Abstract
This investigation determined whether daily strenuous exercise would alter the progression and regression of an allogeneic lymphoid tumor in mice. We also determined whether exercise would alter the cellular composition and vascularity of the tumor. Female BALB/c mice (age 6–8 wk) were randomly assigned to sedentary control (Con) or daily exercised groups (EXH). EXH mice ran on a treadmill at incremental speeds (20–40 m/min) for 3 h or until fatigue. Each mouse was subcutaneously injected with 20 × 106 EL-4 lymphoma cells immediately after the first exercise bout (day 1) and run daily. Tumor volume was measured daily with calipers. In some experiments, mice were euthanized on days 5–10, 12, and 14. Tumors were excised and stained with hematoxylin and eosin or for Factor VIII-associated antigen using immunohistochemistry and analyzed in a blinded fashion under a light microscope. There was no significant treatment main effect found for tumor volumes. Interestingly, a significant treatment × time interaction was found, such that there was a 2-day delay in peak tumor volume and a more rapid tumor regression in EXH. Tumors isolated from Con exhibited significantly higher numbers of apoptotic bodies, blood vessels, macrophages, and neutrophils when compared with EXH. Intratumoral lymphocytes were higher in Con early in tumor growth but higher in EXH at peak tumor size. These data indicate that daily strenuous exercise may influence tumor growth by affecting the microenvironment of the tumor, resulting in a delay in tumor growth and a more rapid regression.
Keywords: angiogenesis, cancer, transplant, mice
Cancer is the second leading cause of death in the United States (1). Despite the progress that has been made in recent years informing the public about healthy lifestyle choices (e.g., regular exercise) and the impact these have on cancer prevention, the incidence rates for some cancers is still rising. Increasing physical activity is one behavioral strategy that has been shown to be beneficial in the fight against cancer. The protective effect of exercise against certain cancers is well established. Epidemiological evidence has linked high levels of physical activity to reduced risk of several cancers, including breast, colon, and ovarian cancers (10, 11, 34, 48). With regards to lymphoma, Zahm et al. (52) found no significant relationship between occupational physical activity and non-Hodgkins lymphoma incidence rates; however, leisure-time activity was not accounted for in this study.
Relatively few studies have examined the role of exercise as an adjunct therapy in cancer treatment. The limited evidence does, however, suggest a beneficial role of exercise in cancer patients (8, 23, 28). Although many mechanisms have been offered to explain why exercise and physical activity are protective against certain cancers (e.g., decreased lifetime exposure to estrogen or other hormones, reduced body fat, enhanced gut motility, improved antioxidant defenses, and stimulation of anti-tumor immune defenses), none have been definitively tested (49).
Animal models have the potential to provide important mechanistic information about the influence of exercise on cancer incidence and progression (for review, see Ref. 49). Animal studies have examined the relationship between forced or voluntary exercise and the growth of transplantable, chemically induced, and/or spontaneous tumors in rodents. Many (15, 33, 39, 45), but not all (12, 47, 50), of these studies have reported some beneficial effect of exercise on tumor incidence or progression. Unfortunately, many of these studies have been descriptive in nature and have failed to examine plausible biological mechanisms as to how exercise might influence tumor growth. Moreover, few have examined the influence of exercise on the structure or composition of the tumor itself.
Neoplastic progression and regression is a complex process regulated by multiple factors such as inflammatory reactions, angiogenic growth factors, cytokines, and hormones (7). Use of the well-established EL-4 allogeneic tumor model (3–5) in this study allowed us to examine the influence of exercise on both progression and, importantly, regression of an experimental tumor. Unlike a syngeneic cancer model, the use of this tumor allowed us to examine tumor regression. In keeping with the theory that prolonged, intense exercise results in immunosuppression (26), we hypothesized that the stressful exercise applied in these experiments would lead to a more rapid progression and a slower (or no) regression of the subcutaneously implanted tumor. This hypothesis was based on the observations that stress, perhaps mediated through immunosuppression, inhibits the rejection of allogeneic tumors (20, 22, 31). Past animal studies that examined the effects of exercise on tumor progression have, in general, not examined the microenvironment of the tumor. Examination of the effects of exercise on the tumor-host interface may shed light on how exercise affects tumor growth and regression. Therefore, the purposes of this study were to 1) determine the effects of daily intense, prolonged exercise on EL-4 tumor progression and regression and 2) to examine whether this exercise paradigm influenced the composition of cells and blood vessels over the course of tumor development.
METHODS
Animals
BALB/cByJ inbred female mice (6–8 wk) were utilized in this study. Mice were housed in a specific pathogen-free animal-containment facility. Mice were all single caged and kept on a 12:12-h reverse light cycle (lights on at 1900) maintained at 23°C. Mice were provided autoclaved food (8640 Harlan Teklad 22-5 Harlan, Madison, WI) and water ad libitum. Running sessions were performed during the dark cycle (1300–1600) at a time when mice are naturally most active. Treatments of animals were approved by the Institutional Animal Care and Use Committee at the University of Illinois at Urbana-Champaign and followed National Institutes of Health guidelines.
Exercise protocol
Treadmill running was the exercise mode used. This mode was selected because exercise intensity and duration could be experimentally manipulated and quantified. Mice were randomly assigned to a sedentary control group (Con) or a daily intense, prolonged exercised group (EXH). EXH exercised for 3 h or until volitional fatigue (e.g., failure to maintain pace with the treadmill) at gradually increasing speeds from 20 to 40 m/min at a 5% grade. The mean time run to exhaustion across all days was 135 ± 25 min. Mice ran without electric shock or prodding. Con were subjected to the noise of the treadmill, housed near the treadmill without food and water (during the time of the exercise bouts), and did not exercise.
EL-4 tumor cells
EL-4 cells are neoplastic lymphoid cells originally induced by 9,10-dimethyl-1,2-benzanthracene in C57/Bl6 mice (14). An allogeneic model was selected to demonstrate a complete cycle of progression and regression of a tumor. EL-4 cells (American Type Culture Collection, Manassas, VA) were cultured in 90% RPMI 1640 medium containing 9% horse serum and 1% penicillin/streptomycin/glutamine (Sigma Chemical, St. Louis, MO). The cultures were initiated at 2 × 105 cells/ml and maintained at ~1 × 105 cells/ml by passage every day. Each mouse was subcutaneously injected (in the back behind the neck) with 0.25 ml of viable 20 × 106 EL-4 cells in sterile phosphate-buffered saline immediately after the first exercise session with a sterile 25-gauge needle and 1-ml syringe.
Experiment 1
Our first experiment was designed to examine the effects of exercise on tumor growth and rejection by measuring tumor volume in Con (n = 7) and EXH (n = 8) mice. Measurement of tumor volume is a common technique used to repeatedly measure growing tumors in rodents. Tumor volume was evaluated beginning the first day the tumor was measurable (approximately day 4) and then daily before each exercise session for both EXH and Con. The tumors were measured in a blinded fashion with Vernier calipers and recorded in millimeters. Duplicate measures were taken and then averaged. Many different formulas of area and volume have been used in studies; however, the formula π/6 × length × width × height has the highest correlation to tumor mass (40). This is the formula of an ellipsoid, which was the shape of the subcutaneous EL-4 tumors in our experiments.
Experiments 2–4
Three follow-up experiments were performed to examine the kinetic effects of exercise on tumor histology. In experiment 2, we euthanized mice on days 6–10 (n = 4 in each treatment each day). In experiment 3, we euthanized mice on days 5, 6, 8, and 10 (n = 6 in each treatment each day; except for day 7, where n = 7 for EXH). In experiment 4, we euthanized mice on days 10, 12, and 14 (n = 6 in each treatment each day). This gave us a total subject number of 6 in each group (e.g., Con and EXH) at days 5, 12, and 14; 4 in each group at days 7 and 9; 10 in each group on days 8 and 10 in Con and 11 in EXH at day 6; and 16 in each group at day 10. In these experiments, mice were euthanized by rapid CO2 asphyxiation 24 h after their last exercise bout on days 5–10, 12, and 14 after injection of EL-4 tumor cells. The animals were weighed, and tumors were removed and weighed. Tumors were divided in half for fluid-content analysis or for histological analysis. Because differences in tumor volume can be influenced by the fluid content of the tumor and because prolonged exercise can affect hydration status, it was important to measure whether exercise affected tumor fluid volume and thus overall tumor volumes. This was done by weighing harvested wet tumors on days 5, 6, 8, 10, 12, and 14 immediately after exercise (or control) and then heating the tumors for 72 h at 40°C and measuring dry weight. The difference in weight was calculated as the tumor fluid content. For histological analysis, the tumors were fixed in 10% neutral buffered formalin for 12 h under constant gentle agitation. The fixed tissues were then stored in 80% ethyl alcohol. Tissues were dehydrated in graded alcohol, embedded in paraffin blocks, and then cut in 3-μm sections with a microtome.
Histological analysis
After staining with hematoxylin and eosin, all slides were analyzed under a light microscope in a blinded fashion by a trained observer. Verification of the results was performed by an independent blinded observer on approximately one-half of the slides for each variable assessed. There were no significant differences between the results obtained from the two different observers, and interrater reliability coefficients were high (>0.85) for all variables measured. For tumor sections from each mouse, histological parameters were measured in six predetermined areas (4 peripheral and 2 central) at ×400 magnification (0.196-mm2 field of view). Cells and structures were counted in each viewing area, and the average was taken from the six viewing sites and adjusted to number per square millimeter. All hematoxylin and eosin slide results were analyzed in three separate experiments on days 5–10, 12, and 14.
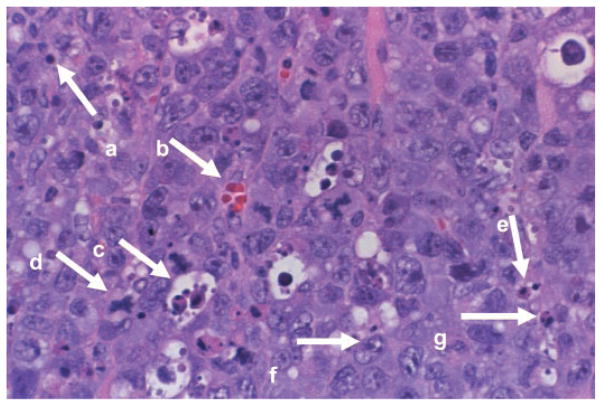
Hematoxylin and eosin-stained slides were used to evaluate the number of EL-4 cells, neutrophils, macrophages, blood vessels, apoptotic bodies, mitotic figures, and necrotic area. Figure 1 shows an example of a hematoxylin and eosin-stained slide identifying many of the components assessed in this study. Briefly, any endothelial cell cluster that was clearly separate from surrounding blood vessels was considered a single blood vessel and counted. Neutrophils were counted as cells of 10–12 μm in diameter with a multilobed nucleus and containing lightly stippled, purplish azurophilic primary granules. Macrophages were counted as cells of 15–80 μm in diameter with an oval or reniform nucleus, gray-blue cytoplasm, and/or small vacuoles and occasional granules. Apoptotic bodies were counted if there was a rounding up and fragmentation of an individual cell with nuclear chromatin in a crescent formation along one edge of the intact nuclear membrane. Necrotic tissue was classified when the cytoplasm became hypereosinophilic and hyalinized, the cytoplasm became fragmented, or chromatin clumps were found in an irregular fashion with the integrity of the nuclear membrane being lost. These necrotic areas were counted by the percentage of each viewing area that was necrotic on a 1–5 scale corresponding to 1 = 0–19%, 2 = 20–39%, 3 = 40–59%, 4 = 60–79%, and 5 = 80–100%. EL-4 tumor cells were counted as large (20–80 μm in diameter) cells with irregular growth. Any cell undergoing mitosis and corresponding to a metaphase, anaphase, or early telophase figure was counted as a mitotic figure. For each mouse, tumor-infiltrating lymphocytes were assessed in six surrounding areas (not a central region) of the section of each tumor where lymphocytes are most prevalent. Any cell that was of the size 6–9 μm in diameter with a round, densely stained nucleus and a relatively small amount of pale basophilic, nongranular cytoplasm was counted as a lymphocyte.
Fig. 1.
A representative hematoxylin and eosin-stained slide (×400 magnification) from a subcutaneous EL-4 tumor from a control mouse. Individual components of the tumor were identified by a blinded observer. a, Lymphocyte; b, blood vessel; c, macrophage (with ingested cellular debris); d, mitotic figure; e, apoptotic body; f, EL-4 cell; g, neutrophil.
Immunohistochemical staining of factor VIII-associated antigen
Deparaffinized tissue sections of 3-μm thickness were stained with antibody against von Willebrand factor VIII-associated antigen (Dako, Carpinteria, CA) and counterstained with hematoxylin. Factor VIII-associated antigen-stained slides were analyzed on days 5 and 10 to verify the results of the visual determination of blood-vessel density in tumors from six mice per group at each time point.
Statistical analysis
All data are reported as means ±SE. A repeated-measures two-way (time × treatment) ANOVA was used to determine significant differences between groups for time and treatment and their interactions for tumor volume data (experiment 1; Fig. 1). The SPSS general linear model multivariate procedure was used to analyze tumor histology data from experiments 2–4 (Figs. 3–7 and 9–11). Significant overall multivariate effects were followed up by a series of 2 (group) by 8 (days since death) ANOVAs with Bonferroni adjust for number of tests conducted (P < 0.006 was considered statistically significant). Pearson’s correlation was used to determine the relationship between the blood-vessel data obtained with visual analysis and that of immunohistochemically determined factor VIII-related antigen staining. Significance level was set at P < 0.05.
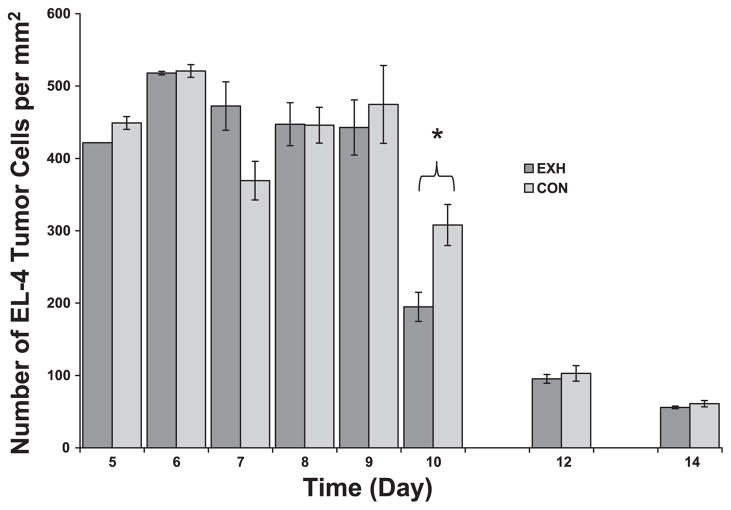
Fig. 3.
EL-4 density in tumor cross sections obtained from Con and EXH. There was a significant time main effect and a significant time × treatment interaction, such that Con tumors contained lower densities of EL-4 cells early in development (day 7) but a higher density at day 10. *Significant difference between Con and EXH (P < 0.006).
Fig. 7.
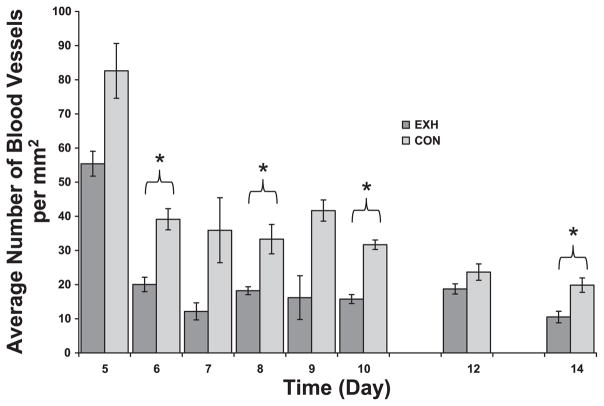
Visually determined blood-vessel density in tumor cross sections obtained from Con and EXH. There were significant main effects for time and treatment. *Significant difference between Con and EXH (P < 0.006).
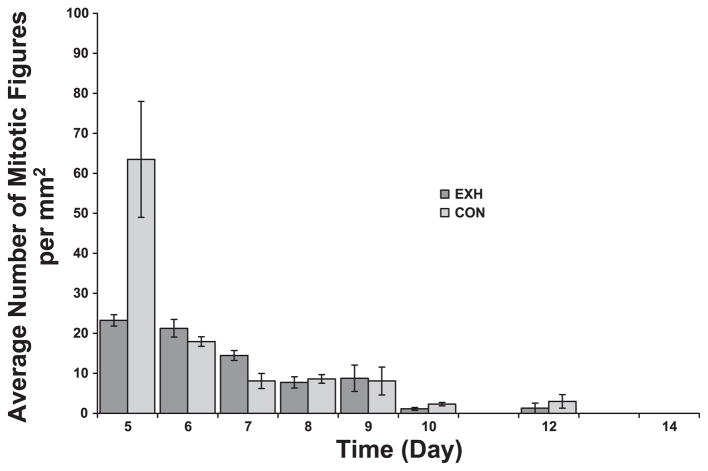
Fig. 9.
Density of mitotic figures (a marker for cell division) in tumor sections from Con and EXH. There were significant main effects for time, treatment, and their interaction. However, post hoc analysis failed to detect any significant differences between groups on any given day after Bonferroni adjustment.
Fig. 11.
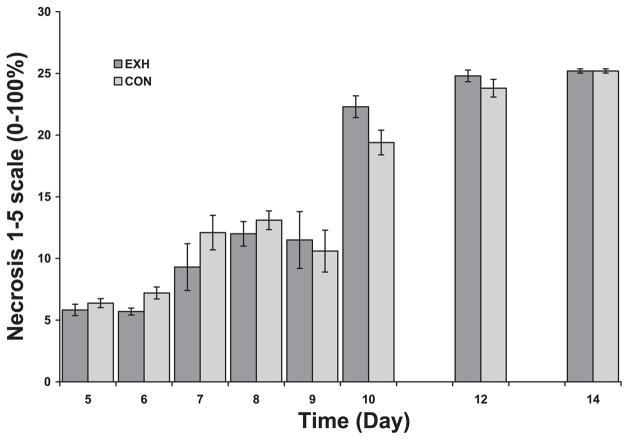
Necrotic area of tumor sections from Con and EXH. There was a significant time main effect but no treatment or time by treatment interaction. *Significant difference between Con and EXH (P < 0.006).
RESULTS
Effects of exhaustive exercise on EL-4 tumor progression and regression
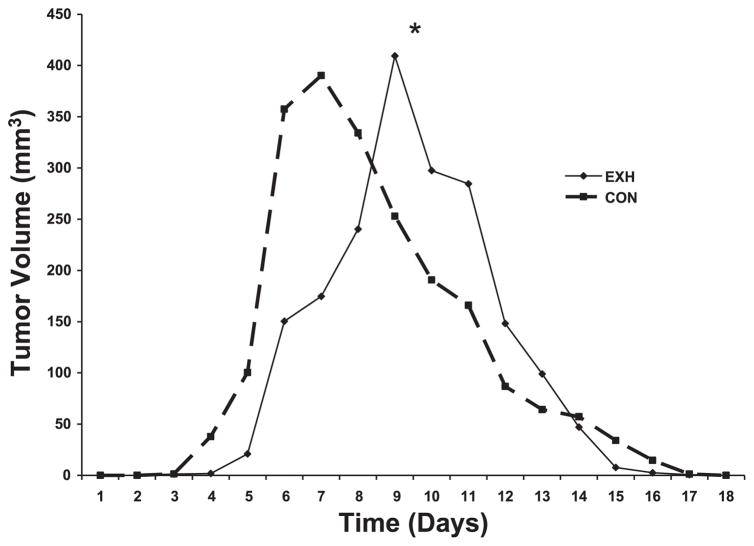
The effects of daily intense, prolonged exercise on tumor volume relative to Con mice can be found in Fig. 2. Results of the two-way repeated-measures ANOVA indicated that, although there were no significant differences in tumor volume due to treatment (e.g., treatment main effect), there was a significant main effect for time and a significant interaction (e.g., time × treatment) effect. In other words, EXH exhibited a different tumor growth pattern compared with Con. Specifically, tumor growth in EXH was delayed relative to Con. Peak tumor volume was achieved on day 7 in Con vs. day 9 in EXH, and there was a slight delay in tumor appearance in EXH (day 5) vs. Con (day 4). Contrary to our hypothesis, the tumors were rejected faster in the EXH group compared with Con. It took Con ~10 days to reject the tumor graft, whereas rejection occurred in ~7 days in EXH (Fig. 2). Unlike other exercise studies (30, 42, 44), we found no significant differences in peak tumor volume (390 ± 96 and 409 ± 140 mm3 for Con and EXH, respectively) between groups in this study using the EL-4 tumor.
Fig. 2.
Effects of daily exhaustive exercise on the growth of an EL-4 allogeneic tumor in mice [n = 8 exercise group (EXH) and n = 7 control group (Con)]. Error bars were excluded for clarity. *Two-way repeated-measures ANOVA results revealed a significant treatment × time interaction indicating that the 2 groups exhibited different tumor growth patterns (P < 0.05).
Effects of exhaustive exercise body weight and tumor fluid volume
There were no significant differences between preexperiment (day 0) and terminal body weights for either treatment group (18.2 ± 1.5 vs. 18.6 ± 1.3 g for EXH pre- and postexperiment, respectively, and 18.1 ± 1.2 vs. 18.4 ± 1.3 g for Con pre- and postexperiment, respectively). This indicates that the EXH animals were not in an energy-deficient state relative to Con. This was important to demonstrate because nutritional energy deficits have been shown to reduce the growth of tumors in mice (19).
Exercise affects hydration status, which could have an impact on the volume of fluid within a tumor and contribute to exercise-induced changes in tumor volume. We assessed tumor fluid content by measuring the difference between wet and dry tumor weights on days 5, 6, 8, 10, 12, and 14. Although the fluid content of the tumors decreased in both groups as a function of time, there were no significant differences in the amount of fluid lost between Con and EXH. For example, on day 8, the percentage of tumor weight that was fluid was 21 ± 2.5 and 24 ± 6% for Con and EXH, respectively. This suggests that there were no exercise-induced differences in hydration (e.g., edema) in the tumors from EXH compared with Con.
Exercise-induced changes in intratumoral cellular composition
Solid tumors contain many cell types, including tumor cells and host leukocytes like inflammatory cells (macrophages and neutrophils) and tumor-infiltrating lymphocytes (usually CD8+ T lymphocytes) (2). The host cellular infiltrate influences the growth and regression of the tumor (2, 6). Therefore, we examined the density of EL-4 tumor cells, macrophages, neutrophils, and lymphocytes in tumor sections obtained from EXH and Con on days 5–10, 12, and 14 after implantation.
EL-4 cells are large lymphocytes with a distinct nuclear-to-cytoplasmic ratio easily identifiable at ×400 magnification (see Fig. 1). EL-4 cells made up a large amount of the tumor volume and area in each cross section. Results of the multivariate ANOVA revealed that there was a significant time main effect, no treatment main effect, and a significant time × treatment interaction (Fig. 3). As can be see in Fig. 3, the density of EL-4 cells decreased as a function of time after implant. With regards to the interaction effect, on day 7, there was a lower EL-4 density in Con compared with EXH; however, on day 10, the pattern was reversed. There were no differences between groups on days 5–9, 12, and 14. It is important to remember that the number of EL-4 cells can be influenced by other cell types present in the cross sections and not just by the size or compactness of the EL-4 cells. In this regard, it is interesting to note that there were a significantly higher number of host lymphocytes in the tumors from the exercised mice at day 10 (Fig. 6). Their presence and the fact that these host lymphocytes kill EL-4 cells (see Refs. 4 and 5) could be the reason the number of EL-4 cells counted in the exercised group was lower than Con.
Fig. 6.
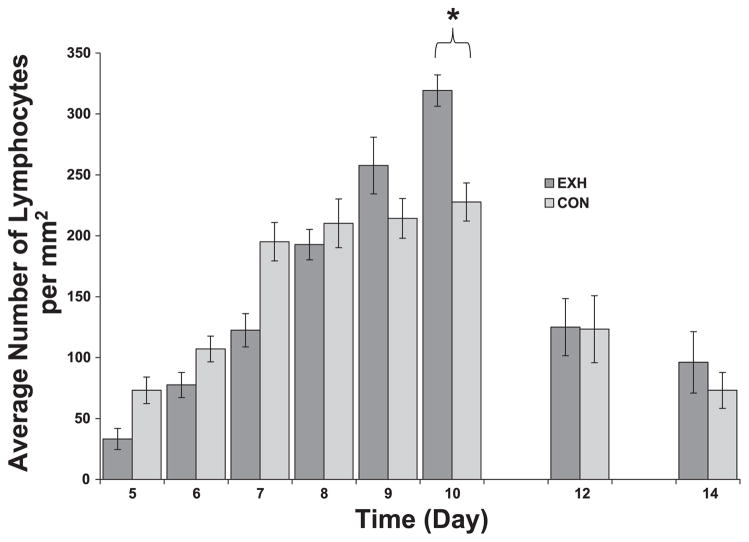
Lymphocyte density in tumor cross sections obtained from Con and EXH. There was a significant time effect and a time × treatment interaction. *Significant difference between Con and EXH (P < 0.006).
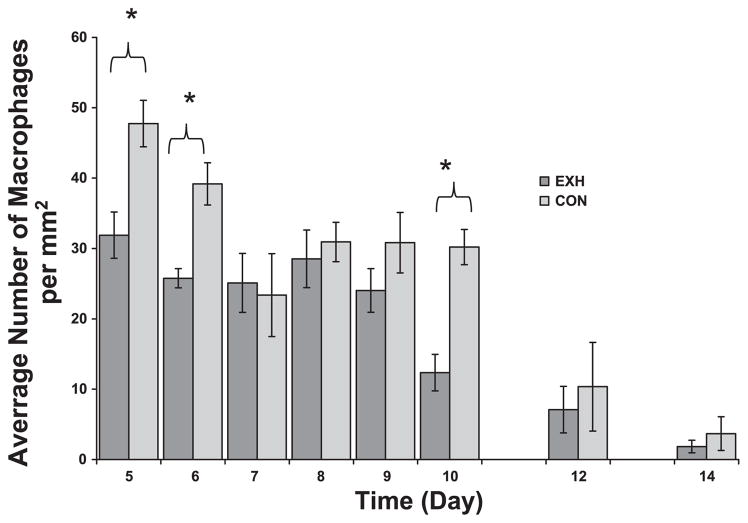
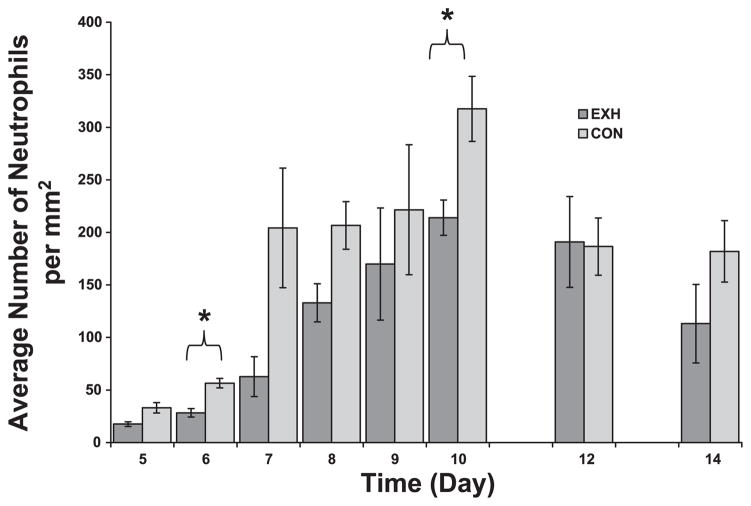
The microenvironment of a tumor is characterized by the presence of host leukocytes both in the supporting stroma and in the tumor area (25). Based on their unique morphology (see Fig. 1), we were able to distinguish and count intratumoral neutrophils and macrophages, which are two cells of the innate immune system involved in inflammation. There were significant time and treatment main effects but no interaction for both cell types. In both groups, macrophage density decreased as a function of time (Fig. 4), whereas neutrophil density increased (Fig. 5). We found consistently higher densities of macrophages (Fig. 4) and neutrophils (Fig. 5) in tumor cross sections of Con compared with EXH, especially at early time points. Post hoc analysis indicated significant differences between groups on days 5, 6, and 10 for macrophages (Fig. 4) and days 6 and 10 for neutrophils (Fig. 5).
Fig. 4.
Macrophage density in tumor cross sections obtained from Con and EXH. There was a significant time and treatment main effect. *Significant difference between Con and EXH (P < 0.006).
Fig. 5.
Neutrophil density in tumor cross sections obtained from Con and EXH. There was a significant time and treatment main effect. *Significant difference between Con and EXH (P < 0.006).
Host lymphocytes can also be found in tumors. These cells are typically CD8+ T lymphocytes that express a memory phenotype (2). In the EL-4 model, these killer T cells are responsible for rapid tumor regression (3). We visualized T lymphocytes based on their small size and low nuclear-to-cytoplasmic ratio. Two-way multivariate ANOVA revealed a significant time main effect and a significant time × treatment interaction. Lymphocyte density increased as a function of time up to day 8–10 (peaking at the same time of largest tumor volume) and then decreased as the tumors regressed (Fig. 6). Regarding the significant interaction effect, post hoc analysis revealed lower lymphocyte densities early in tumor growth (days 5 and 7) in EXH compared with Con. This pattern reversed itself on day 10, when significantly higher lymphocyte densities were found in EXH compared with Con (Fig. 6).
Exercise reduces blood-vessel density within tumors
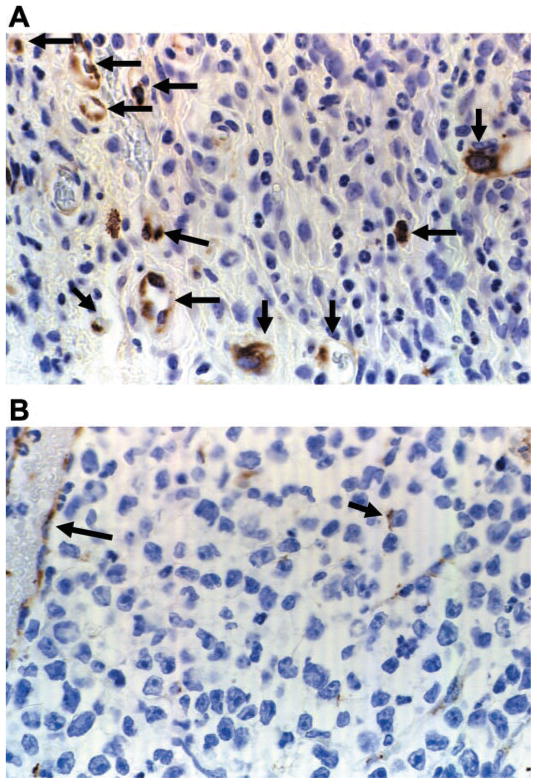
We evaluated blood-vessel density in tumor sections from Con and EXH mice both visually (on days 5–10, 12, and 14) and via immunohistochemistry with an antibody directed against factor VIII-associated antigen (days 5 and 10). There were significant time and treatment main effects and a significant time × treatment interaction. Blood-vessel density decreased as a function of time in both groups (Figs. 7 and 8). Post hoc analysis revealed significant differences between groups on days 6, 8, 10, and 14 (Fig. 7). Tumors from EXH consistently exhibited a 25–50% reduction in blood-vessel density compared with Con. Moreover, there was a high correlation (r = 0.969, P < 0.0001) between the visual technique for determining blood-vessel number and immunohistochemical staining for factor VIII-associated antigen.
Fig. 8.
Tumor sections from Con (A) and EXH (B) on day 10 stained with antibody against factor VIII-associated antigen. Arrows indicate factor VIII-associated antigen staining.
Effects of exercise on markers of cell proliferation and death
Mitotic figures in hematoxylin and eosin-stained slides are easily identifiable by the tangled chromosomes and dark-staining chromatin threads. Mitotic figures are used histologically to predict how extensively cells are undergoing division (36). The multivariate ANOVA revealed significant main effects for time, treatment, and an interaction. As expected, the density of mitotic figures decreased as a function of time in both groups, and levels were very low during tumor regression (Fig. 9). The treatment main effect arose as a consequence of the higher density of mitotic figures in the control group on day 5. This higher density of mitotic figures on day 5 in Con suggests a higher level of cell division in Con compared with EXH early in tumor development.
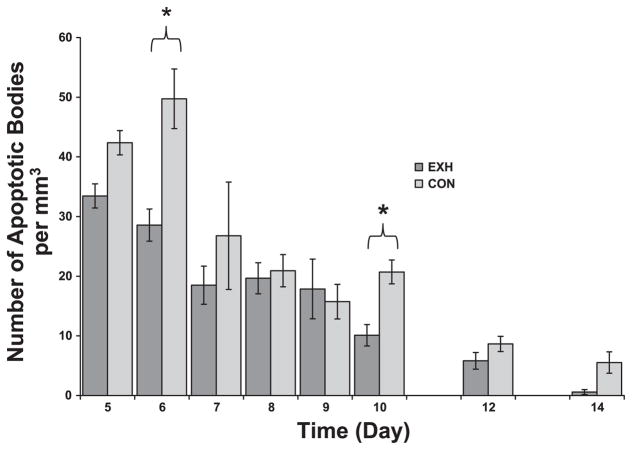
Apoptosis is generally defined as a type of genetically programmed cell death. It is one of the major mechanisms by which tissue growth is regulated and a way in which tissues are rejected. An apoptotic cell has marked nuclear and cytoplasmic condensation and fragments into multiple apoptotic bodies bound by an intact and functional plasma membrane. There were significant main effects for time, treatment, and an interaction effect. The density of apoptotic bodies decreased as a function of time in both groups (Fig. 10). However, significant group differences existed on days 6 and 10, when the density of apoptotic bodies was higher in Con compared with EXH. In contrast to apoptosis, necrosis is a pathological response to irreversible injury. There was a significant time main effect but no treatment effect or interaction. As seen in Fig. 11, necrotic area in the tumors increased as a function of time with no consistent differences observed between groups.
Fig. 10.
Density of apoptotic bodies in tumor sections from Con and EXH. There were significant main effects for time, treatment, and their interaction. *Significant difference between Con and EXH (P < 0.006).
DISCUSSION
We found that daily strenuous exercise, when performed during the progression and regression of an allogeneic tumor, did not alter peak tumor size but caused a delay in growth and a more rapid regression of the tumor. Although the physiological significance of a 2-day delay in tumor growth is unknown, it is interesting to note that the mice were able to reject the tumor and do so more rapidly than their sedentary counterparts. This was contrary to our hypothesis, based on previous literature (20, 22, 31), that stress would promote tumor growth and slow regression of this allogeneic tumor due perhaps to immunosuppression.
Many studies have found that prolonged, intense exercise or overtraining can suppress many aspects of immune function, including the development of T helper 1 responses (37, 38). This is of relevance to the present findings in that the generation of CD8+ T cells (e.g., the cells responsible for rejection of the EL-4 tumors) is dependent on proper signals (e.g., T helper 1 cytokines IL-2, IL-12, IFN-γ) produced by T helper 1 cells (41). Based on our findings, it appeared that prolonged exercise in this instance had no negative effect on the ability of mice to generate an anti-EL-4 CD8+ T cell-mediated immune response and may have had a positive one. The delay in peak tumor volume was not associated with any exercise-induced changes in fluid content of the tumor or body weight loss. Consistent with the delay in tumor growth, on day 5, we found a lower number of mitotic figures (an indication of proliferation) in tumors from the exercised mice. Although not statistically significant, the apparent slower mitotic rate in the exercised group early in tumor progression may have contributed to the delay in peak tumor volume, suggesting that exercise may have slowed the progression of the tumor through some mitotic cell cycle checkpoint, such as p53 or pRB (16).
Early animal studies have reported an exercise-induced inhibition of tumor growth in transplantable tumor systems (15, 33) or tumor occurrence in chemically induced or spontaneous tumor models (13, 24, 32). Unfortunately, interpretation of these early studies is confounded by the fact that the animals were subjected to very large (e.g., 16 h/day in Ref. 33) amounts of exercise. A primary concern in the interpretation of these early data was whether exercise exerted an effect on caloric energy status; the thinking here was that tumor cells were less likely to develop or continue to grow if there was little or no excess food energy available. In the present study, we documented no exercise-induced changes in body weight relative to Con and concluded that the exercise employed in this study did not drastically affect energy status and, therefore, contribute to the delay in tumor growth.
In more recent animal studies (30, 39, 42, 44–46), more realistic exercise protocols have been utilized, and results have tended to be less dramatic in terms of reducing tumor growth compared with the earlier studies. In some instances, exercise had no effect on tumor growth (12, 47). For example, in a previous study (50), our laboratory demonstrated that moderate or exhaustive treadmill exercise, when performed during (but not before) tumor implantation, did not significantly alter the incidence or growth of a syngeneic mammary adenocarcinoma in mice. Unlike most of the studies that found significant exercise effects, in the present study, we document a delay in growth but not a significant reduction in tumor size. One reason for the differences in tumor growth between the present study and others was that, in this study, we used an allogeneic tumor.
A major finding in our study was that exercise significantly reduced (~33%) the density of macrophages and neutrophils within the tumors, especially during the early stages of tumor development (days 5–7). The presence of inflammatory cells within tumors constitutes a double-edged sword. On the one hand, these cells have the capacity to kill tumor cells, inhibit their growth, and clean up debris from dying cells (2, 7). On the other hand, macrophages and neutrophils secrete growth and angiogenic factors that promote tumor growth and proliferation (2, 7). Indeed, recent evidence suggests that a high content of macrophages within breast tumors dictates a poor prognosis (21). To our knowledge, only one other study has examined the effect of exercise on macrophage content within a tumor (50). In that study, our laboratory found that moderate exercise (30 min/day) increased, whereas prolonged, exhaustive exercise (2–3 h/day) slightly decreased the content of phagocytic cells (~85% macrophages and 15% neutrophils) within a transplanted syngeneic tumor. Our previous study supports the findings of the present study, despite the fact that two different tumor cell lines were used.
A tumor cannot grow beyond 2–3 mm3 without blood-vessel formation (angiogenesis), which provides oxygen and nutrients to the rapidly dividing cells. Moreover, high-blood vessel density in tumors is correlated with poorer prognosis and an enhanced potential for metastasis (17). Indeed, recent studies in cancer treatment have targeted the process of angiogenesis as a means of combating tumors. It was interesting to note that, in addition to reducing intratumoral macrophage and neutrophil content, exercise also significantly reduced tumor blood-vessel density by ~25–50%. This effect was seen with both visual blood-vessel identification and immunohistochemistry against factor VIII-associated antigen (von Willebrand Factor). Based on our data, a scenario can be offered where exercise reduced the content of inflammatory cells in the tumor, which in turn reduced angiogenic growth factor levels leading to a reduction in blood-vessel density within the tumor and a delay in its growth. In agreement with other studies (43), we found a significant positive correlation of 0.59 (P < 0.001) between blood-vessel and macrophage numbers when both groups were analyzed throughout the experiment. Prolonged exercise is known to result in elevated glucocorticoid levels (51) that may act as anti-inflammatory signals to cells like macrophages inhibiting their migration into sites of inflammation (29). Future experiments are planned to dissect the relationships described above and whether intratumoral expression of angiogenic growth factors like vascular endothelial growth factor is affected by exercise. In support of this latter contention, Radak et al. (30) found vascular endothelial growth factor expression in solid leukemia tumors to be slightly (but not statistically significantly) lower in mice exposed to 1 h of swimming per day.
It was interesting to note that, even though the exercise employed in this experiment was intense and prolonged, there was no difference in the ability of the mice to reject the tumors. In fact, the tumors were rejected slightly faster in the exercised mice. This was contrary to our hypothesis that the tumors would be rejected slower or not at all. There are several factors that could be responsible for this more rapid rejection in EXH vs. Con, including lack of oxygen due to a reduction in blood-vessel density and/or a higher density of lymphocytes in the tumors at peak tumor volume. The differences in apoptosis and necrosis within the tumors suggest that alterations in tumor regression may have occurred through different mechanisms in the two groups. It is known that rejection of this EL-4 tumor corresponds to the generation of anti-EL-4 specific cytotoxic CD8+ T lymphocytes (4, 5, 18), although lymphokine-activated killer cells may also play a role (35). To our knowledge, no studies have examined the effect of exercise on the generation of antigen-specific cytotoxic T lymphocytes. In this study, we clearly show that the content of intratumoral lymphocytes increases as a function of time, peaking at time when the tumors are largest. What we do not know is whether these lymphocytes were CD8+ cytolytic T cells or lymphokine-activated killer cells. We speculate that they were cytolytic T cells and not lymphokine-activated killer cells based on their small size and lack of granules.
In conclusion, our results are the first to demonstrate that prolonged, intense exercise has an effect on the microenvironment of a subcutaneously transplanted allogeneic tumor. We found that daily intense, prolonged exercise caused a delay in tumor growth, a decrease in the number of inflammatory cells (macrophages and neutrophils), and a decrease in the number of blood vessels within the tumors. Contrary to our hypothesis, the tumors were rejected faster in EXH compared with Con. It is the decreased angiogenesis observed in EXH that may be the most significant finding in this study, perhaps accounting for the slower growth of the tumor and its rapid regression. Future experiments will examine whether angiogenic growth factors are differentially expressed in tumors from strenuously exercised mice. Moreover, we will also determine whether shorter duration, moderate exercise also impacts allogeneic tumor growth and composition.
Acknowledgments
GRANTS
This work supported by National Institute on Aging Grant AG-13928 (to J. A. Woods).
References
- 1.American Cancer Society. Cancer Facts and Figures. 2003 www.cancer.org.
- 2.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 3.Berke G. Debate: the mechanism of lymphocyte-mediated killing. Lymphocyte-triggered internal target disintegration. Immunol Today. 1991;12:396–399. doi: 10.1016/0167-5699(91)90138-j. [DOI] [PubMed] [Google Scholar]
- 4.Berke G, Sullivan K, Amos B. Rejection of ascites tumor allografts I. Isolation, characterization, and in vitro reactivity of peritoneal lymphoid effector cells from Balb/c mice. J Exp Med. 1972;135:1334–1350. doi: 10.1084/jem.135.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berke G, Sullivan K, Amos B. Rejection of ascites tumor allografts II. A pathway for cell-mediated tumor destruction in vitro by peritoneal exudate lymphoid cells. J Exp Med. 1972;136:1594–1604. doi: 10.1084/jem.136.6.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bingle L, Brown NJ, Lewis CE. The role of tumor-associated macrophages in tumor progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 7.Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis. 2002;19:247–258. doi: 10.1023/a:1015587423262. [DOI] [PubMed] [Google Scholar]
- 8.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 9.Folkman T. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 10.Fraser GE, Shavlik D. Risk factors, lifetime risk, and age at onset of breast cancer. Ann Epidemiol. 1997;7:375–382. doi: 10.1016/s1047-2797(97)00042-2. [DOI] [PubMed] [Google Scholar]
- 11.Friedenreich CM. Physical activity and cancer: lessons learned from nutritional epidemiology. Nutr Rev. 2001;59:349–357. doi: 10.1111/j.1753-4887.2001.tb06962.x. [DOI] [PubMed] [Google Scholar]
- 12.Gillette CA, Zhu Z, Westerlind KC, Melby CL, Wolfe P, Thompson HJ. Energy availability and mammary carcinogenesis: effects of calorie restriction and exercise. Carcinogenesis. 1997;18:1183–1188. doi: 10.1093/carcin/18.6.1183. [DOI] [PubMed] [Google Scholar]
- 13.Good RA, Fernandes G. Enhancement of immunologic function and resistance to tumor growth in Balb/c mice by exercise (Abstract) Fed Proc. 1981;10:1040. [Google Scholar]
- 14.Gorer PA, Amos DB. Passive immunity in mice against C57BL leukosis EL-4 by means of iso-immune serum. Cancer Res. 1956;16:338–343. [PubMed] [Google Scholar]
- 15.Hoffman SA, Paschkis KE, DeBias DA, Cantarow A, Williams TL. The influence of exercise on the growth of transplanted rat tumors. Cancer Res. 1962;22:597–599. [PubMed] [Google Scholar]
- 16.Hudson JD, Shaibi MA, Maestro R, Carnero A, Gannon GH, Beach DH. A pro-inflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato T, Kameoka S, Kimura T, Nishikawa T, Kobayashi M. The combination of angiogenesis and blood vessel invasion as a prognostic indicator in primary breast cancer. Br J Cancer. 2003;88:1900–1908. doi: 10.1038/sj.bjc.6600921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemp A, Berke G, Crowell J, Amos B. Induction of cell-mediated immunity against leukemia EL4 in C57BL mice. J Natl Cancer Inst. 1973;51:1877–1882. doi: 10.1093/jnci/51.6.1877. [DOI] [PubMed] [Google Scholar]
- 19.Kritchevsky D. Caloric restriction and experimental carcinogenesis. Toxiol Sci. 1999;52(Suppl 2):13–16. doi: 10.1093/toxsci/52.2.13. [DOI] [PubMed] [Google Scholar]
- 20.Li T, Harada M, Tamada K, Abe K, Nomoto K. Repeated restraint stress impairs the antitumor T cell response through its suppressive effect on Th1-type CD4+ T cells. Anticancer Res. 1997;17:4259–4268. [PubMed] [Google Scholar]
- 21.Lin EY, Gouon-Evans G, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002;7:147–162. doi: 10.1023/a:1020399802795. [DOI] [PubMed] [Google Scholar]
- 22.Mazur-Kolecka B, Machala O, Skowron-Cendrzak A, Kubera M, Bubak-Satora M, Basta-Kaim A, Roman A. Effect of immobilization stress on tumor growth in mice. Neoplasma. 1994;41:183–186. [PubMed] [Google Scholar]
- 23.McTiernan A, Schwartz RS, Potter J, Bowen D. Exercise clinical trials in cancer prevention research: a call to action. Cancer Epidemiol Biomarkers Prev. 1999;8:201–207. [PubMed] [Google Scholar]
- 24.Moore C, Tittle P. Muscular activity, body fat, and induced rat mammary tumors. Surgery. 1973;73:329–332. [PubMed] [Google Scholar]
- 25.Negus RPK, Stamp GWQ, Hadley J, Balkwill FR. Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship ot the expression of C-C chemokines. Am J Pathol. 1997;150:1723–1734. [PMC free article] [PubMed] [Google Scholar]
- 26.Nieman DC. Current perspective on exercise immunology. Curr Sports Med Rep. 2003;2:239–242. doi: 10.1249/00149619-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Ono M, Torisu H, Fukushi J, Nishie A, Kuwano M. Biological implications of macrophage infiltration in human tumor angiogenesis. Cancer Chemother Pharmacol. 1999;43:S69–S71. doi: 10.1007/s002800051101. [DOI] [PubMed] [Google Scholar]
- 28.Pickett M, Mock V, Ropka ME, Cameron L, Coleman M, Podewils L. Adherence to moderate-intensity exercise during breast cancer therapy. Cancer Pract. 2002;10:284–292. doi: 10.1046/j.1523-5394.2002.106006.x. [DOI] [PubMed] [Google Scholar]
- 29.Poon M, Gertz SD, Fallon JT, Wiegman P, Berman JW, Sarembock IJ, Taubman MB. Dexamethasone inhibits macrophage accumulation after balloon arterial injury in cholesterol fed rabbits. Atherosclerosis. 2001;155:371–380. doi: 10.1016/s0021-9150(00)00605-5. [DOI] [PubMed] [Google Scholar]
- 30.Radak Z, Gaal D, Taylor AW, Kaneko T, Tahara S, Nakamoto H, Goto S. Attenuation of the development of murine solid leukemia tumor by physical exercise. Antioxid Redox Signal. 2002;4:213–219. doi: 10.1089/152308602753625979. [DOI] [PubMed] [Google Scholar]
- 31.Radosevic-Stasic B, Udovic-Sirola M, Stojanov L, Ribaric L, Rukavina D. Growth of allogeneic sarcoma in mice subjected to halothane anesthesia and/or surgical stress. Anesth Analg. 1989;69:570–574. [PubMed] [Google Scholar]
- 32.Rashkis HA. Systemic stress as an inhibitor of experimental tumors in Swiss mice. Science. 1952;116:169–171. doi: 10.1126/science.116.3007.169. [DOI] [PubMed] [Google Scholar]
- 33.Rusch HP, Kline BE. The effect of exercise on the growth of a mouse tumor. Cancer Res. 1944;4:116–118. [Google Scholar]
- 34.Slattery ML, Potter JD. Physical activity and colon cancer: confounding or interaction? Med Sci Sports Exerc. 2002;34:913–919. doi: 10.1097/00005768-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Smart YC, Farrelly ML, Burton MC. Correlation of growth of tumours in NC-cell-depleted mice with NC- and NK-cell-mediated lysis in vitro. Int J Cancer. 1992;50:817–821. doi: 10.1002/ijc.2910500526. [DOI] [PubMed] [Google Scholar]
- 36.Smith SH, Goldschmidt MH, McManus PM. A comparative review of melanocytic neoplasms. Vet Pathol. 2002;39:651–678. doi: 10.1354/vp.39-6-651. [DOI] [PubMed] [Google Scholar]
- 37.Steensberg A, Toft AD, Bruunsgaard H, Sandmand M, Halkjaer-Kristensen J, Pedersen BK. Strenuous exercise decreases the percentage of type 1 cells in the circulation. J Appl Physiol. 2001;91:1708–1712. doi: 10.1152/jappl.2001.91.4.1708. [DOI] [PubMed] [Google Scholar]
- 38.Sukuki K, Nakaji S, Kurakake S, Totsuka M, Sato K, Kuriyana T, Fujimoto H, Shibusawa K, Machida K, Sugawara K. Exhaustive exercise and type-1/type-2 cytokine balance with special focus on interleukin-12 p40/p70. Exerc Immunol Rev. 2003;9:48–57. [PubMed] [Google Scholar]
- 39.Thompson HJ, Westerlind KC, Snedden J, Briggs S, Singh M. Exercise intensity dependent inhibition of 1-methyl-1-nitrosourea induced mammary carcinogenesis in female F-344 rats. Carcinogenesis. 1995;16:1783–1786. doi: 10.1093/carcin/16.8.1783. [DOI] [PubMed] [Google Scholar]
- 40.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 41.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 42.Uhlenbruck G, Order U. Can endurance sports stimulate immune mechanisms against cancer and metastasis? Int J Sports Med. 1991;12:S53–S68. doi: 10.1055/s-2007-1024753. [DOI] [PubMed] [Google Scholar]
- 43.Valkovic T, Dobrila F, Melato M, Sasso F, Rizzardi C, Jonjic N. Correlation between vascular endothelial growth factor, angiogenesis, and tumor-associated macrophages in invasive ductal breast carcinoma. Virchows Arch. 2002;440:583–588. doi: 10.1007/s004280100458. [DOI] [PubMed] [Google Scholar]
- 44.Welsch MA, Cohen LA, Welsch CW. Inhibition of growth of human breast carcinoma xenografts by energy expenditure via voluntary exercise in athymic mice fed a high-fat diet. Nutr Cancer. 1995;23:309–318. doi: 10.1080/01635589509514385. [DOI] [PubMed] [Google Scholar]
- 45.Westerlind KC, McCarty HL, Schultheiss PC, Story R, Reed AH, Baier ML, Strange R. Moderate exercise training slows mammary tumour growth in adolescent rats. Eur J Cancer Prev. 2003;12:281–287. doi: 10.1097/00008469-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Whittal KS, Parkhouse WS. Exercise during adolescence and its effects on mammary gland development, proliferation, and nirtosomethylurea (NMU) induced tumorigenesis in rats. Breast Cancer Res Treat. 1996;37:21–27. doi: 10.1007/BF01806628. [DOI] [PubMed] [Google Scholar]
- 47.Whittal-Strange KS, Chadan S, Parkhouse WS, Chadau S. Exercise during puberty and NMU induced mammary turmorigenesis in rats. Breast Cancer Res Treat. 1998;47:1–8. doi: 10.1023/a:1005838721890. [DOI] [PubMed] [Google Scholar]
- 48.Willer A. Reduction of the individual cancer risk by physical exercise. Onkologie. 2003;26:283–289. doi: 10.1159/000071626. [DOI] [PubMed] [Google Scholar]
- 49.Woods JA. Exercise and resistance to neoplasia. Can J Physiol Pharmacol. 1998;76:581–588. doi: 10.1139/cjpp-76-5-581. [DOI] [PubMed] [Google Scholar]
- 50.Woods JA, Davis JM, Kohut ML, Ghaffar A, Mayer EP, Pate RR. Effects of exercise on the immune response to cancer. Med Sci Sports Exerc. 1994;26:1109–1115. [PubMed] [Google Scholar]
- 51.Woods JA, Davis JM, Mayer EP, Ghaffar A, Pate RR. Exercise increases inflammatory macrophage anti-tumor cytotoxicity. J Appl Physiol. 1993;75:879–886. doi: 10.1152/jappl.1993.75.2.879. [DOI] [PubMed] [Google Scholar]
- 52.Zahm SH, Hoffman-Goetz L, Dosemeci M, Cantor KP, Blair A. Occupational physical activity and non-Hodgkin’s lymphoma. Med Sci Sports Exerc. 1999;31:566–571. doi: 10.1097/00005768-199904000-00012. [DOI] [PubMed] [Google Scholar]