Abstract
Designing an ophthalmic drug delivery system is one of the most difficult challenges for the researchers. The anatomy and physiology of eye create barriers like blinking which leads to the poor retention time and penetration of drug moiety. Some conventional ocular drug delivery systems show shortcomings such as enhanced pre-corneal elimination, high variability in efficiency, and blurred vision. To overcome these problems, several novel drug delivery systems such as liposomes, nanoparticles, hydrogels, and in situ gels have been developed. In situ-forming hydrogels are liquid upon instillation and undergo phase transition in the ocular cul-de-sac to form viscoelastic gel and this provides a response to environmental changes. In the past few years, an impressive number of novel temperature, pH, and ion-induced in situ-forming systems have been reported for sustain ophthalmic drug delivery. Each system has its own advantages and drawbacks. Thus, a combination of two drug delivery systems, i.e., nanoparticles and in situ gel, has been developed which is known as nanoparticle laden in situ gel. This review describes every aspects of this novel formulation, which present the readers an exhaustive detail and might contribute to research and development.
Keywords: Ion-activated in situ gel, in situ gel, ocular irritancy test, pH-based hydrogel, temperature sensitive in situ gel
INTRODUCTION
In last few decades, significant attention has been focused on development of controlled and sustained drug delivery systems. The unique structure of the eye restricts the entry of drug molecules at the site of action. Drug delivery to the eye can be broadly classified into anterior and posterior segments. Conventional systems such as eye drops, suspensions, and ointments cannot be considered optimal in the treatment of vision, threatening ocular diseases.[1,2] Among them the extensive research has been carried in designing of polymeric drug delivery systems. The development of in situ gel systems has received considerable attention. Ocular in situ gels are the delivery system, which can be instilled as eye drops and undergo an immediate gelation when in contact with the eye. In situ-forming hydrogels are liquid upon instillation and undergo phase transition in the ocular cul-de-sac to form viscoelastic gel and this provides a response to environmental changes.[3] The stimuli that induces various responses to form hydrogels includes: Physical stimuli such as change in temperature, electric fields, light, pressure, sound, and magnetic fields; chemical stimuli such as change in pH and ion activation from biological fluids; and biological or biochemical stimuli such as change in glucose level. Out of these different environmental conditions only pH, ion activated, and temperature stimuli are used for ophthalmic drug delivery system.[4]
In situ drug delivery system offers advantages such as reduced frequency of administration and improved patient compliance and comfort. An in situ gel formulation provides an interesting alternative for achieving effective plasma drug concentration, an advantage over conventional delivery systems. In situ gelling system delivers accurate dose as well as prolongs residence time of drug in contact with mucosal membrane, thus overcomes the problems generally encountered in semisolid dosage forms. It is interesting that in situ gel is in sol form outside the body and once administered in the body, it changes to the gel form. The dosage form has sought attention because of many advantages like increased accurate dosing. Thus the in situ gelling system overcomes the side effects of pulsed dosing produced by conventional drug delivery systems. The drug delivery system provides sustained and controlled drug delivery. In situ gels increase the ocular bioavailability of drug by increasing the drug retention time. This can be achieved by effective adherence to corneal surface by the use of suitable polymers. The dosage form enables the targeting within the ocular globe so as to prevent the loss to other ocular tissues, and also provide comfort, better compliance to the patient, and improves therapeutic performance of drug.[5–8]
Any medicament when achieve minimum therapeutic concentration at the intended site of action is able to elicit pharmacological response. A major problem being faced in ocular therapeutics is the attainment of an optimal concentration at the site of action. Tear production, transient residence time, and non-permeability of corneal epithelium create major problem resulting in poor bioavailability and absorption of ocular dosage form. Binding to the lachrymal proteins, limited corneal surface, and metabolism are the other problems associated with the poor bioavailability of ocular dosage forms. Failure to attain minimum therapeutic concentration by conventional ophthalmic solutions is the result of rapid pre-corneal elimination of drug that can be minimized and overcome by the use of a sol-gel system.[9,10]
In situ gel upon administration does not cause blurred vision or irritation. These are delivered as solution in cul-de-sac and depending upon the physiological stimuli, they undergo sol to gel transition.[11] Topical route of administration is preferred in treatment of ocular problems because a lesser fraction of the dose will effectively pass the blood–retinal barrier. Thus, the eye seems an ideal and easily accessible target organ for topical treatment, but the absorption of drug moiety from the corneal surface is also restricted.[12] In situ gelling system provides the increased pre-corneal residence time of drugs and consequently, their bioavailability by the use of polymeric solutions which change to a gel as a result of exposure to the physiological temperature, pH, or ionic composition of the lachrymal fluid. For in situ gels-based ocular drug delivery, natural polymers such as gellan gum, alginic acid, and xyloglucan are most commonly used. Topical delivery of the formulation to the eye is a complicated issue because of the numerous protective mechanisms that are present in the eye to prevent the visual pathway from foreign particles.[13–16]
Problems Associated with Intraocular Drug Transportation
Tear
One of the main pre-corneal barriers for ocular dosage form is the tear production. It reduces the effective concentration of the instilled drugs due to dilution by the tear turnover which is approximately 1 μL/min. Tear production also causes rapid clearance and binding of the drug molecule to the tear proteins.[17]
Cornea
Cornea is the outermost transparent layer of eye. It consists of three layers: Outer epithelium, middle stroma, and inner endothelium. Cornea consists of a mechanical barrier to inhibit transport of exogenous substances into the eye. Each possesses a rate-limiting structure for drug permeation. The corneal epithelium is of a lipophilic nature, and tight junctions among cells are formed to restrict paracellular drug permeation from the tear film.[18] Stroma, a highly hydrated structure, composed of an extracellular matrix of a lamellar arrangement of collagen fibrils acts as a barrier to permeation of lipophilic drug molecules. The innermost single layer corneal endothelium is made up of hexagonal-shaped cells, and acts as a separating barrier between the stroma and aqueous humor. The endothelial junctions are leaky and facilitate the passage of macromolecules between the aqueous humor and stroma.[19]
Conjunctiva
Conjunctiva is a thin and transparent membrane, involved in the formation and maintenance of the tear film. Conjunctiva has a rich supply of capillaries and lymphatics; hence, the drugs administered in the conjunctival or episcleral space are cleared through blood and lymph. The conjunctival blood vessels do not form a tight junction barrier, which means drug molecules can enter into the blood circulation by pinocytosis and/or convective transport through paracellular pores in the vascular endothelial layer. The conjunctival lymphatics act as an efflux system for the efficient elimination from the conjunctival space.[20]
Sclera
The sclera mainly consists of collagen fibers and proteoglycans embedded in an extracellular matrix. Hydrophobicity of drugs affects scleral permeability. An increase in lipophilicity shows a lower permeability, while hydrophilic drugs may diffuse through the aqueous medium of proteoglycans in the fiber matrix pores more easily than lipophilic drugs. Posterior sclera is composed of a looser weave of colla gen fibers than the anterior sclera.[21,22]
Novel approaches for in situ gelling Ocular Targeting
Hydrogels are high molecular weight hydrophilic and cross-linked polymers that form a three-dimensional network in water or aqueous media. Hydrogels have relatively a longer residence time in the cul-de-sac with an increased bioavailability. These dosage forms are more acceptable by the patients and increases patient compliance. The polymers used for these gelling systems exhibit reversible phase transitions. The most common way to improve drug retention on the corneal surface is undoubtedly using polymers to increase solution viscosity.[23] Hydrogels are polymers endowed with an ability to swell in water or aqueous solvents and induce a liquid-gel transition. Many mechanisms have been employed to cause reversible sol-gel phase transition, i.e., in situ gel-forming system under the influence of biological conditions.[24] The stimuli that induce various responses to form hydrogels include physical stimuli such as change in temperature, electric fields, light, pressure, sound, and magnetic fields; chemical stimuli such as change in pH and ion activation from biological fluids; and biological or biochemical stimuli such as change in glucose level. Out of these different conditions, only pH, ion activated, and temperature stimuli are used for ophthalmic drug delivery system.[25–27]
In situ gelling based on change in pH
Sol to gel transition is induced by the pH change of the physiological environment. The pH sensitive polymers contain pendant acidic or basic group which is able to either accept or to release a proton in response to change in the pH.[28] The polymers with a large number of ionizable groups are called as polyelectrolytes. Weakly acidic polymers, containing anionic groups which swell on increase in the pH, while swelling decreases in case of weakly basic drugs. Buffer plays an important role in designing the ophthalmic drops. They contribute significantly to chemical stability and clinical response. They also influence the comfort and safety of the product. In this mechanism, the sol to gel transition is pH triggered. Potential ophthalmic in situ gels reported in the literatures include gelling triggered by a change in pH. The viscosities increase when the pH is raised from its native value to the eye environment (pH 7.4) like cellulose acetophthalate (CAP) latex, cross-linked polyacrylic acid derivatives such as carbomers and polycarbophil. The pH of eye works as stimuli for the conversion of polymeric solution into the gel form. Polyacrylic acids are used as the gelling agent in combination with other polymers like hydroxypropyl methylcellulose (HPMC), which also help in enhancement of viscosity.[29,30] The formulation with pH-triggered in situ gel is therapeutically efficient than the conventional dosage forms since these are stable, non-irritant, and provide sustain release of the drug for longer duration. CAP is a polymer undergoing coagulation when the original pH of the solution 4.5 is raised to 7.4 by the tear fluid.
In situ gelling based on Temperature Modulation
These hydrogels are liquid at room temperature (20-25°C) and forms gel when in contact with body fluids (35-37°C), due to an increase in temperature. The use of biopolymers whose transition from sol to gel is triggered by an increase in the temperature is an attractive way to approach in situ gel formation.[31] Temperature-sensitive polymers have a lower critical solvent temperature (LCST). These polymers contract when heated above LCST. LCST is a temperature below which the components of the mixture are miscible in all proportions. The ideal critical temperature for this system is ambient and physiological temperature so that phase transition does not require any external source of heat other than the body heat. Different thermo-setting gels have been described in this review, including pluronics, cellulose derivatives, and xyloglucan. This system is designed to use poloxamer as a vehicle for ocular drug targeting using in situ gel formation characteristic of polymer. The gelation temperature of graft copolymers can be determined by measuring the temperature at which immobility of the meniscus in each solution was first noted. The bioadhesive and thermo-gelling of these graft copolymers expected to be an excellent drug carrier for the prolonged delivery to surface of the eye. Poloxamer-407 (polyoxyethylene polyoxypropylene block copolymer, Pluronic F-127®) is a polymer with a solution viscosity that increases when its temperature is raised to the eye temperature.[32,33]
Ion-activated in situ Gelation
Ion-activated in situ systems are able to cross-link with cations present in the tear fluid on the corneal surface and prolongs the retention time of the drug. Sodium alginate is a sodium salt of alginic acid.[34] It is a natural hydrophilic polysaccharide and contains two types of monomers, d-mannuronic acid (M) and l-guluronic acid (G). The characteristic properties of these hydrogels, such as mechanical strength and porosity, are dependent upon the G:M ratios, type of ionic cross-linker (bio- or polyvalent cations), concentration, and viscosity of the initial alginate solution. The polymer forms three-dimensional matrices of hydro gels and the high G content alginate forms a low viscosity, free-flowing liquid at concentrations suitable for gel formation in the lacrimal fluid. Polymer like alginate is used as the gelling agent in combination with HPMC which acts as a viscosity-modifying agent.[35] Gelrite gellan gum is a novel ophthalmic vehicle that gels in the presence of mono or divalent cations, present in the lachrymal fluid can be used alone, and in combinations with sodium alginate as the gelling agent.
Nanoparticles
Over the past few decades, concept of nanoparticle has been emerged considerably. Various polymeric nanoparticles are being used in drug delivery effectively to the specific site of action at therapeutically optimal rate and dosage regimen. Nanoparticles vary in size from 10 to 1000 nm. Drug is dissolved and entrapped in a polymeric matrix. Nanoparticles have shown a great potential in ocular targeting drug delivery [Figure 1].[36]
Figure 1.
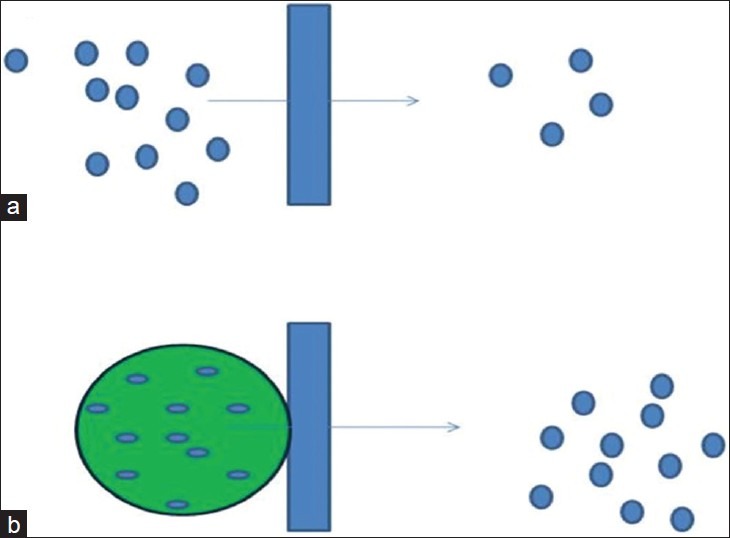
Drug uptake from corneal membrane through (a) free drug and (b) through Nanoparticles
Polymeric nanoparticles can be prepared using some suitable methods. In solvent evaporation method, the polymer is dissolved in any organic solvent. Drug is dissolved or dispersed into the polymer solution and emulsified into an aqueous solution to make a W/O (water in oil) emulsion. Organic solvent is then evaporated by increasing the temperature under pressure or by continuous stirring. Solvent evaporation method implies the use of organic solvent that may be hazardous to the physiological system. US FDA specified the residual amount of organic solvent in injectable colloidal system. However, salting out method and use of supercritical fluid are also widely used for the production of polymeric nanoparticles.[37,38]
Drug loading in the nanoparticles can be achieved by two methods, either by incorporating the drug at the time of nanoparticle production or by adsorbing the drug after the formation of nanoparticles by incubating them in drug solution. Incorporation method entrapped large amount of drug and is thus efficient method than the later one. These drug containing nanoparticles are then incorporated to the gels for ocular treatment.
MATERIALS USED FOR PREPARING NANOPARTICLES LADEN IN SITU GEL
Biopolymer as Drug Carrier
Biopolymers are produced by living organisms. Biopolymers, contain monomeric units that are covalently bonded to form larger chain. There are three main classes of biopolymers based on the differing monomeric units used and the structure of the biopolymer formed. Polynucleotides are long polymers composed of 13 or more nucleotide monomers; polypeptides are short polymers of amino acids; and polysaccharides are often linear-bonded polymeric carbohydrate structures. The concept of using polymer in ocular drug deliveries is not new. Ocular drug delivery systems using polymers especially biodegradable polymer have already been studied. Polymers were added to the vehicle to increase the viscosity of the preparation that may reduce the drainage rate and subsequently improve the therapeutic efficacy. High molecular weight polymers with different functional groups such as carboxyl, hydroxyl, amino, and sulfate are capable of forming hydrogen bonds and not crossing the biological membrane; have already been tried as vehicle in ocular drug deliveries. Nanoparticles of polymers are having many advantages like when used as carrier, the potency of the drug is not affected by pH and enzymes and also the drug develop high interaction with tissues and biological fluids. All these factors ultimately lead to increase bioavailability of the drug. But the major developmental issues in the case of nanoparticles include the formulation stability, particle size uniformity, control of drug release rate, and large-scale manufact ure of sterile preparations.[39,40]
Poly (dl-lactic acid) Nanoparticles
Poly (dl-lactic acid) (PLA) is a glassy material, occurring as white to golden-yellow pellets or granules. PLA is stable under dry conditions. However, it typically biodegrades over a period of 10-15 months depending on the molecular weight. Increasing moisture and temperature enhance biodegradation; the onset of degradation in water at 25°C is 6 months. In contrast to many other biodegradable polymers, PLA degrades through a two-step mechanism. PLA is used in drug delivery systems in implants, injections, and oral solid dispersions. It is also used as a coating agent. PLA nanoparticles have shown promising results in rabbit model for the sustained release of various drugs and increased corneal penetration of acyclovir eye ointment for antiviral treatment. The use of PLA results in several fold increase in the aqueous humor concentration of antiviral drug acyclovir as compared to the conventional preparations of acyclovir.[41]
Chitosan Nanoparticles
Chitosan is a natural polymer which is being implied in colloidal carrier preparations like nanoparticle. Chitosan is basically a cationic polysaccharide copolymer of 1,4-(2-amino-2-deoxy-D-glucopyranose) and 1,4-(2-acetamide-2-deoxy-D-glucopyaranose. Recent researches have been demonstrated that indomethacin when coated with chitosan and polylysine obtain a positive electrical charge in order to interact with anionic ocular mucin layer of pre-corneal tear film and thus caused a significant increase in the bioavailability of the indomethacin.[42]
Acrylic Nanoparticles
Acrylic acid-derived polymers are also investigated for the ocular targeting. Polyalkyl cyanoacrylate and polymethacrylates are the most commonly used carriers in the range of 200-500 nm size. Mucoadhesive properties of acrylates created more interest for their implementation. Kreuter[43] had experimentally proved the increased drug binding to the cornea and conjunctiva after instillation, while Diepold et al.[44] have been reported higher concentrations of nanoparticles in inflamed eye of rabbit in comparison to normal eye. Few other studies have been reported that use of acrylic nanoparticles are helpful in glaucoma therapy and reduced side effects of the drug by the use of nanocarriers.
Poloxamer Nanoparticles
Poloxamer has been widely used in pharmaceutical industry as dispersing agent, emulsifying agent, solubilizing agent, tablet lubricant, and as wetting agent. Poloxamers are non-ionic polyoxyethylene-polyoxypropylene copolymers used primarily in pharmaceutical formulations as emulsifying or solubilizing agents. Poloxamers generally occur as white, waxy, free-flowing prilled granules, or as cast solids. They are practically odorless and tasteless. At room temperature, poloxamer 124 occurs as a colorless liquid.[45]
Carbomer Nanoparticles
Carbomers are used in liquid or semisolid pharmaceutical formulations as rheology modifiers. Formulations include creams, gels, lotions, and ointments for use in ophthalmic, rectal, topical, and vaginal preparations. It has been used as gelling agent at a concentration of 0.5-2.0%. Carbomers are used as controlled release agents and/or as binders. In contrast to linear polymers, higher viscosity does not result in slower drug release with carbomers. Lightly cross-linked carbomers (lower viscosity) are generally more efficient in controlling drug release than highly cross-linked carbomers (higher viscosity).[46]
Nanoparticles from Cationic Polymers
Retinal surface bears negative charge that created an interest in developer to search for cationic polymers for the carrier for ocular ointments pest, and gels. Many cationic polymers have been studied to prepare nanoparticles with positive charge to facilitate an effective adhesion to ocular surface that bears negative charge. Pignatello et al.[47] employed copolymers of poly (ethacrylate), poly (methyl methacrylate), and poly (chlorotrimethylaminoethyl methacrylate) with quaternary groups.[47,48] In vivo experiments in rabbit eyes showed good tolerance with no inflammation and/or discomfort. Nanoparticles resulted in a prolonged release of the drug and an increased retention to the corneal surface resulted in the persistent higher concentration of the drug in aqueous humour.[49,50]
Nanoparticles of Hyaluronic Acid
Hyaluronic acid also known as hyase has also been incorporated as nanoparticle carrier for ocular disease treatment. Kyyronen et al.[51] have studied the release of methyl prednisolone from particles consisting of hyaluronic acid esters both in vitro and in rabbit eyes. They demonstrated that the polymer bound drug itself increased the penetration to aqueous humor but when combined with hyaluronic acid, the pre-corneal resident time of methyl prednisolone increased many folds that helped in sustain release of the drug.[51]
RECENT ADVANCEMENTS
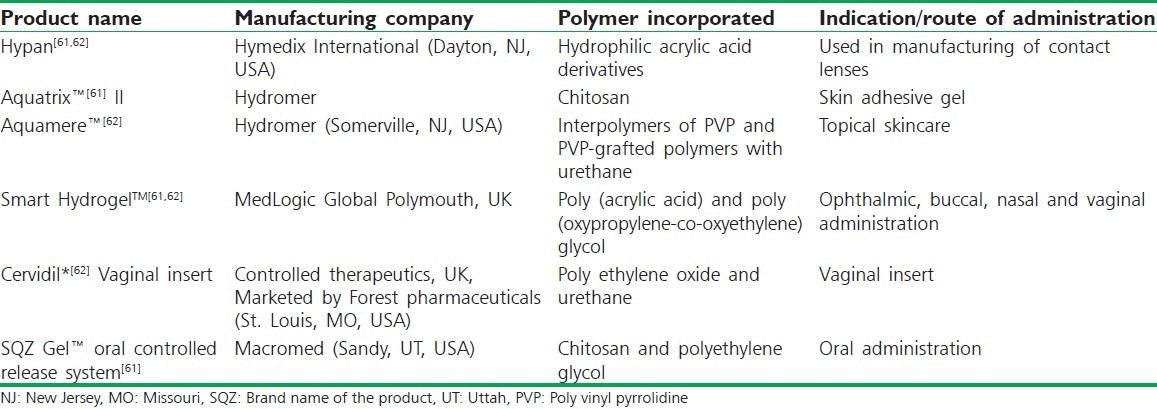
Hasan Sathali et al.[52] developed a niosomal in situ gel ocular delivery system of brimonidine tartrate. The objective of the study was to develop brimonidine tartrate niosomal in situ gels for glaucoma treatment. Niosomes were formulated using different ratios of span series and cholesterol. The niosomal formulation was transformed into gel when it instilled into the eye. All the gel formulations exhibited pseudo-plastic rheological behavior and controlled drug release pattern.[52] Bhalerao et al.[53] formulated an in situ gelling ophthalmic drug delivery system for the treatment of glaucoma. Sodium alginate in combination with hydroxypropyl cellulose (HPC) was used as gelling agent, which also acted as viscosity-enhancing agent. Darwhekar et al.[54] developed and optimized dorzolamide hydrochloride and timolol maleate in situ gel for the treatment of glaucoma. in situ gel was prepared using various concentrations of Pluronic F-127 (15-20% w/v) as a temperature-induced gelling system in combination with varying concentrations of HPMC (0.5, 1.0, 1.5% w/v) (Methocel K15M) as a viscosity-enhancing agent with an objective of increasing contact time, achieving controlled release, reduction in frequency of administration, and greater therapeutic efficacy of drug. Pandey et al.[55] developed and optimized in situ gel of levobunolol hydrochloride for the treatment of glaucoma. Levobunolol HCl in situ gel was prepared using various concentrations of polymers such as Carbopol-940 (0.1%, 0.2%, 0.3%, and 0.4% w/v), HPMC-E50 LV (0.5%, 1.0%, 1.5%, and 2.0% w/v), and HPMC E4M (0.2%, 0.4%, and 0.6% w/v) by pH-induced gelling system and shown a prolong release characteristics. Liu et al.[56] developed in situ gelling gelrite/alginate formulations as vehicles for ophthalmic drug delivery. The objective of this study was to develop an ion-activated in situ gelling vehicle for ophthalmic delivery of matrine. Bharath et al.[57] formulated and evaluated sustained ophthalmic delivery of ofloxacin from an ion-activated in situ gelling system. Sodium alginate was used as the gelling agent in combination with HPC that acted as a viscosity-enhancing agent. In vitro release studies indicated that the alginate/HPC solution retained the drug better than the alginate or HPC solutions alone. The formulations were therapeutically efficacious, sterile, and stable, and provided sustained release of the drug over a period of time. Hiremath et al.[58] formulated and evaluated a novel in situ gum-based ophthalmic drug delivery system of linezolid. Hydroxypropyl guar and xanthan were used as gum with the combination of hydroxyethyl cellulose, carbopol, and sodium alginate as viscosity-enhancing agents. Suitable concentrations of buffering agents were used to adjust the pH to 7.4. The developed formulations exhibited sustained release of drug from formulation over a period of 6 h, thus increasing residence time of the drug.[58] Mishra et al.[59] studied the design and characterization of bio-adhesive in situ gelling ocular inserts of gatifloxacin sesquihydrate. Polymeric ocular inserts of Gatifloxacin sesquihydrate were composed using sodium alginate and chitosan with glycerin as plasticizer by solvent casting method. Gupta, Jain, et al.[60] prepared and evaluated sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system using Pluronic F-127 (a thermo-sensitive polymer) in combination with chitosan (pH sensitive polymer also acts as permeation enhancer) was used as gelling agent with timolol maleate. Table 1 comprises the recent developments in the field of in situ gelling systems for different uses and indications.
Table 1.
In situ gelling systems available in market
Characterization of in situ Gelling System
In situ gel for ocular treatments should be evaluated for the following parameters.
Gelling capacity
The gelling capacity of the prepared formulation is determined by placing a drop of the formulation in a vial containing 2.0 ml of freshly prepared simulated tear fluid and visually observed.[63,64]
Rheological studies
The viscosity measurements can be calculated using Brookfield viscometer. The formulation before gelling should have a viscosity of 5-1000 mPa s and after ion gel activation by the eye should have a viscosity of 50-50,000 mPa s. The samples should be analyzed both at room temperature at 25°C and thermo stated at 37 ± 0.5°C by a circulating bath connected to the viscometer adaptor prior to each measurement.[8,65]
In vitro drug release studies
In vitro release study of in situ gel solutions should be carried out using Franz diffusion cell. The formulation placed in donor compartment and freshly prepared simulated tear fluid in receptor compartment. Between donor and receptor compartment, dialysis membrane is placed (0.22 μm pore size). The whole assembly should be placed on the thermostatically controlled magnetic stirrer. The temperature of the medium should be maintained at 37 ± 0.5°C.[66]
Texture analysis
The consistency, firmness, and cohesiveness of in situ gel are assessed using texture profile analyzer which mainly indicated gel strength and easiness in administration in vivo. Higher values of adhesiveness of gels are needed to maintain an intimate contact with mucus surface.[67]
Isotonicity evaluation
Isotonicity is an important characteristic of the ophthalmic preparations. Isotonicity has to be maintained to prevent tissue damage or irritation of eye. All ophthalmic preparations are subjected to isotonicity testing, since they exhibited good release characteristics and gelling capacity and the requisite viscosity. Formulations are mixed with few drops of blood and observed under microscope at ×45 magnification and compared with standard marketed ophthalmic formulation.[68]
Drug polymer Interaction study and Thermal Analysis
Interaction study can be performed with Fourier transform infra-red spectroscopy. During gelation process, the nature of the interacting forces can be evaluated using the technique by employing KBr pellet method. Thermo-gravimetric analysis can be conducted for in situ forming polymeric system to quantitate the percentage of water in hydrogel. Differential Scanning calorimetry conducted to observe whether there are any changes in thermograms as compared with pure active ingredients used for gelation.[69]
Ocular Irritancy Test
The Draize irritancy test is used for the ocular irritation potential of the ophthalmic product prior to marketing. According to the Draize test, the amount of substance applied to the eye is normally 100 μl placed into the lower cul-de-sac of male rabbit with observation of the various criteria made at a designed required time interval of 1 h, 24 h, 48 h, 72 h, and 1 week after administration. The sterile formulation is instilled twice a day for a period of 7 days, and a cross-over study is carried out (a 3-day washing period with saline was carried out before the cross-over study). Rabbits are observed periodically for redness, swelling, and watering of the eyes.[70]
Antibacterial Activity
The formulated in situ gels for treating infectious diseases of eye are studied for their antimicrobial spectrum. The microbiological growth of bacteria should be measured by concentration of antibiotics and this has to be compared with that produced by known concentration of standard preparation of antibiotic. To carryout microbiological assay, serial dilution method is employed.[71,72]
Accelerated Stability Studies
Formulations should be placed in ambient color vials and sealed with aluminum foil for a short-term accelerated stability study at 40 ± 2°C and 75 ± 5% RH (Relative Humidity) as per International Conference on Harmonization states Guidelines. Samples should be analyzed every month for clarity, pH, gelling capacity, drug content, rheological evaluation, and in vitro release.[73]
CONCLUSION
Advancement in the understanding of principles and processes governing ocular drug absorption and elimination and continuing technological advances brought some improvements in the efficacy of ophthalmic delivery systems. Use of polymeric nanoparticle loaded in situ gel provides a number of advantages over the conventional ocular dosage forms. Sustain and prolonged release makes the delivery system more reliable. Use of biodegradable and water soluble polymers for the nanoparticle loaded in situ gel formulations makes them more acceptable and excellent drug delivery systems. in situ activated gel-forming systems seem to be favored as they can be administered in drop form and produce appreciably less inconvenience with vision. The dosage form due to its control release of drug is more acceptable to the patients and increases the patient compliance. This drug delivery system is now being in use to treat glaucoma, dry eye syndrome, Sjogren's syndrome, etc.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Gaudana R, Jwala J, Boddu SH, Mitra AK. Recent perspectives in ocular drug delivery. Pharm Res. 2009;26:1197–216. doi: 10.1007/s11095-008-9694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang JC. Ocular drug delivery: Conventional ocular formulations. Adv Drug Deliv Rev. 1995;16:39–45. [Google Scholar]
- 3.Kumar S, Haglund BO, Himmelstein KJ. In situ forming gels for ophthalmic drug delivery. J Ocul Pharmacol. 1994;10:47–56. doi: 10.1089/jop.1994.10.47. [DOI] [PubMed] [Google Scholar]
- 4.Nanjawade BK, Manvi FV, Manjappa AS. In situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122:119–34. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Nirmal HB, Bakliwal SR, Pawar SP. In situ gel: New trends in controlled and sustained drug delivery system. Int J Pharm Tech Res. 2010;2:1398–408. [Google Scholar]
- 6.Kaur IP, Kanwar M. Ocular preparations: The formulation approach. Drug Dev Ind Pharm. 2002;28:473–93. doi: 10.1081/ddc-120003445. [DOI] [PubMed] [Google Scholar]
- 7.Kaur IP, Singh M, Kanwar M. Formulation and evaluation of ophthalmic preparations of acetazolamide. Int J Pharm. 2000;199:119–27. doi: 10.1016/s0378-5173(00)00359-8. [DOI] [PubMed] [Google Scholar]
- 8.Mitan RG, Jolly RP, Megha B, Dharmesh MM. A pH triggered in situ gel forming ophthalmic drug delivery system for tropicamide. Drug Deliv Technol. 2007;5:44–9. [Google Scholar]
- 9.Ganguly S, Dash AK. A novel in situ gel for sustained drug delivery and targeting. Int J Pharm. 2004;276:83–92. doi: 10.1016/j.ijpharm.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Meqi SA, Deshpande SG. Ocular drug delivery. In: Jain NK, editor. Controlled and Novel Drug Delivery. New Delhi: CBS Publishers; 2002. pp. 82–4. [Google Scholar]
- 11.Ding S. Recent developments in ophthalmic drug delivery. Pharm Sci Technol Today. 1998;1:328–35. [Google Scholar]
- 12.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–5. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Urtti A, Salminen L. Minimizing systemic absorption of topically administered ophthalmic drugs. Surv Ophthalmol. 1993;37:435–56. doi: 10.1016/0039-6257(93)90141-s. [DOI] [PubMed] [Google Scholar]
- 14.Shell JW. Pharmacokinetics of topically applied ophthalmic drugs. Surv Ophthalmol. 1982;26:207–18. doi: 10.1016/0039-6257(82)90081-9. [DOI] [PubMed] [Google Scholar]
- 15.Menqi SA, Deshpandey SG. Ocular Drug Delivery. In: Jain NK, editor. Controlled and Novel Drug Delivery. 1st ed. New Delhi: CBS Publishers and Distributors; 2005. pp. 82–99. [Google Scholar]
- 16.Thakur RR, Kashiv M. Modern delivery system for ocular drug formulations. Int J Res Pharm Biomed Sci. 2011;2:8–18. [Google Scholar]
- 17.Kuno N, Fuji S. Recent advances in ocular drug delivery systems. Polymers. 2003;3:193–221. [Google Scholar]
- 18.Schoenwald RD. Ocular drug delivery.Pharmacokinetic considerations. Clin Pharmacokinet. 1990;18:255–69. doi: 10.2165/00003088-199018040-00001. [DOI] [PubMed] [Google Scholar]
- 19.Tortora GJ, Derrikson B. 11th ed. USA: John Willey and Sons, Inc; 2007. Principles of Anatomy and Physiology; pp. 575–93. [Google Scholar]
- 20.Singh D. Conjunctival lymphatic system. J Cataract Refract Surg. 2003;29:632–3. doi: 10.1016/s0886-3350(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 21.Maurice DM, Polgar J. Diffusion across the sclera. Exp Eye Res. 1977;25:577–82. doi: 10.1016/0014-4835(77)90136-1. [DOI] [PubMed] [Google Scholar]
- 22.Ambati J, Canakis CS, Miller JW, Gragoudas AE, Weissgold DJ, Kim I, et al. Diffusion of high molecular weight compounds through sclera. Invest Ophthalmol Vis Sci. 2000;41:1181–5. [PubMed] [Google Scholar]
- 23.Nanjawade BK, Manvi FV, Manjappa AS. In situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122:119–34. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Kreuter J. Colloidal Drug Delivery Systems. 2nd ed. New York: Marcel Dekker; 2012. Nanoparticles; pp. 288–310. [Google Scholar]
- 25.Srividya B, Cardoza RM, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J Control Release. 2001;73:205–11. doi: 10.1016/s0168-3659(01)00279-6. [DOI] [PubMed] [Google Scholar]
- 26.Edsman K, Carlfors J, Petersson R. Rheological evaluation of poloxamer as an in situ gel for ophthalmic use. Eur J Pharm Sci. 1998;6:105–12. doi: 10.1016/s0928-0987(97)00075-4. [DOI] [PubMed] [Google Scholar]
- 27.Johan C, Katarina E, Roger P, Katarina J. Rheological evaluation of gelrite in situ gel for ophthalmic use. Eur J Pharm Sci. 1998;6:113–6. doi: 10.1016/s0928-0987(97)00074-2. [DOI] [PubMed] [Google Scholar]
- 28.Nanjawade BK, Manjappa AS. A novel pH triggered in situ gel for sustained ophthalmic delivery of Ketorlac tromithamine. Asian J Pharm Sci. 2009;4:189–99. [Google Scholar]
- 29.Kumar S, Himmelstein KJ. Modification of in situ gelling behavior of carbopol solutions by hydroxypropyl methylcellulose. J Pharm Sci. 1995;84:344–8. doi: 10.1002/jps.2600840315. [DOI] [PubMed] [Google Scholar]
- 30.Haglund BO, Joshi R, Himmelstein KJ. An in situ gelling system for parenteral delivery. J Control Release. 1996;41:229–35. [Google Scholar]
- 31.Ruel-Gariepy E, Leroux JC. In situ-forming hydrogels – Review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–26. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar N. Thermal gelation properties of methyl and hydroxyl propyl methyl cellulose. J Appl Polym Sci. 1979;24:1073–87. [Google Scholar]
- 33.Hoffman AS, Chen G, Kaang S, Priebe D. New bioadhesive polymer compositions for prolonged drug release in the eye. Proc Int Symp Control Rel Bioact Mater. 1995;22:159–60. [Google Scholar]
- 34.Wamorkar V, Varma M. Formulation and evaluation of stomach specific in-situ gel of metoclopramide using natural, bio-degradable polymers. Int J Res Pharm Biomed Sci. 2011;2:193–201. [Google Scholar]
- 35.Rathore KS, Nema RK. An insight into ophthalmic drug delivery system. Int J Pharm Sci Drug Res. 2009;1:1–5. [Google Scholar]
- 36.Cohen S, Lobel E. A novel in situ forming ophthalmic drug delivery system for alginates undergoing gelation in the eye. J Control Rel. 1997;44:201–8. [Google Scholar]
- 37.Duan X, Haiban LI. Nanoparticles for drug delivery to the central nervous system. Nanoscience. 2006;11:207–9. [Google Scholar]
- 38.Ansari SH, Islam F, Sameem M. Influence of nanotechnology on herbal drugs: A Review. J Adv Pharm Technol Res. 2012;3:142–6. doi: 10.4103/2231-4040.101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahoo S, Sahoo R, Nanda R, Tripathy MK, Nayak PL. Mucoadhesive nanopolymer-A novel drug carrier for topical ocular drug delivery. Eur J Sci Res. 2010;46:401–9. [Google Scholar]
- 40.Haynes RJ, Tighe PJ, Dua HS. Antimicrobial defensin peptides of the human ocular surface. Br J Ophthalmol. 1999;83:737–41. doi: 10.1136/bjo.83.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giannavola C, Bucolo C, Maltese A, Paolino D, Vandelli MA, Puglisi G, et al. Influence of preparation conditions on acyclovir-loaded poly-D, L-lactic acid nanospheres and effect of PEG coating on ocular drug bioavailability. Pharm Res. 2003;20:584–90. doi: 10.1023/a:1023290514575. [DOI] [PubMed] [Google Scholar]
- 42.De Campos AM, Sánchez A, Gref R, Calvo P, Alonso MJ. The effect of a PEG versus a chitosan coating on the interaction of drug colloidal carriers with the ocular mucosa. Eur J Pharm Sci. 2003;20:73–81. doi: 10.1016/s0928-0987(03)00178-7. [DOI] [PubMed] [Google Scholar]
- 43.Zimmer A, Kreuter J. Microspheres and nanoparticles used in ocular delivery systems. Adv Drug Deliv Rev. 1995;16:61–73. [Google Scholar]
- 44.Kreuter J. Particulates (nanoparticles and microparticles) In: Mitra AK, editor. Ophthalmic Drug Delivery Systems. New York: Marcel Dekker; 1993. pp. 257–97. [Google Scholar]
- 45.Nause RG, Daugherity PD. Handbook of Pharmaceutical Excipients. In: Rowe RC, Sheshkey PJ, Quinn ME, editors. 6th ed. London: RPS Publication; 2009. p. 143. [Google Scholar]
- 46.Cho HJ, Balakrishnan P, Park EK, Song KW, Hong SS, Jang TY, et al. Poloxamer/cyclodextrin/chitosan-based thermoreversible gel for intranasal delivery of fexofenadine hydrochloride. J Pharm Sci. 2011;100:681–91. doi: 10.1002/jps.22314. [DOI] [PubMed] [Google Scholar]
- 47.Pignatello R, Bucolo C, Puglisi G. Ocular tolerability of Eudragit RS100 and RL100 nanosuspensions as carriers for ophthalmic controlled drug delivery. J Pharm Sci. 2002;91:2636–41. doi: 10.1002/jps.10227. [DOI] [PubMed] [Google Scholar]
- 48.Pignatello R, Bucolo C, Ferrara P, Maltese A, Puleo A, Puglisi G. Eudragit RS100 nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci. 2002;16:53–61. doi: 10.1016/s0928-0987(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 49.Maria CP, Mariana SA, Ruben HM, Alvaro FJK. Polyelectrolytes as drug carriers.analysis by dynamic light scattering of reconstituted and in-situ prepared model polymethacrylate-drug aqueous dispersions. Open Nanoscience J. 2010;4:1–7. [Google Scholar]
- 50.Calvo P, Vila-Jato JL, Alonso MJ. Evaluation of cationic polymer-coated nanocapsules as ocular drug carriers. Int J Pharm. 1997;153:41–50. [Google Scholar]
- 51.Kyyronen K, Hume L, Benedetti L, Urtti A, Topp E, Stella V, et al. Methyl prednisolone esters of hyaluronic acid in ophthalmic drug delivery: In vitro and in vivo release. Int J Pharm. 1992;80:161–9. [Google Scholar]
- 52.Hasan Sathali AA, Sangeetha T. Formulation and evaluation of ocular Niosomal in situ gel of levofloxacin hemihydrates. J Pharmacy Res. 2011;4:4331–7. [Google Scholar]
- 53.Bhalerao AV, Singh SS. In situ gelling ophthalmic drug delivery system for glaucoma. International J Pharma Biosci. 2011;2:7–14. [Google Scholar]
- 54.Darwhekar G, Jain P, Jain DK, Agrawal G. Development and optimization of dorzolamide hydrochloride and timolol maleate in situ gel for glaucoma treatment. Asian J Pharm Anal. 2011;1:93–7. [Google Scholar]
- 55.Pandey A, Prashant YM, Sachdeva D, Patel DK, Ramesh R. Development and optimization of levobunolol hydrochloride in-situ gel for glaucoma treatment. Int J Pharmaceutical Biol Arch. 2010;1:134–9. [Google Scholar]
- 56.Liu Y, Liu J, Zhang X, Zhang R, Huang Y, Wu C. In situ gelling gelrite/alginate formulations as vehicles for ophthalmic drug delivery. AAPS Pharm Sci Tech. 2010;11:610–20. doi: 10.1208/s12249-010-9413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abraham S, Furtado S, Bharath S, Basavaraj BV, Deveswaran R, Madhavan V. Sustained ophthalmic delivery of ofloxacin from an ion-activated in situ gelling system. Pak J Pharm Sci. 2009;22:175–9. [PubMed] [Google Scholar]
- 58.Hiremath SS, Nadaf A, DasanKoppa FS, Jamakandi VG, Mulla JS, Sreenivas SA, et al. Formulation and evaluation of a novel in situ gum based ophthalmic drug delivery system of linezolid. Sci Pharm. 2008;76:515–32. [Google Scholar]
- 59.Mishra DN, Gilhotra RM. Design and characterization of bioadhesive in-situ gelling ocular inserts of gatifloxacin sesquihydrate. DARU. 2008;16:1–8. [Google Scholar]
- 60.Nanjundswami NG, Dasankoppa FS, Sholapur HN. A review on hydrogels and its use in in situ ocular drug delivery. Indian J Novel Drug Deliv. 2009;1:11–7. [Google Scholar]
- 61.Gupta P, Vermani K, Garg S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov Today. 2002;7:569–79. doi: 10.1016/s1359-6446(02)02255-9. [DOI] [PubMed] [Google Scholar]
- 62.Gupta H, Jain S. Ion- and pH-activated novel in-situ gel system for sustained ocular drug delivery. Drug Deliv. 2007;14:507–15. doi: 10.1080/10717540701606426. [DOI] [PubMed] [Google Scholar]
- 63.Senthil V, Kumar RS, Nagaraju CV, Jawahar N, Ganesh GN, Gowthamarajan K. Design and development of hydrogel nanoparticles for mercaptopurine. J Adv Pharm Technol Res. 2010;1:334–7. doi: 10.4103/0110-5558.72431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balasubramaniam J, Kant S, Pandit JK. In vitro and in vivo evaluation of the Gelrite gellan gum-based ocular delivery system for indomethacin. Acta Pharm. 2003;53:251–61. [PubMed] [Google Scholar]
- 65.Kumari A, Sharma PK, Garg VK, Garg G. Ocular inserts-Advancement in therapy of eye diseases. J Adv Pharm Technol Res. 2010;1:291–6. doi: 10.4103/0110-5558.72419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kashyap N, Viswanad B, Sharma G, Bhardwaj V, Ramarao P, Ravi Kumar MN. Design and evaluation of biodegradable, biosensitive in situ gelling system for pulsatile delivery of insulin. Biomaterials. 2007;28:2051–60. doi: 10.1016/j.biomaterials.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 67.Miyazaki S, Kawasaki N, Kubo W, Endo K, Attwood D. Comparison of in situ gelling formulations for the oral delivery of cimetidine. Int J Pharm. 2001;220:161–8. doi: 10.1016/s0378-5173(01)00669-x. [DOI] [PubMed] [Google Scholar]
- 68.Hatefi A, Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Control Release. 2002;80:9–28. doi: 10.1016/s0168-3659(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 69.Ashim KM. Vol. 58. New York: Marcel Dekker; 1993. Ophthalmic Drug Delivery System; pp. 105–10. [Google Scholar]
- 70.Sautou-Miranda V, Libert F, Grand-Boyer A, Gellis C, Chopineau J. Impact of deep freezing on the stability of 25 mg/ml vancomycin ophthalmic solutions. Int J Pharm. 2002;234:205–12. doi: 10.1016/s0378-5173(01)00961-9. [DOI] [PubMed] [Google Scholar]
- 71.Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances. J Pharmacol Exp Ther. 1944;82:377–90. [Google Scholar]
- 72.Charoo NA, Kohli K, Ali A. Preparation of in situ-forming ophthalmic gels of ciprofloxacin hydrochloride for the treatment of bacterial conjunctivitis: In vitro and in vivo studies. J Pharm Sci. 2003;92:407–13. doi: 10.1002/jps.10290. [DOI] [PubMed] [Google Scholar]
- 73.Proceedings of the International Conference on Harmonisation. Geneva: 1993. Oct, ICH QIA. Stability testing of new drug substances and products. [Google Scholar]


