and cocaine cues [93, 94]. There have been two main explanations for cerebellar activations in cue-reactivity paradigms. First, it has been proposed that the cerebellum (through its connections with the prefrontal cortex) is active as part of a distributed memory network, subserving emotional and cognitive links between the environment and drug craving [93]. Second, it has been proposed that the cerebellum (through its connections with motor and premotor neocortical areas) is active as part of a distributed sensorimotor network, subserving automatized behavioral reactions towards drug-related stimuli [95, 96]. Co-activations between the cerebellum and the basal ganglia in cue-induced craving studies have been observed in several studies (e.g., [90, 96–98]). Therefore, future accounts may benefit from considering interactions with the basal ganglia as another potential neural substrate for cerebellar involvement in cue-induced craving and addiction.
Cerebellar interactions with the basal ganglia have been shown to contribute to the symptoms of certain motor disorders, particularly Parkinson's disease and dystonia (for reviews, see [99–101]). Briefly, in Parkinson's disease, the loss of dopaminergic neurons of the substantia nigra pars compacta results in the manifestation of tremor, rigidity, bradykinesia and akinesia [102]. However, cerebellar activity is also abnormal in Parkinson's disease [103–105]. In parkinsonian patients [106, 107] and in monkey models of the disease [108], oscillatory activity at tremor frequencies has been recorded in regions of the thalamus that receive cerebellar, not basal ganglia, efferents. Furthermore, the cerebellar receiving thalamus is one of the most effective surgical sites for treating parkinsonian tremor [109]. These results suggest that abnormal activity in cerebellar circuits may account for parkinsonian tremor. Furthermore, deep brain stimulation of the STN is not only highly effective in reducing the motor symptoms in Parkinson's disease [110], but also normalizes cerebellar activity and function [111–115]. The disynaptic connection from the STN to the cerebellum may be the anatomical substrate that mediates this effect of STN stimulation. Overall, these lines of evidence suggest that interactions between the basal ganglia and cerebellum contribute to the expression of the motor abnormalities observed in Parkinson's disease.
Dystonia is another motor disorder that is often attributed to the basal ganglia [116]. Dystonia is characterized by involuntary muscle contractions, twisting movements and abnormal postures [117]. However, dystonia can also arise from cerebellar dysfunction and may be better described as a network disorder involving the basal ganglia and cerebellum [116, 118]. Human carriers of genetic mutations associated with dystonia exhibit abnormalities in both the basal ganglia and the cerebellum [119–125]. In normal mice with pharmacological excitation of the cerebellum or mutant tottering mice, abnormal cerebellar activity drives dystonic movements [126–130]. Additional subclinical lesions of the basal ganglia in these animals exaggerate the expression of dystonia, indicating that basal ganglia contributes to the manifestation of motor abnormalities even when the primary defect originates in the cerebellum [116, 130]. In fact, aberrant cerebellar activity may even cause dystonic movements through its effects on the basal ganglia. This view is supported by a mouse model of rapid-onset Dystonia-Parkinsonism in which abnormal cerebellar activity can influence the basal ganglia, via the disynaptic pathway through the thalamus that we have described above [131]. Overall, these findings support important functional interactions between the cerebellum and the basal ganglia in the manifestation of motor disorders typically associated with the basal ganglia.
Disturbances of basal ganglia circuits are associated with a wide range of conditions including not only the motor disorders discussed above, but also disorders with non-motor components such as Tourette syndrome, attention-deficit/hyperactivity disorders and schizophrenia (for a review, see [87]). There is evidence that the cerebellum is involved in these conditions as well (for reviews, see [17, 60, 132, 133]). For example, in Tourette syndrome, the cerebellum and the basal ganglia are likely to be concurrently involved in tic generation (Figure 8d) [133]. Tourette syndrome patients can also be differentiated from controls by an abnormal metabolic pattern that includes increased cerebellar and decreased basal ganglia metabolism [134]. Thus, cerebellar interactions with the basal ganglia are likely to be as important for neuropsychiatric disturbances, as they are in the motor disorders.
Overall, multiple lines of evidence provide support for functionally relevant interactions of the cerebello-basal ganglia network. We have provided evidence that the cerebellum and basal ganglia operate concurrently in the process of associative reward-based learning, that they interact in the manifestation of motor disorders (Parkinson's disease and dystonia) and that their interaction may contribute to neuropsychiatric disorders (addiction and Tourette syndrome). Further work is needed to explore how the communication between the cerebellum and the basal ganglia contributes to these and other normal and abnormal behaviors (Box 2).
Summary and conclusions
The dominant view of cerebellar function over the past century has been that it is concerned with the coordination and control of motor activity through its connections with M1. Here, we have reviewed the data that has led to a radical change in our concepts about cerebellar structure and function. The cerebellum not only receives input from a vast array of areas of the cerebral cortex, but it also has outputs that influence many, if not all of these neocortical areas. Indeed, a significant portion of the output from the dentate nucleus of the cerebellum projects to non-motor areas of the cerebral cortex, including regions of prefrontal and posterior parietal cortex. Motor and non-motor functions are spatially separated with distinct domains in the dentate nucleus and cerebellar cortex. Thus, the anatomical substrate exists for cerebellar output to influence the cognitive and visuospatial computations performed in prefrontal and posterior parietal cortex, as well as the generation and control of movement at the level of the cortical motor areas.
Another dramatic change in our view of cerebellar circuits is the observation that this major subcortical structure is densely interconnected with the basal ganglia. The interconnections between the cerebellum and the basal ganglia link the motor and non-motor domains of one subcortical system with the corresponding domain in the other system. Thus, the anatomical substrate exists for cerebellar output to influence the input stage of basal ganglia, and vice-versa. These interconnections provide the neural basis for cerebellar involvement in what have typically been considered to be basal ganglia operations, such as reward-related learning, and in so-called basal ganglia disorders, such as Parkinson’s disease, dystonia, or Tourette syndrome. These new results challenge us to discover the entire range of behavior that is influenced by the cerebro-cerebellar-basal ganglia network and the neural computations that are subserved by these interconnections.
Figure 7. STN projection to the cerebellar hemisphere.
(a) Rabies virus injection sites into the cerebellar cortex. Left, flattened map of the cerebellar cortex in cebus monkeys. Right, shaded region on the left side is expanded to show two injection sites, one into Crus IIp (AB2, red) and one into HVIIB (AB3, blue). (b) Charts of retrogradely labeled neurons in STN after rabies virus injections into Crus IIp (red dots) and HVIIB (blue dots) are overlapped to illustrate the topographic differences in distribution of STN second-order neurons in the two cases. (c) Schematic representation of STN organization, according to the tripartite functional subdivisions of the basal ganglia. (d) Schematic summary of the known connections between STN and areas of the cerebral cortex. C: caudal; D: dorsal; M: medial; STN: subthalamic nucleus. Adapted from [14].
Acknowledgements
This work was supported in part by funds from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and by National Institutes of Health Grants R01 NS24328 (P.L.S.), R01 MH56661 (P.L.S.), P40 OD010996 (P.L.S.), P30 NS076405 (P.L.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glickstein M, et al. Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J. Comp. Neurol. 1985;235:343–359. doi: 10.1002/cne.902350306. [DOI] [PubMed] [Google Scholar]
- 2.Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum. Brain Mapp. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Allen GI, Tsukahara N. Cerebrocerebellar communication systems. Physiol. Rev. 1974;54:957–1006. doi: 10.1152/physrev.1974.54.4.957. [DOI] [PubMed] [Google Scholar]
- 4.Akkal D, et al. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J. Neurosci. 2007;27:10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dum RP, et al. Motor and nonmotor domains in the monkey dentate. Ann. N. Y. Acad. Sci. 2002;978:289–301. doi: 10.1111/j.1749-6632.2002.tb07575.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J. Neurosci. 1999;19:1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schell GR, Strick PL. The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J. Neurosci. 1984;4:539–560. doi: 10.1523/JNEUROSCI.04-02-00539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clower DM, et al. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J. Neurosci. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clower DM, et al. Basal ganglia and cerebellar inputs to 'AIP'. Cereb. Cortex. 2005;15:913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- 10.Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch JC, et al. Input to the primate frontal eye field from the substantia nigra, superior colliculus, and dentate nucleus demonstrated by transneuronal transport. Exp. Brain. Res. 1994;100:181–186. doi: 10.1007/BF00227293. [DOI] [PubMed] [Google Scholar]
- 12.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Percheron G, et al. The primate motor thalamus. Brain Res. Brain Res. Rev. 1996;22:93–181. [PubMed] [Google Scholar]
- 14.Bostan AC, et al. The basal ganglia communicate with the cerebellum. Proc. Natl. Acad. Sci. USA. 2010;107:8452–8456. doi: 10.1073/pnas.1000496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshi E, et al. The cerebellum communicates with the basal ganglia. Nat. Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 16.Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- 17.Strick PL, et al. Cerebellum and nonmotor function. Annu. Rev. Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 18.Allen GI, et al. Convergence of cerebral inputs onto dentate neurons in monkey. Exp. Brain Res. 1978;32:151–170. doi: 10.1007/BF00239724. [DOI] [PubMed] [Google Scholar]
- 19.Stanton GB. Topographical organization of ascending cerebellar projections from the dentate and interposed nuclei in Macaca mulatta: an anterograde degeneration study. J. Comp. Neurol. 1980;190:699–731. doi: 10.1002/cne.901900406. [DOI] [PubMed] [Google Scholar]
- 20.Rispal-Padel L, et al. Cerebellar nuclear topography of simple and synergistic movements in the alert baboon (Papio papio) Exp. Brain Res. 1982;47:365–380. doi: 10.1007/BF00239355. [DOI] [PubMed] [Google Scholar]
- 21.Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J. Neurophysiol. 2003;89:634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto M, et al. Motor and non-motor projections from the cerebellum to rostrocaudally distinct sectors of the dorsal premotor cortex in macaques. Eur. J. Neurosci. 2010;31:1402–1413. doi: 10.1111/j.1460-9568.2010.07151.x. [DOI] [PubMed] [Google Scholar]
- 23.Middleton FA, Strick PL. Dentate output channels: motor and cognitive components. Prog. Brain. Res. 1997;114:553–566. doi: 10.1016/s0079-6123(08)63386-5. [DOI] [PubMed] [Google Scholar]
- 24.Prevosto V, et al. Cerebellar inputs to intraparietal cortex areas LIP and MIP: functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb. Cortex. 2010;20:214–228. doi: 10.1093/cercor/bhp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picard N, Strick PL. Imaging the premotor areas. Curr. Opin. Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 26.Fortin M, et al. Calcium-binding proteins in primate cerebellum. Neurosci. Res. 1998;30:155–168. doi: 10.1016/s0168-0102(97)00124-7. [DOI] [PubMed] [Google Scholar]
- 27.Voogd J. The human cerebellum. J. Chem. Neuroanat. 2003;26:243–252. doi: 10.1016/j.jchemneu.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Matano S, et al. Volume comparisons in the cerebellar complex of primates. II. Cerebellar nuclei. Folia Primatol. 1985;44:182–203. doi: 10.1159/000156212. [DOI] [PubMed] [Google Scholar]
- 29.Matano S. Brief communication: Proportions of the ventral half of the cerebellar dentate nucleus in humans and great apes. Am. J. Phys. Anthropol. 2001;114:163–165. doi: 10.1002/1096-8644(200102)114:2<163::AID-AJPA1016>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Diedrichsen J, et al. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. NeuroImage. 2011;54:1786–1794. doi: 10.1016/j.neuroimage.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 31.Küper M, et al. Structural and functional magnetic resonance imaging of the human cerebellar nuclei. Cerebellum. 2012;11:314–324. doi: 10.1007/s12311-010-0194-5. [DOI] [PubMed] [Google Scholar]
- 32.Allen G, et al. Magnetic resonance imaging of cerebellar-prefrontal and cerebellar-parietal functional connectivity. NeuroImage. 2005;28:39–48. doi: 10.1016/j.neuroimage.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Habas C, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J. Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habas C. Functional imaging of the deep cerebellar nuclei: a review. Cerebellum. 2010;9:22–28. doi: 10.1007/s12311-009-0119-3. [DOI] [PubMed] [Google Scholar]
- 35.Küper M, et al. Evidence for a motor and a non-motor domain in the human dentate nucleus--an fMRI study. NeuroImage. 2011;54:2612–2622. doi: 10.1016/j.neuroimage.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Brodal P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain. 1978;101:251–283. doi: 10.1093/brain/101.2.251. [DOI] [PubMed] [Google Scholar]
- 37.Vilensky JA, Van Hoesen GW. Corticopontine projections from the cingulate cortex in the rhesus monkey. Brain Res. 1981;205:391–395. doi: 10.1016/0006-8993(81)90348-6. [DOI] [PubMed] [Google Scholar]
- 38.Leichnetz GR, et al. Cortical projections to the paramedian tegmental and basilar pons in the monkey. J. Comp. Neurol. 1984;228:388–408. doi: 10.1002/cne.902280307. [DOI] [PubMed] [Google Scholar]
- 39.Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J. Neurosci. 1997;17:438–458. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmahmann JD, Pandya DN. Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J. Comp. Neurol. 1991;308:224–248. doi: 10.1002/cne.903080209. [DOI] [PubMed] [Google Scholar]
- 41.Schmahmann JD, Pandya DN. Prelunate, occipitotemporal, and parahippocampal projections to the basis pontis in rhesus monkey. J. Comp. Neurol. 1993;337:94–112. doi: 10.1002/cne.903370107. [DOI] [PubMed] [Google Scholar]
- 42.Asanuma C, et al. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res. 1983;286:237–265. doi: 10.1016/0165-0173(83)90015-2. [DOI] [PubMed] [Google Scholar]
- 43.Batton RR, 3rd, et al. Fastigial efferent projections in the monkey: an autoradiographic study. J. Comp. Neurol. 1977;174:281–305. doi: 10.1002/cne.901740206. [DOI] [PubMed] [Google Scholar]
- 44.Kalil K. Projections of the cerebellar and dorsal column nuclei upon the thalamus of the rhesus monkey. J. Comp. Neurol. 1981;195:25–50. doi: 10.1002/cne.901950105. [DOI] [PubMed] [Google Scholar]
- 45.Anand BK, et al. Cerebellar projections to limbic system. J. Neurophysiol. 1959;22:451–457. doi: 10.1152/jn.1959.22.4.451. [DOI] [PubMed] [Google Scholar]
- 46.Snider RS, Maiti A. Cerebellar contributions to the Papez circuit. J. Neurosci. Res. 1976;2:133–146. doi: 10.1002/jnr.490020204. [DOI] [PubMed] [Google Scholar]
- 47.Manni E, Petrosini L. A century of cerebellar somatotopy: a debated representation. Nat. Rev. Neurosci. 2004;5:241–249. doi: 10.1038/nrn1347. [DOI] [PubMed] [Google Scholar]
- 48.Brodal P, Brodal A. The olivocerebellar projection in the monkey. Experimental studies with the method of retrograde tracing of horseradish peroxidase. J. Comp. Neurol. 1981;201:375–393. doi: 10.1002/cne.902010306. [DOI] [PubMed] [Google Scholar]
- 49.Grodd W, et al. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum. Brain Mapp. 2001;13:55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buckner RL, et al. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiestler T, et al. Integration of sensory and motor representations of single fingers in the human cerebellum. J. Neurophysiol. 2011;105:3042–3053. doi: 10.1152/jn.00106.2011. [DOI] [PubMed] [Google Scholar]
- 52.Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb. Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Reilly JX, et al. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb. Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balsters JH, et al. Evolution of the cerebellar cortex: the selective expansion of prefrontal-projecting cerebellar lobules. NeuroImage. 2010;49:2043–2052. doi: 10.1016/j.neuroimage.2009.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramnani N, et al. The evolution of prefrontal inputs to the cortico-pontine system: diffusion imaging evidence from Macaque monkeys and humans. Cereb. Cortex. 2006;6:811–818. doi: 10.1093/cercor/bhj024. [DOI] [PubMed] [Google Scholar]
- 56.Stoodley CJ. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;11:352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- 57.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 58.E K-H, et al. A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum. Brain Mapp. doi: 10.1002/hbm.22194. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat. Rev. Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 60.Ito M. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 61.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J. of Neuropsychiatry Clin. Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 62.Fiez JA, et al. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115:155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- 63.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 64.Allen G, Courchesne E. Differential effects of developmental cerebellar abnormality on cognitive and motor functions in the cerebellum: an fMRI study of autism. Am. J. Psychiatry. 2003;160:262–273. doi: 10.1176/appi.ajp.160.2.262. [DOI] [PubMed] [Google Scholar]
- 65.Gottwald B, et al. Evidence for distinct cognitive deficits after focal cerebellar lesions. J. Neurol. Neurosurg. Psychiatr. 2004;75:1524–1531. doi: 10.1136/jnnp.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr. Opin. Neurobiol. 2000;10:732–739. doi: 10.1016/s0959-4388(00)00153-7. [DOI] [PubMed] [Google Scholar]
- 67.Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr. Opin. Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Sakai ST, et al. Comparison of cerebellothalamic and pallidothalamic projections in the monkey (Macaca fuscata): a double anterograde labeling study. J. Comp. Neurol. 1996;368:215–228. doi: 10.1002/(SICI)1096-9861(19960429)368:2<215::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 69.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Brain Res. Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 70.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res. Brain Res. Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 71.Houk JC. Agents of the mind. Biol. Cybern. 2005;92:427–437. doi: 10.1007/s00422-005-0569-8. [DOI] [PubMed] [Google Scholar]
- 72.Schultz W, et al. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 73.Blaiss CA, Janak PH. The nucleus accumbens core and shell are critical for the expression, but not the consolidation, of Pavlovian conditioned approach. Behav. Brain Res. 2009;200:22–32. doi: 10.1016/j.bbr.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parkinson JA, et al. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J. Neurosci. 1999;19:2401–2411. doi: 10.1523/JNEUROSCI.19-06-02401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O'Doherty JP, et al. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38:329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 76.O'Doherty J, et al. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 77.Thompson RF, et al. Intracerebellar conditioning--Brogden and Gantt revisited. Behav. Brain Res. 2000;110:3–11. doi: 10.1016/s0166-4328(99)00196-5. [DOI] [PubMed] [Google Scholar]
- 78.Swain RA, et al. The cerebellum: a neural system for the study of reinforcement learning. Front. Behav. Neurosci. 2011;5:8. doi: 10.3389/fnbeh.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brogden WJ, Gnatt WH. Intraneural conditioning: cerebellar conditioned reflexes. Arch. Neurol. Psychol. 1942;48:18. [Google Scholar]
- 80.Seymour B, et al. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- 81.Pohlack ST, et al. Activation of the ventral striatum during aversive contextual conditioning in humans. Biol. Psychol. 2012;91:74–80. doi: 10.1016/j.biopsycho.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 82.Tanaka SC, et al. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat. Neurosci. 2004;7:887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 83.Liljeholm M, O'Doherty JP. Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cog. Sci. 2012;16:467–475. doi: 10.1016/j.tics.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bellebaum C, et al. Focal basal ganglia lesions are associated with impairments in reward-based reversal learning. Brain. 2008;131:829–841. doi: 10.1093/brain/awn011. [DOI] [PubMed] [Google Scholar]
- 85.Thoma P, et al. The cerebellum is involved in reward-based reversal learning. Cerebellum. 2008;7:433–443. doi: 10.1007/s12311-008-0046-8. [DOI] [PubMed] [Google Scholar]
- 86.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharm. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maia TV, Frank MJ. From reinforcement learning models to psychiatric and neurological disorders. Nat. Neurosci. 2011;14:154–162. doi: 10.1038/nn.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miquel M, et al. Why should we keep the cerebellum in mind when thinking about addiction? Curr. Drug Abuse Rev. 2009;2:26–40. doi: 10.2174/1874473710902010026. [DOI] [PubMed] [Google Scholar]
- 89.Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J. Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.David SP, et al. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;58:488–494. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schneider F, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. Am. J. Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- 92.Yang Z, et al. Dynamic neural responses to cue-reactivity paradigms in heroin-dependent users: an fMRI study. Hum. Brain Mapp. 2009;30:766–775. doi: 10.1002/hbm.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grant S, et al. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonson KR, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 95.Yalachkov Y, et al. Sensory and motor aspects of addiction. Behav. Brain Res. 2010;207:215–222. doi: 10.1016/j.bbr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 96.Yalachkov Y, et al. Brain regions related to tool use and action knowledge reflect nicotine dependence. J. Neurosci. 2009;29:4922–4929. doi: 10.1523/JNEUROSCI.4891-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mcclernon FJ, et al. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology. 2009:25–35. doi: 10.1007/s00213-008-1436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Olbrich HM, et al. Brain activation during craving for alcohol measured by positron emission tomography. Aust. N. Z. J. Psychiatry. 2006;40:171–178. doi: 10.1080/j.1440-1614.2006.01765.x. [DOI] [PubMed] [Google Scholar]
- 99.Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain. doi: 10.1093/brain/aws360. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Filip P, et al. Dystonia and the cerebellum: A new field of interest in movement disorders? Clin. Neurophysiol. doi: 10.1016/j.clinph.2013.01.003. (in press) [DOI] [PubMed] [Google Scholar]
- 101.Sadnicka A, et al. The cerebellum in dystonia - help or hindrance? Clin. Neurophysiol. 2012;123:65–70. doi: 10.1016/j.clinph.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 102.Wichmann T, et al. Milestones in research on the pathophysiology of Parkinson's disease. Mov. Disord. 2011;26:1032–1041. doi: 10.1002/mds.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rascol O, et al. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain. 1997;120:103–110. doi: 10.1093/brain/120.1.103. [DOI] [PubMed] [Google Scholar]
- 104.Catalan MJ, et al. A PET study of sequential finger movements of varying length in patients with Parkinson's disease. Brain. 1999;122:483–495. doi: 10.1093/brain/122.3.483. [DOI] [PubMed] [Google Scholar]
- 105.Ghaemi M, et al. Monosymptomatic resting tremor and Parkinson's disease: a multitracer positron emission tomographic study. Mov. Disord. 2002;17:782–788. doi: 10.1002/mds.10125. [DOI] [PubMed] [Google Scholar]
- 106.Ohye C, et al. An analysis of the spontaneous rhythmic and non-rhythmic burst discharges in the human thalamus. J. Neurol. Sci. 1974;22:245–259. doi: 10.1016/0022-510x(74)90249-4. [DOI] [PubMed] [Google Scholar]
- 107.Lenz FA, et al. Single unit analysis of the human ventral thalamic nuclear group: correlation of thalamic "tremor cells" with the 3–6 Hz component of parkinsonian tremor. J. Neurosci. 1988;8:754–764. doi: 10.1523/JNEUROSCI.08-03-00754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guehl D, et al. Tremor-related activity of neurons in the 'motor' thalamus: changes in firing rate and pattern in the MPTP vervet model of parkinsonism. Eur. J. Neurosci. 2003;17:2388–2400. doi: 10.1046/j.1460-9568.2003.02685.x. [DOI] [PubMed] [Google Scholar]
- 109.Narabayashi H, et al. Long-term follow-up study of nucleus ventralis intermedius and ventrolateralis thalamotomy using a microelectrode technique in parkinsonism. Applied Neurophys. 1987;50:330–337. doi: 10.1159/000100736. [DOI] [PubMed] [Google Scholar]
- 110.Krack P, et al. Postoperative management of subthalamic nucleus stimulation for Parkinson's disease. Mov. Disord. 2002;17:S188–197. doi: 10.1002/mds.10163. [DOI] [PubMed] [Google Scholar]
- 111.Hilker R, et al. Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson's disease. J. Cereb. Blood Flow Metab. 2004;24:7–16. doi: 10.1097/01.WCB.0000092831.44769.09. [DOI] [PubMed] [Google Scholar]
- 112.Payoux P, et al. Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch. Neurol. 2004;61:1307–1313. doi: 10.1001/archneur.61.8.1307. [DOI] [PubMed] [Google Scholar]
- 113.Grafton ST, et al. Normalizing motor-related brain activity: subthalamic nucleus stimulation in Parkinson disease. Neurology. 2006;66:1192–1199. doi: 10.1212/01.wnl.0000214237.58321.c3. [DOI] [PubMed] [Google Scholar]
- 114.Trost M, et al. Network modulation by the subthalamic nucleus in the treatment of Parkinson's disease. NeuroImage. 2006;31:301–307. doi: 10.1016/j.neuroimage.2005.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Geday J, et al. STN-stimulation in Parkinson's disease restores striatal inhibition of thalamocortical projection. Hum. Brain Mapp. 2009;30:112–121. doi: 10.1002/hbm.20486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Neychev VK, et al. The functional neuroanatomy of dystonia. Neurobiol. Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- 118.LeDoux MS. Animal models of dystonia: Lessons from a mutant rat. Neurobiol. Dis. 2011;42:152–161. doi: 10.1016/j.nbd.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eidelberg D. Functional brain networks in movement disorders. Curr. Opin. Neurol. 1998;11:319–326. doi: 10.1097/00019052-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 120.Trost M, et al. Primary dystonia: is abnormal functional brain architecture linked to genotype? Ann. Neurol. 2002;52:853–856. doi: 10.1002/ana.10418. [DOI] [PubMed] [Google Scholar]
- 121.Ghilardi M-F, et al. Impaired sequence learning in carriers of the DYT1 dystonia mutation. Ann. Neurol. 2003;54:102–109. doi: 10.1002/ana.10610. [DOI] [PubMed] [Google Scholar]
- 122.Carbon M, et al. Increased cerebellar activation during sequence learning in DYT1 carriers: an equiperformance study. Brain. 2008;131:146–154. doi: 10.1093/brain/awm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carbon M, Eidelberg D. Abnormal structure-function relationships in hereditary dystonia. Neuroscience. 2009;164:220–229. doi: 10.1016/j.neuroscience.2008.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carbon M, et al. Functional imaging in hereditary dystonia. Eur. J. Neurosci. 2010;17:58–64. doi: 10.1111/j.1468-1331.2010.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Argyelan M, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J. Neurosci. 2009;29:9740–9747. doi: 10.1523/JNEUROSCI.2300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Campbell DB, Hess EJ. Cerebellar circuitry is activated during convulsive episodes in the tottering (tg/tg) mutant mouse. Neuroscience. 1998;85:773–783. doi: 10.1016/s0306-4522(97)00672-6. [DOI] [PubMed] [Google Scholar]
- 127.Campbell DB, et al. Tottering mouse motor dysfunction is abolished on the Purkinje cell degeneration (pcd) mutant background. Exp. Neurol. 1999;160:268–278. doi: 10.1006/exnr.1999.7171. [DOI] [PubMed] [Google Scholar]
- 128.Pizoli CE, et al. Abnormal cerebellar signaling induces dystonia in mice. J. Neurosci. 2002;22:7825–7833. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen G, et al. Low-frequency oscillations in the cerebellar cortex of the tottering mouse. J. Neurophysiol. 2009;101:234–245. doi: 10.1152/jn.90829.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Neychev VK, et al. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Calderon DP, et al. The neural substrates of rapid-onset Dystonia-Parkinsonism. Nat. Neurosci. 2011;14:357–365. doi: 10.1038/nn.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ito M. The Cerebellum: Brain for an Implicit Self. FT Press; 2011. [Google Scholar]
- 133.O'Halloran CJ, et al. The cerebellum and neuropsychological functioning: a critical review. J. Clin. Exp. Neuropsychol. 2012;34:35–56. doi: 10.1080/13803395.2011.614599. [DOI] [PubMed] [Google Scholar]
- 134.Lerner A, et al. Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndrome. Neurology. 2007;68:1979–1987. doi: 10.1212/01.wnl.0000264417.18604.12. [DOI] [PubMed] [Google Scholar]
- 135.Yeo BTT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stoodley CJ, et al. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol. Rev. 2010;30:261–270. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J. Neurosci. Methods. 2000;103:63–71. doi: 10.1016/s0165-0270(00)00296-x. [DOI] [PubMed] [Google Scholar]
- 139.Dum RP, Strick PL. Transneuronal tracing with neurotropic viruses reveals network macroarchitecture. Curr. Opin. Neurobiol. doi: 10.1016/j.conb.2012.12.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]