Abstract
Squamous metaplasia (SQM) is a specific phenotype in response to estrogen in the prostate and estrogen receptor (ER) α is required to mediate this response. Previous studies utilizing tissue recombination with seminal vesicle (SV) mesenchyme and prostatic ductal tips from Wt and ERαKO mice suggested that both epithelial and stromal ERα are necessary for SQM. However, tissue recombination is conducted in the renal capsule of immune-deficient mouse, in which the microenvironment is different from normal prostate microenvironment in the intact mouse. Furthermore, whether the requirement of stromal ERα in the SV for developing SQM is same as in the prostate is unknown. Therefore, there is a clear need to evaluate the respective roles of ERα in prostate epithelial versus stromal compartments in the intact mouse. Here we generated a mouse model that has selectively lost ERα in either stromal (FSP-ERαKO) or epithelial prostate cells (pes-ERαKO) to determine the requirements of ERα for estrogen stimulated prostate proliferation and SQM. Our results indicated that FSP-ERαKO prostates develop a full and uniform SQM, which suggests that loss of the majority (~65%) of stromal ERα will not influence estrogen mediated SQM. In contrast, loss of epithelial ERα inhibits estrogen mediated prostate growth and SQM evidenced by decreasing CK10 positive squamous cell stratification and differentiation, by reducing the up-regulated ERα, and by the presence of normal proliferative activities in the estrogen treated pes-ERαKO prostates. These in vivo results suggest that epithelial ERα is required for estrogen mediated proliferative response and could be an appropriate target for preventing aberrant estrogen signaling in the prostate.
Keywords: estrogen receptor α, squamous metaplasia, prostate, tissue specific knockout, Cre-loxP
Introduction
The prostate is primarily considered to be an androgen target organ [1], but estrogen can also influence its development and pathogenesis [2]. Estrogens exert both indirect and direct effects on the prostatic organ. The indirect estrogen action is mediated at the hypothalamic level to suppress circulating androgens and produce a “castration like” effect [3–6]. The direct actions of estrogens are mediated by two distinct receptors, estrogen receptor (ER) α and β in the prostate [7–10]. Studies from ERα knockout (ERαKO) mouse prostates have consistently shown that ERα is involved in the prostatic branching morphogenesis and required for normal prostate development [11–13]. Studies from conventional ERβKO/neo knock-in mouse prostates showed inconsistent phenotypes in the hyperplastic prostates [14–16]. Recently, a updated cre-loxP ERβKO mouse model was developed via mating CMV-cre with floxed ERβ mice (CMV-cre-ERβKO) [17]. The CMV-cre-ERβKO males appear to be a complete ERβ null mice, yet there is no prostatic phenotype. Taken together, ERβ does not seem to have a critical role in prostate development.
Besides the normal physiological roles of estrogen signaling in the prostate development, exposure of perinatal or neonatal male mice to estrogens leads to a reduced responsiveness to androgens during adulthood and an increased incidence of severe dysplasia with aging in the prostate gland, which is similar to high grade prostatic intraepithelial neoplasia (PIN) in the human prostate [18, 19]. The effect on prostate development, following estrogen exposure in the prenatal or neonatal rodent, is called developmental estrogenization of the prostate (also called estrogen imprinting effect), a process by which genomic signals generated in utero can induce a greater propensity for cancer in later life. In contrast, exposure of adult prostate to estrogens leads to squamous metaplasia (SQM) in the prostate gland. SQM is associated with a stratified basal epithelial layer, onset of cytokeratin 10 (CK10) expression, and enhanced proliferative activities in the estrogen treated prostates [20]. Together, developmental estrogenization of the prostate and SQM are specific responses to estrogen in the prostate gland and appear to be mediated by ERα, but not ERβ [19, 21].
The prostate develops in the context of stromal-epithelial cell interaction. The reciprocal interaction between prostatic epithelial and stromal cells plays a role in homeostasis and maintaining the growth quiescence in the adult prostatic gland [22]. It has been reported that ERα could be detected in both prostate epithelial and stromal cells [12, 18, 19, 21]. There are some controversies about whether epithelial versus stromal ERα plays a role in the developmental estrogenization of the prostate. Based on the immunohistochemical staining profile of ERα, one study results suggested that stromal ERα, but not epithelial ERα, is required for the estrogen imprinting effect on the prostate [19], while the other showed that both epithelial and stromal ERα are required for the estrogen imprinting effect [12].
In an effort to determine the respective roles of epithelial versus stromal ERα in estrogen meditated SQM, the earlier study utilized tissue recombination techniques with newborn seminal vesicle (SV) mesenchyme and adult prostatic ductal tips from Wt and ERαKO mice showing that both epithelial and stromal ERα are necessary for estrogen mediated SQM in the prostate [21]. However, tissue recombination is conducted in the renal capsule of immune-deficient mice, in which the microenvironment is different from the prostate microenvironment in immune-intact mice. In addition, the experimental method used prostate duct tips rather than prostate epithelial cells as the epithelial compartment to prepare the tissue recombinants, therefore the ductal tips could have been contaminated by some stromal cells. Furthermore, whether the function of stromal ERα in the SV mesenchyme is the same as in prostate mesenchyme in estrogen mediated SQM is unknown.
Until recently there have been no models to elucidate the respective roles of epithelial versus stromal ERa in vivo within the murine prostate during prostatic pathogenesis, or to demonstrate a direct implication for understanding prostatic disease and pharmaceutical targets. Here we generated a mouse model that has lost ERα selectively in either stromal cells (FSP-ERαKO) or prostate epithelial cells (pes-ERαKO) to determine the temporal and spatial requirements of ERα in the estrogen stimulated proliferation and SQM in prostate. Our results indicated that FSP-ERαKO prostates develop a full and uniform SQM, which indicates that loss of the majority (~65%) of stromal ERα will not influence estrogen mediated SQM in prostate. In contrast, in pes-ERaKO male mice, the loss of epithelial ERα inhibits estrogen mediated prostate responses evidenced by decreasing CK10 positive squamous cell stratification and differentiation, by reducing the up-regulated ERα expression, and by the presence of normal proliferative activities in the estrogen treated pes-ERαKO prostates. These in vivo results suggest that epithelial ERα is required for estrogen mediated proliferative response in the adult prostate and could be an appropriate target for preventing aberrant estrogen signaling in the adult prostate.
Materials and Methods
Generation of tissue specific ERαKO Mice and Hormone Treatment
The ACTB-ERαKO, pes-ERαKO, and FSP-ERαKO mice were generated by mating floxed ERα female mice with ACTB-Cre, probasin-Cre, and FSP1-Cre transgenic male mice, in which cre recombinase is under the control of the β-actin, probasin, and FSP1 promoter, respectively [23–25]. Genomic DNA was used as a template for PCR genotyping of DNA isolated from tail biopsies. Genotyping and primers used were as previously described [24]. The adult ACTB-ERαKO, FSP-ERαKO, and pes-ERαKO male mice, or wild-type (Wt) littermate controls were treated with s.c. implants of 20-mg pellets containing 2 mg diethylstilbestrol (DES) and 18 mg cholesterol at 12 weeks of age [21]. Groups of five Wt or ERαKO animals were studied at intervals of up to 3 weeks following DES treatment. The mice were sacrificed and prostate tissues were harvested at 15 weeks of age. All animal procedures were approved by the Animal Care and Use Committee of the University of Rochester Medical Center, in accordance with National Institutes of Health guidelines.
Immunohistochemistry (IHC) and immunofluorescent (IF) staining
IHC and IF staining were carried out as described previously [11, 26]. Sections were incubated with the following primary antibodies and dilutions: anti-CK10 (DE-K10, 1:50), anti-p63 (A4A, 1:100), anti-ERα antibodies (MC-20, 1:400), anti-CK5 (AF138, 1:200), anti-androgen receptor (AR) (N-20, 1:400), anti-p27 (C-19, 1:200), and anti-Ki67 (NCL-Ki67p, 1:1000) in 3% BSA in PBS overnight at 4°C followed by respective secondary antibodies. The stained slides were mounted and visualized by a bright-field fluorescent microscope.
Statistical analysis
Results are expressed as mean ± SEM. Statistical analysis was performed by using an independent Student t-test for two groups of data and analysis of variance (ANOVA) followed by Scheffe’s test for multiple comparisons. A p-value less than 0.05 was considered significant.
Results
Loss of epithelial ERα inhibits estrogen mediated SQM in pes-ERαKO prostates
In order to address the respective roles of epithelial versus stromal ERα in the SQM, we generated mice that conditionally lack of ERα in the prostate epithelial cells or stromal cells by mating floxed ERα female mice with male mice expressing Cre recombinase driving by probasin or fibroblast specific protein 1 (FSP1) promoter. The promoter of probasin is well characterized and widely applied to delete loxP gene in several mouse models [25, 27, 28]. The expression of probasin-Cre is post-natal and prostatic epithelium-specific, which is gradually increased and has nearly 100% activity in adult mice to delete floxed gene expression (suppl. Fig. 1) [25, 27–29]. The expression of FSP1-Cre is first detected in the cells of fibroblastic phenotype after embryonic day 8.5 [31–33]. FSP1-cre is able to knock out ~65 % of floxed genes in the mouse prostate stromal compartment (suppl. Fig. 2) and has been widely used to knockout a variety of target genes [23, 34]. We generated 3 ERαKO mouse models: (i) flox ERα gene deletion in every tissue by ACTB-cre (ACTB-ERαKO); (ii) selective deletion in stromal cells by FSP1-cre (FSP-ERαKO), and (iii) selective deletion in prostate epithelial cells by probasin-cre (pes-ERαKO) to determine the temporal and spatial requirements of ERα in the estrogen stimulated proliferation and SQM in prostate. Our data indicate that FSP-ERαKO prostates have less branching morphogenesis compared to Wt littermates; In contrast, pes-ERαKO mice develop a normal prostate (Chan and Yeh, paper submission). We chose 10 to 12 weeks old adult mice to perform our experiments since the expression of Cre develops into full activity in the adult.
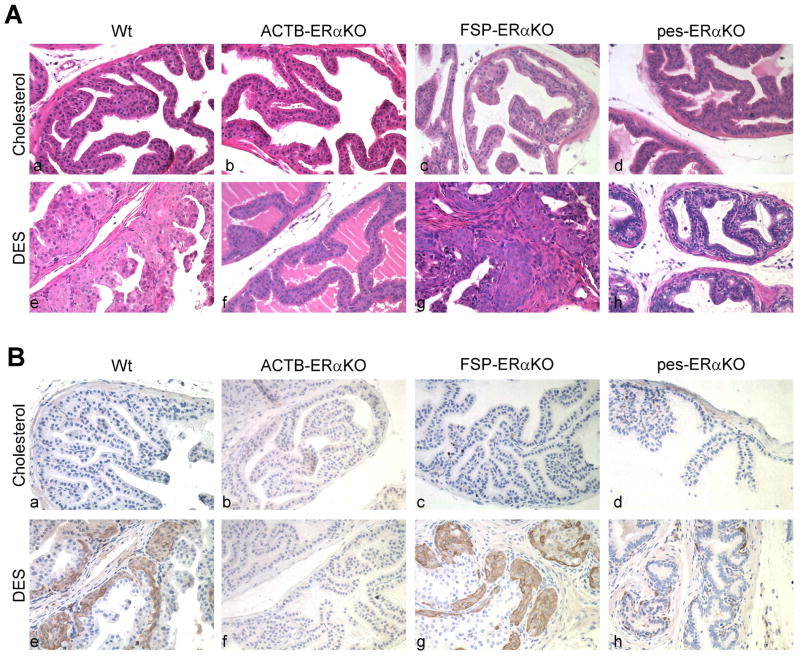
Previous studies have shown that the effects of DES on the three prostatic lobes revealed a hierarchy of responses, with the anterior prostate (AP) being the most responsive, the dorso-lateral prostate (DLP) less responsive, and the ventral prostate (VP) the least responsive. Fig. 1 showed representative AP histology sections of DES treated Wt, ACTB-ERαKO, pes-ERαKO, and FSP-ERαKO mice. The normal prostate ducts were lined by a layer of simple columnar epithelial cells in different cholesterol-treated mouse genotypes (Fig. 1A, a, b, c, and d). DES treatment resulted in uniform stratified basal epithelial layers in the AP of Wt and FSP-ERαKO mice (Fig. 1A, e and g). Those stratified basal epithelial cells were positive for the squamous epithelial marker, CK10 (Fig. 1B, e and g), which was not presented in the cholesterol-treated mouse prostates (Fig. 1B, a–d). In contrast, DES treated ACTB-ERαKO prostates appeared histologically identical with cholesterol treated ACTB-ERαKO prostates and did not express CK10 (Fig. 1, A and B, f vs b), which is consistent with previous studies showing that conventional ERαKO-neo knockin mice (αERKO) did not respond to DES treatment [19, 21]. Importantly, our data showed that loss of prostate epithelial ERα inhibited SQM in the pes-ERαKO mice as the prostatic ducts from DES treated pes-ERαKO were mainly lined by a single layer of epithelial cells (Fig. 1A, h vs d), in which CK10 was ether not detected or only focally, single-layer detected (Fig 1B, h vs d). These residual CK10 positive cells could be due to lack of probasin-Cre activity in some prostate basal epithelial cells to knockout floxed ERα gene. Our current studies confirmed that ERα is required for estrogen-mediated response in the mouse prostate [19, 21]. Furthermore, epithelial ERα is required to mediate this estrogenic effect in the murine prostate.
Fig. 1.
PES-ERαKO mice fail to respond to DES treatment. H& E staining (A) and IHC staining of CK10 (B) from Wt, ACTB-ERαKO, FSP-ERαKO, and pes-ERαKO AP tissue treated with vehicle (cholesterol) or DES. Note that DES treatment in Wt and FSP- ERαKO mice resulted in uniform stratified basal epithelial layer, in which squamous epithelial marker, CK10, is expressed (A and B, e and g), but not in the DES treated ACTB- ERαKO and pes- ERαKO mice (A and B, f and h).
Decreased SQM phenotype and basal cell proliferation in DES treated pes-ERαKO prostates
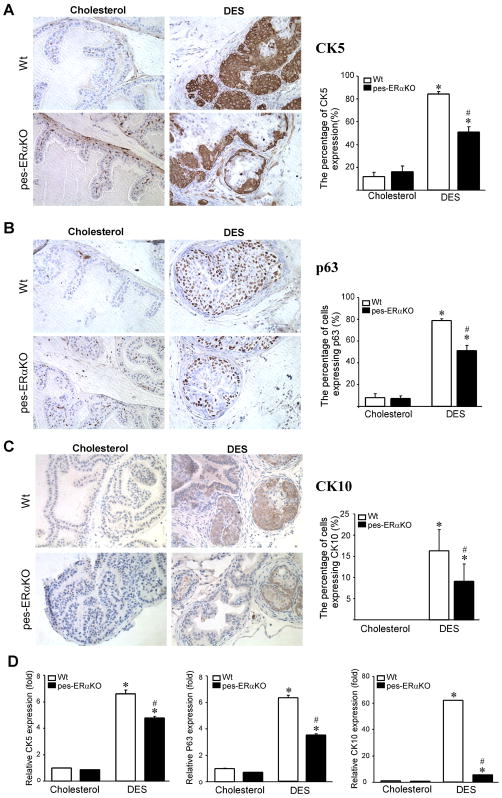
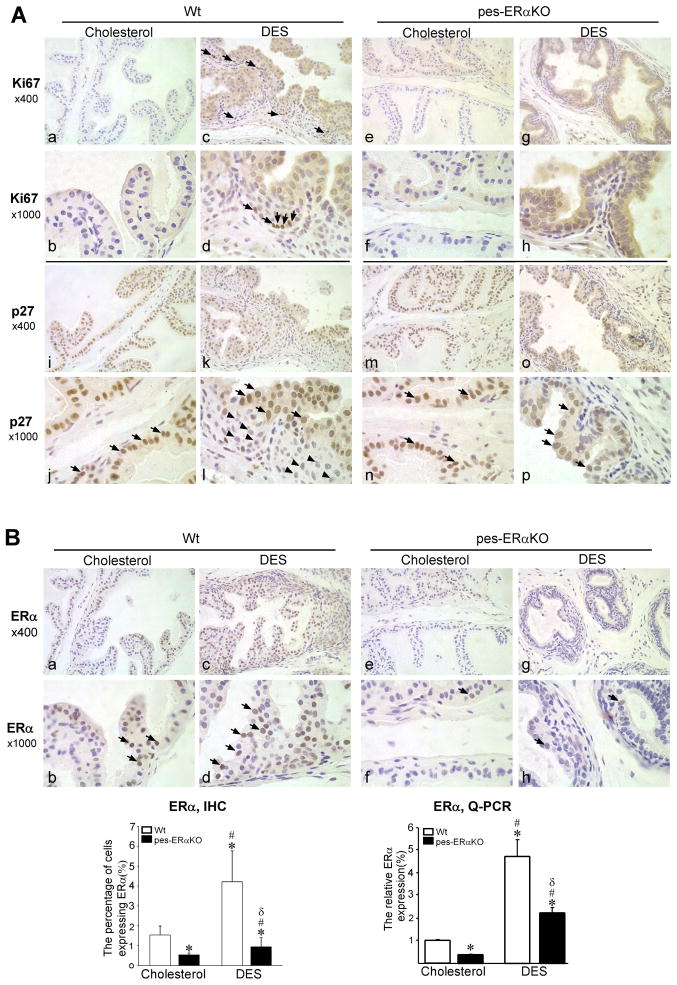
To better characterize SQM defects and cell type changes in DES treated pes-ERαKO and Wt mice, we further used IHC and real-time quantitative PCR (Q-PCR) assays to detect CK5, CK10, and p63 markers. CK10 is the marker for SQM cells. CK5 and p63 are markers for basal epithelial cells. As indicated by both IHC and Q-PCR analyses, the percentage of cells expressing CK5, CK10, and p63 are profoundly increased in DES treated Wt mice, however, the changes in DES treated pes-ERαKO mice are much less as compared to those in DES treated Wt mice (Fig. 2, A–D). Meanwhile, we also co-stained CK5/CK10, ERα/p63, and ERα/CK10 to examine how those markers colocalize and spatially change during SQM. The normal mouse prostate contains few basal cells, which are intermittently expressed at the basement membrane in both vehicle (cholesterol) treated Wt and pes-ERαKO control mice (Fig. 3, A and B, a and f, arrow), and CK10 is not expressed in those basal cells (Fig. 3, A and B, b). In Wt mice, DES treatment resulted in CK5 positive basal cells expansion, stratification, and multi-layering (Fig. 3C-a). These CK5 positive basal cells also expressed squamous epithelial marker, CK10 (Fig. 3C-b). In contrast, pes-ERαKO mice response to DES had a very limited basal cell expansion as compared to DES treated Wt mice (Fig. 3D-a vs Fig. 3C-a). DES treatment only resulted in a continuous, but not stratified, CK5 positive basal layer in the pes-ERαKO prostates (Fig. 3D-a). Consistently, limited expansion of basal type cells in DES pes-ERαKO prostates were also observed when stained with another basal cell marker, p63 (Fig. 3D-f vs Fig. 3C-f). Furthermore, only a few CK5 positive cells co-expressed CK10 in the DES treated pes-ERαKO prostates (Fig. 3D-b, arrowhead), which is consistent with the results in Fig. 2D showing that the up-regulation of CK10 positive cells is much less than that in DES treated Wt prostates. Together, our results indicated that loss of epithelial ERα in the pes-ERαKO prostates inhibits basal cell expansion and SQM phenotype evidenced by significantly decreased CK10-positive squamous cells and CK5 (or p63) positive basal cells.
Fig. 2.
IHC staining and Q-PCR quantification of the cytokeratin and basal cell markers in prostates of DES and vehicle treated Wt and pes-ERαKO mice. Results were examined and quantified by using both IHC staining (A, B, C), and real-time Q-PCR assay (D). Note that the percentage of cells expressing CK5 (A), p63 (B), and CK10 (C) and the relative level of those markers are profoundly up-regulated in DES treated Wt mice, however, the changes in DES treated pes-ERαKO mice are significantly less (A-D) (p< 0.05, ✱: compared to cholesterol treated mice; ♯: compared to DES treated Wt mice).
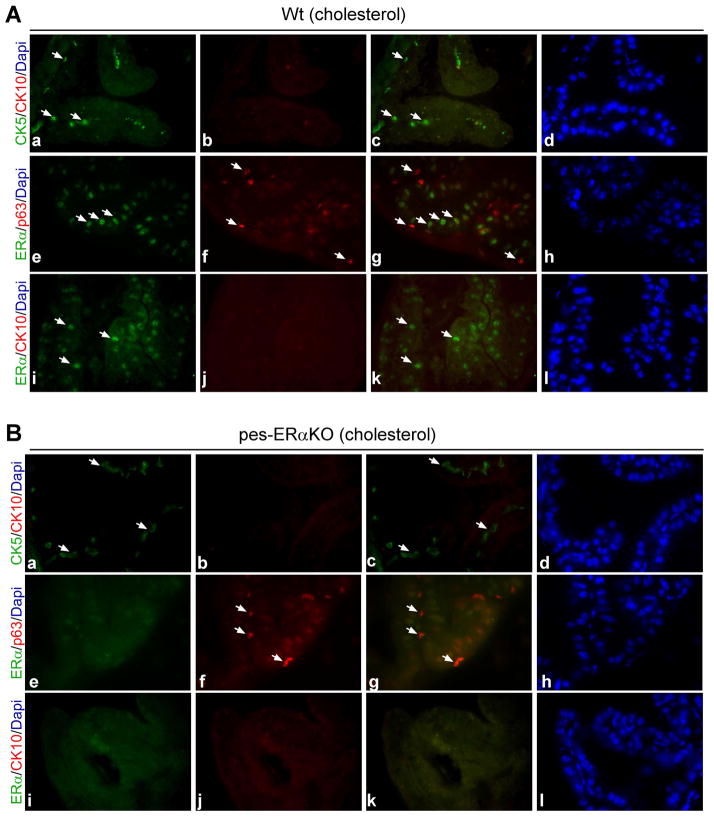
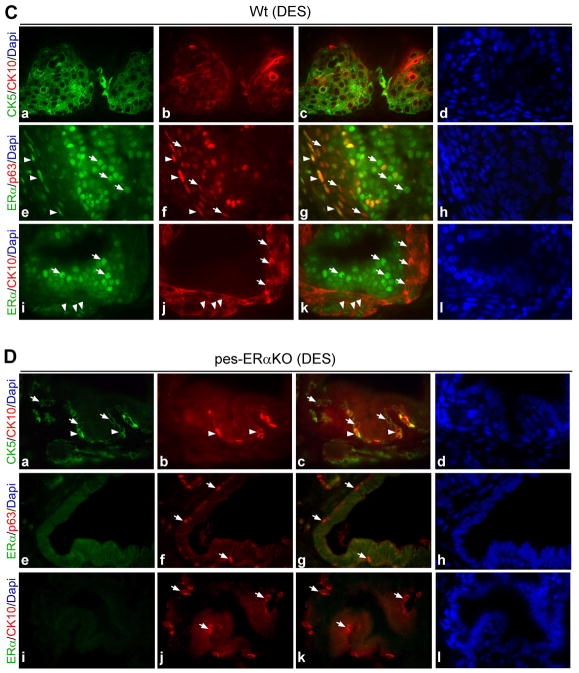
Fig. 3.
Immunofluorescence double staining of cytokeratin marker and ERα in prostates of Wt and pes-ERαKO mice. Immunofluorescence analyses were carried out using AP tissue sections from DES and vehicle treated Wt (A, C) and pes-ERαKO mice (B, D). The tissue sections were double-stained for CK5/CK10, ERα/p63, and ERα/CK10, and followed by counterstaining with DAPI to visualize the nuclei. Arrows show the cells that only expressed indicated genes. c, g, and k are merged images. d, h, and i are for DAPI staining. The others are the images for specific gene expressions. Note that in vehicle treated Wt and pes-ERαKO mice, basal cells are intermittently scattered at the basement membrane as indicated by CK5 and p63 staining (A and B, a and f), and CK10 are not expressed in those basal cells (A and B, b). ERα is expressed in the luminal cells, but not in the basal cells, of Wt prostates as ERα is not co-expressed with basal cell marker, p63 (A, e-g). There is almost no detectable epithelial ERα expression in pes-ERαKO prostates (B, e and i). In DES treated Wt prostate, CK5 positive cells are stratified and co-express squamous epithelial marker, CK10 (C, a–c). In contrast, in DES treated pes-ERαKO prostates, DES treatment resulted in a continuous, but not stratified, CK5 positive basal layer, in which only a few cells express CK10 (D, a–c). p63 staining results also indicated that basal cell expansion occurs focally in the single-layer basal epithelial cells of pes-ERαKO prostates, but it occurs in the multi-layer basal epithelial cells of Wt prostates (D–f vs C–f). ERα is up-regulated in both luminal and basal cells in DES treated Wt mice (C, e and i, arrows and arrowheads), but not in DES treated pes-ERαKO mice (D, e and i).
Estrogen exposure stimulates ERα expression in the luminal and basal epithelial cells in Wt prostates, not in pes-ERαKO mouse prostates
Previous studies have shown that the induction of SQM is accompanied by the upregulation of ERα expression in the prostate epithelial cells, which suggests that ERα might be involved in the estrogen mediated SQM [21]. Therefore, we were interested in examining how ERα changes in the pes-ERαKO prostates in response to DES. The prostate epithelial compartment contains 2 major types of cells, luminal cells and basal cells, and few intermediate cells. We found that ERα is expressed in the luminal cells, but not in the basal cells of Wt prostates as ERα is not co-expressed with basal cell marker, p63 (Fig. 3A, e and f, arrows). As expected, there is almost no ERα expression in epithelial compartment of pes-ERαKO prostates (Fig. 3B, e and i). In Wt mice, DES treatment resulted in upregulation of ERα not only in the luminal cells, but also in the basal cells as well as squamous cells, as some of the ERα positive cells also co-expressed p63 or CK10 in the basal layer (Fig. 3C, g and k, arrowhead). In contrast, epithelial ERα upregulation was not observed in DES treated pes-ERαKO prostates (Fig. 3D, e and i). We further validated the expression level and the distributed cell types of ERα in DES treated Wt vs. pes-ERαKO using IHC staining. Consistent with the immunofluorescence results in Fig. 3C and D, ERα was upregulated in Wt mice after DES treatment (Fig. 4B, a and b, c and d, arrows), but ERα was rarely expressed and not upregulated in DES treated pes-ERαKO mice (Fig. 4B, e and f, g and h, arrows). In addition, our Q-PCR results indicated that although the level of ERα mRNA are up-regulated in both DES treated Wt and pes-ERαKO mice, the ERα mRNA in DES treated pes-ERαKO mice is significantly less than that in DES treated Wt mice (Fig. 4B). Our results indicated that the inhibited SQM phenotype is due to loss of prostate epithelial ERα in DES pes-ERαKO mice, this conclusion is also evidenced by reducing the up-regulated ERα mRNA and protein expression in prostate basal cells.
Fig. 4.
The expression of cell growth markers and ERα of Wt and pes-ERaKO prostates were examined by IHC staining. IHC staining of Ki67, p27Kip1, and ERα was carried out using AP tissue sections of cholesterol (vehicle) or DES treated Wt and pes-ERαKO mice. Arrows indicate cells positive for the gene expression. Note that basal cells in DES treated Wt prostates have increased cell proliferation as indicated by Ki67 staining (c vs a; d vs b, arrow), but decreased p27Kip1 expression (k vs I; l vs j, arrowheads) in contrast to constantly positive p27Kip1 expression in the luminary epithelial cells (j and l, arrow). However, DES treated pes-ERαKO prostates rarely proliferate (g vs e; h vs f) and also constantly maintain high p27Kip1 expression in the prostate epithelial cells prior and after DES treatment (o vs m; p vs n). Up-regulation of ERα is observed in the Wt prostate epithelial cells after DES treatment (s vs q; t vs r), but not in the DES treated pes-ERαKO prostate (w vs u; x vs v). The change in the expression of ERα in cholesterol and DES treated mouse prostates was further examined by real-time Q-PCR. Note that the level of ERα mRNA are profoundly upregulated in DES treated Wt prostates, the increases in DES treated pes-ERαKO prostates are much less (✱: compared to control Wt mice; #: compared to control Wt and pes-ERKO mice, δ: compared to DES treated Wt mice).
DES treated pes-ERαKO prostates have a significantly reduced basal cell growth as compared to that of DES treated Wt prostates
DES stimulated prostate proliferation is further characterized by IHC staining of Ki67 and p27Kip1. Staining of the cell proliferation marker, Ki67, indicated that the adult prostate is a quiescent organ. The Ki67 positive proliferative cell was rarely detected in vehicle treated Wt and pes-ERαKO mice (Fig. 4A, a and b; e and f). In the Wt mice, DES stimulated the cell proliferation, This proliferation was mainly located in the basal cell compartment (Fig. 4A, c and d, arrows). In addition, in DES treated Wt mice the stratified squamous/basal cells lack expression of the cell cycle inhibitor p27Kip1 (Fig. 4A, l, arrowheads), which is still maintained and expressed in quiescent luminary epithelial cells in vehicle treated prostates (Fig. 4A, j and l, arrows). The results indicated that DES induces proliferation of basal cells, with concomitant loss of p27Kip1. In contrast to DES treated Wt mice, prostate cell proliferation is rarely observed in the DES treated pes-ERαKO mice (Fig. 4A, g and h) and p27Kip1 still remains positive in the majority of epithelial cells (Fig. 4A, m and n, o and p, arrow). This staining data indicated that the DES treated pes-ERαKO prostates remains quiescent, suggesting that epithelial ERα is important for responding the estrogen stimulated proliferative event in the mouse prostate.
Discussion
We generated mouse models that have selectively lost ERα in either prostate epithelial or stromal cells to study their respective roles in the adult prostate in response to estrogen. During characterization of male reproductive and normal prostate development in FSP-ERαKO and pes-ERαKO mice, our unpublished data indicate that the circulating levels of testosterone and fertility are not altered in male mice of either model. However, FSP-ERαKO prostates have less branching morphogenesis compared to Wt littermates; In contrast, pes-ERαKO mice maintain normal homeostasis and develop a normal prostate without changing branching morphogenesis [35], highlighting the critical role of stromal ERα in prostate development. In our present study, we found that FSP-ERαKO prostates develop full and uniform SQM as in Wt mice after DES treatment, which indicates that loss of the majority (~65%) of stromal ERα results in developmental defects with reduced prostate branch, yet, will not influence estrogen-stimulated SQM in prostate. However, full and uniform SQM observed in FSP-ERαKO prostates may also suggest that the remainder of stromal ERα retains sufficient capacity to support estrogen mediated SQM in the prostate. Further studies utilizing more efficient stromal Cre transgenic mice are needed to confirm the roles of stromal ERα in estrogen mediated SQM in vivo. In contrast to FSP-ERαKO mice, loss of epithelial ERα in the pes-ERαKO prostates maintain a normal prostate histological structure, yet, result in an inhibition of estrogen stimulated SQM, in which prostatic ducts were lined by a single layer of columnar epithelial cells and exhibited rare and focal CK10 staining (Fig. 1). Together, our cre-loxP models prove a direct in vivo evidence that epithelial ERα is dispensable for the prostate development, yet, is critical for estrogen mediated prostate proliferation and SQM in adult prostate.
Further characterization of SQM in Wt mice showed that DES treatment resulted in CK5 positive basal cells expansion, stratification, and CK10 squamous cell differentiation (Fig. 3C). However, pes-ERαKO mice fail to develop a full and uniform SQM evidenced by a continuous, but not stratified, CK5 positive basal layer, with very few CK5 positive cells co-expressing CK10 (Fig. 3D). These results indicate that loss of epithelial ERα in the prostates inhibits both basal cell proliferation and squamous cell differentiation. Even though both prostatic luminal and basal cells are know to express significant level of probasin Cre [30], the few residual CK10 positive cells observed in the DES treated pes-ERαKO could be due to incomplete knockout ERα in prostate basal cells by probasin-Cre. There are 2 major types of prostate epithelial cells, luminal type and basal type, and a few intermediate type cells. Previous reports have shown that PB-cre can knockout floxed gene in luminal epithelial cell and basal-intermediate epithelial cell [28, 29]. The other characteristic of SQM is the upregulation of ERα in both prostatic luminal and basal cells (Fig. 3C). One interesting observation is that ERα expression is not found in the basal cells of normal Wt prostates (Fig. 3A), but it appears in the basal cells of DES treated Wt prostates (Fig. 3C), which could directly contribute to the response of the basal cells to estrogens and result in the basal cell proliferation and metaplasia. In contrast, epithelial ERαKO prostates have a profoundly reduced ERα up-regulation in response to DES treatment, which could contribute to the phenotypic loss of SQM in pes-ERαKO mice (Fig. 3D and Fig. 4). The underlying mechanisms by which ERα promotes the basal cell proliferation and squamous cell differentiation need to be further investigated.
The find that DES treated pes-ERαKO mice lacking SQM is also supported by SQM biomarker CK10 staining. The adult rodent prostate is a growth quiescent organ. One of the SQM characteristics is estrogen induced basal proliferation. In the Wt males, luminal epithelial cells were positive for the cell cycle inhibitor p27 and were growth quiescent. After DES treatment, a continuous layer of proliferating basal cells emerged and were positive for Ki67, whereas p27 expression was not detected in the multilayered basal epithelial cells (Fig. 4). In contrast, increase of Ki67 positive basal cells and loss of p27 were not observed in the basal cells of DES treated pes-ERαKO mice (Fig. 4). The results indicated that the mitogenic effect of estrogen in the adult mouse prostate is mediated by epithelial ERα. Although the increased proliferation activity is observed in the SQM, the SQM is also characterized by downregulation of AR expression in DES treated Wt mice (suppl. Fig. 3, c vs a; d vs b). In the DES treated pes-ERαKO, the expression of AR was also significantly inhibited compared to untreated pes-ERαKO (suppl. Fig. 3, g vs e; h vs f), which indicated that AR downregulation might not directly contribute to estrogen mediated SQM and proliferative activities in the mouse prostates.
Previous studies have also shown that the effects of DES in the rodent prostate lobes revealed a hierarchy of responses, with AP being the most responsive, the DLP less responsive, and VP lobe the least responsive [20]. Indeed, we observed that pes-ERαKO prostates failed to develop SQM in all lobes compared to Wt mice, of which are evident by complete loss of CK10 in pes-ERαKO VP and DLP after DES treatment (data not shown). Together, our current studies indicate that epithelial ERα plays critical roles in the estrogen mediated SQM and proliferative activities in the prostate. An earlier study utilizing tissue recombination techniques with mesenchyme of newborn mouse seminal vesicle (SV) and adult prostatic ductal tips from Wt and ERαKO mice stated that both epithelial and stromal ERα are necessary for estrogen mediated SQM in the prostate. In an attempt to validating the roles of the stromal ERα in SQM using in vivo FSP-ERαKO model, we observed that the majority loss of stromal ERα will not influence estrogen mediated SQM in prostate, but our results might be compounded by the incomplete ablation of stromal ERα in the mouse prostate. As mentioned above, a more efficient stromal Cre transgenic mouse model is needed to confirm the role of stromal ERα in SQM. The advantage of our in vivo assay is that we utilize the intact mice as an experimental tool, in which a functional immune system exists. During prostatic regrowth of castrated adult mice, data showed that estrogen exposure results in cyclin D1-positive cells in the epithelium and the recruitment of FGF10-positive immune cells in prostates, suggesting the immune system is an important factor contributing to the prostatic regrowth and homeostasis [13].
We summarize the SQM model in current studies (Fig. 5). In this model, estrogens via ERα exert direct actions on the prostate by up-regulating ERα signaling, enhancing the basal cell proliferation, and promoting the expansion of abnormal squamous cells. In contrast, loss of ERα in the prostate epithelial cells results in the failure of estrogen response in the mouse prostate. As a consequence, DES treated pes-ERαKO prostates can not develop a full and uniform SQM. These in vivo results suggest that epithelial ERα is important in the mediation of the proliferative response to estrogen. It has been reported that the expression of ERα was upregulated during prostatic pathogenesis, including BPH and prostate cancer [36–38]. The higher expression of ERα suggests the involvement of estrogen signaling in the prostatic pathogeneses. Studies also showed that during prostatic regrowth, there is a window in time when ERα is upregulated in the prostatic epithelium between day 3 and 5 after testosterone replacement and selective stimulation of epithelial ERα can induce ductal branching, which suggests that epithelial ERα is essential for ductal branching during postnatal prostate growth and homeostasis [13]. Since ERα signaling is involved in prostatic pathogeneses, how to target ERα has a direct implication for understanding prostatic disease and the developmental of pharmaceuticals for the treatment of BPH and prostate cancer. Our studies suggest that epithelial ERα in the prostate could be the appropriate target for preventing aberrant estrogen signaling during prostate pathogenesis.
Fig. 5.
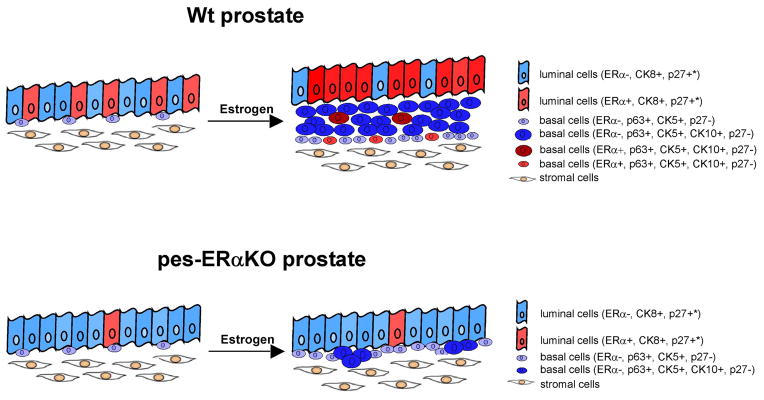
The estrogen stimulated prostate proliferation and SQM compared in Wt and PES-ERαKO mice. Our current studies indicate that loss of ERα in the prostate epithelial cells results in the failure of estrogen response in the mouse prostate as demonstrated by limited stratifying basal cells, squamous metaplasia, and upregulating ERα expression. As a consequence, DES treated pes-ERαKO prostates have a continuous, but not stratified, CK5 positive basal layer, in which only a few cells express CK10, indicating a limited and scattered SQM development. The distribution of different type of prostate cells with different expression of ERα, CK10, CK5, Ki67, p27 are depicted in figure. (*: majority of luminal epithelial cells of adult prostate are p27 positive).
Acknowledgments
We thank Dr. Chawnshang Chang for the helpful suggestions; Drs. Moses HL and Bhowmick NA for providing FSP 1-cre transgenic mice; Karen Wolf for the assistance in manuscript preparation. This work was supported by NCI grant CA137474.
Footnotes
The authors confirm that there are no conflicts of interest.
Author contribution
Shuyuan Yeh, Ming Chen and Hong-Chiang Chang conceived the study design and data interpretation. Ming Chen and Chiuan-Ren Yeh, and Spencer Slavin conceived and carried out experiments. All authors were involved in data discussion, paper preparation, and had approval of the final version of manuscript.
References
- 1.Cunha GR, Donjacour AA, Cooke PS, et al. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- 2.Mawhinney MG, Neubauer BL. Actions of estrogen in the male. Invest Urol. 1979;16:409–420. [PubMed] [Google Scholar]
- 3.Jarred RA, Cancilla B, Prins GS, et al. Evidence that estrogens directly alter androgen-regulated prostate development. Endocrinology. 2000;141:3471–3477. doi: 10.1210/endo.141.9.7648. [DOI] [PubMed] [Google Scholar]
- 4.Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130:3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- 5.Rajfer J, Coffey DS. Sex steroid imprinting of the immature prostate. Long-term effects Invest Urol. 1978;16:186–190. [PubMed] [Google Scholar]
- 6.Taylor RA, Cowin P, Couse JF, et al. 17beta-estradiol induces apoptosis in the developing rodent prostate independently of ERalpha or ERbeta. Endocrinology. 2006;147:191–200. doi: 10.1210/en.2005-0683. [DOI] [PubMed] [Google Scholar]
- 7.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y, Liu G, Zhang Y, et al. Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development. 2007;134:723–734. doi: 10.1242/dev.02765. [DOI] [PubMed] [Google Scholar]
- 9.Lazennec G, Bresson D, Lucas A, et al. ER beta inhibits proliferation and invasion of breast cancer cells. Endocrinology. 2001;142:4120–4130. doi: 10.1210/endo.142.9.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paech K, Webb P, Kuiper GG, et al. Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- 11.Chen M, Hsu I, Wolfe A, et al. Defects of Prostate Development and Reproductive System in the ER{alpha} Null Male Mice. Endocrinology. 2009;150:251–259. doi: 10.1210/en.2008-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omoto Y, Imamov O, Warner M, et al. Estrogen receptor alpha and imprinting of the neonatal mouse ventral prostate by estrogen. Proc Natl Acad Sci U S A. 2005;102:1484–1489. doi: 10.1073/pnas.0409168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omoto Y. Estrogen receptor-alpha signaling in growth of the ventral prostate: comparison of neonatal growth and postcastration regrowth. Endocrinology. 2008;149:4421–4427. doi: 10.1210/en.2007-1413. [DOI] [PubMed] [Google Scholar]
- 14.Dupont S, Krust A, Gansmuller A, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 15.Weihua Z, Makela S, Andersson LC, et al. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc Natl Acad Sci U S A. 2001;98:6330–6335. doi: 10.1073/pnas.111150898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krege JH, Hodgin JB, Couse JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antal MC, Krust A, Chambon P, et al. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc Natl Acad Sci U S A. 2008;105:2433–2438. doi: 10.1073/pnas.0712029105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–1809. doi: 10.1210/endo.138.5.5106. [DOI] [PubMed] [Google Scholar]
- 19.Prins GS, Birch L, Couse JF, et al. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- 20.Risbridger GP, Wang H, Frydenberg M, et al. The metaplastic effects of estrogen on mouse prostate epithelium: proliferation of cells with basal cell phenotype. Endocrinology. 2001;142:2443–2450. doi: 10.1210/endo.142.6.8171. [DOI] [PubMed] [Google Scholar]
- 21.Risbridger G, Wang H, Young P, et al. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev Biol. 2001;229:432–442. doi: 10.1006/dbio.2000.9994. [DOI] [PubMed] [Google Scholar]
- 22.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–434. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 23.Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 24.Chen M, Wolfe A, Wang X, et al. Generation and characterization of a complete null estrogen receptor alpha mouse using Cre/LoxP technology. Mol Cell Biochem. 2009;321:145–153. doi: 10.1007/s11010-008-9928-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X, Wu J, Huang J, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 26.Chen M, Ni J, Chang HC, Lin CY, et al. CCDC62/ERAP75 functions as a coactivator to enhance estrogen receptor beta-mediated transactivation and target gene expression in prostate cancer cells. Carcinogenesis. 2009;30:841–50. doi: 10.1093/carcin/bgn288. [DOI] [PubMed] [Google Scholar]
- 27.Wu CT, Altuwaijri S, Ricke WA, et al. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci U S A. 2007;104:12679–1268428. doi: 10.1073/pnas.0704940104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Gao J, Lei Q, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 29.Niu Y, Altuwaijri S, Lai KP, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci U S A. 2008;34:12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Garcia AJ, Wu M, et al. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci U S A. 2006;103:1480–1485. doi: 10.1073/pnas.0510652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwano M, Fischer A, Okada H, et al. Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Mol Ther. 2001;3:149–159. doi: 10.1006/mthe.2000.0251. [DOI] [PubMed] [Google Scholar]
- 32.Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strutz F, Okada H, Lo CW, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trimboli AJ, Cantemir-Stone CZ, Li F, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen M, Yeh CR, Yeh S. Reduced Branching Morphogenesis in Mice Lacking Stromal Estrogen Receptor alpha but not in Mice Lacking Epithelial Estrogen Receptor alpha. 2011. submission. [Google Scholar]
- 36.Bonkhoff H, Fixemer T, Hunsicker I, et al. Estrogen receptor expression in prostate cancer and premalignant prostatic lesions. Am J Pathol. 1999;155:641–647. doi: 10.1016/S0002-9440(10)65160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Royuela M, de Miguel MP, Bethencourt FR, et al. Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. J Endocrinol. 2001;168:447–454. doi: 10.1677/joe.0.1680447. [DOI] [PubMed] [Google Scholar]
- 38.King KJ, Nicholson HD, Assinder SJ. Effect of increasing ratio of estrogen: androgen on proliferation of normal human prostate stromal and epithelial cells, and the malignant cell line LNCaP. Prostate. 2006;66:105–114. doi: 10.1002/pros.20327. [DOI] [PubMed] [Google Scholar]