Abstract
Purpose of review
Apolipoprotein (apo) A-V functions to modulate intracellular and extracellular triacylglycerol metabolism. The present review addresses molecular mechanisms underlying these effects. The relevance of apoA-V to human disease conditions is illustrated by the strong correlation between single nucleotide polymorphisms in APOA5, elevated plasma triacylglycerol and dyslipidemic disease.
Recent findings
Despite undergoing processing for secretion from hepatocytes, a portion of apoA-V escapes this destiny and accumulates as a component of cytosolic lipid droplets. Expression of recombinant apoA-V in hepatocarcinoma cells results in increased lipid droplet size and number at the expense of triacylglycerol secretion.
ApoA-V modulates atherosclerosis in hypercholesterolemic apoE null mice. ApoE null/human apoA-V transgenic mice had reduced levels of triacylglycerol and cholesterol in plasma along with decreased aortic lesion size.
Summary
ApoA-V modulates triacylglycerol metabolic fate. Following its synthesis, apoA-V enters the endoplasmic reticulum and associates with membrane defects created by triacylglycerol accumulation. Association of apoA-V with endoplasmic reticulum membrane defects promotes nascent lipid droplets budding toward the cytosol. Despite its low concentration in plasma (~150 ng/ml), apoA-V modulates lipoprotein metabolism by binding to glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1. This interaction effectively localizes triacylglycerol-rich lipoproteins in the vicinity of glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1’s other ligand, lipoprotein lipase.
Keywords: apolipoprotein A5, lipid droplet, triacylglycerol, very low-density lipoprotein
INTRODUCTION
The discovery of apolipoprotein (apo) A-V has proven to be one of the most significant advances in lipoprotein research of this millennium. The impact of apoA-V on plasma triacylglycerol levels is vividly illustrated by the seminal report of Pennacchio et al. [1]. Using genetically engineered mice, these authors showed that plasma triacylglycerol concentrations in APOA5 transgenic mice are three-fold lower than control littermates. At the same time, apoa5 (−/−) mice display a four-fold increase in plasma triacylglycerol.
Enhancing the clinical relevance of apoA-V, human population studies have identified single nucleotide polymorphisms (SNPs) in APOA5 that correlate with hypertriglyceridemia (HTG) [2]. To date 147 SNPs have been identified [http://www.ncbi.nlm.nih.gov/SNP/ (search term=Human APOA5)]. Whereas most APOA5 SNPs have not been annotated with respect to functional significance, others are known to impact plasma triacylglycerol concentration. For example, the −1131T>C SNP, located in the promoter region of APOA5 (Fig. 1a), correlates with elevated plasma triacylglycerol levels in different population groups [2]. The coding SNP, c.56C>G (S19W), located in the signal peptide of apoA-V, is also associated with high triacylglycerol levels [1,3–6]. In a recent study, Soufi et al. [7] reported that the c.56C>G variant occurs at a frequency of 14.4% in patients with coronary artery disease. Another SNP (c.553G>T) that substitutes cysteine for glycine at position 162 of mature apoA-V is associated with HTG in Asian populations [8,9]. A recent study by Huang et al. [10] suggested that glycine at position 162 is important for lipoprotein lipase (LPL) mediated VLDL hydrolysis. Association studies have led to grouping of five common APOA5 SNPs into three distinct haplotypes (Fig. 1). The most common haplotype, APOA5*1, is defined by all wild type alleles. APOA5*2 carries four rare alleles (−1131T>C, c.−3A>G, IVS3 + 476G>A, c.1259T>C, whereas the c.56C>G polymorphism defines haplotype APOA5*3. The observation that 25–50% of individuals of White, Hispanic and African–American descent carry at least one copy of APOA5*2 or APOA5*3 [3] indicates the prevalence of these haplotypes. Whereas the APOA5*2 haplotype is strongly associated with apoC3 polymorphisms (i.e. 3238G>C, −482C>T and −455T>C), the APOA5*3 haplotype showed no association with any APOC3 allele [11]. Thus, association of APOA5*2 with triacylglycerol risk may be partly related to its presence within the APOA1/C3/A4/A5 gene cluster.
FIGURE 1.
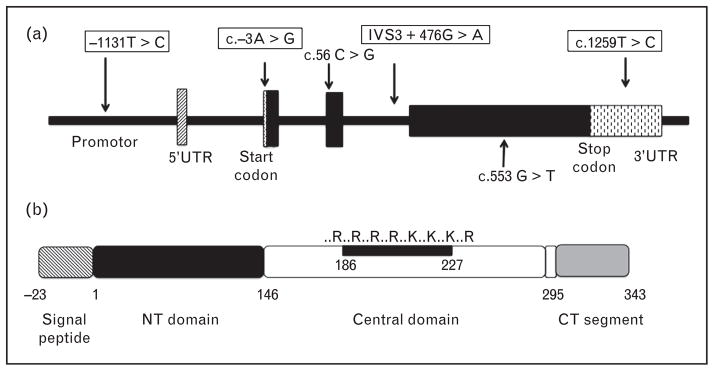
APOA5 gene and protein organization. (a) The APOA5 gene locus is depicted, including intron/exon junctions and the location of prevalent SNPs. Boxed SNPs correspond to haplotype *2. Haplotype *3 is defined by the c.56C>G SNP. The coding SNP, c.553G>T, is associated with elevated plasma triacylglycerol in Asian populations. (b) Domain organization of apoA-V protein. The N-terminal helix bundle domain, the Central domain and the C-terminal segment are shown. Also depicted is the positively charged sequence element in the Central domain. PPPP refers to the tetra proline motif encompassing residues 293–296.
Insofar as human population studies continue to reveal polymorphisms in APOA5, it is critical to determine if a given variant actually displays functional properties that may be related to a given phenotype (e.g. elevated plasma triacylglycerol). Whereas data management efforts can assess the relative abundance of a given SNP and denote the affected region of the gene/protein, only correlations can be drawn from these data. In the case of coding variants, computational tools can be used to classify potentially deleterious or neutral polymorphisms, providing a means to rank different variants in terms of probability for functional relevance. Confirmation that a given SNP is relevant to a specific condition, however, requires examination of the protein in question. In the case of apoA-V, a high throughput triacylglycerol modulation assay using hypertriglyceridemic apoa5 (−/−) mice [12] can provide a rapid in-vivo functional assessment of recombinant apoA-V variants as a prelude to more detailed approaches, such as adeno-associated virus gene transfer studies or transgenic mouse engineering.
APOA-V, TRIACYLGLYCEROL AND ATHEROSCLEROSIS
The role of triacylglycerol in atherosclerosis is still controversial [13▪]. The fact that atherosclerotic plaques possess primarily cholesterol and not triacylglycerol is consistent with the premise that triacylglycerol has no direct role in plaque formation. It is likely, however, that triacylglycerol has an indirect role in disease progression through its association with other genetically regulated components, as suggested by genome-wide association studies [2]. These studies indicate apoA-V is strongly associated with plasma triacylglycerol levels, further implying that APOA5 polymorphisms correlated with elevated plasma triacylglycerol have a role in the atherosclerotic process, although any role in plaque formation is likely to be indirect.
An athero-protective role for apoA-V was suggested by studies in which combined dyslipidemic apoE2 knock-in mice (i.e. increased triacylglycerol and cholesterol) were crossed with human apoA-V transgenic mice [14]. Increased apoA-V expression in mice fed a Western diet significantly lowered triacylglycerol along with cholesteryl ester-rich remnant particles, LDL and VLDL. Concomitantly, compared with apoE2 knock-in mice, a two-fold reduction in atherosclerotic lesion area in the aorta was noted. It is plausible that reductions in cholesterol containing lipoproteins that result from apoA-V-dependent remnant clearance [15] are responsible for the observed reductions in lesion size.
A recent study evaluated the ability of apoA-V to reduce atherosclerotic lesions in apoE (−/−) mice [16▪▪]. This mouse model is characterized by rampant accumulation of proatherogenic cholesterol-rich remnants and atherosclerotic lesions. ApoE deficient mice were bred to mice overexpressing human apoA-V. Overexpression of apoA-V in apoE (−/−) mice led to a significant decrease in VLDL and remnant lipoproteins, together with a 70% reduction in aortic lesion area. These authors also showed that overexpression of apoA-V results in decreased triacylglycerol secretion and enhanced removal of triacylglycerol-rich lipoproteins from plasma. In addition, proatherogenic cytokines, including TNFα, IL-1β and IL-6, were significantly reduced. The results suggest apoA-V exerts an indirect atheroprotective effect by modulating triacylglycerol secretion, lipolysis of triacylglycerol-rich lipoproteins and removal of remnant particles. Although it has yet to be determined if apoA-V (−/−) mice themselves are prone to atherosclerosis, future studies should provide insight with respect to the role of apoA-V in atherogenesis.
HOW APOA-V STRUCTURE RELATES TO ITS FUNCTION
Characterization studies indicate apoA-V has unique structural properties [2,17]. Sequence analysis predicts a hydrophobic protein with significant amounts of α-helical secondary structure [18]. This prediction was confirmed by far ultraviolet (UV) circular dichroism spectroscopy analysis that also provided evidence for a multidomain structure (Fig. 1b) [19]. In a manner similar to other multidomain apolipoproteins (e.g. apoE), the N-terminal 146 amino acids of apoA-V adopt an amphipathic α-helix bundle conformation [20]. Insofar as truncation mutants (e.g. Q139X) in humans correlate with severe HTG [21], it appears that residues beyond the aminoterminal (NT)domain are required for its triacylglycerol modulation activity [22].
Adjacent to the NT domain is a 144 residue Central domain that harbors a sequence motif that is enriched in positively charged amino acids. The Central domain of apoA-V corresponds roughly to residues beyond the N-terminal helix bundle up to the tetraproline motif (i.e. position 292). This region contains three predicted amphipathic α-helices with high surface activity potential. Furthermore, analysis of amino acid sequence variability across species revealed that this region of the protein is the most conserved among mammalian species [18]. A key feature of the Central domain is the sequence between residues 186 and 227. This 42 amino acid segment contains eight positively charged amino acids and zero negatively charged amino acids. Given the fact that positively charged sequence elements in apolipoproteins are often involved in binding to cell surface receptors and/or heparan sulfate proteoglycans (HSPG), binding studies have been conducted with apoA-V. Using heparin as a surrogate for HSPG, ionic strength dependent binding was observed [15]. It is likely that this segment of apoA-V exists as an independently folded structural domain that harbors residues that are critical for binding interactions known to affect plasma lipoprotein metabolism.
Four consecutive prolines near the C-terminus of apoA-V (residues 293–296) demarcate the junction between a loosely folded C-terminal segment and the Central domain. A peptide corresponding to this sequence (residues 296–343) was studied by nuclear magnetic resonance spectroscopy [23], revealing an extended amphipathic α-helix, consistent with a role in lipoprotein binding as a component of full-length apoA-V. These data support the concept that the C-terminal segment is not an integral part of the N-terminal or Central domain but, rather, may function independently to modulate the lipid-binding properties of apoA-V.
APOA-V AND HEPATIC LIPID DROPLETS
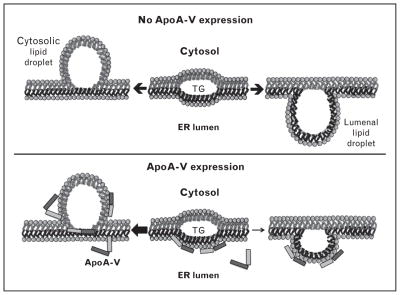
Recent evidence indicates apoA-V does not elicit its biological effects exclusively in the plasma compartment. Seemingly disparate findings have led to the concept that apoA-V plays an active role in intrahepatic triacylglycerol homeostasis. van der Vliet et al. [24] reported a 3.5-fold upregulation of rat apoA-V mRNA in response to partial hepatectomy, a condition that is associated with increased synthesis, and decreased export, of triacylglycerol [25]. In transiently transfected hepatoma cells apoA-V secretion efficiency was low compared with apoA-I, apoB and apoE [26]. Remarkably, despite the presence of a signal peptide, considerable apoA-V was recovered in the cytosol, associated with lipid droplets [26–28]. Pursuing this observation, the presence of apoA-V on lipid droplets was verified in livers of wild type mice and, moreover, livers from APOA5 transgenic mice had increased cellular triacylglycerol compared with wild type mice [29]. Ress et al. [30] reported that knockdown of apoA-V mRNA in HepG2 cells led to a decrease in cellular triacylglycerol content. Consistent with this, Blade et al. [31▪] reported that McA7777 cells stably transfected with apoA-V secrete lipoproteins that are smaller than those from control cells. By the same token, Gao et al. [32▪] showed that McA7777 cells stably expressing apoA-V had greater numbers and larger lipid droplets than control cells. Thus, it may be concluded that apoA-V expression promotes lipid droplet accumulation in these cells. A model depicting how apoA-V modulates the fate of hepatic triacylglycerol is shown in Figure 2. Intrahepatic lipid droplet biogenesis occurs between leaflets of the ER bilayer, initiated by formation of a lens of triacylglycerol. As this triacylglycerol lens increases in size, it can bud either toward the cytosol or the ER lumen. Formation of a lumenal lipid droplet promotes VLDL assembly and triacylglycerol secretion from the cell, whereas cytosolic lipid droplets promote lipid storage. ApoA-V appears to influence the directionality of the triacylglycerol budding process, promoting cytosolic budding as opposed to lumenal intrusion. The model predicts that apoA-V binds to ER membrane defects created by the accumulation of triacylglycerol between leaflets of the bilayer. By stabilizing the lumenal leaflet in the region of this triacylglycerol lens, cytosolic budding is promoted, resulting in cytosolic lipid droplet formation.
FIGURE 2.
Model depicting the effect of apoA-V on the fate of hepatic triacylglycerol. In the absence of apoA-V (Upper panel) triacylglycerol accrual creates a lens between leaflets of the ER membrane. Expansion of this lens by continued accrual of triacylglycerol precedes nascent lipid droplet budding from the cytoplasmic leaflet (left) or, alternatively, from the lumenal leaflet (right) to create a lumenal lipid droplet for use in VLDL maturation. (Lower panel) When present, apoA-V binding to membrane defects created by triacylglycerol accumulation stabilizes the lumenal leaflet, promoting nascent lipid droplet budding toward the cytosol at the expense of lumenal lipid droplet formation (see arrows). Reproduced with permission from [32▪].
APOA-V MODULATION OF PLASMA TRIACYLGLYCEROL LEVELS: INTERACTION WITH GLYCOSYLPHOSPHATIDYLINOSITOL-ANCHORED HIGH-DENSITY LIPOPROTEIN BINDING PROTEIN 1
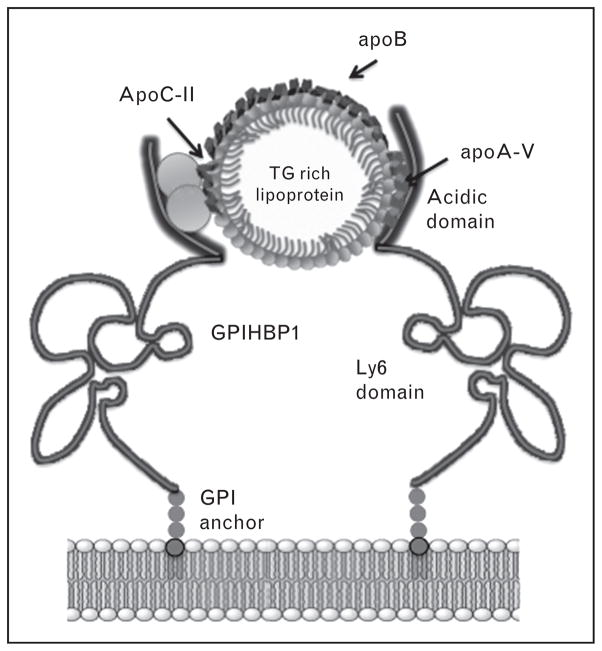
In 2003, a breakthrough in our understanding of triacylglycerol metabolism emerged with discovery of GPIHBP1 [33]. Subsequently, Beigneux et al. [34] showed that gpihbp1 (−/−) mice display severe chylomicronemia. GPIHBP1 contains a N-terminal acidic domain, a cysteine rich lymphocyte antigen 6 (Ly6) domain, and a C-terminal GPI anchor site (Fig. 3). Using GPIHBP1-expressing Chinese hamster ovary (CHO) cells, Gin et al. [35] and Beigneux et al. [36] showed that both the acidic and Ly6 domains are important for GPIHBP1 binding to LPL. Extending this work, Beignuex et al. [37], Franssen et al. [38] and Olivecrona et al. [39] identified point mutations in GPIHBP1 that are associated with defective LPL binding and chylomicronemia while Rios et al. [40] reported on a subject homozygous for deletion of GPIHBP1. In the latter three cases, heparin administration failed to release LPL, consistent with an emerging model wherein GPIHBP1 binds LPL in the capillary subendothelial space and translocates the enzyme to the lumenal surface of endotheial cells [41▪]. On the basis of GPIHBP1’s residence on the lumenal face of capillary endothelial cells, together with its ability to bind LPL, it has been proposed to function as a ‘platform’ for lipolysis of triacylglycerol-rich lipoproteins [42]. Evidence indicates that the highly negatively charged N-terminal sequence (21 of 26 consecutive residues in human GPIHBP1 are aspartate or glutamate) is important for binding LPL and chylomicrons [35]. Indeed, antiserum against GPIHBP1’s acidic domain interferes with LPL and chylomicron binding, a mutant GPIHBP1 lacking the acidic domain does not bind chylomicrons and chylomicron binding to GPIHBP1 is blocked by preincubation with polyaspartate or polyglutamate. Clearly, electrostatic interactions are important for ligand binding to GPIHBP1.
FIGURE 3.
Model of apoA-V interaction with GPIHBP1. Lipoprotein-associated apoA-V binds to the acidic domain of GPIHBP1 molecules tethered to the lumenal surface of capillary endothelial cells via a GPI membrane anchor. Catabolism of triacylglycerol-rich lipoproteins is promoted by clustering of GPIHBP1 molecules that are associated with LPL. Proximity between triacylglycerol-rich lipoprotein substrates and LPL leads to apoC-II activation of LPL and accelerated triacylglycerol hydrolysis. Lipoprotein size not to scale.
In studies of the nature of the ligand responsible for chylomicron binding to GPIHBP1, Gin et al. [43▪] reported that GPIHBP1-expressing CHO cells bind apo-A-V reconstituted HDL. Both the positively charged sequence of apoA-V’s Central domain and the acidic domain in GPIHBP1 are required for binding [35]. This interaction has been postulated to facilitate LPL-mediated hydrolysis of triacylglycerol-rich lipoproteins and provides a plausible explanation for the HTG phenotype associated with apoA-V deficiency. Insofar as apoA-V does not directly activate LPL [17,20], its effects on plasma triacylglycerol are manifest indirectly, via interaction with GPIHBP1. Thus, binding of apoA-V containing triacylglycerol-rich lipoproteins to clusters of GPIHBP1 concentrated in lipid rafts on the surface of endothelial cells [44] facilitates LPL-mediated, apoC-II-dependent, triacylglycerol hydrolysis such that coordinate interactions between LPL, GPIHBP1 and apoA-V promote triacylglycerol-rich lipoprotein processing (Fig. 3). Indeed, when any one of these proteins is missing or defective, HTG ensues.
In keeping with this model, Shu et al. [12] reported that intravenous injection of wild type (WT) apoA-V induces significant triacylglycerol lowering (~60%) in apoa5 (−/−) mice. Whereas apoA-V was rapidly cleared from circulation following injection into apoa5 (−/−) mice, this was not the case after injection into gpihbp1 (−/−) mice, wherein triacylglycerol lowering also failed to occur. These data suggest turnover of apoA-V requires a functional GPIHBP1 – LPL – apoA-V axis. Of interest is the finding that a mutant apoA-V, in which positively charged amino acids in the Central domain were converted to negatively charged or uncharged amino acids, showed a marked attenuation in in-vivo triacylglycerol-lowering activity. This mutation also resulted in loss of GPIHBP1 binding [35], validating the concept that coordination of GPIHBP1, LPL and apoA-V are a prerequisite for maintaining normal plasma triacylglycerol levels.
CONCLUSION
ApoA-V has turned out to be an enigmatic, yet highly potent, modulator of triacylglycerol metabolism. A number of conundrums emerging from research on this protein have yet to be explained. Among these is the exceptionally low concentration of apoA-V in plasma (~150 ng/ml) [45]. In human plasma this is at least 100-fold lower than apoB levels, indicating apoA-V can only be present on a small fraction of circulating triacylglycerol-rich lipoprotein particles. Whereas it has been shown that apoA-V is capable of migrating between lipoprotein particles [46], it is difficult to reconcile its potent triacylglycerol lowering capability with such a low protein concentration. A second conundrum relates to the mechanism whereby apoA-V escapes secretion from hepatocytes and becomes associated with cytosolic lipid droplets. There are few examples of proteins directed to the ER for secretion that are able to elude this fate. How apoA-V achieves this, and redirects triacylglycerol away from secretion in the process, is an important cell biology question with significant relevance of whole body triacylglycerol homeostasis. A third conundrum emerging from studies of apoA-V is an apparent contradiction between results from transgenic and knockout mouse models and human population studies. Whereas genetically engineered mice display an inverse correlation between apoA-V plasma concentration and triacylglycerol levels [1], human population studies show a positive correlation between apoA-V and triacylglycerol, such that individuals with HTG tend to have higher apoA-V levels than normolipidemic patients. For example, Henneman et al. [47] found that apoA-V levels were markedly elevated in patients with severe HTG. Likewise, Talmud et al. [48] reported that apoA-V levels in plasma samples from the Northwick Park Heart Study II correlated positively with triacylglycerol concentration. In another population with type 2 diabetes, Vaessen et al. [49] reported a positive correlation between apoA-V and plasma triacylglycerol and this was the case in controls as well. Nelbach et al. [46] determined apoA-V and triacylglycerol levels in plasma of normolipidemic APOA5 transgenic mice and showed that the plasma concentration of apoA-V positively correlated with triacylglycerol levels. Thus, human APOA5 transgenic mice follow the same trend reported for apoA-V in humans. At present, no satisfactory explanation exists to explain the apparent contradictions between apoA-V concentration and plasma triacylglycerol levels in different settings. In considering how these findings can be reconciled, understanding the effects of insulin on plasma glucose levels may provide a clue. Whereas an absence of insulin results in elevated glucose and its infusion induces a lowering of plasma glucose levels, in the setting of insulin resistance, plasma glucose levels remain elevated despite increased plasma insulin levels. Thus, in this physiological setting a positive correlation between insulin and glucose exists. Extending this analogy to apoA-V and triacylglycerol, a complete lack of apoA-V results in elevated triacylglycerol, whereas acute administration of apoA-V causes a decline in plasma triacylglycerol levels [12]. In a chronic setting or a disease situation, it is conceivable that complex interactions may result in a positive correlation between apoA-V and triacylglycerol levels. Undoubtedly, future studies of this unique and perplexing protein will reveal new insights with profound relevance to dyslipidemic disease processes.
KEY POINTS.
Prevalent SNPs in APOA5 correlate with elevated plasma triacylglycerol levels.
ApoA-V in the endoplasmic reticulum (ER) regulates intracellular triacylglycerol trafficking.
In plasma, lipoprotein-associated apoA-V serves as a ligand for glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 (GPIHBP1).
Acknowledgments
The authors thank Jennifer A. Beckstead and Lisa Nelbach for contributing to research on apoA-V. Work from the authors’ laboratory was supported by an American Heart Association Postdoctoral Fellowship Award (12POST12030008) and the NIH (HL64159). Funding: American Heart Association Postdoctoral Fellowship Award (12POST12030008) and NIH (HL64159).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 180).
- 1.Pennacchio LA, Olivier M, Hubacek JA, et al. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294:169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 2.Sharma V, Ryan RO, Forte TM. Apolipoprotein A-V dependent modulation of plasma triacylglycerol: a puzzlement. Biochim Biophys Acta. 2012;1821:795–799. doi: 10.1016/j.bbalip.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennacchio LA, Olivier M, Hubacek JA, et al. Two independent apolipoprotein A5 haplotypes influence human plasma triglyceride levels. Hum Mol Genet. 2002;11:3031–3038. doi: 10.1093/hmg/11.24.3031. [DOI] [PubMed] [Google Scholar]
- 4.Hubacek JA, Bohuslavova R, Skodova Z, et al. Polymorphisms in the APOA1/C3/A4/A5 gene cluster and cholesterol responsiveness to dietary change. Clin Chem Lab Med. 2007;45:316–320. doi: 10.1515/CCLM.2007.056. [DOI] [PubMed] [Google Scholar]
- 5.Chandak GR, Ward KJ, Yajnik CS, et al. Triglyceride associated polymorphisms of the APOA5 gene have very different allele frequencies in Pune, India compared to Europeans. BMC Med Genet. 2006;10:7–76. doi: 10.1186/1471-2350-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai CQ, Demissie S, Cupples LA, et al. Influence of the APOA5 locus on plasma triglyceride, lipoprotein subclasses, and CVD risk in the Framingham Heart Study. J Lipid Res. 2004;11:2096–2105. doi: 10.1194/jlr.M400192-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Soufi M, Sattler AM, Kurt B, Schaefer JR. Mutation screening of the APOA5 gene in subjects with coronary artery disease. J Investig Med. 2012;7:1015–1019. doi: 10.2310/JIM.0b013e3182686918. [DOI] [PubMed] [Google Scholar]
- 8.Kao JT, Wen HC, Chien KL, et al. A novel genetic variant in the apolipoprotein A5 gene is associated with hypertriglyceridemia. Hum Mol Genet. 2003;12:2533–2539. doi: 10.1093/hmg/ddg255. [DOI] [PubMed] [Google Scholar]
- 9.Pullinger CR, Aouizerat BE, Movsesyan I, et al. An apolipoprotein A-V gene SNP is associated with marked hypertriglyceridemia among Asian-American patients. J Lipid Res. 2008;49:1846–1854. doi: 10.1194/jlr.P800011-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YJ, Lin YL, Chiang CI, et al. Functional importance of apolipoprotein gene cluster on human chromosome 11. Clin Chim Acta. 2012;1–2:246–250. doi: 10.1016/j.cca.2011.09.045. [DOI] [PubMed] [Google Scholar]
- 11.Olivier M, Wang X, Cole R, et al. Haplotype analysis of the apolipoprotein gene cluster on human chromosome 11. Genomics. 2004;83:912–923. doi: 10.1016/j.ygeno.2003.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shu X, Nelbach L, Weinstein MW, et al. Intravenous injection of apolipoprotein A-V reconstituted High-density lipoprotein decreases hypertriglyceridemia in apoav−/− mice and requires glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein. Arterioscler Thromb Vasc Biol. 2010;30:2504–2509. doi: 10.1161/ATVBAHA.110.210815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪.Goldberg IJ, Eckel RH, McPherson R. Triglycerides and heart disease: still a hypothesis? Arterioscler Thromb Vasc Biol. 2011;31:1716–1725. doi: 10.1161/ATVBAHA.111.226100. The authors present a balanced review of basic and clinical research addressing the role of triacylglycerol in cardiovascular disease. Questions exist whether triacylglycerol themselves, or the lipoproteins they are associated with, are directly atherogenic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansouri RM, Baugé E, Gervois P, et al. Atheroprotective effect of human apolipoprotein A5 in a mouse model of mixed dyslipidemia. Circ Res. 2008;103:450–453. doi: 10.1161/CIRCRESAHA.108.179861. [DOI] [PubMed] [Google Scholar]
- 15.Lookene A, Beckstead JA, Nilsson S, et al. Apolipoprotein A-V heparin interactions. Implications for plasma lipoprotein metabolism. J Biol Chem. 2005;280:25383–25387. doi: 10.1074/jbc.M501589200. [DOI] [PubMed] [Google Scholar]
- 16▪▪.Grosskopf I, Shaish A, Afek A, et al. Apolipoprotein A-V modulates multiple atherogenic mechanisms in a mouse model of disturbed clearance of triglyceride-rich lipoproteins. Atherosclerosis. 2012;224:75–83. doi: 10.1016/j.atherosclerosis.2012.04.011. Using genetically engineered mouse models, the authors show that apoA-V modulates atherosclerotic lesion development by affecting lipoprotein production and clearance as well as cytokine levels. These findings identify apoA-V as a potential therapeutic target for treatment of atherosclerosis. [DOI] [PubMed] [Google Scholar]
- 17.Forte TM, Shu X, Ryan RO. The ins (Cell) and outs (Plasma) of plasma apolipoprotein A-V. J Lipid Res. 2009;50:S150–S155. doi: 10.1194/jlr.R800050-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg RB, Cook VR, Beckstead JA, et al. Structure and interfacial properties of human aolipoprotein A-V. J Biol Chem. 2003;278:34438–34444. doi: 10.1074/jbc.M303784200. [DOI] [PubMed] [Google Scholar]
- 19.Beckstead JA, Wong K, Gupta V, et al. The C terminus of apolipoprotein A-V modulates lipid-binding activity. J Biol Chem. 2007;282:15484–15489. doi: 10.1074/jbc.M611797200. [DOI] [PubMed] [Google Scholar]
- 20.Wong K, Lee D, Beckstead JA, et al. The N-terminus of apolipoprotein A-V adopts a helix bundle molecular architecture. Biochemistry. 2008;47:8768–8774. doi: 10.1021/bi800515c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marçais C, Verges B, Charriere S, et al. Apoa5 Q139X truncation predisposes to late-onset hyperchylomicronemia due to lipoprotein lipase impairment. J Clin Invest. 2005;115:2862–2869. doi: 10.1172/JCI24471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong-Mauldin K, Raussens V, Forte TM, Ryan RO. Apolipoprotein A-V N-terminal domain altered lipid interaction properties in vitro explain hypertriglyceridemic phenotype associated with natural truncation mutants. J Biol Chem. 2009;284:33369–33376. doi: 10.1074/jbc.M109.040972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauldin K, Lee BL, Oleszczuk M, et al. The carboxyl terminal segment of apolipoprotein A-V undergoes a lipid-induced conformational change. Biochemistry. 2010;49:4821–4826. doi: 10.1021/bi1005859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Vliet HN, Sammels MG, Leegwater AC, et al. Apolipoprotein A-V: a novel apolipoprotein associated with an early phase of liver regeneration. J Biol Chem. 2001;276:44512–44520. doi: 10.1074/jbc.M106888200. [DOI] [PubMed] [Google Scholar]
- 25.Tijburg LBM, Nyathi CB, Meijer GW, et al. Biosynthesis and secretion of triacylglycerol in rat liver after partial hepatectomy. Biochem J. 1991;277:723–728. doi: 10.1042/bj2770723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shu X, Chan J, Ryan RO, Forte TM. ApoA-V association with intracellular lipid droplets. J Lipid Res. 2007;48:1445–1450. doi: 10.1194/jlr.C700002-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Shu X, Ryan RO, Forte TM. Intracellular lipid droplet targeting by apolipoprotein A-V requires the carboxyl-terminal segment. J Lipid Res. 2008;49:1670–1676. doi: 10.1194/jlr.M800111-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walther TC, Farese RV., Jr Lipid droplets and cellular lipid metabolism. Annu Rev Biochem. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shu X, Nelbach L, Ryan RO, Forte TM. Apolipoprotein A-V associates with intrahepatic lipid droplets and influences triglyceride accumulation. Biochim Biophys Acta. 2010;1801:605–608. doi: 10.1016/j.bbalip.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ress C, Moschen AR, Sausgruber N, et al. The role of apolipoprotein A5 in nonalcoholic fatty liver disease. Gut. 2011;60:985–991. doi: 10.1136/gut.2010.222224. [DOI] [PubMed] [Google Scholar]
- 31▪.Blade AM, Fabritius MA, Hou L, et al. Biogenesis of apolipoprotein A-V and its impact on VLDL triglyceride secretion. J Lipid Res. 2011;52:237–244. doi: 10.1194/jlr.M010793. In stably transfected, doxycycline-inducible, McA-RH7777 cells, apoA-V expression was shown to inhibit triacylglycerol secretion by ~50%. While this was accompanied by a reduction in VLDL particle diameter, there was no effect on apoB secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪.Gao X, Forte TM, Ryan RO. Influence of apolipoprotein A-V on hepatocyte lipid droplet formation. Biochem Biophys Res Commun. 2012;427:361–365. doi: 10.1016/j.bbrc.2012.09.065. Stable transfection of McA-RH7777 with human apoA-V led to increased numbers and size of cytosolic lipid droplets. A molecular model describing the role of apoA-V in intrahepatic triacylglycerol trafficking is presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ioka RX, Kang MJ, Kamiyama S, et al. Expressing cloning and characterization of a novel glycosylphosphatidylinositol-anchored high density lipoprotein binding protein, GPIHBP1. J Biol Chem. 2003;278:7344–7349. doi: 10.1074/jbc.M211932200. [DOI] [PubMed] [Google Scholar]
- 34.Beigneux AP, Davies B, Gin P, et al. Glycosylphosphatidyl-inositol-anchored high density lipoprotein–binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gin P, Yin L, Davies BS, et al. The acidic domain of GPIHBP1 is important for the binding of lipoprotein lipase and chylomicrons. J Biol Chem. 2008;283:29554–29562. doi: 10.1074/jbc.M802579200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beigneux AP, Davies BS, Tat S, et al. Assessing the role of the glycosylphospatidylinositol- anchored high-density lipoprotein-binding protein 1 (GPIHBP1) three-finger domain in binding lipoprotein lipase. J Biol Chem. 2011;286:19735–19743. doi: 10.1074/jbc.M111.242024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beigneux AP, Franssen R, Bensadoun A, et al. Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase. Arterioscler Thromb Vasc Biol. 2009;29:956–962. doi: 10.1161/ATVBAHA.109.186577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franssen R, Young SG, Peelman F, et al. Chylomicronemia with low postheparin lipoprotein lipase levels in the setting of GPIHBP1 defects. Circ Cardiovasc Genet. 2010;3:169–178. doi: 10.1161/CIRCGENETICS.109.908905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivecrona G, Ehrenborg E, Semb H, et al. Mutation of conserved cysteines in the Ly6 domain of GPIHBP1 in familial chylomicronemia. J Lipid Res. 2010;51:1535–1545. doi: 10.1194/jlr.M002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rios JJ, Shastry S, Jasso J, et al. Deletion of GPIHBP1 causing severe chylomicronemia. J Inherit Metab Dis. 2012;35:531–540. doi: 10.1007/s10545-011-9406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Adeyo O, Goulbourne CN, Bensadoun A, et al. GPIHBP1 and the intravascular processing of triglyceride-rich lipoproteins. J Intern Med. 2012 doi: 10.1111/joim.12003. [Epub ahead of print] The author’s describe recent progress in understanding the role of GPIHBP1 in health and disease and discuss unresolved issues with respect to triglyceride-rich lipoprotein processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beigneux AP, Davies BS, Bensadoun A, et al. GPIHBP1, a GPI anchored protein required for the lipolytic processing of triglyceride-rich lipoproteins. J Lipid Res. 2009;50:S57–S62. doi: 10.1194/jlr.R800030-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43▪.Gin P, Beigneux AP, Voss C, et al. Binding Preferences for Glycosylphosphatidylinositol HBP1, a Glycosylphosphatidylinositol-Anchored Protein of Capillary Endothelial Cells. Arterioscler Thromb Vasc Biol. 2011;31:176–182. doi: 10.1161/ATVBAHA.110.214718. This study shows that apoA-V binding to GPIHBP1 requires its amino-terminal acidic domain and is independent of its cysteine-rich, Ly6 domain. GPIHBP1 binds LPL but does not bind other lipase family members, apoA-I or HDL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young SG, Davies BS, Fong LG, et al. GPIHBP1: an endothelial cell molecule important for the lipolytic processing of chylomicrons. Curr Opin Lipidol. 2007;18:389–396. doi: 10.1097/MOL.0b013e3281527914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien PJ, Alborn WE, Sloan JH, et al. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin Chem. 2005;51:351–359. doi: 10.1373/clinchem.2004.040824. [DOI] [PubMed] [Google Scholar]
- 46.Nelbach L, Shu X, Konrad RJ, et al. Effect of apolipoprotein A-V on plasma triglyceride, lipoprotein size, and composition in genetically engineered mice. Lipid Res. 2008;49:572–580. doi: 10.1194/jlr.M700281-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Henneman P, Schaap FG, Havekes LM, et al. Plasma apoAV levels are markedly elevated in severe hypertriglyceridemia and positively correlated with the APOA5 S19W polymorphism. Atherosclerosis. 2007;193:129–134. doi: 10.1016/j.atherosclerosis.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 48.Talmud PJ, Cooper JA, Hattori H, et al. The apolipoprotein A-V genotype and plasma apolipoprotein A-V and triglyceride levels: prospective risk of type 2 diabetes. Results from the Northwick Park Heart Study II. Diabetologia. 2006;49:2337–2340. doi: 10.1007/s00125-006-0387-0. [DOI] [PubMed] [Google Scholar]
- 49.Vaessen SF, Schaap FG, Kuivenhoven JA, et al. Apolipoprotein A-V, triglycerides and risk of coronary artery disease: the prospective Epic-Norfolk Population Study. J Lipid Res. 2006;47:2064–2070. doi: 10.1194/jlr.M600233-JLR200. [DOI] [PubMed] [Google Scholar]