Abstract
Cytokines such as tumor necrosis factor alpha (TNFα) and interleukin-1 beta (IL1β) play a role in sleep regulation in health and disease. TNFα or IL1β injection enhances non-rapid eye movement sleep. Inhibition of TNFα or IL1β reduces spontaneous sleep. Mice lacking TNFα or IL1β receptors sleep less. In normal humans and in multiple disease states, plasma levels of TNFα covary with EEG slow wave activity (SWA) and sleep propensity. Many of the symptoms induced by sleep loss, for example, sleepiness, fatigue, poor cognition, enhanced sensitivity to pain, are elicited by injection of exogenous TNFα or IL1β. IL1β or TNFα applied unilaterally to the surface of the cortex induces state-dependent enhancement of EEG SWA ipsilaterally, suggesting greater regional sleep intensity. Interventions such as unilateral somatosensory stimulation enhance localized sleep EEG SWA, blood flow, and somatosensory cortical expression of IL1β and TNFα. State oscillations occur within cortical columns. One such state shares properties with whole animal sleep in that it is dependent on prior cellular activity, shows homeostasis, and is induced by TNFα. Extracellular ATP released during neuro- and gliotransmission enhances cytokine release via purine type 2 receptors. An ATP agonist enhances sleep, while ATP antagonists inhibit sleep. Mice lacking the P2X7 receptor have attenuated sleep rebound responses after sleep loss. TNFα and IL1β alter neuron sensitivity by changing neuromodulator/neurotransmitter receptor expression, allowing the neuron to scale its activity to the presynaptic neurons. TNFα's role in synaptic scaling is well characterized. Because the sensitivity of the postsynaptic neuron is changed, the same input will result in a different network output signal and this is a state change. The top-down paradigm of sleep regulation requires intentional action from sleep/wake regulatory brain circuits to initiate whole-organism sleep. This raises unresolved questions as to how such purposeful action might itself be initiated. In the new paradigm, sleep is initiated within networks and local sleep is a direct consequence of prior local cell activity. Whole-organism sleep is a bottom-up, self-organizing, and emergent property of the collective states of networks throughout the brain.
Keywords: interleukin-1, tumor necrosis factor, delta power, local sleep, inflammation, sleep loss syndrome
Introduction
Injection of exogenous interleukin-1 beta (IL1β) or tumor necrosis factor alpha (TNFα) into animals and/or humans induces (a) sleepiness or excess sleep, (b) enhanced sensitivity to pain, (c) enhanced sensitivity to kindling stimuli, (d) cognitive and memory impairments, and (e) symptoms associated with metabolic syndrome (reviewed in Krueger, 2008). All of these responses are also characteristic of the syndrome associated with chronic and/or excessive acute sleep loss. Since both IL1β and TNFα are upregulated in the brain during prolonged wakefulness, the injection of exogenous IL1β or TNFα mimics, in part, sleep loss. IL1β and TNFα are well-characterized sleep regulatory substances (SRSs) and are, to date, the only two putative SRSs that have been shown to induce the sleep loss syndrome. There are, however, other endogenous substances involved in sleep regulation and many of those are linked to IL1β or TNFα; this biochemical network and how it is involved in sleep regulation is the focus of this review. Further, knowledge of the biochemical sleep regulatory mechanisms has led to new views concerning brain organization of sleep, where sleep is initiated, and how wakefulness neural/glial activity is kept track of by the brain. Further, it is concluded that these mechanisms cannot be separated from a neural connectivity function for sleep.
IL1β and TNFα in sleep regulation
IL1β was first implicated in sleep regulation in 1984 (Krueger et al., 1984). Rabbits were given purified human IL1β intracerebroventricular (i.c.v.) and then allowed to sleep at will. Large increases in non-rapid eye movement sleep (NREMS) were observed. At the time, IL1β was known as endogenous pyrogen; the IL1β-induced fevers were blocked without affecting the sleep responses. In subsequent years, other species were tested using newer recombinant preparations of IL1β and similar effects were described. The somnogenic actions of TNFα were first characterized in 1987 (Shoham et al., 1987); in this case, human recombinant TNFα was used and it was given i.c.v. to rabbits in the same sleep assay model that was used to test IL1β. Like IL1β, TNFα induced large increases in duration of NREMS, and as with IL1β, the somnogenic actions of TNFα were extended to other animals and humans. These two cytokines were tested because previously we had shown that bacterial peptidoglycan-derived muramyl peptides induced sleep in animal models and they induced IL1β and TNFα production. It was only years later that sleep was first measured over the course of an infection (Toth and Krueger, 1988). Regardless, similar research approaches were used to characterize the sleep regulatory actions of IL1β and TNFα. The results from these studies are summarized together herein. For complete citations, the reader is referred to other reviews (Krueger, 2008; Krueger et al., 2008; Opp, 2005).
Central or systemic injection of either IL1β or TNFα increases time spent in NREMS. These effects usually begin within the first hour after injection and often last 6–10 h. The magnitude of the effects are large, for example, after intraperitoneal (i.p.) injections of TNFα or IL1β about 90 min of extra NREMS occur during the first 6 postinjection hours (e.g., see Fig. 1). If low somnogenic doses are used, NREMS is enhanced without affecting duration of rapid eye movement sleep (REMS). Similarly, low somnogenic doses of either IL1β or TNFα enhance NREMS without inducing fevers. As doses are increased, the magnitude of NREMS responses increase, but REMS begins to become inhibited and fevers are evident. At higher IL1β or TNFα doses, both NREMS and REMS are inhibited and fevers are higher. After central IL1β or TNFα injections, the enhanced NREMS is characterized by electroencephalogram (EEG) delta waves (0.5–4 Hz) of greater than normal magnitude. Similar high amplitude EEG delta waves are observed after sleep deprivation (Pappenheimer et al., 1975), and thus in this regard, the consequences of IL1β and TNFα on sleep mimic those induced by sleep loss. Higher amplitude EEG delta power is considered a measure of NREMS intensity and is used as the parameter to represent the homeostatic component in two process models of sleep (Borbely, 1982). The sleep- or sleepiness-promoting actions of these cytokines have been demonstrated in mice, cats, rats, rabbits, sheep, and humans.
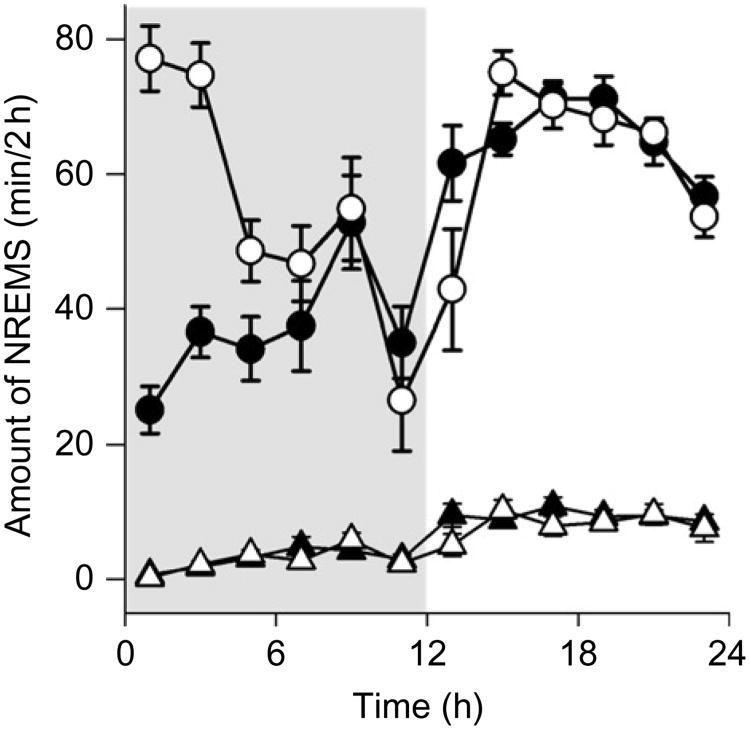
Fig. 1.
Interleukin-1 beta enhances NREMS in mice. IL1β (400 ng) was i.p.-injected at dark (shaded area) onset (time 0) and sleep was recorded for the next 24 h. Duration of NREMS was much greater after IL1β (open circles) than that observed during the control recordings (closed circles) for about 6 h. REMS (triangles) was slightly inhibited by IL1β (open triangles). Data from Krueger et al. (submitted for publication). Similar effects are observed after i.p. TNFα (Fang et al., 1997).
If either IL1β or TNFα is inhibited, duration of spontaneous NREMS is reduced. This occurs whether anti-IL1β or anti-TNFα antibodies are used or if their respective soluble receptors are injected. (Soluble receptors are endogenous in brain and blood; they are the receptor extracellular domains containing the cytokine recognition binding site but lacking the intracellular signaling domain. They are thought to play a role in normal cytokine regulation.) Further, for IL1β there is an endogenous IL1 receptor antagonist; it also inhibits duration of NREMS. Substances that inhibit the transcription or translation or release of the mature forms of IL1β or TNFα also inhibit sleep; these include anti-inflammatory cytokines such as IL4, IL10, and IL13, and hormones such as corticotrophin releasing hormone and glucocorticoids (Fig. 2). Genetic approaches also have implicated IL1β and TNFα in spontaneous physiological sleep regulation. Thus, mice lacking the type 1 IL1β receptor (Fang et al., 1998) or the 55 kDa TNFα receptor (Fang et al., 1997) or both TNFα receptors (Kapas et al., 2008) have less spontaneous NREMS and REMS although the time of day these sleep deficits occur differs in the different mutant mice.
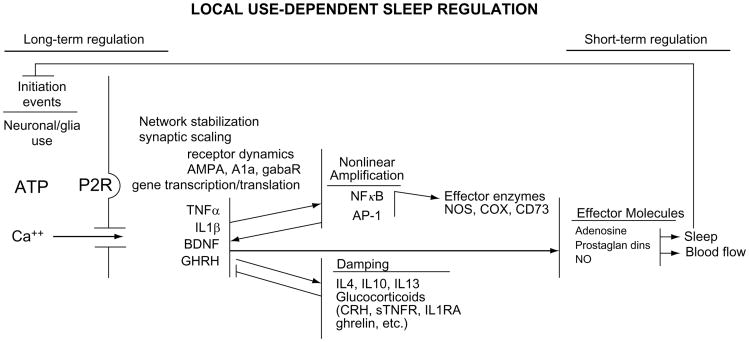
Fig. 2.
Biochemical cascades operate within local networks to alter state. Within any one network, ATP is released in proportion to cell activity. ATP via its P2 receptors drives processing and release of cytokines that in turn induce their own production and thereby create a positive feedback loop useful for amplification. Damping mechanisms are needed for control and are involved in driving oscillations between states. Cytokines activate transcription factors such as nuclear factor kappa B (NFκB) and activator protein-1 (AP-1). These in turn enhance production of multiple substances including the enzymes involved in the production of sleep effector molecules such as adenosine, prostaglandins, and nitric oxide (NO). The sleep state feeds back to alter the activity-dependent initiation events. It is envisioned that multiple local networks are undergoing this process and they synchronize state with each other via the known sleep regulatory circuits. They also influence each other depending upon proximity (diffusion of ATP) and neural connections to each other (Roy et al., 2008). Abbreviations not given elsewhere in this review. CRH, corticotrophin releasing hormone; sTNFR, soluble TNFα receptor; IL1RA, interleukin-1 receptor antagonist; NOS, nitric oxide synthase; COX, cyclooxygenase; CD73, cluster density 73; AMPA, a glutamate receptor; A1a, adenosine 1a receptor; gabaR, gamma-aminobutyric acid receptor.
Injection of either IL1β or TNFα into hypothalamic sleep regulatory areas such as the preoptic area also enhances duration of NREMS (Kubota et al., 2002; Terao et al., 1998). IL1β is also effective at promoting NREMS if injected in the locus coeruleus or into brain stem raphe nuclei containing noradrenergic or serotonergic neurons, respectively, that project widely and promote wakefulness (Imeri and Opp, 2009). Within the hypothalamus, somnogenic doses of IL1β tend to enhance the firing rate of sleep-active neurons and inhibit wake-active neurons (Alam et al., 2004). Local injection of either IL1β or TNFα onto the surface of the cerebral cortex induces unilateral enhancement of EEG delta waves during NREMS but not during wakefulness or REMS. These state-specific effects suggest that the local intensity of NREMS is enhanced by these cytokines; this work is also relevant to the hypotheses developed later in this review concerning local use-dependent sleep.
Brain and circulating levels of IL1β and TNFα vary with sleep propensity. Because accurate measurement of TNFα in plasma has been possible for a longer time than similar IL1β measurements, most of this literature is focused on TNFα. Nevertheless, the very earliest report correlating cytokine levels with changes in sleep showed that IL1β cerebrospinal fluid levels were enhancedduring sleep deprivation (Lue et al., 1988). In the subsequent years, levels of IL1β and TNFα mRNAs and their proteins were shown to increase during sleep deprivation in the hypothalamus and other areas of the brain. An independent literature showed that neuronal and glial expression of IL1β and TNFα in the somatosensory cortex is higher after excessive afferent sensory input induced by whisker stimulation in rats (Hallett et al., 2010). Prolonged wakefulness is associated with enhanced neuronal activity (Vyazovskiy et al., 2009). There are also changes in IL1β and TNFα over the course of the day with higher levels in brain occurring during the onset of daylight and lower levels at the onset of nighttime in rats corresponding to maximum sleep and waking periods, respectively.
IL1β and TNFα are also involved in the sleep responses associated with pathology. Infectious disease is accompanied by large changes in sleep and these changes are mediated by cytokines such as IL1β and TNFα (reviewed in Majde and Krueger, 2005). In fact, as the names imply, IL1β and TNFα were first characterized as key regulators of host defense responses. The involvement of cytokines in the sleep responses associated with inflammation has been characterized using mutant mouse models. For instance, mice lacking both TNFα receptors have much attenuated influenza virus-induced NREMS responses compared to wild-type controls (Kapas et al., 2008). Similarly, the sleep responses induced by bacterial cell wall components such as lipopolysaccharide or muramyl peptides are attenuated if IL1β or TNFα are inhibited (Imeri et al., 1993; Takahashi et al., 1996). Microbes or their components induce a variety of cytokines that in turn are responsible, in part, for regulating inflammatory responses. There is a large literature showing the variation of plasma TNFα or IL1β levels correlating with sleepiness or excess sleep occurring in many pathologies including sleep apnea (Entzian et al., 1996), insomnia, excessive daytime sleepiness (Vgontzas et al., 1997), HIV infection (Reddy et al., 1988), AIDS (Lahdevirta et al., 1988), myocardial infarction (Maury and Teppo, 1989), preeclampsia (Vince et al., 1995), postdialysis fatigue (Sklar et al., 1998), chronic inflammation (Tracey et al., 1988), and rheumatoid arthritis (Franklin, 1999; and reviewed in Kapsimalis, et al., 2005; Krueger et al., 2007; Majde and Krueger, 2005; Obal and Krueger, 2003; Opp, 2005). In fact, if clinically available inhibitors of IL1 (the interleukin-1 receptor antagonist) or TNFα (a TNFα soluble receptor) are given to patients, they reduce the sleepiness and fatigue associated with rheumatoid arthritis (Franklin, 1999; Omdal and Gunnarsson, 2005; Vgontzas et al., 2004). These extensive correlations between pathology, cytokine levels, and sleep have led to the hypothesis that primary sleep disorders such as sleep apnea or insomnia are indeed chronic inflammatory disorders.
Upstream and downstream events in the cytokine sleep regulatory cascade
The data thus far reviewed indicates that IL1β and TNFα are SRSs, however, what it is about wakefulness that enhances their production and subsequent sleep promoting activity remained unknown until recently. It seems that a key signal for release of mature IL1β and TNFα from glia in the brain is extracellular ATP released during neuro- and gliotransmission. There is a large literature showing ATP release during neurotransmission (reviewed in Burnstock, 2009) and another literature showing glia release of Ca2+ in response to ATP and conversely ATP release in response to extracellular Ca2+ (Metea and Newman, 2006). ATP thus released would transiently accumulate in the extracellular space in a manner analogous to neurotransmitter spatial and temporal summation due to repeated action potentials and the short half life of extracellular ATP. This source of extracellular ATP is linked to activity and is distinct from the much larger intracellular ATP compartment. The two ATP compartments, extra- and intracellular, are linked by several mechanisms including adenosine trafficking across the cell membrane.
ATP binds to membrane purine type 2 receptors (P2Rs; there are many subtypes, Y-type receptors are metabotropic while X-type P2 receptors are ionotropic.) The P2X7 receptor is involved in processing pro-IL1β into mature 17 kDa IL1β; this is well characterized within the immunological literature (reviewed in Ferrari et al., 2006). P2 receptors are also involved in IL1β, TNFα, and brain-derived neurotrophic factor (BDNF) release from glia (reviewed in Krueger et al., 2008). An ATP agonist promotes NREMS while ATP antagonists inhibit sleep. Further, the enhanced duration of NREMS and EEG delta power normally observed after sleep deprivation is much attenuated in mice lacking the P2X7 receptor. The P2X7 receptor and the P2Y1 receptor vary with the timeof dayin thesomatosensory cortex (Fig. 3). The involvement of P2Y receptors in sleep regulation has not yet been investigated. In summary, it appears that cell activity-associated release of ATP into the extracellular space is the transient trigger for cytokine processing and release from glia. The cytokines have a longer half life than ATP; this ATP–cytokine mechanism can thereby translate rapid brain cell activity into a long-term sleep regulatory signal. Such a mechanism is fundamentally a local network mechanism and suggests that sleep is initiated locally in response to cell activity.
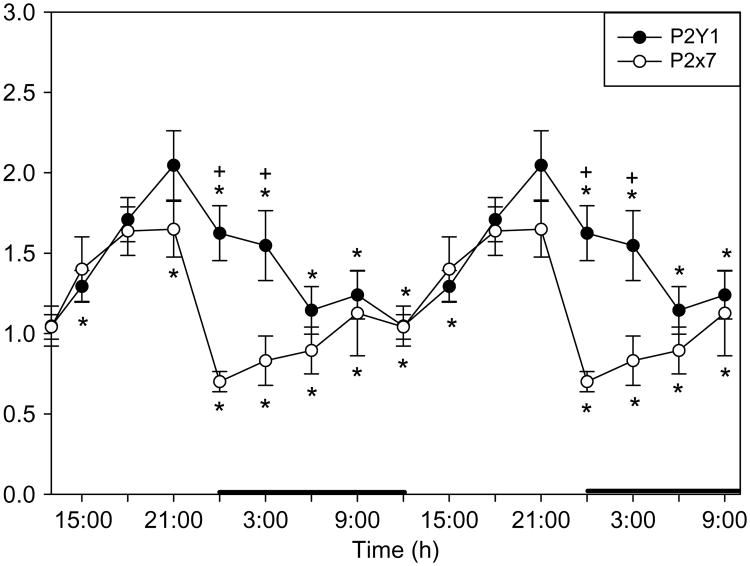
Fig. 3.
Both the P2Y1 and the P2X7 receptor mRNAs vary with the light dark cycle in the rat somatosensory cortex. Highest levels occur during daylight hours when sleep propensity is high. Sleep deprivation also enhances P2X7 and P2Y1 mRNA levels in brain. These receptors bind ATP and are associated with TNFα release and IL1β processing. Data derived in part from Krueger et al. (submitted for publication). Data are double plotted. * indicates p < 0.05.
The translation of extracellular ATP–cytokine signals into sleep involves both rapid and slow downstream events. Some of the extracellular ATP is rapidly catabolized toADP, AMP, and adenosine via ectonucleotidases, CD39 and CD73. CD73 inhibition reduces adenosine tissue concentrations. Interestingly, CD73 knockout mice have more spontaneous NREMS than wild-type controls further implicating ATP and its metabolism to the generation of sleep (Zielinski and Krueger, unpublished). Regardless, ATP is involved in slower downstream sleep mechanisms via its P2 receptor—cytokine release actions. Thus, IL1β and TNFα, among their many actions, activate nuclear factor kappa B(NFκB)totranslocate to the nucleus for enhancing transcriptionof multiple genes including the enzymes involved in production of the effector molecules (e.g., NO; Fig. 2). Further, NFκB activation enhances production of many neuromodulator and neurotransmitter receptors including the adenosine A1a receptor and the gluR1 component of the AMPA glutamate receptor. The change in receptor density will change the sensitivity of the neuron to respective chemical stimuli. Within a network, for example, a single cortical column, such a process will occur in many neurons simultaneously with the net result that the network output induced by an input will change; this is a state shift and it is driven by prior activity as outlined in Fig. 2.
Brain organization of sleep
The biochemical sleep mechanism outlined herein operates within local networks. Yet the dominant sleep regulatory paradigm within sleep research is one of state imposition on the brain by so-called sleep regulatory centers such as the ventrolateral preoptic hypothalamic area (Saper et al., 2005). It is well established that such regulatory circuits influence sleep. We propose that they are involved in the synchronization of state between multiple neuronal networks and are thus important for both sleep and waking cognition (Krueger et al., 2008). Nevertheless, the top-down sleep center imposition of sleep paradigm fails to address many critical issues. For example, it does not provide parsimonious explanations for (a) sleep inertia, (b) sleep homeostasis, (c) reoccurrence of sleep after lesions to the sleep centers, (d) sleep loss-induced performance decrements, and (e) many parasomnias such as sleep walking. The local use-dependent hypothesis outlined herein allows for parts of the brain to be asleep while parts are awake. With this view, it is easy to invoke explanations for these phenomena. For instance, it is possible that, while sleep walking, the parts of the brain necessary to navigate around objects is in the wake state while those parts necessary of consciousness are not. Similarly, upon awakening, some networks may remain in the sleep state thereby causing poor performance fidelity characteristic of sleep inertia.
One of the major collective findings in sleep research is that animals and humans that survive brain lesions sleep regardless of where the lesion is. This is strong evidence for the hypothesis that any viable neuronal/glia network will oscillate between states and that sleep is self-organizing. In fact, there is direct evidence that individual cortical columns (local neuronal networks) oscillate between functional states (Rector et al., 2005). One of those states has characteristics of sleep in that the longer the cortical column is in a wake-like state the higher the probability of it entering the sleep-like state (i.e., sleep homeostasis). Further, column state is dependent on prior activity and if TNFα is applied to a column the probability of that column entering the sleep-like state is greater. When the animal is asleep about 80–90% of the time, individual cortical columns are in the sleep-like state and conversely, when awake most are in the wake-like state. Finally, there is aphilosophical problem of an infinite regress associated with a sleep center telling the brain to sleep; who is telling the teller?
Sleep function
There are several demonstrable functions of sleep. Perhaps, the evolutionary value associated with better physical and cognitive performance gained by sleeping is most evident. By sleeping one also saves calories. Nevertheless, when sleeping one does not eat, drink, reproduce, or socialize and one is subject to predation; these are high evolutionary costs of sleeping. There has thus been intense debate concerning the primordial function of sleep. Most modern considerations of sleep function focus on a neuronal connectivity function (e.g., Benington and Frank, 2003; Kavanau, 1994; Krueger and Obal, 1993; Krueger et al., 2008; Tononi and Cirelli, 2003); such a function is not unrelated to the sleep-associated performance enhancement. A sleep connectivity function is also consistent with the ATP–cytokine–adenosine sleep regulatory mechanism because substances such as TNFα are directly linked to neuronal plasticity mechanisms such as synaptic scaling (Stellwagen and Malenka, 2006; Turrigiano, 2008). Further, epigenetic neuronal plasticity is dependent upon use and the ATP–cytokine–adenosine hypothesis provides a mechanism that tracks rapid neuronal activity and translates that activity into longer-lived molecular signals, for example, cytokines that directly influence state oscillations of local networks and trigger the molecular events associated with neural plasticity. While this is far from an adequate or comprehensive explanation of either sleep function or connectivity, it does directly illustrate that the molecular mechanisms responsible for sleep regulation are also involved in regulating synaptic connectivity. Of importance, the ATP–cytokine–adenosine hypothesis provides insight into the need for reduced consciousness or unconsciousness during sleep. Thus, with repeated use, the extracellular ATP levels spatially and temporally summate within the activated network. ATP in turn releases cytokines that alter receptor expression within the network and thereby changes the sensitivity of the network to transmitters and modulators. Thus, if the original network activity was associated with an adaptive environmental output, after the change in network sensitivities, the same input would have a different output, perhaps one not so adaptive. It would therefore be advantageous to avoid behaving at such times; unconsciousness would promote behavior quiescence.
Acknowledgments
This work was supported by the National Institutes of Health (USA) grant numbers NS25378 and NS31453 and HD36520 and by the W. M. Keck Foundation.
References
- Alam MN, McGinty D, Bashir T, Kumar S, Imeri L, Opp MR, et al. Interleukin-1beta modulates state-dependent discharge activity of preoptic area and basal forebrain neurons: Role in sleep regulation. The European Journal of Neuroscience. 2004;20(1):207–216. doi: 10.1111/j.1460-9568.2004.03469.x. [DOI] [PubMed] [Google Scholar]
- Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Progress in Neurobiology. 2003;69(2):71–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Burnstock G. Purinergic cotransmission. Experimental Physiology. 2009;94(1):20–24. doi: 10.1113/expphysiol.2008.043620. [DOI] [PubMed] [Google Scholar]
- Entzian P, Linnemann K, Schlaak M, Zabel P. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. The American Journal of Respiratory and Critical Care Medicine. 1996;153(3):1080–1086. doi: 10.1164/ajrccm.153.3.8630548. [DOI] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNF alpha treatment. The Journal of Neuroscience. 1997;17(15):5949–5955. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Wang Y, Krueger JM. Effects of interleukin-1 beta on sleep are mediated by the type I receptor. The American Journal of Physiology. 1998;274(3 Pt. 2):R655–R660. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 receptor: a key player in IL-1 processing and release. The Journal of Immunology. 2006;176(7):3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Franklin CM. Clinical experience with soluble TNF p75 receptor in rheumatoid arthritis. Arthritis and Rheumatism. 1999;29(3):172–181. doi: 10.1016/s0049-0172(99)80028-6. [DOI] [PubMed] [Google Scholar]
- Hallett H, Churchill L, Taishi P, De A, Krueger JM. Whisker stimulation increases expression of nerve growth factor- and interleukin-1beta-immunoreactivity in the rat somatosensory cortex. Brain Research. 2010;1333:48–56. doi: 10.1016/j.brainres.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nature Reviews Neuroscience. 2009;10(3):199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imeri L, Opp MR, Krueger JM. An IL-1 receptor and an IL-1 receptor antagonist attenuate muramyl dipeptide- and IL-1-induced sleep and fever. The American Journal of Physiology. 1993;265(4 Pt. 2):R907–R913. doi: 10.1152/ajpregu.1993.265.4.R907. [DOI] [PubMed] [Google Scholar]
- Kapas L, Bohnet SG, Traynor TR, Majde JA, Szentirmai E, Magrath P, et al. Spontaneous and influenza virus-induced sleep are altered in TNF-alpha double-receptor deficient mice. Journal of Applied Physiology. 2008;105(4):1187–1198. doi: 10.1152/japplphysiol.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsimalis F, Richardson G, Opp MR, Kryger M. Cytokines and normal sleep. Current Opinion in Pulmonary Medicine. 2005;11(6):481–484. doi: 10.1097/01.mcp.0000183062.98665.6b. [DOI] [PubMed] [Google Scholar]
- Kavanau JL. Sleep and dynamic stabilization of neural circuitry: A review and synthesis. Behavioural Brain Research. 1994;63(2):111–126. doi: 10.1016/0166-4328(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Krueger JM. The role of cytokines in sleep regulation. Current Pharmaceutical Design. 2008;14(32):3408–3416. doi: 10.2174/138161208786549281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Obal F. A neuronal group theory of sleep function. Journal of Sleep Research. 1993;2(2):63–69. doi: 10.1111/j.1365-2869.1993.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Krueger JM, Rector DM, Churchill L. Sleep and cytokines. Sleep Medicine Clinics. 2007;2(2):161–169. doi: 10.1016/j.jsmc.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Rector DM, Roy S, Van Dongen HP, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nature Reviews Neuroscience. 2008;9(12):910–919. doi: 10.1038/nrn2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Taishi P, De A, Davis CJ, Winters B, Clinton JM, et al. ATP and the purine type 2 X7 receptor are involved in sleep regulation submitted for publication. [Google Scholar]
- Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1) The American Journal of Physiology. 1984;246(6 Pt. 2):R994–R999. doi: 10.1152/ajpregu.1984.246.6.R994. [DOI] [PubMed] [Google Scholar]
- Kubota T, Li N, Guan Z, Brown RA, Krueger JM. Intrapreoptic microinjection of TNF-alpha enhances non-REM sleep in rats. Brain Research. 2002;932(1–2):37–44. doi: 10.1016/s0006-8993(02)02262-x. [DOI] [PubMed] [Google Scholar]
- Lahdevirta J, Maury CP, Teppo AM, Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. The American Journal of Medicine. 1988;85(3):289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- Lue FA, Bail M, Jephthah-Ochola J, Carayanniotis K, Gorczynski R, Moldofsky H. Sleep and cerebrospinal fluid interleukin-1-like activity in the cat. The International Journal of Neuroscience. 1988;42(3–4):179–183. doi: 10.3109/00207458808991595. [DOI] [PubMed] [Google Scholar]
- Majde JA, Krueger JM. Links between the innate immune system and sleep. The Journal of Allergy and Clinical Immunology. 2005;116(6):1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Maury CP, Teppo AM. Circulating tumour necrosis factor-alpha (cachectin) in myocardial infarction. Journal of Internal Medicine. 1989;225(5):333–336. doi: 10.1111/j.1365-2796.1989.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Calcium signaling in specialized glial cells. Glia. 2006;54(7):650–655. doi: 10.1002/glia.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obal F, Jr, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Frontiers in Bioscience. 2003;8:d520–d550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- Omdal R, Gunnarsson R. The effect of interleukin-1 blockade on fatigue in rheumatoid arthritis–A pilot study. Rheumatology International. 2005;25(6):481–484. doi: 10.1007/s00296-004-0463-z. [DOI] [PubMed] [Google Scholar]
- Opp MR. Cytokines and sleep. Sleep Medicine Reviews. 2005;9(5):355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Pappenheimer JR, Koski G, Fencl V, Karnovsky ML, Krueger J. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. Journal of Neurophysiology. 1975;38(6):1299–1311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Research. 2005;1047(1):45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Sorrell SJ, Lange M, Grieco MH. Tumor necrosis factor and HIV P24 antigen levels in serum of HIV-infected populations. Journal of Acquired Immune Deficiency Syndromes. 1988;1(5):436–440. [PubMed] [Google Scholar]
- Roy S, Krueger JM, Rector DM, Wan Y. A network model for activity-dependent sleep regulation. Journal of Theoretical Biology. 2008;253(3):462–468. doi: 10.1016/j.jtbi.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. The American Journal of Physiology. 1987;253(1 Pt. 2):R142–R149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- Sklar AH, Beezhold DH, Newman N, Hendrickson T, Dreisbach AW. Postdialysis fatigue: Lack of effect of a biocompatible membrane. American Journal of Kidney Diseases. 1998;31(6):1007–1010. doi: 10.1053/ajkd.1998.v31.pm9631846. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Kapas L, Krueger JM. A tumor necrosis factor (TNF) receptor fragment attenuates TNF-alpha- and muramyl dipeptide-induced sleep and fever in rabbits. Journal of Sleep Research. 1996;5(2):106–114. doi: 10.1046/j.1365-2869.1996.d01-63.x. [DOI] [PubMed] [Google Scholar]
- Terao A, Matsumura H, Yoneda H, Saito M. Enhancement of slow-wave sleep by tumor necrosis factor-alpha is mediated by cyclooxygenase-2 in rats. Neuroreport. 1998;9(17):3791–3796. doi: 10.1097/00001756-199812010-00005. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: A hypothesis. Brain Research Bulletin. 2003;62(2):143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Toth LA, Krueger JM. Alteration of sleep in rabbits by Staphylococcus aureus infection. Infection and Immunity. 1988;56(7):1785–1791. doi: 10.1128/iai.56.7.1785-1791.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ, Wei H, Manogue KR, Fong Y, Hesse DG, Nguyen HT, et al. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. The Journal of Experimental Medicine. 1988;167(3):1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: Synaptic scaling of excitatory synapses. Cell. 2008;135(3):422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. The Journal of Clinical Endocrinology and Metabolism. 1997;82(5):1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. The Journal of Clinical Endocrinology and Metabolism. 2004;89(9):4409–4413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. British Journal of Obstetrics and Gynaecology. 1995;102(1):20–25. doi: 10.1111/j.1471-0528.1995.tb09020.x. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, et al. Cortical firing and sleep homeostasis. Neuron. 2009;63(6):865–878. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]