Abstract
Purpose.
Elevated intraocular pressure (IOP) is a major risk factor in glaucoma. Various changes in the trabecular meshwork (TM) are responsible for elevated IOP. Glucocorticoids (GCs) increase IOP and mediate biochemical changes in the TM, similar to those associated with primary open-angle glaucoma (POAG). There are differences in steroid responsiveness among the population. Approximately 40% of individuals significantly elevate IOP (i.e., responders) upon GC administration, while others do not (i.e., nonresponders). The responders are at higher risk of developing POAG compared to the nonresponders. In addition, almost all POAG patients are steroid responders. GC responsiveness is regulated by the relative levels of the active GC receptor alpha (GRα) and the alternatively spliced dominant negative regulator isoform GRβ. Glaucomatous TM cell strains have a lower GRβ–GRα ratio compared to normal TM cells, making them more sensitive to GCs. Our purpose was to investigate the role of a special class of natural products called thailanstatins (TSTs) in GR alternative splicing and GC response in cultured human TM cells.
Methods.
Quantitative RT-PCR and Western immunoblotting were used to study the effect of TSTs on GRβ–GRα ratios in human TM cell strains. Effects of TSTs on dexamethasone (DEX) responsiveness were assessed by GRE-luciferase reporter activity assay and fibronectin (FN) induction in TM cells.
Results.
TSTs increased the GRβ–GRα ratio in TM cells. Increased GRβ–GRα ratios were associated with decreased DEX-mediated FN induction and GRE-luciferase activity.
Conclusions.
TSTs modulate the GR splicing process to enhance GRβ levels and thereby decrease the GC response in cultured human TM cells. These TSTs, or similar compounds, may potentially be new glaucoma therapeutic agents.
Keywords: glucocorticoid receptor, splicing, trabecular meshwork, thailanstatins, glaucoma
New class of spliceosome modulators called thailanstatins (TSTs) affect GR alternative splicing to increase GRβ and decrease GC response in TM cells and may potentially be new glaucoma therapeutic agents.
Introduction
Steroid-induced glaucoma and POAG share many phenotypes. They both involve increased extracellular matrix deposition in the trabecular meshwork (TM),1–5 TM cell cytoskeletal reorganization,2,6–8 and elevated IOP due to impaired aqueous humor outflow.2,9 Approximately 40% of general population are steroid responders and are at higher risk for developing either of these forms of glaucoma.1,2,10,11 In addition, the majority of people suffering from POAG develop elevated IOP after glucocorticoid (GC) treatment.10 This limits the anti-inflammatory and anti-allergic use of GCs in these patients. Several different mechanisms may be responsible for differential GC sensitivities among individuals, including polymorphisms in the GR gene (NR3C1)12 and differences in the GR isoform expression due to alternative splicing of the GR gene.13–20 GRβ lacks the GC binding domain, acts as a dominant negative regulator of GC activity,21 and is expressed at higher levels in TM cells of the normal/nonresponder population compared to glaucoma TM cells.19,22 This makes normal TM cells less responsive to GCs compared to glaucoma TM cells. We have also previously shown that the levels and/or activities of different spliceosome proteins can regulate the process of GR alternative splicing in TM cells.23
For the past decade, there has been a significant amount of research to discover and develop novel spliceosome inhibitors and modulators, including FR901464, spliceostatin A, pladienolides, and sudemycins.24–30 Although many of these compounds are targeted for the treatment of various cancers,24–30 they may also be useful for regulating GC responsiveness in the TM of steroid responders and glaucoma patients. Very recently, structurally similar compounds called thailanstatins (TSTs) (Fig. 1) have been isolated from the culture broth of Burkholderia thailandensis.31 These TSTs (TST-A, TST-B, and TST-C) demonstrated potent pre-mRNA splicing inhibitory activities in vitro and antiproliferative activities in a number of human cancer cell lines. The overall goal of our study was to investigate the role of this new class of spliceosome modulators in GR alternative splicing and regulation of GC response in TM cells.
Figure 1.
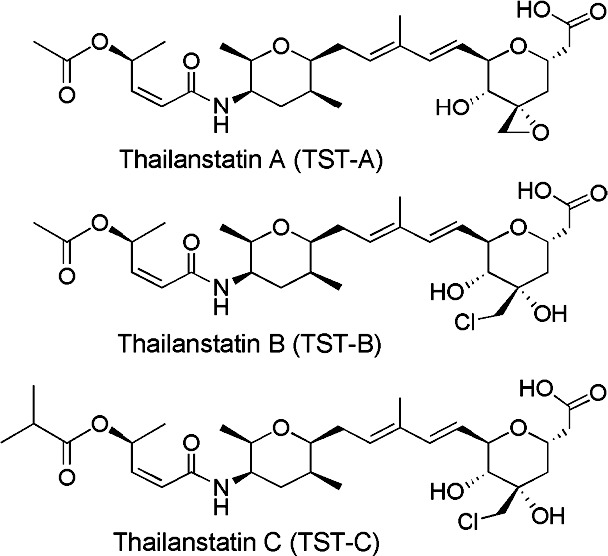
Structure of thailanstatins.
Methods
Chemicals
Thailanstatins were isolated from the fermentation broth of B. thailandensis MSMB4331 and dissolved in dimethyl sulfoxide (DMSO) as 10 mM stock solutions, which were further diluted to varying concentrations for subsequent experiments.
TM Cell Culture
Human TM cells were isolated from carefully dissected human TM tissue explants derived from patients with glaucoma or from normal donors, and the TM cells were characterized as previously described.4–9,19,23,32,33 All donor tissues were obtained from regional eye banks and managed according to the guidelines in the Declaration of Helsinki for research involving human tissue. These isolated TM cells were grown in Dulbecco's modified Eagle's medium (DMEM; Invitrogen-Gibco, Grand Island, NY) containing L-glutamine (0.292 mg/mL; Gibco BRL Life Technologies, Grand Island, NY), penicillin (100 units/mL)/streptomycin (0.1 mg/mL; Gibco BRL Life Technologies), amphotericin B (250 μg/μL; Thermo Scientific, Rockford, IL), and 10% fetal bovine serum (Gibco BRL Life Technologies). The stably transformed human TM cell line GTM334 was also used and cultured in the same medium.
TM Cell Treatment
TM cells were grown to 100% confluency in serum-containing medium. TM cells were incubated with fresh medium containing different doses (10 nM, 100 nM, and 1 μM) of TST-A, TST-B, or TST-C for 6 to 24 hours (GTM3 cells) or for 24 to 48 hours (primary TM cell strains). In some studies, TST (100 nM or 1 μM) treatment was followed by treatment with or without dexamethasone (DEX; 100 nM) for 24 to 72 hours for protein isolation.
RNA Isolation, Reverse Transcription, and Quantitative Real-Time PCR
Total cellular RNA was prepared from cultured TM cells using TRI Reagent RT extraction (MRC, Inc., Cincinnati, OH). iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad Laboratories, Hercules, CA) was used for first-strand cDNA synthesis. Primers for GRα, GRβ, and glyceraldehyde 3-phosphate dehydrogenase, house keeping gene (GAPDH) were designed using Primer3 software (provided in the public domain, http://frodo.wi.mit.edu/primer3/). The primer pairs are listed in Table 1. Quantitative PCR was performed using the BioRad CFX96 real-time system (Bio-Rad Laboratories, Richmond, CA) with the SsoAdvanced SYBR Green master mix (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions for 20-μL samples (95°C for 3 minutes; 40 cycles 95°C for 10 seconds, 62°C for 30 seconds). The cycle threshold (Ct) was assigned as log2 of PCR amplification. Technical triplicates for each sample were averaged and each sample was normalized to internal GAPDH Ct values to give ΔCt values. The difference between the experimental and control ΔCt values was used to determine the relative fold change in gene expression for each sample based on a 2-fold exponential.
Table 1. .
List of PCR Primers
|
Gene Name |
Left Primer Sequence (5′–3′) |
Right Primer Sequence (5′–3′) |
| NR3C1 (GR alpha) | GAACTGGCAGCGGTTTTATC | TTTTGGTATCTGATTGGTGATGA |
| NR3C1 (GR beta) | GAACTGGCAGCGGTTTTATC | TCAGATTAATGTGTGAGATGTGCTT |
| GAPDH | GGGAGCCAAAAGGGTCAT | TTCTAGACGGCAGGTCAGGT |
Protein Extraction and Western Blot (WB) Analysis
Total cellular protein was extracted from the TM cells using mammalian protein extraction buffer (MPER; Pierce Biotech, Rockford, IL) containing protease inhibitor (Pierce Biotech) and phosphatase inhibitor (Pierce Biotech) cocktails. Protein concentrations were determined using the Bio-Rad Dc protein assay system (Bio-Rad Laboratories, Richmond, CA). The cellular proteins were separated on denaturing polyacrylamide gels and then transferred to polyvinylidene fluoride (PVDF) membranes by electrophoresis. Blots were blocked with 10% fat-free dry milk in Tris-buffered saline Tween buffer (TBST) for 1 hour and then incubated overnight with primary antibodies (Table 2). The membranes were washed with TBST and processed with corresponding horseradish peroxidase–conjugated secondary antibodies. The proteins were then visualized in a FluorChem 8900 imager (Alpha Innotech, San Leandro, CA) using ECL detection reagent SuperSignal West Femto Maximum Sensitivity Substrate (Pierce Biotech). To ensure equal protein loading, the same blot was subsequently reprobed for GAPDH expression and used for normalization in densitometry analysis.
Table 2. .
List of Antibodies
|
Protein Name |
Primary Antibody |
Dilution |
| GR alpha | Custom-made rabbit polyclonal | 1:2000 for WB |
| GR beta | Custom-made rabbit polyclonal | 1:5000 for WB |
| FN | Millipore, Billerica, MA AB1945; rabbit polyclonal | 1:500 for WB |
| GAPDH | Cell Signaling, Boston, MA 14C10; rabbit monoclonal | 1:1000 for WB |
Cell Viability Assays
To study effect of TSTs on cell viability, CellTiter-Glo (Promega, Madison, WI) was used according to the manufacturer's protocol. Briefly, primary TM cell strains (5000 cells/well; n = 4) were grown in 96-well opaque-bottom plates. Cells were treated with three concentrations of TSTs (10 nM, 100 nM, and 1 μM) for 24 hours. DMSO (0.1%) vehicle was used as a negative control. Medium was removed; 100 μL CellTiter-Glo reagent (containing 1% Triton X-100) was added and incubated for 10 minutes at room temperature, and luminescence signals were recorded using a M200 plate reader (Tecan, Durham, NC).
GRE-Luciferase Reporter Assays
In a 96-well opaque plate (BD Falcon, Franklin Lakes, NJ), 2 × 104 GTM3 cells/well were transfected with 100 ng cignal GRE reporter plasmid (CCS-006L; SA Biosciences, Frederick, MD) and 0.6 μL Surefect transfection reagent (SA Biosciences) with or without TSTs for 24 hours. Forty-eight hours after transfection, cells were treated with or without 100 nM DEX (in ethanol) in DMEM (Invitrogen-Gibco) containing 10% fetal bovine serum (Invitrogen-Gibco), 1% penicillin + streptomycin, and 2 mM glutamine (Thermo Scientific). Six hours later, Dual-Glow substrate (Promega) was added to each well, and the signal was detected with an M200 plate reader (Tecan). Firefly luciferase activity was normalized to renilla luciferase activity. Experiments were performed in triplicate (n = 3).
Statistical Analysis
For comparing results between two groups, Student's t-test was performed. For comparison of results between more than two groups, one-way ANOVA was employed. Statistical tests used for each individual experiment are listed in the respective figure legends (Figs. 2, 5). A P value < 0.05 was considered statistically significant (*P < 0.05; **P < 0.001; ***P < 0.001).
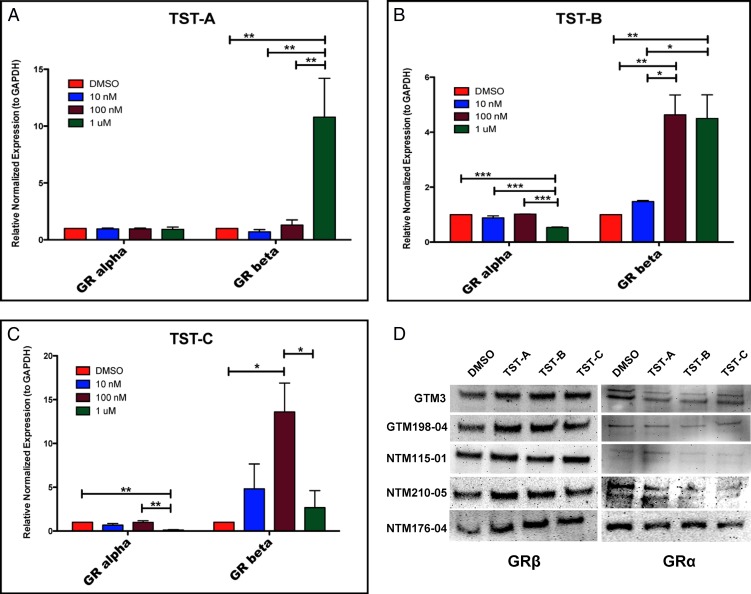
Figure 2.
Thailanstatins increase GRβ levels in TM cells. Primary TM cell strains (n = 4; different TM cell strains) were treated with DMSO or 10 nM, 100 nM, and 1 μM concentrations of TST-A, TST-B, or TST-C for 24 hours. All three TSTs increased GRβ mRNA levels in TM cells. TST-A shows significant induction at 1 μM as compared to the DMSO vehicle control (A) (P < 0.001). TST-B significantly induces GRβ mRNA at 100 nM and 1 μM (B) (P < 0.01) and decreases GRα mRNA levels (B) (P < 0.001). TST-C increases GRβ mRNA levels at 100 nM (C) (P < 0.05) and decreases GRα mRNA levels (C) (P < 0.01). One-way ANOVA was used for statistical analysis. All three TSTs (48 hours) also increased GRβ protein levels in different TM cell strains (D). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5.
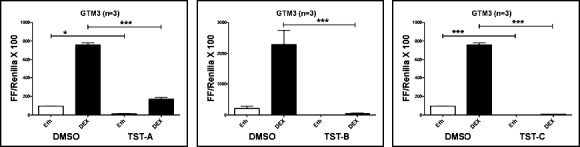
Thailanstatins decrease DEX-mediated GRE-luciferase promoter activity. 1 μM TST-A, 1 μM TST-B, and 100 nM TST-C pretreatment for 24 hours significantly decreased DEX (100 nM, 6 hours)-mediated GRE-luciferase reporter activity in GTM3 cells (n = 3). Mean ± SEM ***P < 0.001 (one-way ANOVA). *P < 0.05.
Results
Effects of Thailanstatins on GRα and GRβ Levels in TM Cells
To investigate the role of TSTs on GR splicing, we treated TM cells (n = 4; different TM cell strains) with different doses of TST-A, TST-B, and TST-C for 24 hours (mRNA) or 48 hours (protein) with DMSO vehicle being used as control. All three TSTs significantly increased GRβ mRNA levels. The concentration for maximum induction depended upon the specific TST. TST-A had a maximum effect at 1 μM, while TST-B and TST-C induced maximally at 100 nM (Figs. 2A, 2B, 2C). Also, 1 μM concentrations of TST-B or TST-C significantly decreased GRα expression as compared to the DMSO vehicle control. Forty-eight-hour treatment with TST-A (1 μM), TST-B (100 nM), and TST-C (100 nM) also significantly (P < 0.05) increased GRβ protein levels (Fig. 2D) with variable effects (same as basal or decrease) on the levels of GRα.
Effects of Thailanstatins on TM Cell Viability
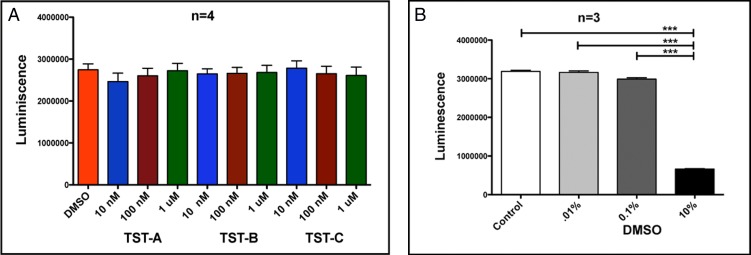
To ensure that the observed changes in the GRα and GRβ mRNA levels were the result of alternative splicing and not due to changes in cell viability cell death, four primary TM cells were treated with 10 nM, 100 nM, and 1 μM each TST for 24 hours compared to the DMSO vehicle control. Cell viability was assessed by the amount of adenosine triphosphate (ATP) produced by metabolically active cells using the CellTiter-Glo system (Promega). The ATP released converts luciferin substrate to luciferin oxide, and released luminescence signals were recorded. Luminescent signals are directly proportional to cell numbers. No significant differences were observed among any treatments (at any concentration) and were similar to the DMSO control (Fig. 3A). There was significant cell death (significant reduction in luminescence activity) at 10% DMSO that served as a positive control (Fig. 3B).
Figure 3.
Thailanstatins do not affect TM cell viability. Twenty-four hours of TST treatment (10 nM, 100 nM, or 1 μM) does not affect TM cell viability as compared to DMSO vehicle control (A), whereas 10% DMSO treatment has significantly lower luminescence signals (B) as compared to 0.1% DMSO (working concentration) serving as positive control. Luminescent readings (y-axis) are directly proportional to cell numbers in a given well. ***P < 0.001.
Effects of Thailanstatins on DEX Activity in TM Cells
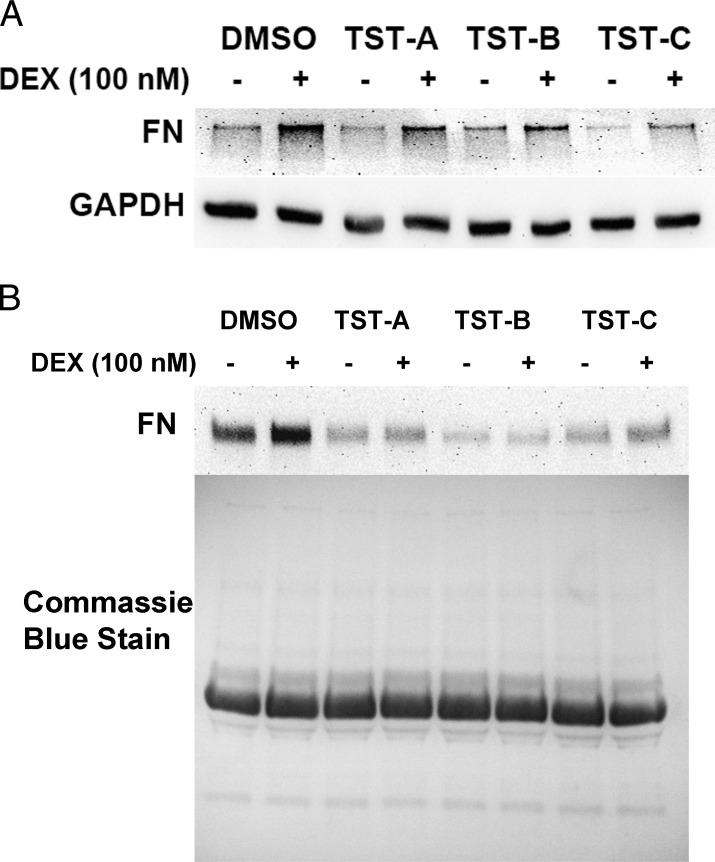
TM cells were pretreated ±TSTs (1 μM for TST-A, 100 nM for TST-B and TST-C) for 48 hours prior to treatment with or without DEX (100 nM) for 3 days. DEX treatment elevated fibronectin (FN) expression compared to its ethanol vehicle control (Figs. 4A, 4B). Pretreatment with TSTs attenuated the DEX-mediated FN induction both in whole cell lysate (Fig. 4A) and in conditioned medium (Fig. 4B).
Figure 4.
Thailanstatins decrease DEX-mediated FN induction in TM cells. Representative data showing 3 days of DEX (100 nM) treatment increases cell-associated FN (n = 4, different primary TM cell strains) (A) and soluble FN in conditioned medium (n = 4) (B). Pretreatment with TST-A (1 μM), TST-B (100 nM), or TST-C (100 nM) for 48 hours decreases DEX-induced FN expression (A, B). Coomassie blue stain was used to stain gels to ensure equal loading in conditioned medium samples.
Effects of Thailanstatins on GRE-Luciferase Reporter Activity
In addition to evaluating the effects of TSTs on the DEX induction of FN, we used a GRE-reporter assay to examine the effects of TSTs on DEX-induced GRE activity. GTM3 cells (n = 3; biological replicates) were transfected with a GRE-luciferase vector followed by treatment with TST-A (1 μM), TST-B (1 μM), TST-C (100 nM), or DMSO vehicle control for 24 hours. The cells were then treated with or without DEX (100 nM), and luciferase activity was determined 6 hours later. Consistent with the FN results, all three TSTs significantly reduced DEX-mediated GRE-luciferase activity as compared to the untreated control (Fig. 5). These results strongly suggest that the TST-mediated increase in GRβ–GRα ratio is associated with decreased DEX responsiveness in TM cells.
Discussion
Long-term GC therapy can lead to ocular hypertension and iatrogenic glaucoma.1,2 However, there are significant differences in GC sensitivities between individuals. Approximately 60% of the population does not develop elevated IOP with GC therapy.10,11 In contrast, almost all glaucoma patients are steroid responders. Expression of the dominant negative isoform GRβ has been associated with a number of steroid-resistant diseases.14–18,35 We have previously shown that GRβ is expressed at lower levels in GTM cells compared to NTM cells and that GTM cells are more responsive to GCs.19 Also, TM cells become more resistant to GCs with increased GRβ expression.
We have also shown that spliceosome SR proteins are involved in GR alternative splicing and GC responsiveness in human TM cells.23 Increased SRp30c and SRp40 expression increased the GRβ–GRα ratios and decreased GC responsiveness in TM cells. The significant decrease in SFRS5.1 (SRp40) gene expression in GTM cells may be responsible for the decreased expression of GRβ in GTM cells.23 Therefore, it may be possible to exploit alternative splicing of the GR to make TM cells more resistant to GCs for therapeutic intervention in steroid-induced glaucoma and POAG.
In our current study, we investigated the role of a special class of spliceosome modulators, the thailanstatins, in GR splicing and GC response in human TM cells. All three TSTs significantly increased GRβ levels in transformed as well as in primary human TM cell strains. In our previous studies, we employed the synthetic bombesin peptide to alter GR splicing.23 However, we now find that the TST compounds are much more potent and efficacious in mediating GRβ expression compared to bombesin. The TSTs work in nanomolar concentrations as compared to micromolar concentrations of bombesin. Increased GRβ levels with these TSTs are associated with decreased DEX response for the induction of FN as well as for GRE-luciferase reporter activity. It is notable that TSTs decrease basal FN levels or GRE-luciferase activity in the absence of DEX. The cell culture medium we used contains cortisol that can contribute to the basal levels of FN (without DEX), so treatment with TSTs alone (that increase GRβ–GRα ratios) may reduce the basal levels of FN as well as GRE activity. Depending upon the GRβ protein levels induced by TSTs, there is variability in inhibitory responses on DEX-induced FN. In addition, the incomplete blockage of DEX-induced FN by TSTs may also be due to GR-independent pathways.
Sharing the pharmacophore similar to that of FR901464 and spliceostatin, we suspect the TSTs may also affect alternative splicing by direct interaction with the SF3b subunit of spliceosome.24,25 Although several of these microbial agents, including FR901464 and pladienolide B, have been proposed to affect mRNA splicing, mechanisms other than direct interaction with RNA splicing machinery may be involved in their activities including increasing mRNA stability, altering the rate of transcription, and/or affecting levels of snRNAs. mRNA splicing is tightly coordinated with mRNA transport and translation, and these agents may also affect these processes. Further studies involving arrays with all known splicing factors, exon junction splice arrays, knockdown studies, and interactions with other known spliceosome inhibitors will help to better understand the mechanisms of action of these TST compounds. Additional studies will also determine whether these compounds can help mitigate GC-induced ocular hypertension and glaucoma.
Acknowledgments
We thank Raghu Krishnamoorthy and Adnan Dibas (University of North Texas Health Science Center) for providing custom-made GR alpha and GR beta antibodies for use. We also thank John Fuller (presently at Johns Hopkins University) and Anirudh Sethi (University of Texas Southwestern Medical Center) for their help in various molecular biological techniques.
Supported by NIH Grants EY016242-06A1 (AFC) and CA152212 (Y-QC).
Disclosure: A. Jain, None; X. Liu, None; R.J. Wordinger, None; T. Yorio, None; Y.-Q. Cheng, None; A.F. Clark, None
References
- 1. Clark AF. Basic sciences in clinical glaucoma: steroids, ocular hypertension, and glaucoma. J Glaucoma. 1995; 4: 354–369 [PubMed] [Google Scholar]
- 2. Wordinger RJ, Clark AF. Effects of glucocorticoids on the trabecular meshwork: towards a better understanding of glaucoma. Prog Retin Eye Res. 1999; 18: 629–667 [DOI] [PubMed] [Google Scholar]
- 3. Yue BY. The extracellular matrix and its modulation in the trabecular meshwork. Surv Ophthalmol. 1996; 40: 379–390 [DOI] [PubMed] [Google Scholar]
- 4. Wordinger RJ, Fleenor DL, Hellberg PE, et al. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007; 48: 1191–1200 [DOI] [PubMed] [Google Scholar]
- 5. Steely HT, Browder SL, Julian MB, Miggans ST, Wilson KL, Clark AF. The effects of dexamethasone on FN expression in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1992; 33: 2242–2250 [PubMed] [Google Scholar]
- 6. Clark AF, Wilson K, McCartney MD, Miggans ST, Kunkle M, Howe W. Glucocorticoid-induced formation of cross-linked actin networks in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 1994; 35: 281–294 [PubMed] [Google Scholar]
- 7. Clark AF, Brotchie D, Read AT, et al. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil Cytoskeleton. 2005; 60: 83–95 [DOI] [PubMed] [Google Scholar]
- 8. Hoare MJ, Grierson I, Brotchie D, Pollock N, Cracknell K, Clark AF. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Invest Ophthalmol Vis Sci. 2009; 50: 1255–1263 [DOI] [PubMed] [Google Scholar]
- 9. Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009; 88: 752–759 [DOI] [PubMed] [Google Scholar]
- 10. Armaly MF, Becker B. Intraocular pressure response to topical corticosteroids. Fed Proc. 1965; 24: 1274–1278 [PubMed] [Google Scholar]
- 11. Lewis JM, Priddy T, Judd J, et al. Intraocular pressure response to topical dexamethasone as a predictor for the development of primary open-angle glaucoma. Am J Ophthalmol. 1988; 106: 607–612 [DOI] [PubMed] [Google Scholar]
- 12. Fingert JH, Alward WL, Wang K, Yorio T, Clark AF. Assessment of SNPs associated with the human glucocorticoid receptor in primary open-angle glaucoma and steroid responders. Mol Vis. 2010; 16: 596–601 [PMC free article] [PubMed] [Google Scholar]
- 13. Hamid QA, Wenzel SE, Hauk PJ, et al. Increased glucocorticoid receptor beta in airway cells of glucocorticoid-insensitive asthma. Am J Respir Crit Care Med. 1999; 159: 1600–1604 [DOI] [PubMed] [Google Scholar]
- 14. Honda M, Orii F, Ayabe T, et al. Expression of glucocorticoid receptor beta in lymphocytes of patients with glucocorticoid-resistant ulcerative colitis. Gastroenterology. 2000; 118: 859–866 [DOI] [PubMed] [Google Scholar]
- 15. Longui CA, Vottero A, Adamson PC, et al. Low glucocorticoid receptor alpha/beta ratio in T-cell lymphoblastic leukemia. Horm Metab Res. 2000; 32: 401–406 [DOI] [PubMed] [Google Scholar]
- 16. Derijk RH, Schaaf MJ, Turner G, et al. A human glucocorticoid receptor gene variant that increases the stability of the glucocorticoid receptor beta-isoform mRNA is associated with rheumatoid arthritis. J Rheumatol. 2001; 28: 2383–2388 [PubMed] [Google Scholar]
- 17. Hamilos DL, Leung DY, Muro S, et al. GRbeta expression in nasal polyp inflammatory cells and its relationship to the anti-inflammatory effects of intranasal fluticasone. J Allergy Clin Immunol. 2001; 108: 59–68 [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Song L, Li B. The expression of glucocorticoid receptor beta messenger RNA in peripheral white blood cells of hormone-resistant nephrotic syndrome patients [in Chinese]. Zhonghua Nei Ke Za Zhi. 2001; 40: 725–728 [PubMed] [Google Scholar]
- 19. Zhang X, Clark AF, Yorio T. Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta. Invest Ophthalmol Vis Sci. 2005; 46: 4607–4616 [DOI] [PubMed] [Google Scholar]
- 20. Zhao YH, Zhou J, Li XX. A study on the relationship between alpha- and beta-isoform of glucocorticoid receptors and glucocorticoid-resistant idiopathic thrombocytopenia purpura [in Chinese]. Zhonghua Nei Ke Za Zhi. 2005; 44: 363–365 [PubMed] [Google Scholar]
- 21. Oakley RH, Sar M, Cidlowski JA. The human glucocorticoid receptor beta isoform. Expression, biochemical properties, and putative function. J Biol Chem. 1996; 271: 9550–9559 [DOI] [PubMed] [Google Scholar]
- 22. Zhang H, Zhao GH, Zhang Q, et al. Relationship between glucocorticoid receptors in the peripheral blood lymphocytes and trabecular meshwork and glucocorticoid induced glaucoma [in Chinese]. Zhonghua Yan Ke Za Zhi. 2006; 42: 431–434 [PubMed] [Google Scholar]
- 23. Jain A, Wordinger RJ, Yorio T, Clark AF. Spliceosome protein (SRp) regulation of glucocorticoid receptor isoforms and glucocorticoid response in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2012; 53: 857–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaida D, Motoyoshi H, Tashiro E, et al. Spliceostatin A targets SF3b and inhibits both splicing and nuclear retention of pre-mRNA. Nat Chem Biol. 2007; 3: 576–583 [DOI] [PubMed] [Google Scholar]
- 25. Kotake Y, Sagane K, Owa T, et al. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat Chem Biol. 2007; 3: 570–575 [DOI] [PubMed] [Google Scholar]
- 26. Albert BJ, Sivaramakrishnan A, Naka T, Czaicki NL, Koide K. Total syntheses, fragmentation studies, and antitumor/antiproliferative activities of FR901464 and its low picomolar analogue. J Am Chem Soc. 2007; 129: 2648–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mizui Y, Sakai T, Iwata M, et al. Pladienolides, new substances from culture of Streptomyces platensis Mer-11107. III. In vitro and in vivo antitumor activities. J Antibiot (Tokyo). 2004; 57: 188–196 [DOI] [PubMed] [Google Scholar]
- 28. Nakajima H, Hori Y, Terano H, et al. New antitumor substances, FR901463, FR901464 and FR901465. II. Activities against experimental tumors in mice and mechanism of action. J Antibiot (Tokyo). 1996; 49: 1204–1211 [DOI] [PubMed] [Google Scholar]
- 29. Lagisetti C, Pourpak A, Goronga T, et al. Synthetic mRNA splicing modulator compounds with in vivo antitumor activity. J Med Chem. 2009; 52: 6979–6990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lagisetti C, Pourpak A, Jiang Q, et al. Antitumor compounds based on a natural product consensus pharmacophore. J Med Chem. 2008; 51: 6220–6224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu X, Biswas S, Berg MG, et al. Genomics-guided discovery of thailanstatins A, B and C as potent pre-mRNA splicing inhibitors and antiproliferative agents from Burkholderia thailandensis MSMB43 [published online ahead of print March 21, 2013] J Nat Prod. doi:10.1021/np300913h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson K, McCartney MD, Miggans ST, Clark AF. Dexamethasone induced ultrastructural changes in cultured human trabecular meshwork cells. Curr Eye Res. 1993; 12: 783–793 [DOI] [PubMed] [Google Scholar]
- 33. Wordinger RJ, Clark AF, Agarwal R, et al. Cultured human trabecular meshwork cells express functional growth factor receptors. Invest Ophthalmol Vis Sci. 1998; 39: 1575–1589 [PubMed] [Google Scholar]
- 34. Pang IH, Shade DL, Clark AF, Steely HT, DeSantis L. Preliminary characterization of a transformed cell strain derived from human trabecular meshwork. Curr Eye Res. 1994; 13: 51–63 [DOI] [PubMed] [Google Scholar]
- 35. Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol. 2003; 85: 457–467 [DOI] [PubMed] [Google Scholar]