Abstract
Purpose.
To document the cyclovertical ocular motor mechanism used for vertical fusion in healthy subjects, and to explore whether vertical vergence training in healthy individuals can produce objectively confirmed vertical deviations that change with head tilt, revealing a basic mechanism that can produce a pattern of misalignment in an otherwise normal ocular motor system that is similar to superior oblique muscle paresis (SOP).
Methods.
Seven subjects with normal orthoptic examinations were adapted to vertical image disparities using our tilting haploscopic eye-tracking apparatus presenting concentric circle targets without torsional cues. Static eye positions were recorded with head straight and when tilted 45 degrees to the left and right, during both binocular and monocular viewing.
Results.
Vertical fusional vergence was accompanied by a cycloversion, with the downward-moving eye intorting and the upward-moving eye extorting, implicating primary involvement of the oblique extraocular muscles. After adaptation to the slowly increasing vertical target separation, all subjects developed a temporary vertical deviation in the straight ahead position that increased with head tilt to one side and decreased with head tilt to the other side.
Conclusions.
These results not only show that head-tilt–dependent changes in vertical deviation are not necessarily pathognomonic for SOP, but also, and more importantly, suggest mechanisms that can mimic SOP and suggest a possible role for vertical vergence training in reducing deviations and thus the amount of head tilt required for fusion. Ultimately, vertical vergence training may provide an adjunct or alternative to extraocular muscle surgery in selected cases.
Keywords: vertical vergence adaptation, superior oblique paresis, Bielschowsky head tilt test, basic cyclovertical deviation, video-oculography
Vertical vergence training in healthy individuals can produce an objective vertical deviation that changes with different head tilt positions, revealing a basic mechanism that can produce head tilt findings similar to those in superior oblique paresis in an otherwise normal ocular motor system.
Introduction
There are two basic types of binocular eye movement: versions, where the eyes move in the same direction, and vergences, where the eyes move in opposite directions. Vergences allow us to align both eyes with each other at distance or near fixation, thus fine-tuning binocular eye alignment in a process called fusional vergence. Small deviations of even 0.3 degree can lead to loss of vertical fusion1 and diplopia. Vertical fusional vergence responds to first-order retinal stimulus disparity.2,3 Vertical fusional vergence used to correct retinal image disparity in healthy subjects has been associated with a characteristic eye movement pattern, that is, a cycloversion of the eyes (torsion of both eyes in the same direction), with the downward-moving eye intorting (superior oblique muscle action) and the upward-moving eye extorting (inferior oblique muscle action), suggesting that the oblique extraocular muscles play a significant role in disparity-induced vertical fusional vergence.4,5 This movement pattern was largely confirmed in another study, with the caveat that part of the cycloversion response was in the form of torsional nystagmus.6
Vertical eye misalignments that vary with gaze direction and head position are termed noncomitant or “incomitant” deviations, and are commonly associated with extraocular muscle paresis. Occasionally, vestibular skew deviations due to unequal vestibular input are also incomitant.7–10 Brainstem lesions near the interstitial nucleus of Cajal (the critical structure for vertical gaze-holding) may result in the ocular tilt reaction, which can also show a head-position–dependent vertical deviation increasing in the roll plane towards the hyperdeviated eye, similar to the finding with the Bielschowsky head tilt (BHT) test for superior oblique muscle paresis (SOP).11
The Parks' three-step test,12 which includes the BHT test, as well as an increase in the hyperdeviation with gaze away from the hyperdeviated eye, has traditionally been used as a criterion for diagnosis of SOP. In blunt head trauma, the fourth cranial nerve, which innervates only the superior oblique muscle (SOM), can be damaged unilaterally, an “acquired SOP,” resulting in a positive Parks' three-step test and a characteristic ocular motility pattern of misalignment. A very similar ocular motility pattern, however, with no such trauma, but with a positive three-step test, can occur in any decade of life, and is of uncertain etiology. The historically assumed cause has been an inborn weakness (paresis) of the SOM not manifest until later in life (“congenital SOP”). Magnetic resonance imaging (MRI) studies, however, have shown that many patients with apparent congenital SOP have normal-appearing SOMs,13,14 suggesting other causes for “congenital SOP” in many cases.15,16
The purpose of this study was to confirm the cyclovertical ocular motor mechanism used for vertical fusion in healthy subjects, and to explore whether vertical vergence training in healthy individuals can produce objectively confirmed vertical deviations that are head-position dependent. Previous studies showed that individuals were subjectively capable of producing head-position–dependent adaptation to small vertical deviations.17,18 In those studies, however, the head-position dependency was a result of using a different adapting stimulus as a function of head posture. Here we show that, with an appropriate choice of the adapting stimulus, head-position–dependent vertical deviations can be induced even when the adaptation phase is limited to a single head posture. This reveals a basic mechanism that can produce head tilt findings, in an otherwise normal ocular motor system, that are similar to those usually associated with SOP.
Methods
Subject Selection
Seven healthy subjects (aged 16–32) participated in this study, which was approved by the Johns Hopkins University Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all subjects after explaining the nature and possible consequences of the study. The seven subjects were all confirmed to have good visual acuity at near (better than or equal to 20/25 in each eye), no more than one prism diopter of vertical phoria on cover test at near, and normal stereoacuity (better than 70 arc seconds on the Randot stereotest; Stereo Optical Company, Chicago, IL). All subjects showed a measureable objective vertical deviation after adaptation, and their eyes had adequate iris detail for accurate torsional tracking. Subjects requiring spectacles for proper target viewing were not studied.
Eye Movement Recordings
Subjects were investigated with our custom mirror haploscope (constructed from an old Bausch and Lomb arc perimeter),19 that allows assessment of simultaneous horizontal, vertical, and torsional eye movements, binocularly, through the use of pupil-based and iris-crypt–based video-oculography,19 as well as providing simultaneous correction for small horizontal, vertical, and torsional in-plane head movements by means of video-based monitoring of black adhesive dots with a white border placed near the subject's inner canthi.20 The entire apparatus, with the subject on a bite-bar/headrest, is capable of tilting up to 45 degrees to the right or left about the naso-occipital axis. A drawing (SolidWorks; Dassault Systèmes S.A., Suresnes, France) and photograph of the haploscopic eye-tracking apparatus are provided in Figure 1.
Figure 1. .
Haploscopic eye-tracking apparatus. Note the overall device design with wide base (left), capable of tilting, with the subject, up to 45 degrees to the subject's right or left (right), with the subject's head tilting with the device, mounted on the bite-bar/headrest.
The subject was seated in front of the haploscope, with the head fixed by means of forehead rests and a dental impression bite bar. An infrared light-emitting diode at the inferotemporal corner of each mirror illuminated the respective eye of the subject. Just behind each mirror was an apparatus-mounted, infrared-sensitive webcam with close-up lens, which was connected to a desktop computer for data acquisition and analysis using custom MATLAB software (Mathworks, Inc., Natick, MA), interfacing with the commercial eye-tracking software IRIS (Chronos Vision, Berlin, Germany).
Visual Stimuli
Vertical fusional vergence was stimulated using concentric circle targets without torsional cues, subtending greater than 54 degrees of visual angle.21 The targets were attached to the arc perimeter's arms on either side of the subject's head, and viewed in two angled cold mirrors, which reflected visible light and thus superimposed the images of the target patterns.
Procedure
Two types of adaptation experiments were conducted, after baseline recordings. In the first, and primary, experiment, subjects were instructed to fuse a gradually increasing target disparity (either right-over-left or left-over-right vertical disparity was introduced) in straight-ahead gaze with head upright for 30 to 45 minutes before the recordings.22 Vertical disparities were created by tilting the perimeter's arc, thus bringing one target upward and the other one downward. The subject could increase or decrease the vertical separation using a lever. Subjects were asked to fixate on the center of the target patterns, but, if possible, to appreciate the stimulus as a whole, achieving a single, fused image. Eye movements were recorded with head straight and when tilted 45 degrees to the left and right, during alternating binocular and monocular viewing sessions of approximately 5 seconds each (sequence: both eyes viewing, right eye viewing, both eyes viewing, left eye viewing, and both eyes viewing) by covering and then uncovering the corresponding target (“haploscopic cover testing”) while subjects were asked to look straight ahead and attempt fusion when both targets were visible. By subsequent analysis of the simultaneous vertical and torsional movements of each eye during fusion acquisition or loss, the predominant cyclovertical ocular motor mechanism used for fusion of the induced vertical deviation was determined in each head position. In addition, subjects were tilted in the fusion-free, dissociated state, with the target for one eye covered, allowing for investigation of static cyclovertical eye positions with different head-tilt positions.
To determine the duration of the induced vertical deviation, eye positions were also measured in a fusion-free, dissociated state with the target for one eye covered (and the other eye looking straight ahead) over several minutes immediately after an adaptation paradigm.
In the second adaptation experiment, performed to confirm the cyclovertical ocular motor mechanism used for vertical fusion in general, subjects slowly alternated (approximately every 15 seconds) the positions of the two targets23 between right-over-left and left-over-right vertical disparities, as much as could be alternately fused, building up the vertical deviation in each direction. Eye movements were recorded during binocular viewing as this task continued.
Results
First Adaptation Experiment
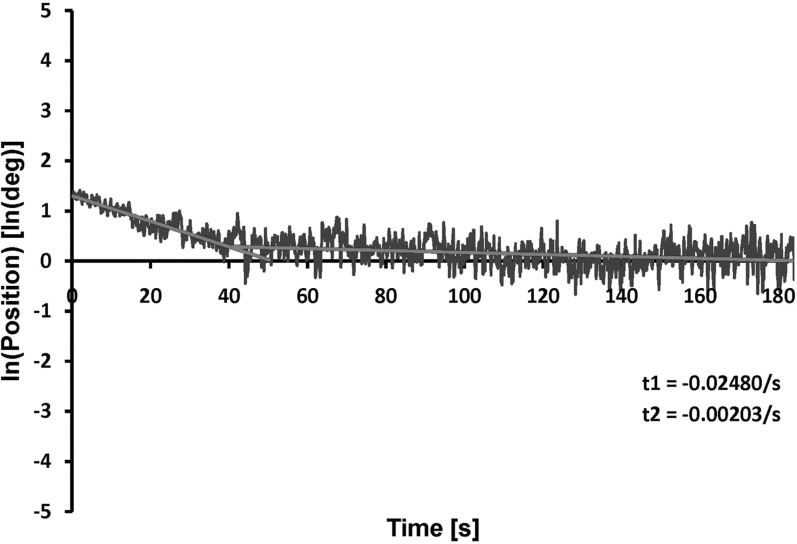
All subjects developed a temporary vertical deviation in the straight-ahead position after 30 to 45 minutes of adaptation to the slowly increasing vertical target separation, with at least 2 degrees (3.5 prism diopters) of vertical deviation, that persisted at least 50 seconds upon dissociation. A slow, double exponential decay of induced vertical vergence was found in our subjects (Fig. 2). Here, the half-life of the induced vertical deviation of approximately 3.5 degrees was 28 seconds.
Figure 2.
Vertical vergence timecourse. The logarithm of the difference between right and left eye vertical positions (the induced vertical vergence) versus time, showing double exponential decay after induced vertical deviation of approximately 3.5 degrees subjectively.
Note that the usual nomenclature of hyperdeviation suggests that the higher eye should move down for fusion, but in this case, because we used visual targets to induce the deviation, the higher eye had moved up for fusion. Thus, we discuss right-over-left vertical vergence, where the right eye moves up and the left eye moves down for fusion (as ordinarily occurs to compensate for a left hyperdeviation), in adapting to a right-over-left stimulus disparity.
During vertical fusional vergence to fuse the induced vertical deviation, all subjects showed a characteristic pattern of torsional eye movements. Vertical fusional vergence, whether right-over-left or left-over-right, was accompanied by a cycloversion, with the downward-moving eye intorting and the upward-moving eye extorting. Specifically, when our subjects showed a right-over-left vertical vergence (relative elevation of the right eye and depression of the left eye to fuse a right-over-left target disparity) this was accompanied by a clockwise cycloversion (from the subject's point of view looking forward: extorsion of the right eye and intorsion of the left eye, Fig. 3) and when they showed left-over-right vertical vergence, this was accompanied by a counterclockwise cycloversion (intorsion of the right eye and extorsion of the left eye).
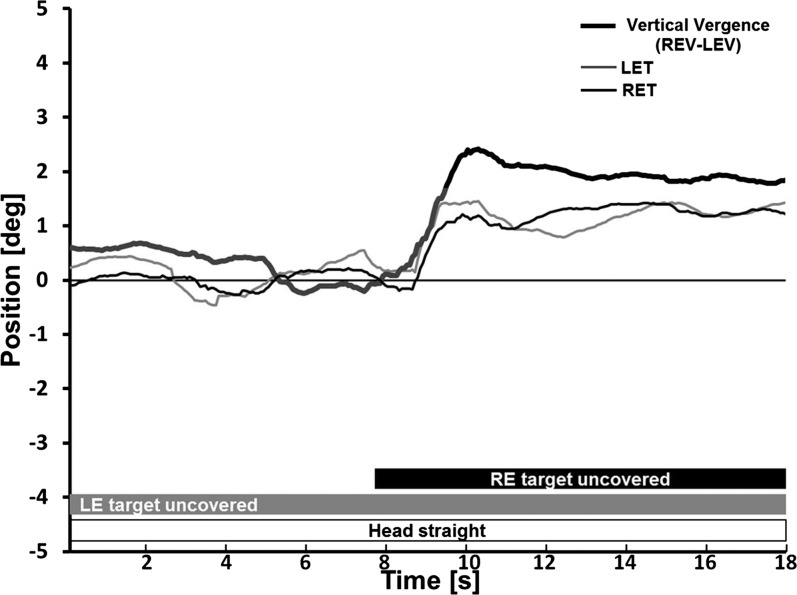
Figure 3.
Video-oculographic recording from a subject during haploscopic cover testing. Initially, the induced right-over-left vertical deviation is slowly decaying during left eye (only) viewing. Then, as the right eye target is uncovered at approximately 8 seconds, a vertical fusional vergence occurs as the right eye moves upward to regain fusion of the presented right-over-left vertical disparity, while the left eye continues fixing. This vertical vergence movement is accompanied by a clockwise cycloversion. Vertical vergence (thick black trace) is shown, with positive representing the right eye higher than the left. Upward deflections of right eye torsion (thin black trace) and of left eye torsion (thin gray trace) represent clockwise eye movements from the subject's perspective looking forward (extorsion of the right eye and intorsion of the left eye). LEV, left eye vertical; REV, right eye vertical; LET, left eye torsion; RET, right eye torsion.
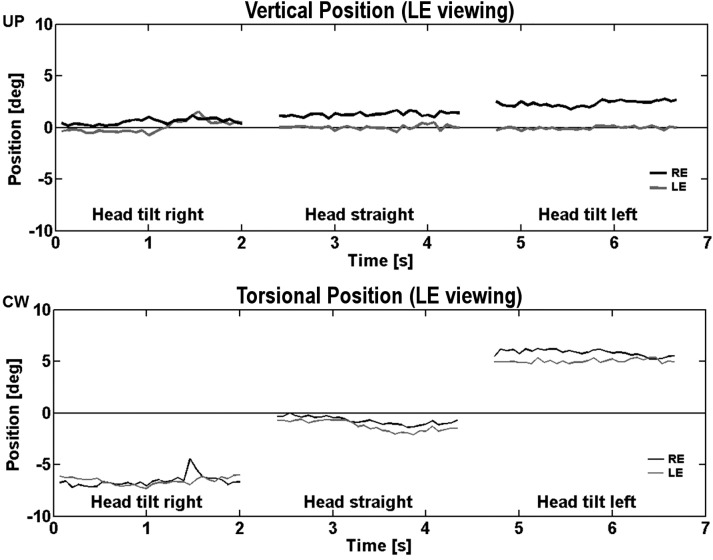
The induced vertical deviation (measured during head straight, and then tilted 45 degrees to the right and left, in the fusion-free, dissociated state with the target for one eye covered) was head-position dependent. For example, right-over-left vertical deviation increased with a 45-degree head tilt to the left, and decreased with a 45-degree head tilt to the right. Torsional eye positions were also measured and showed the typical effect of ocular counter-roll with head tilt (Fig. 4). On the other hand, left-over-right vertical deviation increased with a 45-degree head tilt to the right, and decreased with a 45-degree head tilt to the left. That is, after adaptation to right-over-left or left-over-right stimulus disparity, the left eye elevated more with right head tilt and the right eye elevated more with left head tilt, for five of seven subjects. One subject (#5) showed minimal dependence on head position, and another (#3) showed decreased vertical deviation with head tilt to either side (Table 1).
Figure 4.
Video-oculographic recording from a healthy subject in different head positions. The induced right-over-left vertical deviation and relative torsional divergence increased with left head tilt and decreased with right head tilt. Vertical (upper panel) and torsional (lower panel) eye positions in degrees (black, RE; gray, LE) are plotted versus time in seconds. CW, clockwise from the subject's perspective.
Table 1. .
Induced Vertical Deviation in Different Head Positions
|
Subject No. |
Subject Age |
Motor Deviation (REV–LEV), Degrees |
||
|
STR |
RHT |
LHT |
||
| 1 | 27 | 2.4 | 1.5 | 2.7 |
| 2 | 25 | 1.7 | 0.1 | 2.6 |
| 3 | 25 | 4.0 | 3.3 | 2.9 |
| 4 | 24 | −1.7 | −2.9 | −1.0 |
| 5 | 24 | 3.0 | 3.3 | 3.1 |
| 6 | 32 | 1.3 | 0.9 | 2.1 |
| 7 | 16 | 0.7 | 0.0 | 0.9 |
Incomitance was consistent among subjects. The median motor deviation (i.e., the video-oculographic measured difference between right and left eye vertical position) after adaptation to a right-over-left stimulus was greater with head tilted 45 degrees to the left (LHT) than head straight (STR) or tilted 45 degrees to the right (RHT). For subject 4, who adapted to a left-over-right vertical disparity, the induced left-over-right vertical deviation increased with right head tilt and decreased with left head tilt. Subject 5 showed minimal variation and subject 3 showed decreased deviation with head tilt to either side.
In Table 2, the total subjective induced vertical deviation, that is, the difference between right and left eye target positions that were reportedly fused, is compared with the objective video-oculographic–measured difference between right and left eye vertical positions (motor deviation). Note that the median total subjective vertical deviation was approximately twice the objective motor deviation.
Table 2. .
Comparison of Total Subjective and Objective Induced Vertical Deviation (Motor Deviation)
|
Subject No. |
Subject Age |
Total Deviation, Degrees |
Motor Deviation (REV–LEV), Degrees |
Motor Deviation/ Total Deviation, % |
| 1 | 27 | 6.0 | 2.4 | 40.0 |
| 2 | 25 | 4.0 | 1.7 | 42.5 |
| 3 | 25 | 6.0 | 4.0 | 66.7 |
| 4 | 24 | −3.0 | −1.7 | 56.7 |
| 5 | 24 | 6.0 | 3.0 | 50.0 |
Total subjective vertical deviation, the difference between right and left eye targets that were reportedly fused by healthy subjects after 30 to 45 minutes of vergence training (adaptation) to a gradually increasing vertical target disparity, is compared with the objective induced vertical deviation, that is, the video-oculographic measurement of vertical eye deviation (REV-LEV).
Second Adaptation Experiment
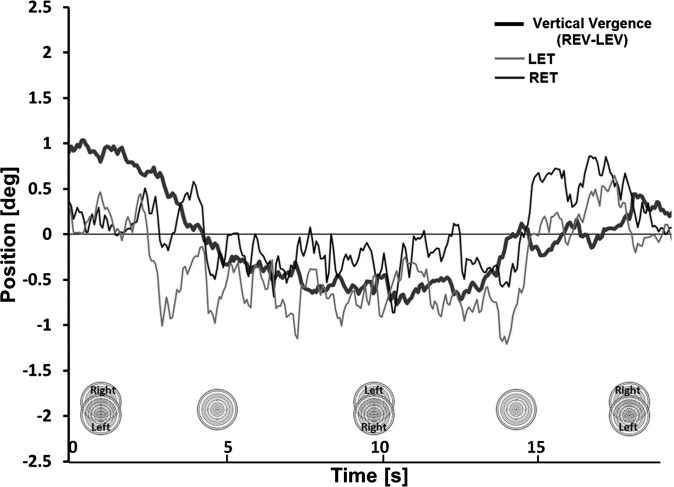
The same characteristic pattern of torsional eye movements was observed during vertical fusional vergence while alternating target positions, with a typical example illustrated in Figure 5. Subjects were asked to slowly alternate the positions of the two targets between maximal right-over-left and left-over-right vertical disparities, building up to as much vertical deviation as could be fused in each direction. As the initial right-over-left vertical deviation changed to a left-over-right vertical deviation, inducing a left-over-right vertical vergence, both eyes rotated counterclockwise, and then rotated clockwise again with right-over-left vertical vergence.
Figure 5.
Video-oculographic recording from a healthy subject during vertical fusional vergence while alternating target positions. Left-over-right and right-over-left vertical fusional vergence is associated with counterclockwise and clockwise cycloversion, respectively. Nomenclature is the same as in Figure 3.
Discussion
In our primary (the first) adaptation experiment, we created a temporary vertical deviation of at least 2 degrees (3.5 prism diopters) that persisted for at least 50 seconds in healthy subjects and was head-position dependent in the roll plane. Vertical fusional vergence to fuse the induced vertical deviation during haploscopic cover testing (first adaptation experiment) or when continuing to fuse while manually alternating the concentric targets (no torsional cues) between right-over-left and left-over-right positions (second adaptation experiment), was accompanied by a cycloversion, with the downward-moving eye intorting and the upward-moving eye extorting.
These combinations of cyclovertical movements during vertical fusional vergence are in agreement with previous studies in healthy subjects,4–6 and may be interpreted as implicating primary contributions of specific muscle actions. With right-over-left vertical vergence, for example, the right eye elevated and extorted, while the left eye depressed and intorted, thus implicating increased action of the right inferior oblique muscle and the left superior oblique muscle. With left-over-right vertical vergence, the right eye depressed and intorted, while the left eye elevated and extorted, implicating increased action of the right superior oblique muscle and the left inferior oblique muscle. Our results in seven healthy subjects thus provide evidence that the oblique extraocular muscles play a significant role in the induced fusional response to a large vertical disparity, extending Enright's findings in 19924 to larger deviations.
After adaptation to the slowly increasing vertical target separation, all subjects developed a temporary vertical deviation in the straight-ahead position that increased with head tilt to one side and decreased with head tilt to the other side. Previous studies using Lancaster red-green testing have indicated that subjects could simultaneously adapt to their vertical phorias by different amounts in right versus left head tilt.17,18 This context-specific adaptation showed that individuals were subjectively capable of producing head-position-dependent adaptation. Here, we show that vertical vergence training to a slowly increasing target disparity in only the head-straight condition produced an objective vertical deviation that then increased with tilt to one side and decreased with tilt to the other side in the roll plane, which has not been previously reported. The vertical deviation in the current study was measured using both subject feedback, to obtain the subjectively induced vertical deviation, and video-oculography to obtain the objective, motor vertical deviation. The total subjective deviation was approximately twice the objective induced motor deviation, with the remaining disparity indicating that the subject must have been suppressing the central area of one eye where Panum's fusional areas are small, while actually fusing peripherally by sensory means.
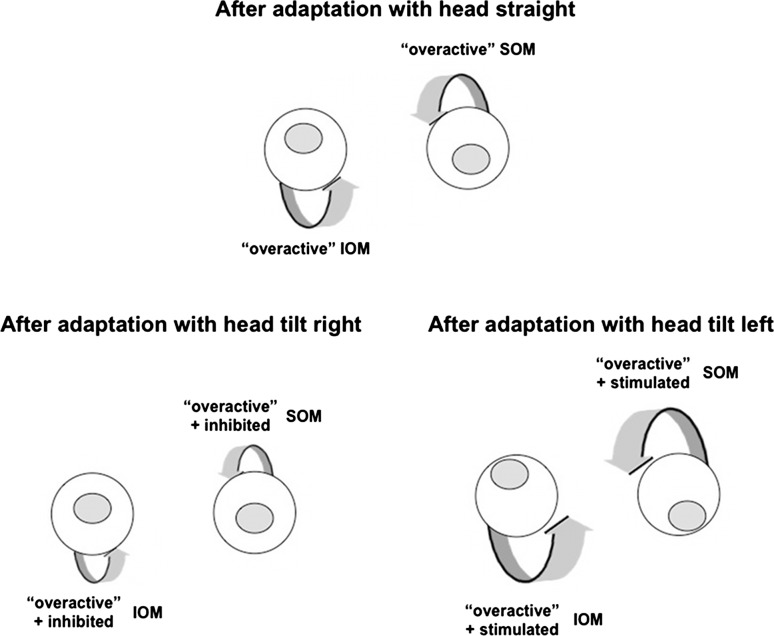
Our subjects showed characteristic head-tilt–dependent changes in vertical ocular deviation after vertical vergence training. After adaptation to right-over-left or left-over-right stimulus disparity, for example, the induced right-over-left vertical deviation increased with a 45-degree head tilt to the left, and decreased with a 45-degree head tilt to the right. We hypothesize that the right-over-left adaptation paradigm created “overaction” of the right inferior oblique muscle (IOM) and “overaction” of the left SOM, resulting in the right-over-left vertical deviation observed with the head straight (see Fig. 6, top). This proposed mechanism (vergence adaptation of the oblique muscles) is in accordance with our and others' findings4–6 of the oblique muscles being primarily used for vertical fusional vergence in healthy subjects.
Figure 6.
Vergence adaptation of oblique muscles as hypothesized explanation of head-tilt-dependent changes in healthy subjects. Adaptation to a right-over-left vertical disparity creates “overaction” of the right IOM and “overaction” of the left SOM in a healthy subject, resulting in a right-over-left vertical deviation with the head straight (top). The vergence-adapted, overactive right IOM and left SOM are specifically stimulated with head tilt to the left, thus increasing the induced vertical deviation (bottom right), whereas the overactive right IOM and SOM are inhibited with head tilt to the right, thus decreasing the vertical deviation (bottom left).
To determine whether this pattern of action was consistent with other behaviors, induction of ocular counter-roll by tilting the head was used to further increase stimulation to certain oblique muscles. When tilting the head in the roll plane to the left (Fig. 6, bottom right), the eyes rolled clockwise from the subject's perspective, largely from vestibular-induced stimulation of the left SOM and right IOM. These were the same muscles that were already overacting because of the adaptation paradigm, so the induced vertical deviation was even greater on left head tilt. When tilting the head to the right in the roll plane (Fig. 6, bottom left), the eyes rolled counterclockwise from the subject's perspective, involving vestibular-induced inhibition of the overactive left SOM and right IOM. Thus the induced vertical deviation was decreased.
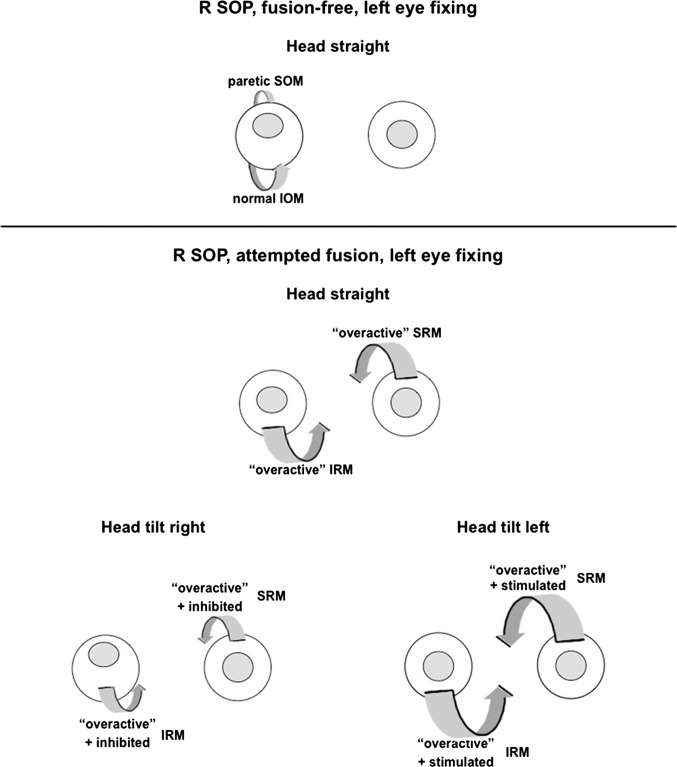
If our interpretation of the effect of vergence training is correct, then this right-over-left adaptation, where both the right IOM and left SOM are overacting, is different from right SOP, where the right SOM is paretic (see Fig. 7, upper panel). With the proposed mechanism involving binocular adaptation in the current study, one would expect a cycloversion response, with no significant torsional deviation between the two eyes created but showing a relatively comitant vertical deviation (which was experimentally confirmed by Lancaster red-green testing in the nine diagnostic positions of gaze; data not shown), unlike the deviation with a “congenital” right SOP, where one finds a relatively comitant torsional deviation and noncomitant vertical deviation.24 Also, most patients with unilateral SOP, in contradistinction to healthy subjects, have been found to primarily use their vertical rectus muscles for vertical fusional vergence.25 Thus, in most patients with right SOP who are able to fuse much of the time, the right inferior rectus muscle (IRM) and left superior rectus muscle (SRM) are relatively overacting, lessening the hyperdeviation with head straight (see Fig. 7: lower panel, top). When tilting the head to the left (Fig. 7: lower panel, bottom right), these specific overacting vertical rectus muscles are further stimulated during ocular counter-roll (while the paretic SOM is inhibited), so the right hyperdeviation is further decreased. When tilting the head to the right (Fig. 7: lower panel, bottom left), the overactive right IRM and left SRM are inhibited during ocular counter-roll (while the paretic SOM is activated) so that the right hyperdeviation is increased.
Figure 7.
Vergence adaptation of vertical rectus muscles as alternative explanation of head-tilt–dependent changes in superior oblique paresis. Upper panel: Patient with right superior oblique paresis (R SOP) and no fusion shows right hyperdeviation with head straight. Lower panel: Patient with R SOP and fusion using the vertical rectus muscles shows “overactive” right IRM and “overactive” left SRM, resulting in lessened hyperdeviation with head straight (top). The overactive right IRM and left SRM are specifically stimulated with head tilt to the left, further decreasing the hyperdeviation (bottom right), whereas the overactive right IRM and left SRM are inhibited with head tilt to the right, so the hyperdeviation is increased (bottom left).
Changes in vertical deviation with head tilt are often taken as evidence for a cyclovertical muscle paresis or occasionally as evidence for vestibular disorders. Indeed, a positive Parks' three-step test12 with extorsion of the hyperdeviated eye in the absence of other neurologic disease is taken as incontrovertible evidence for SOP. Many of these patients, however, appear to have SOMs with normal cross-sectional area and normal contractility,13,14 suggesting other etiologies in these cases.15,16 Our results not only demonstrate that head position-dependent vertical deviations are not necessarily pathognomonic for SOP or vestibular disease, but also illustrate the powerful and rapid adaptability of the ocular motor system, helping to support the possible existence of mechanisms that can mimic SOP. For example, abnormal “adaptive” changes occurring in an intact, otherwise normal ocular motor system may create a pattern of SOP-like misalignment. Guyton,16 for example, has hypothesized that a chronic low level of vertical vergence stimulation with resulting misdirected muscle length adaptation, unchecked in the cyclovertical “plane” because of an abnormality in the cyclovertical fusion mechanism, may actually drive the eyes into a pathologic “basic cyclovertical deviation” over time. This postulated mechanism in the cyclovertical plane is analogous to prolonged accommodative convergence in patients with uncorrected accommodative esotropia driving the eyes into a “basic esotropia” over time in the horizontal plane.16,26 Our demonstration that prolonged vertical vergence can produce a temporary cyclovertical deviation is consistent with this idea, and our finding that the induced vertical deviation changes in magnitude with different head tilt positions helps explain how such a “basic cyclovertical deviation” can masquerade as SOP.
Moreover, the current study of vertical vergence adaptation supports a possible role for vertical vergence training in patients. Just as patients with SOP often tilt their heads away from the hyperdeviation to reduce their vertical deviation, vergence training may reduce the residual deviation or reduce the amount of head tilt (roll) required for fusion. Ultimately, fusional vergence training may provide an adjunct or alternative to extraocular muscle surgery in selected cases.
Limitations of the current study include having only approximately a minute during which the induced vertical deviation could be maintained in the dissociated state, a small sample size (seven subjects), eye tracking performed without precise spectacle correction, and the use of near targets, which encouraged accommodation and convergence.
Future directions include testing with distance targets to see if induced vertical deviations can be increased in amplitude or duration. Because there are probably different etiologies for the ocular motility pattern commonly labeled as SOP, one may expect patients to use different vertical vergence mechanisms for compensation.25 Thus, using the recording haploscope to determine the particular vertical fusional vergence mechanism used by patients diagnosed with congenital SOP may help differentiate among various etiologies.
Acknowledgments
Supported by National Institutes of Health Grant R01 EY019347.
Disclosure: K. Irsch, None; D.L. Guyton, None; N.A. Ramey, None; R.S. Adyanthaya, None; H.S. Ying, None
References
- 1. Hara N, Steffen H, Roberts DC, Zee DS. Effect of horizontal vergence on the motor and sensory components of vertical fusion. Invest Ophthalmol Vis Sci. 1998; 39: 2268–2276 [PubMed] [Google Scholar]
- 2. Sheliga BM, Chen KJ, Fitzgibbon E, Miles FA. Short-latency disparity vergence in humans: evidence for early spatial filtering. Ann N Y Acad Sci. 2005; 1039: 252–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stevenson SB. Visual processing in disparity vergence control. Ann N Y Acad Sci. 2002; 956: 492–494 [DOI] [PubMed] [Google Scholar]
- 4. Enright JT. Unexpected role of the oblique muscles in the human vertical fusion reflex. J Physiol. 1992; 451: 279–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheeseman EW, Guyton DL. Vertical fusional vergence: the key to dissociated vertical deviation. Arch Ophthalmol. 1999; 117: 1188–1191 [DOI] [PubMed] [Google Scholar]
- 6. Van Rijn LJ, Collewijn H. Eye torsion associated with disparity-induced vertical vergence in humans. Vision Res. 1994; 34: 2307–2316 [DOI] [PubMed] [Google Scholar]
- 7. Donahue SP, Lavin PJ, Hamed LM. Tonic ocular tilt reaction simulating a superior oblique palsy: diagnostic confusion with the 3-step test. Arch Ophthalmol. 1999; 117: 347–352 [DOI] [PubMed] [Google Scholar]
- 8. Brodsky MC, Donahue SP, Vaphiades M, Brandt T. Skew deviation revisited. Surv Ophthalmol. 2006; 51: 105–128 [DOI] [PubMed] [Google Scholar]
- 9. Cogan DG. Neurology of the Eye Muscles. 2nd ed. Springfield, IL: CC Thomas; 1958: 133–135 [Google Scholar]
- 10. Smith JL, David NJ, Klintworth G. Skew deviation. Neurology. 1964; 14: 96–105 [DOI] [PubMed] [Google Scholar]
- 11. Hommel M, Bogousslavsky J. The spectrum of vertical gaze palsy following unilateral brainstem stroke. Neurology. 1991; 41: 1229–1234 [DOI] [PubMed] [Google Scholar]
- 12. Parks MM. Isolated cyclovertical muscle palsy. Arch Ophthalmol. 1958; 60: 1027–1033 [DOI] [PubMed] [Google Scholar]
- 13. Özkan SB, Aribal ME, Sener EC, Sanac AS, Gürcan F. Magnetic resonance imaging in evaluation of congenitial and acquired superior oblique palsy. J Pediatr Ophthalmol Strabismus. 1997; 34: 29–34 [DOI] [PubMed] [Google Scholar]
- 14. Chan TK, Demer JL. Clinical features of congenital absence of the superior oblique muscle as demonstrated by orbital imaging. J AAPOS. 1999; 3: 143–150 [DOI] [PubMed] [Google Scholar]
- 15. DeSa LCF, Good WV. Craniofacial anomalies and strabismus. In: Good WV, Hoyt CS. eds Strabismus Management. Oxford, UK: Butterworth-Heinemann; 1995: 128–129 [Google Scholar]
- 16. Guyton DL. Ocular torsion reveals the mechanisms of cyclovertical strabismus: the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2008; 49: 847–857 [DOI] [PubMed] [Google Scholar]
- 17. Maxwell JS, Schor CM. Head-position-dependent adaptation of noncomitant vertical skew. Vision Res. 1997; 37: 441–446 [DOI] [PubMed] [Google Scholar]
- 18. Maxwell JS, Schor CM. Adaptation of vertical eye alignment in relation to head tilt. Vision Res. 1996; 36: 1195–1205 [DOI] [PubMed] [Google Scholar]
- 19. Ramey NA, Ying HS, Irsch K, Müllenbroich MC, Vaswani R, Guyton DL. A novel haploscopic viewing apparatus with a three-axis eye tracker. J AAPOS. 2008; 12: 498–503 [DOI] [PubMed] [Google Scholar]
- 20. Irsch K, Ramey NA, Kurz A, Guyton DL, Ying HS. Video-based head movement compensation for novel haploscopic eye tracking apparatus. Invest Ophthalmol Vis Sci. 2009; 50: 1152–1157 [DOI] [PubMed] [Google Scholar]
- 21. Howard IP, Fang X, Allision RS, Zacher JE. Effect of stimulus size and eccentricity on horizontal and vertical vergence. Exp Brain Res. 2000; 130: 124–132 [DOI] [PubMed] [Google Scholar]
- 22. Rutstein RP, Daum KM, Cho M, Eskridge JB. Horizontal and vertical vergence training and its effect on vergences, fixation disparity curves, and prism adaptation: II. Vertical data. Am J Optom Physiol Opt. 1988; 65: 8–13 [DOI] [PubMed] [Google Scholar]
- 23. Van Rijn LJ, Van der Steen J, Collewijn H. Visually induced cycloversion and cyclovergence. Vision Res. 1992; 32: 1875–1883 [DOI] [PubMed] [Google Scholar]
- 24. Gräf M. Diagnose und Therapie der Trochlearisparese. Klin Monatsbl Augenheilkd. 2009; 226: 806–811 [DOI] [PubMed] [Google Scholar]
- 25. Mudgil AV, Walker M, Steffen H, Guyton DL, Zee DS. Motor mechanisms of vertical fusion in individuals with superior oblique paresis. J AAPOS. 2002; 6: 145–153 [DOI] [PubMed] [Google Scholar]
- 26. Guyton DL. The 10th Bielschowsky Lecture. Changes in strabismus over time: the roles of vergence tonus and muscle length adaptation. Binocul Vis Strabismus Q. 2006; 21: 81–92 [PubMed] [Google Scholar]