Abstract
Objective
The aim of this study was to examine the association between physical activity (PA) and coronary artery calcification (CAC) among two cohorts of postmenopausal (PM) women representing early and late postmenopause.
Methods
The cross-sectional relationship between PA and CAC was examined in 173 younger PM women (mean age T SD, 56.8 T 2.9 y) from the Women on the Move Through Activity and Nutrition (WOMAN) study and 121 older PM women (mean age T SD, 73.9 T 3.8 y) from the Walking Women Follow-up (WWF) study who had complete PA and CAC data. PA was measured objectively using a pedometer over a 7-day period in both cohorts. CAC was assessed using electron beam tomography. Descriptive statistics were used to describe median levels of PA and CAC, as well as proportions of detectable CAC (0 vs 9 0).
Results
Fifty-seven percent of WOMAN study participants and 74% of WWF study participants had detectable CAC. The median (interquartile range) CAC score was 1.4 (0-23.3) for participants in the WOMAN study and 38.8 (0-264.4) among WWF study participants. Median (interquartile range) step counts were 6,447 (4,823-8,722) steps per day in the WOMAN study and 5,466 (3,610-7,576) steps per day for WWF study participants. Among WWF study participants, there was a statistically significant inverse association between pedometer steps and CAC (P for trend = 0.002); no association was found among WOMAN study participants.
Conclusions
Among older PM women, higher levels of PA were associated with lower CAC. However, the relationship was not observed in PM women, likely due to the lower prevalence of CAC in this age group.
Cardiovascular disease (CVD) is the leading cause of death among women (American Heart Association, 2010).1 After menopause, the incidence of CVD in women escalates, which may be due, in part, to the physiological changes that occur during the menopausal process. During menopause, women experience increases in both triglycerides and number of total and small low-density lipoprotein particles, as well as a decrease in high-density lipoprotein particles,2,3 all of which contribute to an increased risk for the development of subclinical CVD. Because of the fact that approximately two thirds of women who die each year of a catastrophic coronary event are asymptomatic,2 it is essential to begin to look at subclinical measures that may predict possible future cardiac events.
Most women who die of myocardial infarction (MI) or coronary heart disease (CHD) do not meet the traditional Framingham Risk Score criteria or are clinically asymptomatic before the initial event, that is, MI or CHD death.4 An alternative approach is necessary to identify women who are at higher risk because of silent or subclinical atherosclerosis, which may lead to risk for future cardiac events. One such method may be electron beam tomography (EBT) or coronary computerized tomography used to measure coronary artery calcification (CAC), a marker of atherosclerosis. The amount of CAC detected by coronary computerized tomography is strongly and directly related to the risk of future cardiac events in numerous studies in both men and 5-7 women and in younger and older individuals. A CAC score of zero is usually considered to place a woman at very low risk of coronary events for at least the next 5 to 10 years.8
There is epidemiological evidence that physical activity (PA) is associated with reduced risk of CVD and cardiovascular mortality9-16; however, the mechanisms underlying the protective effect of PA are many and not fully understood. For example, PA is believed to have beneficial effects on CVD risk factors, such as insulin sensitivity, blood pressure, lipid metabolism, and obesity.14,17 Whether PA is associated with subclinical CVD, specifically CAC, as a measure of atherosclerosis, has not been widely examined. The few studies that have looked at the relationship between PA and CAC in cohorts of both men and women have been inconclusive.5,18-23 20 It has been suggested that duration of PA was associated with less CAC, although other studies reported no significant relationship between the two.6,18,21-23
We were provided with the unique opportunity to examine the association of PA and CAC in two cohorts of postmenopausal women representing early and late post-menopause. The purpose of the current investigation was to examine the role of PA and coronary atherosclerosis in these two different cohorts using an objective measure of PA, the pedometer.
METHODS
Women on the Move Through Activity and Nutrition study
The Women on the Move Through Activity and Nutrition (WOMAN) study was a 5-year randomized clinical trial designed to test whether an aggressive nonpharmacological lifestyle intervention among early postmenopausal women will reduce measures of subclinical CVD. Details regarding the design, recruitment, and objectives of the WOMAN study have been published previously.24,25 Briefly, the WOMAN study included 508 postmenopausal women between the ages of 52 and 62 years from Allegheny County, Pennsylvania. Initial entry criteria for the trial required a woman to have a history of at least 2 years of hormone therapy (HT). Other eligibility criteria for enrollment into the study included having a waist circumference of 80 cm or greater, having a body mass index (BMI) between 25 and 39.9 kg/m2, not currently taking lipid-lowering drugs, having a low-density lipoprotein cholesterol level between 100 and 160 mg/dL, no physical limitations that would preclude walking, no known diabetes, and no diagnosed psychotic disorder or depression.
The study was designed before the release of the results of the Women’s Health Initiative (WHI). Five months into the randomization phase of the WOMAN study, the WHI trial was stopped prematurely in the estrogen and progesterone arm because of adverse effects. Women already enrolled in the WOMAN study were advised to stop HT. Women randomized after the WHI results were published were allowed to enter the WOMAN study after discontinuing HT use. Overall, 204 (39% of the total cohort) women were not taking HT at baseline, with a median time off HT of 7 months before entering the study.25
At baseline, pedometer data were collected on a subsample (33% of the total cohort) of the women enrolled in the WOMAN study because of a limited number of pedometers available. Basically, pedometer data were obtained for the first 15 women who completed a clinic visit each month, for a total of 173 women having worn a pedometer.26 In addition, CAC was measured electron beam computed tomography (EBT).
Walking Women Follow-Up study
The Walking Women Follow-up (WWF) study is a follow-up study to a randomized controlled trial of a walking intervention that took place in Pittsburgh, PA. The original randomized controlled trial was conducted in postmenopausal women between the years of 1982 and 1985. To be eligible for the walking intervention, the women had to meet the following baseline criteria: (1) aged 50 to 65 years, (2) be at least 1 year postcessation of menses, (3) not taking estrogen therapy, and (4) have no limitations that might preclude walking. Based on these criteria, 229 white women were enrolled in the study and randomly assigned to either a walking intervention or an educational control group. At the end of the clinical trial (1985), the intervention women were found to be more physically active than the control women based upon activity surveys and objective activity monitors.
In 1995, the women were recontacted by telephone to examine if the original walking intervention had long-term effects on their activity habits and health 10 years later. Results from this examination indicated that differences in reported walking between the two randomized groups were still present a decade later and that women who comprised the walking intervention group were more active and had less CVD than did the control women.27
In 1999, the women from the original trial were invited to attend a 1-day comprehensive clinic evaluation. The evaluation included measures of body composition, bone density, heart disease, PA, and health status. PA was assessed by pedometer, and CAC was assessed by EBT. Of the original 229 women, 171 completed the clinic visit, 17 completed telephone interviews, 3 refused to participate, 20 were confirmed dead, 8 were considered too sick to participate, and 10 were lost to follow-up.
Both the WOMAN and WWF study protocols were approved by the University of Pittsburgh Institutional Review Board, and written informed consent forms were obtained from all participants before their participation in any part of the study.
PA assessment
PA was assessed objectively in both studies with a pedometer. In the WOMAN study, the Accusplit AE120 pedometer (Accusplit, San Jose, CA) was used to assess PA at baseline (2002-2003), and in the WWF study, the Yamax Digiwalker SW-200 (Yamasa Corporation, Chiba, Japan) pedometer was used at the year 6 follow-up (1999). The Accusplit AE120 pedometer is identical to the Yamax SW-200, and both pedometers have been shown to be a valid and reliable assessment tool for assessing step counts in a variety of laboratory and field settings.28-36 At their clinical examination, participants in both studies received a pedometer, instructions for wearing the pedometer, and an activity diary and were asked to wear the pedometer for seven consecutive days (five weekdays and two weekend days) and record in the activity diary the number of steps taken daily. Participants were asked not to change their normal PA habits during the 7-day monitoring period. Because previous research has suggested that 3 days of activity can provide a sufficient estimate of weekly PA,37 participants with 3 or more days of data were included in the study. Steps per day averaged over the week was calculated for any person who had data for 3 or more days, taking the sum of steps per day divided by the number of available days with a maximum of 7 days.
CAC
EBT was used to assess CAC in both study populations. EBT was assessed at baseline (2002-2003) in the WOMAN study and at the 1999 follow-up visit for the WWF study. Scans were conducted at the Cardiovascular Institute in Pittsburgh, PA, by a trained radiology technician using a GE-Imatron C150 EBT Scanner (GE Medical Systems, South San Francisco, CA). For the coronary arteries, 30 to 40 contiguous 3-mm-thick transverse images were obtained from the level of the aortic root to the apex of the heart. Images were obtained during a maximal breath-holding by using electrocardiogram triggering so that each 100-millisecond exposure is obtained during the same phase of the cardiac cycle (60% of the R-R interval).6 After completion of the coronary scan, an aortic evaluation was conducted using 6-mm contiguous images (300-ms exposure time) of the aortic arch to the iliac bifurcation. Calcium scores were calculated using methods by Agatston et al38 using a densitometric program available on the Imatron C150 scanner. Coronary artery calcium was considered to be present when three contiguous pixels greater than 130 Hounsfield units were detected overlying the vessels of interest.
Statistical analysis
Descriptive statistics were calculated for each cohort separately. Because the two cohorts were recruited from different studies, between-study comparisons were not generated. All continuous data were assessed for normality. Normally distributed data were reported as mean (SD), whereas non-normal variables were reported as median (interquartile range [IQR]). Depending on the normality assessment of the variable, analysis of variance or the Kruskal-Wallis or the W2 test was used to describe and compare differences among CAC scores. To determine the proportion of women with detectable CAC in each cohort, CAC was categorized using the following cut-points: 0, 1 to 10, 11 to 100, and 101 or higher.6 In addition, pedometer steps were categorized into cohort-specific quartiles to examine whether detectable coronary calcification varied across pedometer quartiles. Multivariate logistic regression analyses were performed to determine the association between PA and CAC scores, controlling for potential confounding factors, including age, BMI, total cholesterol, and HT. An interaction term between HT use and PA was also explored. Statistical analyses were performed using Statistical Analysis Software, version 8.2 (SAS Institute, Inc., Cary, NC). Statistical significance was considered as a P value less than 0.05. In addition, to aid in the interpretation of the findings and because of the cross-sectional nature of the study, we limited our analyses to women free from known CVD with a minimum of 3 days of pedometer data, with CAC data, and not taking lipid-lowering drugs.
RESULTS
At baseline, 173 (100% of the total substudy participants) WOMAN study participants had complete PA and CAC data. In addition, at the 1999 follow-up visit, 121 (71% of the total cohort) participants from the WWF study had complete PA and CAC data. The descriptive characteristics of participants of both studies are presented in Table 1. WOMAN study participants were more likely to have higher BMIs (30.6 vs 26.1 kg/m2) and were more likely to use HT (63.0% vs 40.5%) than the older WWF study participants. Median (IQR) step count was 6,446 (4,823-8,722) steps per day for participants in the WOMAN study and 5,466 (3,610-7,576) steps per day among WWF study participants. In addition, among the women in the WWF study, 12 women (10% of the total cohort) reported that they had been told by their physician that they had one or more CHD-related conditions (MI, heart disease, or congestive heart failure). Seven women (5.8%) reported diabetes and 47 (38.8%) reported high blood pressure. Based on exclusion criteria for the WOMAN study, no women reported a CHD condition or diabetes, and all had to have a blood pressure of lower than 160/95 mm Hg at initial screening and lower than 140/90 mm Hg at randomization with or without drug therapy.
TABLE 1.
Descriptive characteristics of the WOMAN and WWF study cohorts
| WOMAN study | WWF study | |
|---|---|---|
| (n = 173) | (n = 121) | |
| Age, y | 56.8 (2.9) | 73.9 (3.8) |
| BMI, kg/m2 | 30.6 (3.6) | 26.1 (4.9) |
| Currently smoking, % | 6.9 | 5.8 |
| Systolic blood pressure, mm Hg |
123.8 (13.7) | 131.0 (15.8) |
| Diastolic blood pressure, mm Hg |
76.4 (8.2) | 74.0 (8.8) |
| Hormone therapy users, % | 63.0 | 40.5 |
| Total cholesterol, mg/dL | 215.0 (27.7) | 218.7 (36.5) |
| Triglycerides, mg/dL | 144.6 (79.6) | 133.5 (62.0) |
| HDL, mg/dL | 60.5 (14.6) | 63.7 (17.3) |
| LDL, mg/dL | 125.6 (24.9) | 128.0 (35.5) |
| Pedometer (steps/day averaged over the week) |
6,447 (4,823, 8,722) |
5,466 (3,610, 7,576) |
| CAC | 1.4 (0, 23.3) | 38.8 (0, 264.4) |
| CAC 90, % | 57.2 | 74.4 |
Data are presented as mean (SD) unless otherwise specified. CAC and pedometer data are presented as median (interquartile range). WOMAN, Women on the Move Through Activity and Nutrition; WWF, Walking Women Follow-up; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; CAC, coronary artery calcification.
With regard to CAC, 57.2% of WOMAN study participants and 74.4% of WWF study participants had detectable CAC, equating CAC scores greater than zero. The median (IQR) CAC score was 1.4 (0-23.3) among WOMAN study participants and 38.8 (0-264.4) among WWF study participants.
Figure 1 presents the distribution of coronary artery calcium scores among participants in the WOMAN and WWF study cohorts. The WOMAN study was more likely than the WWF study to have greater percentages of participants with calcium scores of 0 (42.8% vs 22.2%, respectively), 1 to 10 (24.3% vs 13.9%, respectively), and 11 to 100 (22.5% vs 13.4%, respectively). Conversely, the WOMAN study was more likely than the WWF study to have a lower percentage of participants with calcium scores higher than 100 (10.4% vs. 44.5%, respectively).
FIG. 1.
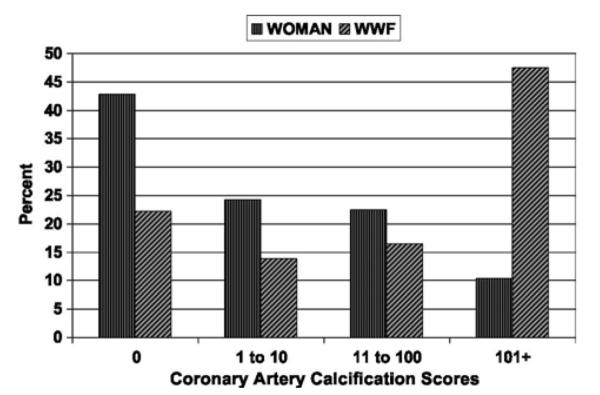
Distribution of coronary artery calcium scores in the WOMAN (n = 173) and WWF (n = 121) study cohorts. WOMAN, Women on the Move Through Activity and Nutrition; WWF, Walking Women Follow-up.
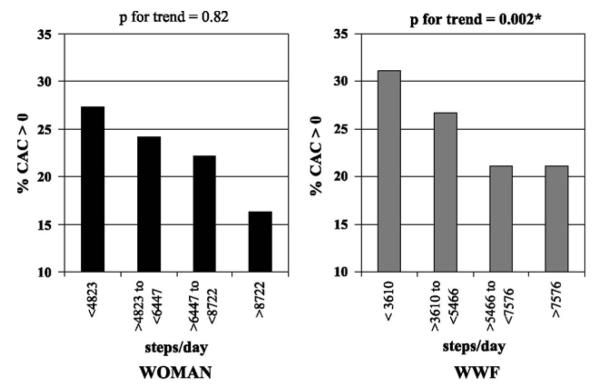
Figure 2 presents the proportion of women with detectable CAC by quartile of pedometer steps among the two cohorts. Among WOMAN study participants, there were no significant associations between the percentage of women with detectable CAC and the quartiles of pedometer steps (P for linear trend = 0.82). However, among WWF study participants, there was a statistically significant inverse association between pedometer steps and CAC: as pedometer steps increased, the percentage of women with detectable CAC decreased (P for linear trend = 0.002).
FIG. 2.
Percentage of women with detectable CAC (90) by quartiles of pedometer steps in the WOMAN (n = 173) and WWF (n = 121) study cohorts. CAC, coronary artery calcification; WOMAN, Women on the Move Through Activity and Nutrition; WWF, Walking Women Follow-up.
This association between PA and the prevalence of CAC among women in the WWF study was examined more thoroughly. Using multivariate analysis controlling for age, BMI, lipids, and HT use at follow-up, WWF study women in the lowest quartile of PA had a 40% (95% CI, 1.09-1.81) (quartile 1) higher likelihood of having detectable CAC compared with women who were in the highest quartile of PA. An additional model was run, which also included systolic blood pressure, and although the model changed slightly, the confidence limits did not (Table 2). A combined two-way interaction term involving PA levels and current HT use did not significantly add to the model, so this interaction term was not included in the fully adjusted model.
Table 2.
Logistic regression models for the WWF Study
| Unadjusted multivariate modela | Adjusted modelb | Fully adjusted multivariate modelc | |
| Quartile 1: >3,610 steps | 1.35 (1.09-1.67) | 1.40 (1.09-1.81) | 1.31 (0.99-1.73) |
| Quartile 2: ≥3,610-<5,466 steps | 1.18 (1.00-1.46) | 1.18 (0.92-1.50) | 1.08 (0.83,1.40) |
| Quartile 3: ≥5,466-<7,576 steps | 0.82 (0.79-1.21) | 0.99 (0.79-1.25) | 0.94 (0.74-1.19) |
| Quartile 4: ≥7,576 steps | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
Data are shown as parameter estimate (odds ratio) and 95% CI (in parentheses).
Fully adjusted model: age, BMI, total cholesterol, current hormone therapy use, and systolic blood pressure; P = 0.058. WWF, Walking Women Follow-up; BMI, body mass index.
Unadjusted model: P = 0.04.
Adjusted model: age, BMI, total cholesterol, and current hormone therapy use: P = 0.049.
Fully adjusted model: age, BMI, total cholesterol, current hormone therapy use, and systolic blood pressure.
Discussion
The present report examined the association of PA and CAC among two cohorts of postmenopausal women representing both early and late postmenopause. The findings of this study suggest that, among late postmenopausal women in the WWF study, PA as assessed by pedometer steps was significantly associated with detectable CAC after accounting for potentially confounding factors such as age, BMI, lipids, and current hormone use. In late postmenopausal women, participants in the lowest quartile of activity measured by pedometer steps were 40% more likely to have detectable CAC compared with those women in the highest quartile of activity.
Conversely, among early postmenopausal women in the WOMAN study, PA as assessed by pedometer was not associated with detectable CAC at baseline. This finding is most probably a result of a lack of power in the WOMAN study, at least partially because of the low prevalence of CAC in this cohort. In addition, all participants in the WOMAN study took HT for a period of 2 or more years at some point before being enrolled in the study. HT may be preventive of heart disease and may have impacted the development of CAC among WOMAN study participants.
To our knowledge, the current study is the first to examine the association between PA and CAC in cohorts of women at different stages of postmenopause (early vs late). Few studies have examined the effect of PA on subclinical atherosclerosissuch as CAC,18,20<23 and most have been conducted on cohorts of both men and women of varying ages and have provided mixed results.
One study by Desai et al 20 suggested that the dose of PA with regard to intensity and duration was inversely associated with CAC among both men (mean age, 54 T 8 y) and women (mean age, 57 T 9 y). Women in this study who were found to be sedentary or engaged in less than 30 minutes of PA one to two times a week had a higher associated prevalence of CAC, severe CAC, and advanced CAC compared with women who participated in 30 minutes or more of PA three or more times a week, independent of confounding variables. This reduction in CAC is in line with findings among older postmenopausal women in the WWF study, despite the fact that the mean age of the study population in the Desai study was approximately 20 years lower than that of the WWF study.
Most studies have reported no significant association between PA and CAC.18,22,23 However, two of these studies were conducted in younger populations ranging in age from 39 to 45 years22 and 45 to 64 years,23 which may not have had significant amounts of detectable CAC present at the time of examination. In addition, all of these studies used a self-report measure of PA. Although subjective measures, such as a questionnaire, accurately assess moderate to higher intensity activity, focusing solely on leisure and/or occupational activity may only be valid for younger and healthier populations. It is well established that certain subgroups of the population (older adults and women) acquire most of their PA in lower intensity activities, which subjective measures tend to do a poor job of accurately estimating.39,40 Therefore, the use of objective measures, such as the pedometer used in the current study, may provide for a more accurate assessment of PA levels in these populations. The use of an objective activity monitor, such as the pedometer, ensures that the entire spectrum of PA, including both structured and unstructured activity, is captured relative to a subjective measure.
Despite the advantages of using the pedometer to capture unstructured and low-intensity PA in the both cohorts, there are, unfortunately, limitations that need to be considered with its use as an assessment tool. First, the pedometer does not accurately measure nonambulatory activities such as resistance training and cycling, and because it cannot get wet, water activities are also not captured via pedometer. In addition, many pedometers, such as the pedometer used in the current study, lack an internal clock and data storage capability; thus, we had to rely on the participants from both studies to accurately record their step counts from the pedometer in their 7-day activity diary. This process may have resulted in reporting errors or lack of data. Furthermore, the pedometers used in this study are unable to discriminate between steps accumulated in walking, running, or stair climbing; therefore, we were unable to determine steps accumulated in varying intensity levels.
The present study has additional limitations that need to be considered. Participants of both the WOMAN and WWF study were volunteers in clinical trials, which may limit the overall generalizability of the findings to more diverse populations. In addition, data from only one time point were used in the analyses: for the WOMAN study, the baseline visit, andfor the WWF study, the year 6 follow-up. Although informative, this cross-sectional analysis is limited because it does not provide information pertaining to the direction of association or allow us to determine how the relationship between PA and CAC changes as a woman changes her PA level.
CONCLUSIONS
In conclusion, among older postmenopausal women, higher levels of PA were associated with lower CAC. However, this relationship was not observed in younger postmenopausal women and was probably due to the lower prevalence of CAC in this age group. These findings warrant future research examining the role of PA and the progression of CAC in early postmenopausal women.
Acknowledgments
We thank the staff of the WOMAN and WWF studies for their contribution.
REFERENCES
- 1.American Heart Association . Heart Disease and Stroke StatisticsV2010 Update. American Heart Association; Dallas, TX: 2010. [Google Scholar]
- 2.Kuller LH. Hyperlipidemia and cardiovascular disease. Curr Opin Lipidol. 2007;18:230–233. doi: 10.1097/MOL.0b013e32805dfb47. [DOI] [PubMed] [Google Scholar]
- 3.Kuller LH, Grandits G, Cohen JD, Neaton JD, Prineas R. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis. 2007;195:122–128. doi: 10.1016/j.atherosclerosis.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews KA, Kuller LH, Chang Y, Edmundowicz D. Premenopausal risk factors for coronary and aortic calcification: a 20-year follow-up in the healthy women study. Prev Med. 2007;45:302–308. doi: 10.1016/j.ypmed.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Newman AB, Naydeck BL, Sutton-Tyrrell K, et al. Relationship between coronary artery calcification and other measures of subclinicalcardiovascular disease in older adults. Arterioscler Thromb Vasc Biol. 2002;22:1674–1679. doi: 10.1161/01.atv.0000033540.89672.24. [DOI] [PubMed] [Google Scholar]
- 6.Kuller LH, Matthews KA, Sutton-Tyrrell K, Edmundowicz D, Bunker CH. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors: the Healthy Women Study. Arterioscler Thromb Vasc Biol. 1999;19:2189–2198. doi: 10.1161/01.atv.19.9.2189. [DOI] [PubMed] [Google Scholar]
- 7.Newman AB, Naydeck BL, Ives DG, et al. Coronary artery calcium, carotid artery wall thickness, and cardiovascular disease outcomes in adults 70 to 99 years old. Am J Cardiol. 2008;101:186–192. doi: 10.1016/j.amjcard.2007.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuller LH, Matthews KA, Edmundowicz D, Chang Y. Incident coronary artery calcium among postmenopausal women. Atherosclerosis. 2008;200:278–285. doi: 10.1016/j.atherosclerosis.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services . Surgeon General’s Report on Physical Activity and Health. US Government Printing Office: Centers for Disease Control and Prevention; Washington, DC: 1996. [Google Scholar]
- 10.Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: review and meta-analysis. Am J Prev Med. 2004;26:407–418. doi: 10.1016/j.amepre.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Richardson CR, Kriska AM, Lantz PM, Hayward RA. Physical activity and mortality across cardiovascular disease risk groups. Med Sci Sports Exerc. 2004;36:1923–1929. doi: 10.1249/01.mss.0000145443.02568.7a. [DOI] [PubMed] [Google Scholar]
- 12.Sesso HD, Paffenbarger RS, Ha T, Lee IM. Physical activity and cardiovascular disease risk in middle-aged and older women. Am J Epidemiol. 1999;150:408–416. doi: 10.1093/oxfordjournals.aje.a010020. [DOI] [PubMed] [Google Scholar]
- 13.Sherman SE, D’Agostino RB, Cobb JL, Kannel WB. Physical activity and mortality in women in the Framingham Heart Study. Am Heart J. 1994;128:879–884. doi: 10.1016/0002-8703(94)90583-5. [DOI] [PubMed] [Google Scholar]
- 14.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB, Belanger A, D’Agostino R, Israel I. Physical activity and physical demand on the job and risk of cardiovascular disease and death: the Framingham Study. Am Heart J. 1986;112:820–825. doi: 10.1016/0002-8703(86)90480-1. [DOI] [PubMed] [Google Scholar]
- 16.Bauman AE. Updating the evidence that physical activity is good for health: an epidemiological review 2000-2003. J Sci Med Sport. 2004;7:6–19. doi: 10.1016/s1440-2440(04)80273-1. [DOI] [PubMed] [Google Scholar]
- 17.Hardman A. Physical activity and cardiovascular risk. J Cardiovasc Risk. 1995;2:285–288. [PubMed] [Google Scholar]
- 18.Bertoni AG, Whitt-Glover MC, Chung H, et al. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444–454. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bild DE, Folsom AR, Lowe LP, et al. Prevalence and correlates of coronary calcification in black and white young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arterioscler Thromb Vasc Biol. 2001;21:852–857. doi: 10.1161/01.atv.21.5.852. [DOI] [PubMed] [Google Scholar]
- 20.Desai MY, Nasir K, Rumberger JA, et al. Relation of degree of physical activity to coronary artery calcium score in asymptomatic individuals with multiple metabolic risk factors. Am J Cardiol. 2004;94:729–732. doi: 10.1016/j.amjcard.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Nishino M, Malloy MJ, Naya-Vigne J, Russell J, Kane JP, Redberg RF. Lack of association of lipoprotein(a) levels with coronary calcium deposits in asymptomatic postmenopausal women. J Am Coll Cardiol. 2000;35:314–320. doi: 10.1016/s0735-1097(99)00555-0. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AJ, Watkins T, Bell D, et al. Physical activity and the presence and extent of calcified coronary atherosclerosis. Med Sci Sports Exerc. 2002;34:228–233. doi: 10.1097/00005768-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Folsom AR, Evans GW, Carr JJ, Stillman AE. Association of traditional and nontraditional cardiovascular risk factors with coronary artery calcification. Angiology. 2004;55:613–623. doi: 10.1177/00033197040550i602. [DOI] [PubMed] [Google Scholar]
- 24.Kuller LH, Kinzel LS, Pettee KK, et al. Lifestyle intervention and coronary heart disease risk factor changes over 18 months in postmenopausal women: the Women on the Move Through Activity and Nutrition (WOMAN study) clinical trial. J Womens Health (Larchmt) 2006;15:962–974. doi: 10.1089/jwh.2006.15.962. [DOI] [PubMed] [Google Scholar]
- 25.Kuller LH, Kriska AM, Kinzel LS, et al. The clinical trial of Women on the Move Through Activity and Nutrition (WOMAN) study. Contemp Clin Trials. 2007;28:370–381. doi: 10.1016/j.cct.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman MA, Pettee KK, Storti KL, Richardson CR, Kuller LH, Kriska AM. Monthly variation in physical activity levels in postmenopausal women. Med Sci Sports Exerc. 2009;41:322–327. doi: 10.1249/MSS.0b013e3181864c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pereira MA, Kriska AM, Day RD, Cauley JA, LaPorte RE, Kuller LH. A randomized walking trial in postmenopausal women: effects on physical activity and health 10 years later. Arch Intern Med. 1998;158:1695–1701. doi: 10.1001/archinte.158.15.1695. [DOI] [PubMed] [Google Scholar]
- 28.Bassett DR, Jr, Ainsworth BE, Leggett SR, et al. Accuracy of five electronic pedometers for measuring distance walked. Med Sci Sports Exerc. 1996;28:1071–1077. doi: 10.1097/00005768-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Bassett DR, Jr, Ainsworth BE, Swartz AM, Strath SJ, O’Brien WL, King GA. Validity of four motion sensors in measuring moderate intensity physical activity. Med Sci Sports Exerc. 2000;32:S471–S480. doi: 10.1097/00005768-200009001-00006. [DOI] [PubMed] [Google Scholar]
- 30.Bassett DR, Jr, Cureton AL, Ainsworth BE. Measurement of daily walking distanceVquestionnaire versus pedometer. Med Sci Sports Exerc. 2000;32:1018–1023. doi: 10.1097/00005768-200005000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Crouter SE, Schneider PL, Karabulut M, Bassett DR., Jr Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc. 2003;35:1455–1460. doi: 10.1249/01.MSS.0000078932.61440.A2. [DOI] [PubMed] [Google Scholar]
- 32.Schneider PL, Crouter SE, Bassett DR. Pedometer measures of freeliving physical activity: comparison of 13 models. Med Sci Sports Exerc. 2004;36:331–335. doi: 10.1249/01.MSS.0000113486.60548.E9. [DOI] [PubMed] [Google Scholar]
- 33.Schneider PL, Crouter SE, Lukajic O, Bassett DR., Jr Accuracy and reliability of 10 pedometers for measuring steps over a 400-m walk. Med Sci Sports Exerc. 2003;35:1779–1784. doi: 10.1249/01.MSS.0000089342.96098.C4. [DOI] [PubMed] [Google Scholar]
- 34.Nelson TE, Leenders NYJM, Sherman WM. Comparison of activity monitors worn during treadmill walking [abstract] Med Sci Sports Exerc. 1998;30:S11. [Google Scholar]
- 35.Leenders NY, Nelson TE, Sherman WM. Ability of different physical activity monitors to detect movement during treadmill walking. Int J Sports Med. 2003;24:43–50. doi: 10.1055/s-2003-37196. [DOI] [PubMed] [Google Scholar]
- 36.Behrens TK, Dinger MK, Vesely SK, Fields DA. Accuracy of step recording in free-living adults. Res Q Exerc Sport. 2007;78:542–547. doi: 10.1080/02701367.2007.10599453. [DOI] [PubMed] [Google Scholar]
- 37.Tudor-Locke C, Burkett L, Reis JP, Ainsworth BE, Macera CA, Wilson DK. How many days of pedometer monitoring predict weekly physical activity in adults? Prev Med. 2005;40:293–298. doi: 10.1016/j.ypmed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 39.Kriska AM, Caspersen CJ. Introduction to the collection of physical activity questionnaires. Med Sci Sports Exerc. 1997;29:S5–S9. [PubMed] [Google Scholar]
- 40.Jacobs DRJ, Ainsworth BE, Hartman T, Leon AS. A simultaneous evaluation of 10 commonly used questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]