Abstract
The cyclin dependent kinase (CDK) inhibitor flavopiridol has demonstrated promising clinical results in relapsed CLL patients leading to efforts to develop improved CDK inhibitors. Dinaciclib (SCH727965) is a pan-CDK inhibitor, derived from a detailed screen in ovarian xenograft mouse models for therapeutic index, whose toxicity in solid tumor phase I studies appears favorable. Dinaciclib in CLL cells demonstrates concentration dependent apoptosis that is superior to flavopiridol following a clinically relevant 2-hour exposure. Dinaciclib potently down-regulates expression of Mcl-1 in CLL cells and antagonizes protection mediated by multiple soluble proteins important in the microenvironment of CLL including TNF-α IL-4, BAFF, and CD40-ligand. In contrast, contact with stromal cells or fibronectin abrogates the cytotoxicity of dinaciclib that is antagonized by a pan inhibitor and p110 alpha isoform specific inhibitor of the phosphatidylinositol 3-kinase pathway suggesting potential for combination strategies. These data justify clinical development of dinaciclib in CLL.
Keywords: Dinaciclib, CDK, chronic lymphocytic leukemia
Introduction
CLL represents the most prevalent type of adult leukemia and is currently incurable with available therapies. The introduction of fludarabine (F), fludarabine/cyclophosphamide (FC), and either of these combined with rituximab (FR or FCR) has improved outcome for younger patients with CLL. Treatment options available for patients in the setting of relapsed disease following receipt of chemoimmunotherapy are less where most patients have high risk genomic findings including IgVH un-mutated disease, del(17p13.1), and del(11q22.3) associated with poor treatment response (reviewed in(1)). Identifying therapies with novel mechanisms of action for this patient group is important.
One class of drugs that has promise for the treatment of relapsed CLL is the cyclin dependent kinases (CDK inhibitors). Flavopiridol is the first member of this group to be extensively tested based upon pre-clinical work by several groups(2–4) which while having a narrow therapeutic window, was shown to be a clinically active in high risk genomic patients with a dose limiting side effect of hyper-acute tumor lysis syndrome (TLS)(5, 6). A multicenter phase II trial confirmed activity of flavopiridol including in patients with del(17p13.1) but also toxicity associated with its narrow therapeutic index (American Society of Hematology Annual meeting 2010). These results provide support for development of CDK inhibitors that have an improved therapeutic index.
Dinaciclib (SCH 727965)(7) is a selective inhibitor of CDK 1, 2 and 9 (IC50 of < 5nM) that was selected pre-clinically by an in vivo screen that identified it as having a favorable therapeutic index of maximally tolerated dose to effective dose in an ovarian carcinoma xenograft mouse model. Specifically, the therapeutic index of dinaciclib was 10 versus 2 for BMS-387032 (now known as SNS-032) and <1 for flavopiridol(8). Dinaciclib has completed phase I testing in solid tumors where the dose limiting side effect of neutropenia and cytokine release syndrome was observed with a relatively favorable therapeutic index (i.e. no diarrhea and less fatigue as compared to flavopiridol (American Society of Clinical Oncology annual meeting 2009). Herein, we describe dinaciclib has dramatic pre-clinical activity in CLL justifying its development as a potential clinical candidate agent in CLL.
Materials and Methods
Patients, Cell Separation, Culture Conditions, and Reagents
Blood was obtained from CLL patients(9) with written informed consent in accordance with the Declaration of Helsinki and under a protocol approved by the Institutional Review Board of The Ohio State University (Columbus, OH). CLL cell selection, interphase cytogenetics, and IVGH mutational analysis was done as previously reported(10). The HS-5 cell line was obtained from ATCC (Manassas, VA) Dinaciclib was obtained from Merck & Co. (Whitehouse Station, NJ). Fluorescein isothiocyanate-labeled annexin V and propidium iodide (PI) were purchased from BD Pharmingen (San Diego, CA). LY294002 was purchased from BIOMOL (Plymouth Meeting, PA). IL-4 and BAFF were purchased from R&D Systems (Minneapolis, MN). CD40L was purchased from PeproTech (Rocky Hill, NJ). IC87114 was synthesized according to international patent and published structure(11). TGX-221 was purchased from Calbiochem (Gibbstown, NJ). PIK-75 was purchased from Selleck Chemicals (Houston, TX).
Viability, Western Blot, and PCR Assays
MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) assays were performed as previously reported. Apoptosis was determined by staining with annexin V-FITC and PI. Experiments examining survival signals used 1mg/mL CD40L, 800U/mL IL-4, 50ng/mL BAFF, 20ng/mL TNFα or co-culturing on fibronectin or HS-5 cell line coated plates. Immunoblot was performed for MCL-1 as previously described by our group(2). Quantitative RT-PCR was performed using manufacturer’s instructions (Applied Biosystems, Foster City, CA).
Statistical Analysis
To stabilize the variance, the raw Ct value of real time PCR data was normalized to internal control, and the standardized data were analyzed using linear mixed effects models. Holm’s procedure was used to correct for multiple comparisons when appropriate(12). Type I error is strongly controlled at α=0.05 for single comparisons and after adjustment for multiple comparisons or endpoints.
Results and Discussion
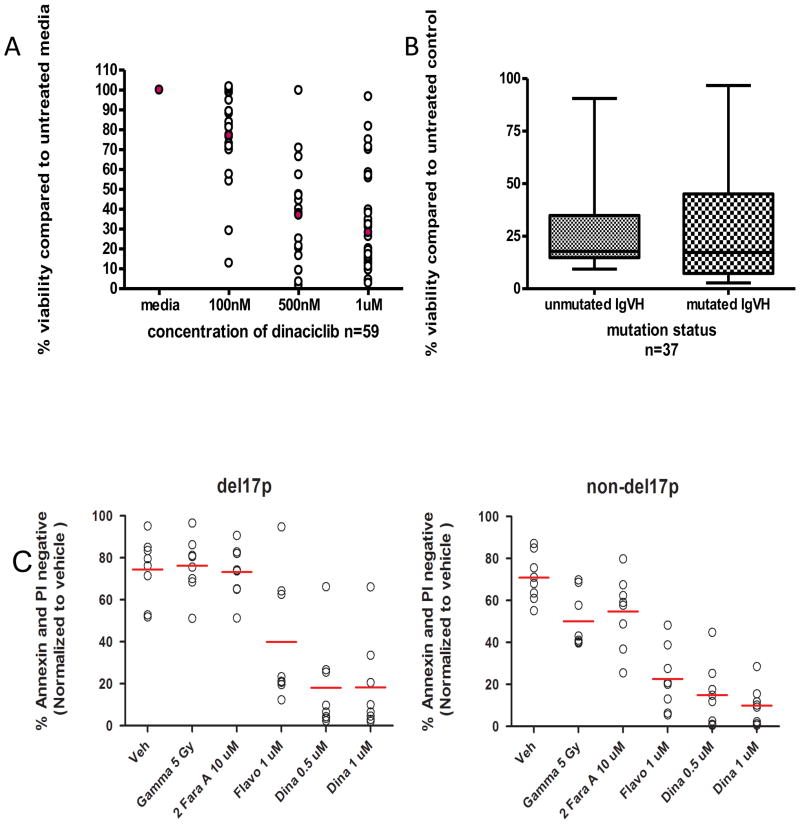
The phase I clinical trials performed with dinaciclib have administered this agent using a 2-hour infusion (American Society of Clinical Oncology annual meeting 2008 and 2009). We therefore sought to determine if a 2-hour exposure of dinaciclib, readily achievable in the clinic, would promote apoptosis in CLL cells. Figure 1A demonstrates that dinaciclib mediates potent apoptosis in CLL cells with an IC50 of 352.3 nM (95% CI of 351.06, 353.48; p<0.0001). Given the importance of identifying therapeutics that work independent of del(17p13.1) and IgVH un-mutated status, we next sought to determine if there was variable sensitivity to dinaciclib based upon these features. Figures 1B and 1C demonstrate that dinaciclib mediates similar apoptosis irrespective of IgVH mutational status or del17p.
Figure 1. Dinaciclib induces apoptosis in CLL patient cells.
A) CD19+ cells from CLL patients were incubated with or without dinaciclib (100nM-1μM) for 2 hours. Viability was measured at 24 hours by annexin V/propidium iodide staining and flow cytometry. B) CD19+ cells from CLL patients with unmutated or mutated IgVH were incubated with or without 1μM dinaciclib for 2 hours. Viability was measured at 24 hours by annexin V/propidium iodide staining and flow cytometry. C) CD19+ cells from CLL patients with or without the del17p chromosomal deletion were incubated with or without 0.5μM or 1μM dinaciclib, 10μM F-ara-A, or 5Gy gamma irradiation. Viability was measured at 24 hours by annexin V/propidium iodide staining and flow cytometry.
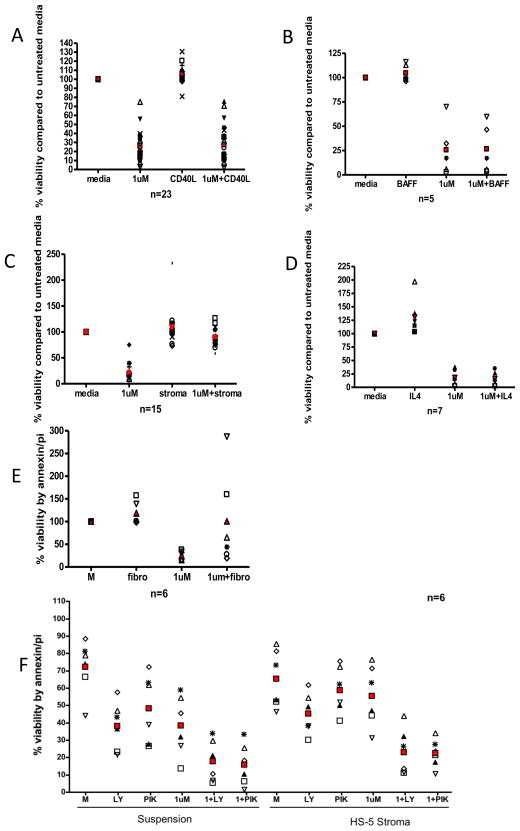
A variety of cytokines produced by microenvironment cells including CD40L, BAFF, and IL-4 promote survival of CLL cells in vitro. We demonstrate that co-treatment of CD40L (Figure 2A), BAFF (Figure 2B), and IL-4 (Figure 2C) all protect CLL cells from spontaneous apoptosis although this effect is abrogated by dinaciclib treatment. These results collectively suggest that soluble survival signals provided by the microenvironment are antagonized by dinaciclib. Direct contact with stromal cells exert a profound effect on preventing spontaneous and drug induced apoptosis. Concurrent treatment of CLL cells on stromal cells with dinaciclib demonstrated loss of adhesion of stromal cells and their protective effect (data not shown). This prompted dinaciclib pre-treatment of CLL cells followed by incubation on HS-5 stromal cells. Figure 2D demonstrates that a 2-hour exposure of dinaciclib on HS-5 stromal cells almost completely abrogates the cytotoxicity mediated by this agent (p<0.0001). Similar findings were observed with fibronectin fixed plates (Figure 2E) (p=0.0465). Previous studies have shown that stromal protection is mediated by the phosphatidylinositol 3-kinase (PI3-kinase) p110-alpha isoform(13) mediated pathway that are supported by studies in Figure 2F where the pan-PI3-kinase inhibitor LY294002 and the p110-alpha isoform specific inhibitor PIK-75 can in fact abrogate the protective effect of stromal attachment toward dinaciclib. In contrast, the p110-delta specific inhibitor and the p110-beta specific inhibitors of PI3-kinase cannot (Supplemental Figure 1).
Figure 2. Dinaciclib Abrogates Protection of CD40-ligand, BAFF, and IL-4 but not Stromal Cells or Fibronectin.
A) CD19+ cells from CLL patients were incubated with or without 1μM dinaciclib and with or without human recombinant 500 ng/mL CD40L for 24 hours. Viability was measured at 24 hours by annexin V/propidium iodide staining and flow cytometry. B) 50 ng/mL BAFF (24 hours). C) 800 U/mL IL-4 (24 hours). D) CD19+ cells from CLL patients were pre-treated with or without 1μM dinaciclib in suspension for 2 hours and then incubated in suspension or on an HS-5 cell layer for 24 hours. Viability was measured at 24 hours by annexin V/propidium iodide staining and flow cytometry. E) CD19+ cells from CLL patients were incubated for 2 hours with or without 1μM dinaciclib in suspension or on a fibronectin-coated plate. Viability was measured at 24 hours by annexin V/propidium iodide staining and flow cytometry. F) CD19+ cells from CLL patients were pre-treated with or without 1μM dinaciclib in suspension for 2 hours and then incubated in suspension or on an HS-5 cell layer for 24 hours with or without 25μM LY294002 or 35nM PIK-75. Viability was measured at 24 hours by annexin V/propidium iodide staining and flow cytometry.
Herein, we have described that the pan-CDK inhibitor dinaciclib has potent pre-clinical in vitro activity against CLL cells independent of high-risk genomic features. We also have demonstrated that dinaciclib down-regulates mRNA and protein expression of the important anti-apoptotic protein MCL-1 independent of caspases, which has been shown to be essential for CLL cell survival (Supplemental Figure 2A–B)(14). While MCL-1 modulation may serve as a pharmacodynamic marker of CDK inhibition as we have shown with flavopiridol previously, it was not demonstrated to correlate with response suggesting alternative mechanisms are relevant(15). In this regard we demonstrate that dinaciclib similar to flavopiridol abrogates the protective anti-apoptotic effect of several microenvironment cytokines including CD40L, BAFF and IL-4. However, treatment of CLL cells with dinaciclib followed by incubation on stromal cell or fibronectin co-culture protects from dinaciclib-mediated cytotoxicity. Of great interest, treatment of CLL cells with a p110-alpha specific PI3-kinase inhibitor but not p110-beta or delta inhibitors abrogates this effect. Collectively this points to rational combination strategies of dinaciclib with pan-PI3 kinase inhibitors in CLL to enhance the cytotoxic potential of this agent.
Based upon these promising pre-clinical data and also a favorable therapeutic index observed in pre-clinical and solid tumor studies, a phase I disease specific study of dinaciclib has been initiated in relapsed and refractory CLL. A preliminary report of this study has noted favorable tolerability and clinical activity in high risk genomic patients as dose escalation in the trial continued (American Society of Hematology annual meeting 2010). Collectively, these pre-clinical data support continued development of dinaciclib as a single agent and also in combination strategies for the treatment of CLL.
Supplementary Material
Acknowledgments
This work was supported by Specialized Center of Research from the Leukemia and Lymphoma Society, K12 CA133250, P50-CA140158, P01 CA95426, P01 CA8153 and P01 CA101956 from the National Cancer Institute, Pelotonia Fellowship Program, and The D. Warren Brown Foundation. AJJ is a Paul Calabresi Scholar and YYY is a Pelotonia post-doctoral fellow.
The authors wish to thank all of the patients who donated blood for these studies and also the clinical support staff who collected them. All statistical evaluation was done by the OSU Center for Biostatistics.
Footnotes
Authorship Contribution: LLS, AJW, JH, and YYY performed experiments; LLS, AJJ, and JCB analyzed results and made the figures; XZ performed statistical analysis; JF, JJ, MRG, and JCB provided clinical samples; AJJ, RB, MRG, and JCB designed the research and wrote the paper.
Conflict-of-interest disclosure: RB is an employee of Merck and Co.
References
- 1.Grever MR, Lucas DM, Johnson AJ, Byrd JC. Novel agents and strategies for treatment of p53-defective chronic lymphocytic leukemia. Best Pract Res Clin Haematol. 2007 Sep;20( 3):545–556. doi: 10.1016/j.beha.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Byrd JC, Shinn C, Waselenko JK, Fuchs EJ, Lehman TA, Nguyen PL, et al. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood. 1998 Nov 15;92(10):3804–3816. [PubMed] [Google Scholar]
- 3.Konig A, Schwartz GK, Mohammad RM, Al-Katib A, Gabrilove JL. The novel cyclin-dependent kinase inhibitor flavopiridol downregulates Bcl-2 and induces growth arrest and apoptosis in chronic B-cell leukemia lines. Blood. 1997 Dec 1;90(11):4307–4312. [PubMed] [Google Scholar]
- 4.Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000 Jul 15;96(2):393–397. [PubMed] [Google Scholar]
- 5.Phelps MA, Lin TS, Johnson AJ, Hurh E, Rozewski DM, Farley KL, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009 Mar 19;113(12):2637–2645. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin TS, Ruppert AS, Johnson AJ, Fischer B, Heerema NA, Andritsos LA, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009 Dec 10;27(35):6012–6018. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paruch K, Dwyer MP, Alvarez C, Brown C, Chan T-Y, Doll RJ, et al. Discovery of Dinaciclib (SCH 727965): A Potent and Selective Inhibitor of Cyclin-Dependent Kinases. ACS Medicinal Chemistry Letters. 2010;1(5):204–208. doi: 10.1021/ml100051d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parry D, Guzi T, Shanahan F, Davis N, Prabhavalkar D, Wiswell D, et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010 Aug;9(8):2344–2353. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- 9.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008 Jun 15;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011 Jun 9;117(23):6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadhu C, Dick K, Tino WT, Staunton DE. Selective role of PI3K delta in neutrophil inflammatory responses. Biochemical and biophysical research communications. 2003 Sep 5;308(4):764–769. doi: 10.1016/s0006-291x(03)01480-3. [DOI] [PubMed] [Google Scholar]
- 12.Gilboa N, Durante D, Guggenheim S, Lacher J, Holman R, Schorr W, et al. Immune deposit nephritis and single-component cryoglobulinemia associated with chronic lymphocytic leukemia. Evidence for a role of circulating IgG-anti-IgG immune complexes in the pathogenesis of the renal lesion. Nephron. 1979;24(5):223–231. doi: 10.1159/000181721. [DOI] [PubMed] [Google Scholar]
- 13.Niedermeier M, Hennessy BT, Knight ZA, Henneberg M, Hu J, Kurtova AV, et al. Isoform-selective phosphoinositide 3′-kinase inhibitors inhibit CXCR4 signaling and overcome stromal cell-mediated drug resistance in chronic lymphocytic leukemia: a novel therapeutic approach. Blood. 2009 May 28;113(22):5549–5557. doi: 10.1182/blood-2008-06-165068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain SR, Lucas DM, Johnson AJ, Lin TS, Bakaletz AP, Dang VX, et al. Flavopiridol causes early mitochondrial damage in chronic lymphocytic leukemia cells with impaired oxygen consumption and mobilization of intracellular calcium. Blood. 2008 Mar 15;111(6):3190–3199. doi: 10.1182/blood-2007-10-115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woyach JA, Lozanski G, Ruppert AS, Lozanski A, Blum KA, Jones JA, et al. Outcome of Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) Treated with Flavopiridol: Impact of Genomic Features. Leukemia. 2011 doi: 10.1038/leu.2011.375. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.