Abstract
Non-gastrointestinal stromal soft tissue sarcomas are uncommon neoplasms that have a dismal prognosis due to a high incidence of metastases and a poor response to conventional chemotherapy. The identification of characteristic genetic alterations in several of these tumors has opened the window for molecular targeted therapies in patients who have failed conventional chemotherapy. Imaging plays a critical role in assessing the response to these novel therapeutic agents. Just like the response of gastrointestinal stromal tumors to imatinib, the response of non-gastrointestinal stromal soft tissue sarcomas to molecular targeted drugs is better evaluated on imaging by alternate tumor response criteria such as the Choi criteria. In addition, these drugs are associated with distinct class-specific drug toxicities that can come to attention for the first time on imaging. The purpose of this article is to provide a primer for the radiologist on the various molecular targeted therapies in advanced/metastatic non-gastrointestinal stromal soft tissue sarcomas with emphasis on the role of imaging in assessing treatment response and complications.
Keywords: Soft tissue sarcomas, molecular targeted therapies, CT, MRI, PET/CT, drug toxicities
Introduction
In 2002, imatinib mesylate (Gleevec), a c-KIT tyrosine kinase inhibitor (TKI), which was initially used to treat chronic myeloid leukemia, was granted accelerated US Food and Drug Administration (FDA) approval for the treatment of advanced or metastatic gastrointestinal stromal tumor (GIST). Sunitinib, a vascular endothelial growth factor receptor (VEGFR) inhibitor, approved for use in metastatic renal cell carcinoma, was the next drug found to have a beneficial effect in imatinib-resistant GISTs. Understanding the molecular pathways of GISTs and the identification of potential new molecular targets led to a quest to replicate the success of imatinib in non-GIST soft tissue sarcomas (STS). More than a decade later, the usefulness of several new TKIs that are close congeners of imatinib is being explored in the treatment of advanced and metastatic STS. As more of these drugs are being developed and approved by the FDA, there is a greater need for radiologists to be aware of the molecular pathways involved in sarcomagenesis, treatment response patterns and frequently encountered toxicities.
STSs are uncommon tumors of mesenchymal origin; they account for about 1% of all malignancies[1]. They arise from connective tissue virtually anywhere in the body, but most commonly in the extremities and the retroperitoneum. Several histologic subtypes of sarcomas exist (more than 50) of which the common types in adults are liposarcoma (LPS), leiomyosarcoma (LMS), GIST, and synovial sarcoma (SS)[2]. STSs are clinically challenging to manage. More than 50% of patients have metastatic disease at presentation, frequently in the lungs and liver[3]. Considerable progress has been made in the treatment of GISTs with the advent of imatinib and its congeners. However, the management of most non-GIST STSs has not met with similar success. Surgery with wide resection margins with or without radiotherapy is the standard of care for the primary non-GIST STS[2,4]. Anthracyclines and ifosfamide-based regimens are still the mainstay of therapy of advanced and metastatic STSs, with gemcitabine, docetaxel and dacarbazine acting as second-line agents[2,4]. However, most STSs have poor chemosensitivity resulting in high treatment failure rates, with mean survival rates approaching 1 year[5]. In nonresponsive cases, several molecular targeted therapies (MTT) have shown variable responses in phase II clinical trials[6–10] (Table 1). Imatinib was found to have activity in aggressive fibromatosis, dermatofibrosarcoma protuberans (DFSP) and malignant pigmented villonodular synovitis (PVNS)[11–13]. Inhibitors of mammalian target of rapamycin (mTOR) have shown dramatic efficacy in mTOR-driven tumors such as perivascular epithelioid cell tumor (PEComas)[14]. Trabectedin or ET743 has been approved in the European Union for the treatment of advanced LPS and LMS[15]. Imaging plays a critical role in assessing the efficacy of treatment and detecting drug toxicity in these non-GIST STSs treated with MTT. Accordingly, the aim of this article is to provide a review of established molecular pathways behind some of the non-GIST STSs and the various MTTs for their treatment, emphasizing the role of imaging in assessing tumor response and complications of therapy (Table 2).
Table 1.
Summary of molecular targeted drugs used in non-GIST STS, their targets and class-specific drug toxicities
| Molecular targeted drug | Mechanism of action | Soft tissue sarcoma | Class-specific drug toxicities |
|---|---|---|---|
| Imatinib mesylate | Inhibits ABL, c-KIT, PDGF, CSF1 kinases | Aggressive fibromatosis, dermatofibrosarcoma protuberans, pigmented villonodular synovitis | Fluid retention |
| Tyrosine kinase inhibitors and antibodies (non-imatinib) | Inhibit VEGF, PDGF, Src kinases | Leiomyosarcoma, solitary fibrous tumor, synovial sarcoma | Hepatobiliary, pancreatic and bowel-related complications, thromboembolic phenomena, pleural effusions (dasatinib) |
| mTOR inhibitors | Inhibit the PI3K-AKT-mTOR pathway | PEComa | Non-infectious pneumonitis, acute cholecystitis |
| Trabectedin | Unknown (probably inactivates FUS-CHOP oncogene) | Myxoid liposarcoma, leiomyosarcoma | Capillary leak syndrome |
GIST, gastrointestinal stromal tumor; STS, soft tissue sarcoma; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor; CSF1, colony-stimulating factor 1.
Table 2.
Summary of what radiologists should know about molecular targeted therapies (MTT) in advanced and metastatic non-GIST STS
| Imaging findings which indicate response to MTT | Decrease in tumor density |
Decrease in enhancement
| |
| Decrease in size | |
| Ancillary findings: hemorrhage, cystic change, transient increase in size | |
| Treatment response criteria | Understand limitations of RECIST |
| Alternative criteria: Choi, size and attenuation CT criteria, MASS criteria | |
| Imaging findings indicating tumor recurrence | Increase in size |
| Increase in tumor density | |
| Enhancing intratumoral nodule | |
| Imaging findings of drug toxicity | Fluid retention: anasarca, pleural effusions, pulmonary edema |
| Pneumonitis | |
| Hepatic steatosis, hepatitis | |
| Acute cholecystitis | |
| Pancreatitis | |
| Bowel-related complications: ischemic colitis, pneumatosis intestinalis | |
| Arterial and venous thromboembolic phenomenon | |
| Capillary leak syndrome: unilateral pulmonary edema |
MTT, molecular targeted therapy; GIST, gastrointestinal stromal tumor; STS, soft tissue sarcoma; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor; TKI, tyrosine kinase inhibitor; RECIST, Response Evaluation Criteria in Solid Tumors; MASS, morphology, attenuation, size and structure.
Cancer pathways of non-GIST STSs and potential targets for therapies
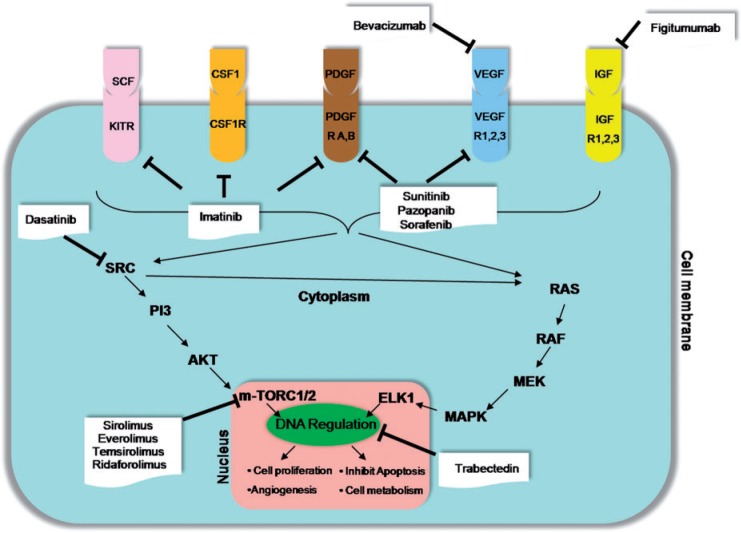
STSs are broadly classified at a molecular level into those with simple diploid karyotypes consisting of specific predictable molecular aberrations and those with complex karyotypes consisting of non-specific genetic alterations, which are random and unpredictable[16]. STSs with complex karyotypes are associated with a high frequency of chromosomal instability, p53 and retinoblastoma pathway mutations. On the other hand, STSs with simple karyotypes are associated with specific genetic alterations such as translocations, deletions, gene amplifications and mutations[2,17]. Reciprocal translocations result in the formation of chimeric fusion proteins that affect the intracellular signal cascades. Examples of fused genes resulting from translocations in STS include the FUS-CHOP [t(12; 16)(q13; q11)] seen in 95% of cases of myxoid LPS, EWS-FLI1 [t(11; 22)(q24; q12)] seen in 85% of cases of Ewing sarcoma and ASPSCR1-TFE3 [t(X; 17)(p11; q25)] seen in 99% of cases of alveolar soft part sarcoma[2,16,17]. Both simple and complex karyotypes in STS eventually cause dysregulation of several downstream signal pathways. The major pathways that play a vital role in sarcomagenesis are the VEGF pathway, the insulin-like growth factor (IGF) pathway and the mTOR pathway, each of which could potentially act as targets for several MTTs[18] (Fig. 1). The VEGF pathway plays a key role in angiogenesis mediated by several kinases including VEGF and platelet-derived growth factor (PDGF) and their receptors[18,19]. The IGF pathway is important for promoting cellular proliferation and growth and is mediated by 4 types of IGF receptors that activate intracellular signaling cascades through either the RAS-RAF-MEK-MAPK pathway or the PI3K-AKT-mTOR pathway[16]. The mTOR pathway is the final merging point for several intracellular signal cascades and can be intrinsically activated by several mutations and deletions[16].
Figure 1.
Cancer pathways in non-GIST STS and potential targets for therapies. VEGF/VEGFR, vascular endothelial growth factor/receptor; PDGF/PDGFR, platelet-derived growth factor/receptor; CSF1/CSF1R, colony-stimulating factor 1/receptor, IGF/IGFRm insulin-like growth factor/receptor.
Imatinib in non-GIST STS
Initially approved for treatment of metastatic GISTs, imatinib subsequently showed efficacy in several other STSs. Imatinib is the prototype for the class of drugs that inhibit multiple intracellular tyrosine kinases with activity against ABL, c-KIT, PDGFR, CSF1R kinases[20] (Fig. 1). The activity of imatinib in GIST is mainly due to its ability to inhibit c-KIT mediated kinases. Another tumor where imitanib acts through c-KIT-mediated kinases is aggressive fibromatosis (Fig. 2). Aggressive fibromatosis or desmoid tumor is a locally aggressive non-metastasizing mesenchymal neoplasm, which commonly presents as a firm intra-abdominal or abdominal wall mass in young females, often associated with familial adenomatous polyposis (FAP) syndrome[21]. Surgical resection remains the treatment of choice for primary desmoid tumors if present in favorable locations[21]. Non-operable and recurrent desmoids may be treated by radiotherapy or with systemic therapy with anthracyclines or hormonal therapy[2,4]. Immunohistochemistry of desmoid tumors shows strong positivity to c-KIT and PDGFB, which is the basis of successful therapy with imatinib[11,21] (Fig. 2). In a study of 40 patients with unresectable or progressive fibromatosis, Penel et al.[11] found non-progression rates of 89% and 67% for patients with previously progressive disease after 3 and 12 months of treatment, respectively.
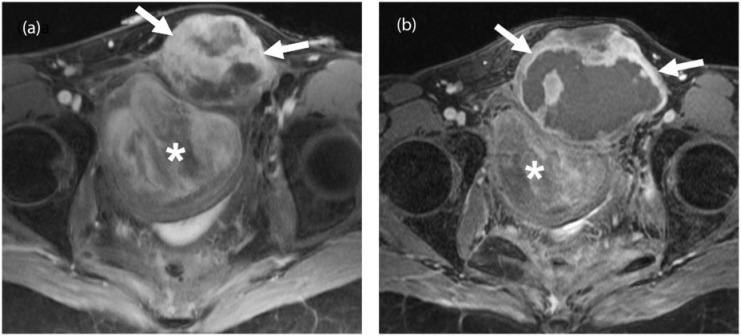
Figure 2.
A 33-year-old woman with familial adenomatous polyposis (FAP)-associated desmoid fibromatosis. (a) Axial fat-suppressed post-gadolinium T1-weighted MR image of the pelvis demonstrates 2 large heterogeneously enhancing pelvic desmoid masses (arrows and asterisk). (b) Follow-up axial fat-suppressed post-gadolinium T1-weighted MR image 6 months after treatment with imatinib shows a mild increase in the size of the anteriorly located mass (arrows) with marked central necrosis and a mild decrease in the size and enhancement of the posterior mass (asterisk) consistent with treatment response.
Two other non-GIST STSs where imatinib acts through completely different targets are DFSP and PVNS. DFSP, a slow-growing mesenchymal tumor, is historically the second STS after GIST to be treated with MTT[22]. DFSP is a locally aggressive tumor of the dermis mainly encountered in the trunk and proximal extremities of early and middle-aged men. Although a benign tumor, it has a tendency to recur locally if incompletely resected. Rarely, DFSP can have overgrowth of fibrosarcomatous components, and presents with lung metastasis in up to 4% of cases[12]. The target for imatinib in DFSP is the PDGFB receptor, which is upregulated by the chimeric protein COL1A1-PDGFB resulting from the translocation t(17;22)[16,22]. This translocation is seen in excess of 90% of cases of DFSP and is the rationale behind the efficacy of imatinib[16]. In a study of 10 patients with DFSP, McArthur et al.[12] were able to achieve clinical response in 8 patients with locally advanced disease and partial response in 1 case of metastatic DFSP. The response in these 9 patients was attributed to the inhibition of t(17;22)-mediated activation of PDGFB.
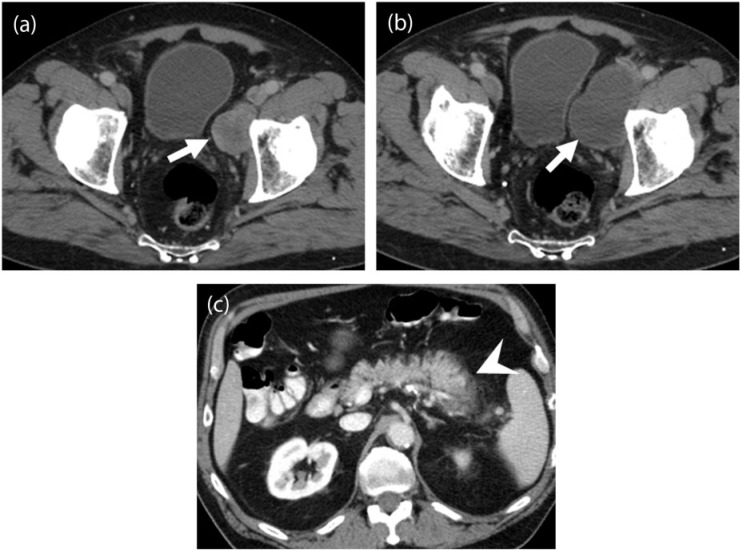
PVNS refers to a benign neoplastic process affecting the synovium of mainly large joints such as the knee and characterized by the presence of multi-nucleated giant cells and hemosiderin deposition on histopathology[23]. Malignant transformation in PVNS is a rare phenomenon occurring usually in the later decades of life with less than 5% incidence and seen at imaging as pulmonary and lymph nodal metastasis[23] (Fig. 3). At a molecular level, PVNS has a specific translocation t(1;2), which results in overexpression of the macrophage colony-stimulating factor (CSF1) gene and recruitment of cells expressing CSF1 receptor. Imatinib has been shown to have an inhibitory effect on CSF1R, independent of its activity against other kinases[24] (Fig. 3). In a study of 29 patients with locally advanced or metastatic PVNS, Cassier et al.[13] found objective response in 20% patients using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0.
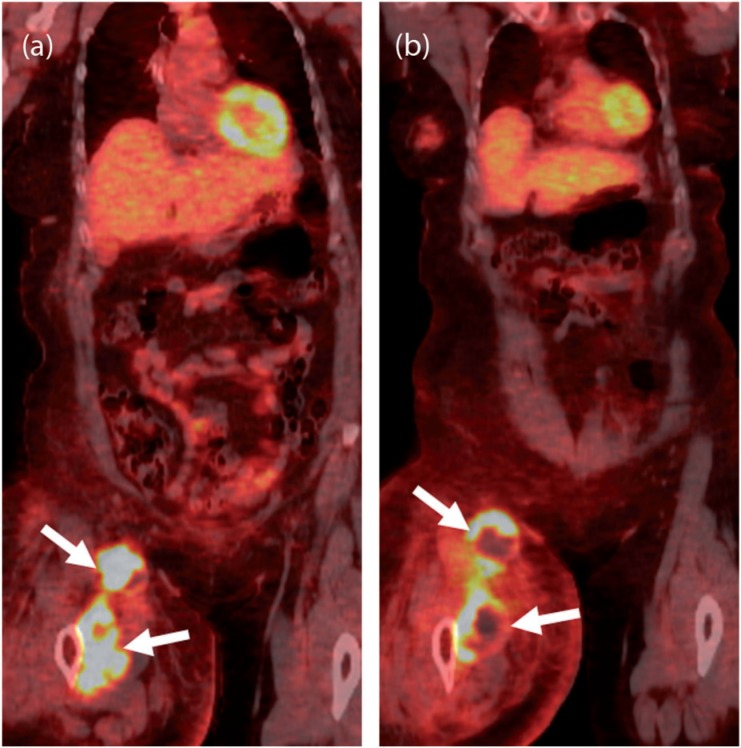
Figure 3.
A 56-year-old woman with pigmented villonodular synovitis (PVNS) metastatic to the inguinal nodes and muscles of the thigh after resection of the primary tumor in the knee joint 1 year before. (a) Coronal [18F]FDG-PET/CT image of the right lower limb demonstrates an intensely FDG-avid (maximum standardized uptake value (SUVmax) of 22) mass in the anterior compartment of the right thigh (arrows). (b) Coronal [18F]FDG-PET/CT image obtained 1 month after treatment with imatinib shows decreased FDG uptake of the mass with central photopenia (arrows), suggesting interval necrosis and treatment response.
The role of imaging in these tumors, apart from making the primary diagnosis, is to assess the response to treatment and detect drug toxicity. The assessment of response with the RECIST in these soft tissue tumors treated with MTT may have pitfalls and limitations[25,26]. Similar to GIST, the response to imatinib in these tumors is better evaluated by personalized tumor response criteria involving changes in density with or without changes in size. A few studies have shown the applicability of the Choi criteria even in non-GIST STS[27] (Fig. 2). In a study of 37 patients, Stacchiotti et al.[27] compared RECIST and Choi criteria for assessing the response to treatment in high-grade STS on contrast-enhanced computed tomography (CT) and dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI). The RECIST and Choi criteria were adapted to MRI in their study by calculating the percentage of enhancement on DCE-MRI on subtracted contrast-enhanced T1-weighted images (matrix, 254 × 384; slice thickness, 5 mm; inter-slice gap, 2 mm; repetition time (TR), 616 ms; echo time (TE), 11 ms) using serial regions of interest encompassing the entire tumor and muscle as reference. A partial response was indicated by either a decrease in size of more than 10% or a decrease in attenuation on CT or enhancement on contrast-enhanced MRI by greater than 15%. In addition, functional imaging techniques such as fluoro-2-deoxy-d-glucose (FDG)-positron emission tomography (PET)/CT are also useful in assessing the response to imatinib (Fig. 3). Imatinib causes dose-dependent fluid retention and edema, occasionally progressing to ascites and pleural effusions, which may be misinterpreted as disease progression[28,29]. Fluid retention may be seen in up to 80% of patients, especially elderly female patients with low serum albumin levels[28]. Response to diuretics and occasionally drug discontinuation can help in differentiating toxicity from disease progression.
Other TKIs in non-GIST STS
Sunitinib is used as a second-line agent in GIST, following primary or secondary resistance to imatinib[2]. It is a multi-targeted TKI which is FDA approved for imatinib-resistant GIST and metastatic renal cell carcinoma. In sunitinib-resistant GIST, a host of other TKIs including sorafenib, pazopanib, dasatinib and nilotninib are under research in several phase I and II trials[30]. Expecting results similar to imatinib and sunitinib in GISTs, the efficacy of multi-targeted TKI is being studied in advanced non-GIST STSs in several phase II trials. In a study of 142 patients with advanced or relapsed STS treated with pazopanib after failure with second-line chemotherapeutic agents, Sleijfer et al.[9] found that the 12-week progression-free rate (PFR) was 44%, 49% and 39% in LMS, SS and other STS types, respectively, which were better than the response to second-line chemotherapeutic agents. In April 2012, pazopanib was approved by the FDA for the treatment of metastatic renal cell carcinoma and advanced STS (excluding adipocytic STS and GIST) that fail to respond to first-line chemotherapeutic agents.
The activity of TKIs, which are potent antiangiogenic agents, is based on the rationale that angiogenesis is key to the growth and dissemination of tumors, including STS. The formation of neovascular channels in tumors occurs in response to ischemia, which upregulates the expression of angiogenic factors such as VEGF and PDGF. VEGF is overexpressed in most sarcomas, reflecting their malignant potential and poor outcome[31]. Studies on various STSs have shown that there is a direct correlation between the tumor concentration of angiogenic factors and tumor grade, local recurrence, metastasis and overall survival[31]. Receptors of VEGF (VEGF-R1, 2, 3) and PDGF (alpha-, beta-) are targets for TKI[18] (Fig. 1). Monoclonal antibodies to VEGF have an effect similar to TKI. Bevacizumab, a humanized anti-VEGF antibody that is FDA approved for combination treatment of metastatic colorectal, renal and lung cancers, is being evaluated in phase II trials for combination treatment in STS[32].
TKI knock off the neoangiogenic pathways in sarcomas causing progressive decrease in density and enhancement on contrast-enhanced CT without necessarily causing shrinkage of the tumor (Fig. 4). This is explained by the fact that MTTs are cytostatic agents rather than cytotoxic[33,34]. There may be an apparent increase in the size of the tumor, which may be misinterpreted as tumor progression (Fig. 5). Similarly, tumor progression can occur without actual increase in size and may be seen as new intratumoral nodules or increase in tumor attenuation[35] (Fig. 6). Thus, density changes on post-treatment imaging rather than size alone are more critical for assessing treatment response. The pitfalls and limitations of using RECIST alone for response evaluation at imaging in non-GIST STSs are similar to that of GIST and metastatic renal cell carcinomas (RCC)[25,34] (Figs. 5 and 6). Alternate tumor response criteria such as the size and attenuation CT criteria, morphology, attenuation, size, and structure (MASS) criteria for metastatic RCC and the Choi criteria for GIST have been proposed to incorporate a subjective component to response evaluation[26,36,37]. On similar lines, the Choi criteria have been evaluated for assessment of treatment response in high-grade STS. In a study of 37 patients with advanced STS treated with chemoradiotherapy, Stacchiottti et al.[27] found that the Choi criteria had greater sensitivity than RECIST for assessing tumor response. Analyzing the metabolic tumor response based on [18F]FDG uptake according to the EORTC (European Organisation for Research and Treatment of Cancer) criteria is an attractive option, but limited by lack of standardization of uptake values[38].
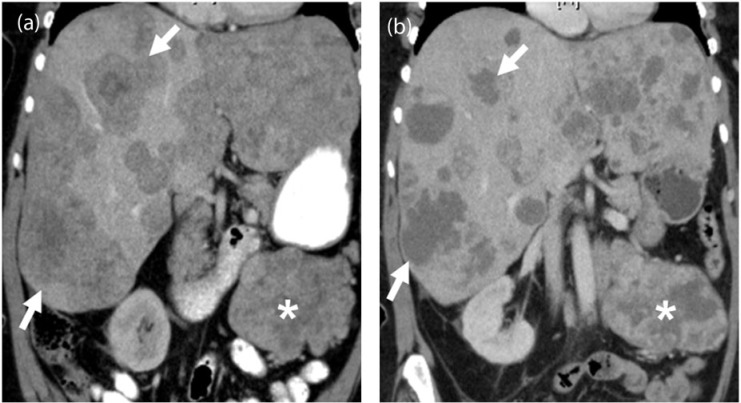
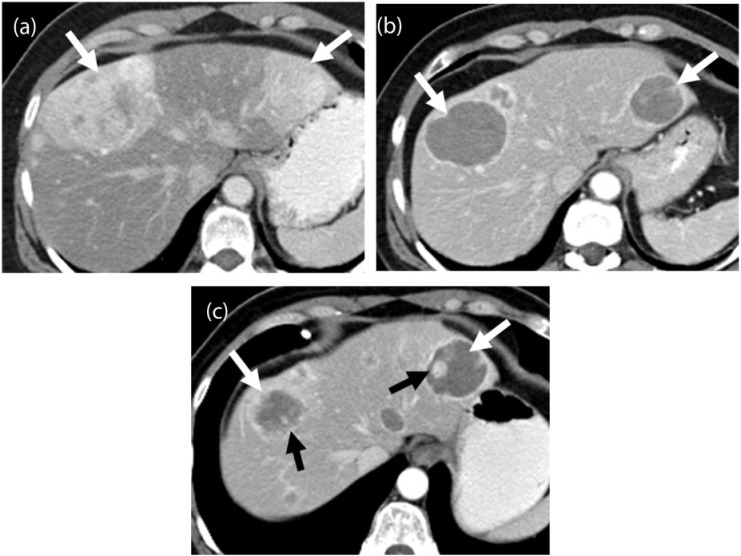
Figure 4.
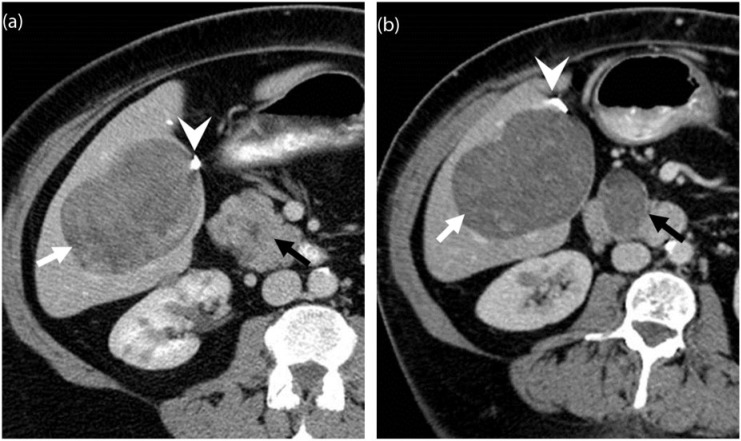
A 67-year-old woman with metastatic leiomyosarcoma. (a) Coronal contrast-enhanced CT image before the start of treatment demonstrates multiple heterogeneous solid liver lesions almost replacing the hepatic parenchyma (arrows) and a large mesenteric mass (asterisk) representing widespread metastatic disease. (b) Coronal contrast-enhanced CT image after 1 month of treatment with pazopanib reveals significantly decreased density and size of the liver metastases (arrows), many of which have become cystic, representing treatment response according to the Choi criteria. Similar changes are noted in the mesenteric mass (asterisk).
Figure 5.
A 78-year-old man with metastatic leiomyosarcoma. (a) Axial contrast-enhanced CT image demonstrates a lobulated heterogeneous mass along the left pelvic sidewall (arrow) representing metastatic disease. (b) Axial contrast-enhanced CT image after 6 months of treatment with pazopanib reveals increased size of the mass with a concurrent significant decrease in the density (arrow). (c) Axial contrast-enhanced CT image of the abdomen during an episode of acute abdominal pain during the course of treatment reveals a bulky and edematous pancreas with peripancreatic stranding consistent with acute pancreatitis (arrowhead), a class-specific drug toxicity of tyrosine kinase inhibitors (TKIs).
Figure 6.
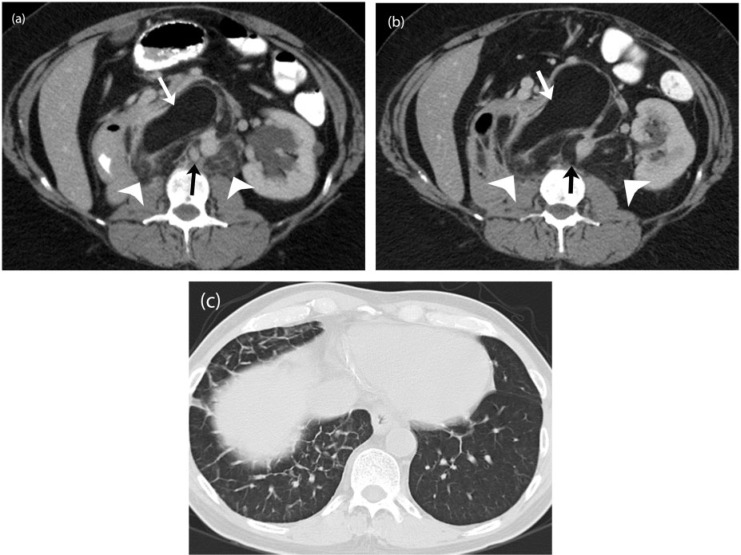
A 55-year-old woman with a metastatic solitary fibrous tumor. (a) Axial contrast-enhanced CT image demonstrates large, heterogeneous and hypervascular metastases in the liver (white arrows). (b) Axial contrast-enhanced CT image obtained 12 months after initiating treatment with pazopanib shows progressive decrease in the enhancement of the lesions, which have become almost cystic in appearance (white arrows). (c) Follow-up axial contrast-enhanced CT image 18 months after the start of treatment shows new nodular enhancement within the cystic-appearing lesions (black arrows) termed nodule within a cyst development representative of tumor recurrence according to the Choi criteria.
Imaging also plays a key role in recognizing drug toxicity. TKI can cause transient increase in aminotransferases, amylase and lipase[39]. Imaging evaluation may be requested in such patients to exclude hepatitis or pancreatitis (Fig. 5). Pazopanib and bevacizumab have been reported to cause hepatic steatosis and hepatitis when used as monotherapy or in combination with non-hepatotoxic drugs[39]. Sorafenib has been reported to cause painless pancreatitis diagnosed based on CT findings and increased serum lipase levels[40]. TKI can also cause acute acalculous cholecystitis due to gall bladder ischemia[41]. Bowel-related complications are known with both TKI and anti-VEGF antibodies[42]. TKI can cause ischemic colitis, pneumatosis and bowel perforation, which may come to attention for the first time at imaging. Recognizing these complications, especially in the perioperative period, becomes important and may necessitate withdrawal of the drug. Given that TKIs cause endothelial injury, it is not unusual to see arterial and venous thromboembolic phenomena, which may necessitate drug discontinuation[29].
Src intracellular kinases, which are the common point for several tyrosine kinase pathways determine tissue invasion and metastasis. Dasatinib is a new novel therapeutic agent that can inhibit multiple kinases including the Src family, BCR-ABL kinase and PDGFR[43]. Dasatinib has been shown to inhibit cell migration and promote apoptosis in sarcoma cell lines[43]. Dasatinib has been found to be useful for chronic myeloid leukemia resistant to imatinib and Philadelphia chromosome-positive acute lymphoblastic leukemia[44]. Several phase II trials are exploring the efficacy of dasatinib in metastatic STS[45] (Fig. 7). The toxicity profile of dasatinib includes myelosuppression, neuropathy, gastrointestinal effects (diarrhea, nausea, vomiting) and fluid retention. Exudative pleural effusions can occur with dasatinib in 20% of patients, requiring dose reduction, diuretics or steroid therapy[46] (Fig. 7).
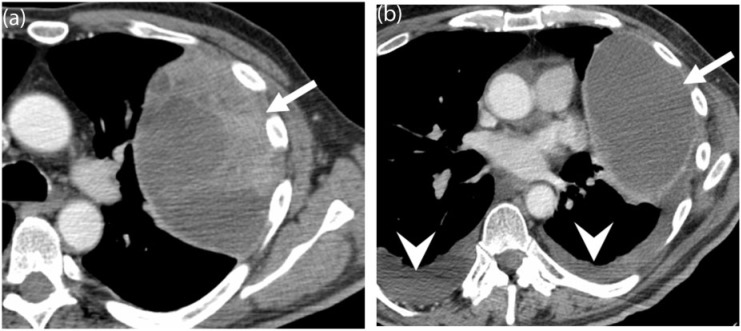
Figure 7.
A 24-year-old man with metastatic synovial sarcoma. (a) Baseline contrast-enhanced CT image of the chest demonstrates a large, heterogeneous left-sided pleural-based metastasis (arrow). (b) Follow-up CT after 6 months of treatment with dasatanib shows treatment response, with a decrease in the size and soft tissue component of the mass (arrow). Note the interval development of small bilateral pleural effusions (arrowheads), a common side effect of dasatinib therapy.
mTOR driven non-GIST STS
The mTOR serine/threonine kinase is an integral part of the PI3K pathway that is activated in several mTOR driven cancers such as renal cell carcinoma, either intrinsically or by mutations in regulatory genes such as TSC1 and 2, VHL, PTEN[47]. mTOR inhibitors are a group of drugs that inhibit the mTOR cascade by binding to the mTOR complex 1 protein and include sirolimus, temsirolimus, ridaforolimus and everolimus[18] (Fig. 1). Temsirolimus and everolimus are currently FDA approved for treatment of advanced RCC. Based on the rationale that the mTOR pathway is critical even in sarcomagenesis, mTOR inhibitors are being evaluated in non-GIST STS. For example, ridaforolimus has shown encouraging results in the SUCCEED phase III trial for advanced sarcomas[48]. Using a combination of drugs to block both the mTOR cascade and its upstream regulators such as the IGF pathway may have a synergistic effect. Quek et al.[49] reported encouraging results with a combination of everolimus and figitumumab, IGF-1R monoclonal antibody, in a phase I trial in a sarcoma-predominant population.
A classic example for mTOR driven sarcoma is PEComa, a tumor of the perivascular endothelial cells. PEComas typically arise in the genitourinary tract, liver, pancreas, retroperitoneum and mesentery and affect young adult females[50]. They can range from seemingly benign to malignant variants. Malignant PEComas tend to behave as aggressively as other STSs with high recurrence and metastatic rates[14] (Fig. 8). Preoperative diagnosis at imaging is often difficult due to their non-specific appearance. In the uterus, benign tumors may mimic leiomyomas, whereas the malignant variants can mimic other uterine sarcomas[51]. Malignant PEComas are usually large heterogeneous masses with variable enhancement and infiltration of adjacent structures. Treatment of malignant PEComas is surgical excision as they are usually resistant to chemotherapy and radiotherapy[14]. In addition, targeted drugs against the mTOR cascade are under research (Fig. 8). In a study of 3 patients with PEComas treated with the mTOR inhibitor sirolimus, Wagner et al.[14] found significant clinical response in all the patients.
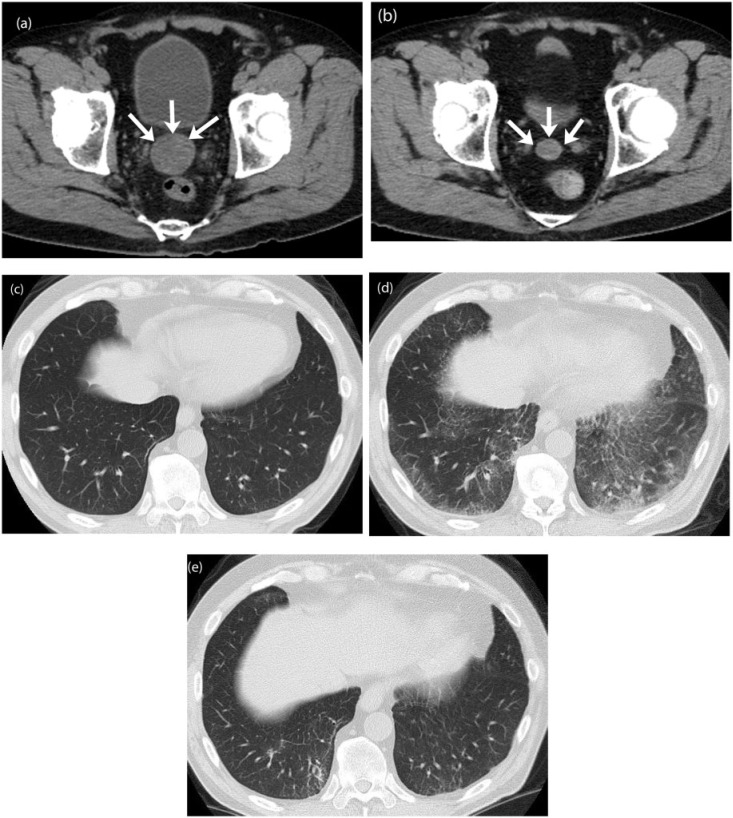
Figure 8.
A 68-year-old man with metastatic malignant renal PEComa 3 years after nephrectomy. (a,b) Axial unenhanced CT images of the pelvis before (a) and 1 month after the start of treatment with everolimus (b) reveals a decrease in the size of a metastatic deposit in the rectovesical pouch (arrows). (c) Axial CT image of the lung bases at baseline demonstrates no abnormality. (d) Axial CT image of the lung bases 1 month after the start of everolimus treatment shows rapid interval development of interlobar septal thickening and ground glass opacities with basilar and peripheral distribution consistent with everolimus-induced interstitial pneumonitis, a class-specific drug toxicity of mTOR inhibitors. The patient was symptomatic, with shortness of breath. (e) Follow-up CT of the chest 3 months after discontinuing everolimus and initiating steroid therapy demonstrates improvement in the interstitial lung toxicity.
The major toxicities of mTOR inhibitors include myelosuppression, asthenia and gastrointestinal side effects (nausea, vomiting, diarrhea, enteritis). Everolimus is rarely associated with acute cholecystitis, which is postulated to be due to ischemia related to endothelial injury[52]. Discontinuation of the drug with antibiotic therapy is usually sufficient with surgery reserved for complicated cases. mTOR inhibitors are also known to cause dose-dependent non-infectious pneumonitis (NIP) in 2% to 36% of patients[53] (Fig. 8). Patients can be asymptomatic or present with cough, fever and dyspnea. Imaging features usually precede clinical features and progress from non-specific ground glass opacities and septal thickening to multifocal consolidation with basilar and peripheral predominance (Fig. 8). Less commonly, diffuse alveolar hemorrhage, alveolar proteinosis and desquamative interstitial pneumonia may be encountered. NIP associated with mTOR inhibitors is graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (v4.0). Grade 1 and 2a NIP, defined as abnormal radiological findings in asymptomatic patients or patients with cough, do not need dose adjustment, but grade 2b, 3 and 4 NIP, defined as severe cough requiring oxygen may need dose reduction or drug withdrawal and steroid treatment[53]. Bronchioalveolar lavage to exclude infection and pulmonary function testing with weekly high-resolution chest CT are required for monitoring the patient and excluding a malignant disease process. Immunosuppression can result in opportunistic infections. Bowel toxicity is known with mTOR inhibitors and includes enteritis and bowel perforation[42].
Trabectedin (ET743): the drug from the sea
Trabectedin (ET743) is a tetrahydroisoquinoline alkaloid, originally extracted from a marine tunicate, that binds to and inhibits DNA transcription preventing the progression of the cell cycle beyond the G2/M phase[54] (Fig. 1). Several phase II clinical trials have evaluated the efficacy of trabectedin in non-GIST STS including LPS, LMS, SS but the maximum response was seen in myxoid LPS and LMS[7,55] (Fig. 9). Approved for second-line treatment of metastatic STS in Europe, trabectedin has been shown to result in more than 20% progression-free survival after 6 months of treatment although the objective response as per the World Health Organization (WHO) criteria was not satisfactory[56].
Figure 9.
A 51-year-old woman with metastatic myxoid liposarcoma. (a) Axial contrast-enhanced CT image of the abdomen demonstrates a large heterogeneous metastatic mass in the liver (white arrow) and the pancreas (black arrow). Note the surgical clip related to previous cholecystectomy (arrowhead). Note the incidental renal cortical cysts. (b) Axial contrast-enhanced CT image 6 months after treatment with trabectedin reveals a mild increase in the size of both the lesions with homogeneous decreased internal density suggestive of treatment effect (white and black arrows). The increase in size represents pseudo-progression rather than true progression, wherein a lesion may increase in size but is in fact responding to therapy as shown by the density changes.
Myxoid/round cell LPS is the second most common variant of LPS and is associated with aggressive behavior and higher risk of metastasis, especially if the round cell component is higher[57]. Round cell LPS is now considered as a spectrum of myxoid LPS as both have a common translocation t(12; 16)(q13; q11) in excess of 90% of cases resulting in the formation of a chimeric fusion protein FUS-CHOP[2,16]. Myxoid LPS is a well-demarcated tumor at imaging with fluid characteristics on both CT and MRI[57]. The pathognomonic features at MRI include microscopic fat-containing septae and diffuse enhancement after contrast administration. The amount of macroscopic fat is, however, minimal. Myxoid LPS has a tendency to metastasize to serosal membranes, paraspinal locations and the retroperitoneum[54].
Localized LPSs are treated with surgery and radiotherapy with a relapse rate of about 40%[54]. For advanced and metastatic LPS, trabectedin has shown significant efficacy in most phase II trials. The response rate of LPS to trabectidin by RECIST is variably reported as 24–51%[7,58]. The mechanism of action of trabectidin is unknown, but in the case of myxoid LPS, it is postulated that trabectedin inactivates the fusion oncogene FUS-CHOP and removes the block in the cell cycle, thereby promoting differentiation of tumor cells[59]. This manifests pathologically as replacement of the cellular tumor component by acellular stroma and a decrease in tissue density[7]. At imaging, treatment response is indicated by a decrease in tumor density with or without tumor shrinkage[54] (Fig. 9). An unusual form of treatment response observed in some cases of LPS is a progressive increase in the amount of macroscopic fat with no appreciable change in tumor size, in contrast to minimal or no fat on the pretreatment scan, representing differentiation in dedifferentiated components (Fig. 10). Trabectedin is usually well tolerated; neutropenia, thrombocytopenia and transient increase in transaminases are common adverse effects. Capillary leak syndrome has been described with the use of trabectedin, which manifests as unilateral pulmonary edema and anasarca[51] (Fig. 10).
Figure 10.
A 54-year-old woman with well-differentiated liposarcoma containing dedifferentiated components. (a) Axial contrast-enhanced CT image before the start of treatment demonstrates well-differentiated fat anteriorly (white arrows). The dedifferentiated component is seen as ill-defined soft tissue attenuation areas posteriorly (white arrowheads) with enhancing solid nodules (black arrow). (b) Axial contrast-enhanced CT image 12 months after treatment with trabectedin reveals replacement of the dedifferentiated component (white arrowheads) as well as the soft tissue nodule (black arrow) with fatty tissue suggestive of treatment effect or further differentiation in previously dedifferentiated components. The well-differentiated component (white arrow) is unchanged. (c) Axial CT image of the chest during the course of treatment when the patient developed an episode of fever and cough demonstrates interlobar interstitial thickening in the right lung base representing unilateral pulmonary edema, seen with capillary leak syndrome, a known complication of trabectedin therapy.
MTT in miscellaneous STSs
Alveolar soft part sarcoma (ASPS) is an uncommon vascular mesenchymal tumor affecting the lower extremities in young adults[60]. They have a high tendency to metastasize most often to the lungs, liver and central nervous system. Metastatic lesions respond poorly to conventional therapy. ASPSs belong to the group of MiT tumors that are associated with activation of microphthalmia-associated transcription factor (MIFT) and upregulation of the c-Met gene. A characteristic translocation t(X; 17) seen in more than 90% of cases of ASPS is responsible for upregulation of c-Met, which plays a key role in the angiogenesis of ASPS[16,61]. Inhibitors of c-Met and antiangiogenic factors are under investigation in various trials[62].
The heterogeneous group of small round cell tumors including skeletal and extraskeletal Ewing sarcoma constitutes the Ewing sarcoma family of tumors (ESFT)[63]. Adult ESFTs often tend to be extraskeletal and can arise anywhere from the head to the extremities. Metastases are present in up to 31% of patients at the initial diagnosis, usually in the lungs and bone/bone marrow[64]. The standard of care for ESFT is neoadjuvant chemotherapy with conventional agents followed by radiation and surgery. Recognition of a characteristic translocation t(11; 22) in 85% of cases of ESFT has created interest in MTT for these tumors[16]. The transcriptional product of this translocation EWS-FLI1 causes upregulation of IGF1. Figitumumab, a monoclonal antibody against the IGF1-receptor has shown benefit in the preliminary phase I data[65] (Fig. 1).
Conclusion
Advanced and metastatic STSs are challenging to manage due to poor response to conventional chemotherapy. Several non-GIST STSs have characteristic genetic alterations that can be targeted at a molecular level in patients who have failed conventional chemotherapy. Following the success of imatinib in GISTs, many more sarcomas are being treated with MTT. The use of these drugs will increase in the future and make the role of radiologist critical in assessing treatment response. Similar to GIST, response of non-GIST STS to MTT tends to be different from other solid tumors treated with conventional chemotherapeutic agents, which necessitates the use of alternate response criteria. A decrease in tumor density with or without tumor shrinkage is often seen with most of the targeted therapies. Recognizing class-specific drug toxicities at imaging is important for appropriate patient management.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Teicher BA. Searching for molecular targets in sarcoma. Biochem Pharmacol. 2012;84:1–10. doi: 10.1016/j.bcp.2012.02.009. . PMid:22387046. [DOI] [PubMed] [Google Scholar]
- 2.Demetri GD, Antonia S, Benjamin RS, et al. Soft tissue sarcoma. J Natl Compr Cancer Network. 2010;8:630–674. doi: 10.6004/jnccn.2010.0049. [DOI] [PubMed] [Google Scholar]
- 3.Italiano A, Mathoulin-Pelissier S, Cesne AL, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117:1049–1054. doi: 10.1002/cncr.25538. . PMid:20945333. [DOI] [PubMed] [Google Scholar]
- 4.Casali PG, Blay JY. Soft tissue sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):198–203. doi: 10.1093/annonc/mdq209. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Younus J, Stys-Norman D, Haynes AE, Blackstein M. Meta-analysis of ifosfamide-based combination chemotherapy in advanced soft tissue sarcoma. Cancer Treat Rev. 2008;34:339–347. doi: 10.1016/j.ctrv.2008.01.005. . PMid:18313854. [DOI] [PubMed] [Google Scholar]
- 6.George S, Merriam P, Maki RG, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009;27:3154–3160. doi: 10.1200/JCO.2008.20.9890. . PMid:19451429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronchi A, Bui BN, Bonvalot S, et al. Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol. 2012;23:771–776. doi: 10.1093/annonc/mdr265. . PMid:21642514. [DOI] [PubMed] [Google Scholar]
- 8.Maki RG, D'Adamo DR, Keohan ML, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27:3133–3140. doi: 10.1200/JCO.2008.20.4495. . PMid:19451436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European Organisation for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC study 62043) J Clin Oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. . PMid:19451427. [DOI] [PubMed] [Google Scholar]
- 10.Tariq Mahmood S, Agresta S, Vigil CE, et al. Phase II study of sunitinib malate, a multitargeted tyrosine kinase inhibitor in patients with relapsed or refractory soft tissue sarcomas. Focus on three prevalent histologies: leiomyosarcoma, liposarcoma and malignant fibrous histiocytoma. Int J Cancer. 2011;129:1963–1969. doi: 10.1002/ijc.25843. . PMid:21154746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penel N, Le Cesne A, Bui BN, et al. Imatinib for progressive and recurrent aggressive fibromatosis (desmoid tumors): an FNCLCC/French Sarcoma Group phase II trial with a long-term follow-up. Ann Oncol. 2011;22:452–457. doi: 10.1093/annonc/mdq341. . PMid:20622000. [DOI] [PubMed] [Google Scholar]
- 12.McArthur GA, Demetri GD, van Oosterom A, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol. 2005;23:866–873. doi: 10.1200/JCO.2005.07.088. . PMid:15681532. [DOI] [PubMed] [Google Scholar]
- 13.Cassier PA, Gelderblom H, Stacchiotti S, et al. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer. 2012;118:1649–1655. doi: 10.1002/cncr.26409. . PMid:21823110. [DOI] [PubMed] [Google Scholar]
- 14.Wagner AJ, Malinowska-Kolodziej I, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835–840. doi: 10.1200/JCO.2009.25.2981. . PMid:20048174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornton KA. Trabectedin: the evidence for its place in therapy in the treatment of soft tissue sarcoma. Core Evid. 2010;4:191–198. doi: 10.2147/ce.s5993. PMid:20694075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quesada J, Amato R. The molecular biology of soft-tissue sarcomas and current trends in therapy. Sarcoma. 2012 doi: 10.1155/2012/849456. doi:10.1155/2012/849456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oda Y, Tsuneyoshi M. Recent advances in the molecular pathology of soft tissue sarcoma: implications for diagnosis, patient prognosis, and molecular target therapy in the future. Cancer Sci. 2009;100:200–208. doi: 10.1111/j.1349-7006.2008.01024.x. . PMid:19076980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin Liberal J, Lagares-Tena L, Sainz-Jaspeado M, Mateo-Lozano S, Garcia Del Muro X, Tirado OM. Targeted therapies in sarcomas: challenging the challenge. Sarcoma. 2012 doi: 10.1155/2012/626094. doi:10.1155/2012/626094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annu Rev Pathol. 2007;2:251–275. doi: 10.1146/annurev.pathol.2.010506.134925. . PMid:18039100. [DOI] [PubMed] [Google Scholar]
- 20.Judson I. Targeted therapies in soft tissue sarcomas. Ann Oncol. 2010;21(Suppl 7):277–280. doi: 10.1093/annonc/mdq288. [DOI] [PubMed] [Google Scholar]
- 21.Mace J, Sybil Biermann J, Sondak V, et al. Response of extraabdominal desmoid tumors to therapy with imatinib mesylate. Cancer. 2002;95:2373–2379. doi: 10.1002/cncr.11029. . PMid:12436445. [DOI] [PubMed] [Google Scholar]
- 22.Maki RG, Awan RA, Dixon RH, Jhanwar S, Antonescu CR. Differential sensitivity to imatinib of 2 patients with metastatic sarcoma arising from dermatofibrosarcoma protuberans. Int J Cancer. 2002;100:623–626. doi: 10.1002/ijc.10535. . PMid:12209598. [DOI] [PubMed] [Google Scholar]
- 23.Murphey MD, Rhee JH, Lewis RB, Fanburg-Smith JC, Flemming DJ, Walker EA. Pigmented villonodular synovitis: radiologic-pathologic correlation. Radiographics. 2008;28:1493–1518. doi: 10.1148/rg.285085134. . PMid:18794322. [DOI] [PubMed] [Google Scholar]
- 24.Blay JY, El Sayadi H, Thiesse P, Garret J, Ray-Coquard I. Complete response to imatinib in relapsing pigmented villonodular synovitis/tenosynovial giant cell tumor (PVNS/TGCT) Ann Oncol. 2008;19:821–822. doi: 10.1093/annonc/mdn033. . PMid:18296418. [DOI] [PubMed] [Google Scholar]
- 25.Nishino M, Jagannathan JP, Krajewski KM, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198:737–745. doi: 10.2214/AJR.11.7483. . PMid:22451534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. . PMid:17470865. [DOI] [PubMed] [Google Scholar]
- 27.Stacchiotti S, Collini P, Messina A, et al. High-grade soft-tissue sarcomas: tumor response assessment–pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology. 2009;251:447–456. doi: 10.1148/radiol.2512081403. . PMid:19261927. [DOI] [PubMed] [Google Scholar]
- 28.Van Glabbeke M, Verweij J, Casali PG, et al. Predicting toxicities for patients with advanced gastrointestinal stromal tumours treated with imatinib: a study of the European Organisation for Research and Treatment of Cancer, the Italian Sarcoma Group, and the Australasian Gastro-Intestinal Trials Group (EORTC-ISG-AGITG) Eur J Cancer. 2006;42:2277–2785. doi: 10.1016/j.ejca.2006.03.029. . PMid:16876399. [DOI] [PubMed] [Google Scholar]
- 29.Chikarmane SA, Khurana B, Krajewski KM, et al. What the emergency radiologist needs to know about treatment-related complications from conventional chemotherapy and newer molecular targeted agents. Emerg Radiol. 2012 doi: 10.1007/s10140-012-1052-1. doi:10.1007/s10140-012-1052-1. [DOI] [PubMed] [Google Scholar]
- 30.Riedel RF. Targeted agents for sarcoma: is individualized therapy possible in such a diverse tumor type? Semin Oncol. 2011;38(Suppl 3):S30–S42. doi: 10.1053/j.seminoncol.2011.09.003. . PMid:22055970. [DOI] [PubMed] [Google Scholar]
- 31.Yudoh K, Kanamori M, Ohmori K, Yasuda T, Aoki M, Kimura T. Concentration of vascular endothelial growth factor in the tumour tissue as a prognostic factor of soft tissue sarcomas. Br J Cancer. 2001;84:1610–1615. doi: 10.1054/bjoc.2001.1837. . PMid:11401313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon SS, Duda DG, Karl DL, et al. Phase II study of neoadjuvant bevacizumab and radiotherapy for resectable soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2011;81:1081–1090. doi: 10.1016/j.ijrobp.2010.07.024. . PMid:20932656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Contractor KB, Aboagye EO. Monitoring predominantly cytostatic treatment response with 18F-FDG PET. J Nucl Med. 2009;50(Suppl 1):97S–105S. doi: 10.2967/jnumed.108.057273. . PMid:19403880. [DOI] [PubMed] [Google Scholar]
- 34.Yaghmai V, Miller FH, Rezai P, Benson AB, 3rd, Salem R. Response to treatment series: part 2, tumor response assessment–using new and conventional criteria. AJR Am J Roentgenol. 2011;197:18–27. doi: 10.2214/AJR.11.6581. . PMid:21701006. [DOI] [PubMed] [Google Scholar]
- 35.Shankar S, vanSonnenberg E, Desai J, Dipiro PJ, Van Den Abbeele A, Demetri GD. Gastrointestinal stromal tumor: new nodule-within-a-mass pattern of recurrence after partial response to imatinib mesylate. Radiology. 2005;235:892–898. doi: 10.1148/radiol.2353040332. . PMid:15833985. [DOI] [PubMed] [Google Scholar]
- 36.Smith AD, Lieber ML, Shah SN. Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. AJR Am J Roentgenol. 2010;194:157–165. doi: 10.2214/AJR.09.2941. . PMid:20028918. [DOI] [PubMed] [Google Scholar]
- 37.Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM. Morphology, Attenuation, Size, and Structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol. 2010;194:1470–1478. doi: 10.2214/AJR.09.3456. . PMid:20489085. [DOI] [PubMed] [Google Scholar]
- 38.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/S0959-8049(99)00229-4. . PMid:10673991. [DOI] [PubMed] [Google Scholar]
- 39.Howard SA, Krajewski KM, Thornton E, et al. Decade of molecular targeted therapy: abdominal manifestations of drug toxicities–what radiologists should know. AJR Am J Roentgenol. 2012;199:58–64. doi: 10.2214/AJR.11.7432. . PMid:22733894. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi Y, Kanemitu T, Kamoto A, et al. Painless acute pancreatitis associated with sorafenib treatment: a case report. Med Oncol. 2011;28:463–465. doi: 10.1007/s12032-010-9479-2. . PMid:20300970. [DOI] [PubMed] [Google Scholar]
- 41.Gomez-Abuin G, Karam AA, Mezzadri NA, Bas CA. Acalculous cholecystitis in a patient with metastatic renal cell carcinoma treated with sunitinib. Clin Genitourin Cancer. 2009;7:62–63. doi: 10.3816/CGC.2009.n.011. . PMid:19213671. [DOI] [PubMed] [Google Scholar]
- 42.Shinagare A, Howard S, Krajewski K, Zukotynski K, Jagannathan J, Ramaiya N. Pneumatosis intestinalis and bowel perforation associated with molecular targeted therapy: an emerging problem and the role of radiologists in its management. AJR Am J Roentgenol. 2012;199:1259–1265. doi: 10.2214/AJR.12.8782. . PMid:23169717. [DOI] [PubMed] [Google Scholar]
- 43.Shor AC, Keschman EA, Lee FY, et al. Dasatinib inhibits migration and invasion in diverse human sarcoma cell lines and induces apoptosis in bone sarcoma cells dependent on SRC kinase for survival. Cancer Res. 2007;67:2800–2808. doi: 10.1158/0008-5472.CAN-06-3469. . PMid:17363602. [DOI] [PubMed] [Google Scholar]
- 44.Steinberg M. Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Clin Ther. 2007;29:2289–2308. doi: 10.1016/j.clinthera.2007.11.005. . PMid:18158072. [DOI] [PubMed] [Google Scholar]
- 45.Kim LC, Rix U, Haura EB. Dasatinib in solid tumors. Expert Opin Investig Drugs. 2010;19:415–425. doi: 10.1517/13543781003592097. . PMid:20113198. [DOI] [PubMed] [Google Scholar]
- 46.Brixey AG, Light RW. Pleural effusions due to dasatinib. Curr Opin Pulm Med. 2010;16:351–356. doi: 10.1097/MCP.0b013e328338c486. . PMid:20375898. [DOI] [PubMed] [Google Scholar]
- 47.Yuan R, Kay A, Berg WJ, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol. 2009;2:45. doi: 10.1186/1756-8722-2-45. . PMid:19860903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chawla SP, Blay J, Ray-Coquard IL, et al. Results of the phase III, placebo-controlled trial (SUCCEED) evaluating the mTOR inhibitor ridaforolimus (R) as maintenance therapy in advanced sarcoma patients (pts) following clinical benefit from prior standard cytotoxic chemotherapy (CT) J Clin Oncol. 2011;29(Suppl) abstr 10005. [Google Scholar]
- 49.Quek R, Wang Q, Morgan JA, et al. Combination mTOR and IGF-1R inhibition: phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res. 2011;17:871–879. doi: 10.1158/1078-0432.CCR-10-2621. . PMid:21177764. [DOI] [PubMed] [Google Scholar]
- 50.Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. PEComas: the past, the present and the future. Virchows Arch. 2008;452:119–132. doi: 10.1007/s00428-007-0509-1. . PMid:18080139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah SH, Jagannathan JP, Krajewski K, O'Regan KN, George S, Ramaiya NH. Uterine sarcomas: then and now. AJR Am J Roentgenol. 2012;199:213–223. doi: 10.2214/AJR.11.7287. . PMid:22733915. [DOI] [PubMed] [Google Scholar]
- 52.Cetin B, Coskun U, Yildiz R, Buyukberber S, Baykara M, Benekli M. Acute cholecystitis in a patient with metastatic renal cell carcinoma treated with everolimus. J Oncol Pharm Pract. 2011;17:274–278. doi: 10.1177/1078155210363317. . PMid:20215482. [DOI] [PubMed] [Google Scholar]
- 53.Albiges L, Chamming's F, Duclos B, et al. Incidence and management of mTOR inhibitor-associated pneumonitis in patients with metastatic renal cell carcinoma. Ann Oncol. 2012;23:1943–1953. doi: 10.1093/annonc/mds115. . PMid:22689175. [DOI] [PubMed] [Google Scholar]
- 54.Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. . PMid:17586092. [DOI] [PubMed] [Google Scholar]
- 55.Demetri GD, Chawla SP, von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol. 2009;27:4188–4196. doi: 10.1200/JCO.2008.21.0088. . PMid:19652065. [DOI] [PubMed] [Google Scholar]
- 56.Yovine A, Riofrio M, Blay JY, et al. Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J Clin Oncol. 2004;22:890–899. doi: 10.1200/JCO.2004.05.210. . PMid:14990645. [DOI] [PubMed] [Google Scholar]
- 57.O'Regan KN, Jagannathan J, Krajewski K, et al. Imaging of liposarcoma: classification, patterns of tumor recurrence, and response to treatment. AJR Am J Roentgenol. 2011;197:W37–W43. doi: 10.2214/AJR.10.5824. . PMid:21700993. [DOI] [PubMed] [Google Scholar]
- 58.Grosso F, Sanfilippo R, Virdis E, et al. Trabectedin in myxoid liposarcomas (MLS): a long-term analysis of a single-institution series. Ann Oncol. 2009;20:1439–1444. doi: 10.1093/annonc/mdp004. . PMid:19465423. [DOI] [PubMed] [Google Scholar]
- 59.Forni C, Minuzzo M, Virdis E, et al. Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma tumors. Mol Cancer Ther. 2009;8:449–457. doi: 10.1158/1535-7163.MCT-08-0848. . PMid:19190116. [DOI] [PubMed] [Google Scholar]
- 60.Portera CA, Jr, Ho V, Patel SR, et al. Alveolar soft part sarcoma: clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer. 2001;91:585–591. doi: 10.1002/1097-0142(20010201)91:3<585::AID-CNCR1038>3.0.CO;2-0. . PMid:11169942. [DOI] [PubMed] [Google Scholar]
- 61.Ladanyi M, Lui MY, Antonescu CR, et al. The der(17)t(X; 17)(p11; q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene. 2001;20:48–57. doi: 10.1038/sj.onc.1204074. . PMid:11244503. [DOI] [PubMed] [Google Scholar]
- 62.Vistica DT, Hollingshead M, Borgel SD, et al. Therapeutic vulnerability of an in vivo model of alveolar soft part sarcoma (ASPS) to antiangiogenic therapy. J Pediatr Hematol Oncol. 2009;31:561–570. doi: 10.1097/MPH.0b013e3181a6e043. . PMid:19636271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Javery O, Krajewski K, O'Regan K, et al. A to Z of extraskeletal Ewing sarcoma family of tumors in adults: imaging features of primary disease, metastatic patterns, and treatment responses. AJR Am J Roentgenol. 2011;197:W1015–W1022. doi: 10.2214/AJR.11.6667. . PMid:22109315. [DOI] [PubMed] [Google Scholar]
- 64.El Weshi A, Allam A, Ajarim D, et al. Extraskeletal Ewing's sarcoma family of tumours in adults: analysis of 57 patients from a single institution. Clin Oncol (R Coll Radiol) 2010;22:374–381. doi: 10.1016/j.clon.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 65.Juergens H, Daw NC, Geoerger B, et al. Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol. 2011;29:4534–4540. doi: 10.1200/JCO.2010.33.0670. . PMid:22025154. [DOI] [PMC free article] [PubMed] [Google Scholar]