Abstract
Purpose.
To investigate the impact on visual performance of modifying monovision with monocularly induced spherical aberration (SA) to increase depth of focus (DoF), thereby enhancing binocular through-focus visual performance.
Methods.
A binocular adaptive optics (AO) vision simulator was used to correct both eyes' native aberrations and induce traditional (TMV) and modified (MMV) monovision corrections. TMV was simulated with 1.5 diopters (D) of anisometropia (dominant eye at distance, nondominant eye at near). Zernike primary SA was induced in the nondominant eye in MMV. A total of four MMV conditions were tested with various amounts of SA (±0.2 and ±0.4 μm) and fixed anisometropia (1.5 D). Monocular and binocular visual acuity (VA) and contrast sensitivity (CS) at 10 cyc/deg and binocular summation were measured through-focus in three cyclopledged subjects with 4-mm pupils.
Results.
MMV with positive SA had a larger benefit for intermediate distances (1.5 lines at 1.0 D) than with negative SA, compared with TMV. Negative SA had a stronger benefit in VA at near. DoF of all MMV conditions was 3.5 ± 0.5 D (mean) as compared with TMV (2.7 ± 0.3 D). Through-focus CS at 10 cyc/deg was significantly reduced with MMV as compared to TMV only at intermediate object distances, however was unaffected at distance. Binocular summation was absent at all object distances except 0.5 D, where it improved in MMV by 19% over TMV.
Conclusions.
Modified monovision with SA improves through-focus VA and DoF as compared with traditional monovision. Binocular summation also increased as interocular similarity of image quality increased due to extended monocular DoF.
Keywords: aberrations, presbyopia, monovision, binocular adaptive optics
Through-focus binocular visual performance is improved in modified monovision by introducing spherical aberration to the nondominant eye.
Introduction
Presbyopia, the age-related loss of accommodation, mostly affects the population aged over approximately 50 years.1–3 Due to the prevalence of presbyopia, there has been a great deal of effort toward its correction. While a true restoration of accommodation does not yet exist, there are many strategies that aim to optically recover near vision, the most common of which include reading glasses and bifocals. Accommodative intraocular lenses (IOLs) represent an option with promise of dynamic power change of the eye, yielding a genuine restoration of accommodation; however, their results are inconsistent.4,5 The other common approach to presbyopic correction is pseudoaccommodation, or the optical increase of depth of focus (DoF). This can be achieved by restricting the pupil with a small-aperture corneal inlay,6–8 or with multifocal optical corrections that can be implemented with contact lenses,9,10 corneal refractive surgery,11,12 or IOLs.13–15 Multifocal optics can improve presbyopic through-focus visual performance; however, many previous studies evaluating their efficacy are based on monocular visual performance.16,17
Monovision is a technique that utilizes the binocular nature of the visual system. In traditional monovision, anisometropia is induced to the nondominant eye, thereby correcting it for near vision, whereas the dominant eye remains corrected for distant vision.18–20 Via suppression of the blurred retinal image of the defocused eye,21 through-focus binocular visual acuity (VA) is approximately determined by the monocular acuity of the optically superior eye.22,23 Monovision can be implemented with contact lenses,22,24,25 corneal refractive surgery,26–29 or monofocal IOLs following crystalline lens removal in cataract surgery.20,30–32
Despite decades of clinical application, the ideal degree of anisometropia in monovision is still a topic of active research.19,20,33,34 The optimal power difference between the two eyes has been reported to vary between 1.0 and 2.0 diopters (D), depending on the need according to the patient's lifestyle or occupation.19,33,34 It has been shown that anisometropia greater than 1.0 D is effective for improving distance-corrected near VA.33,34 However, anisometropia greater than 2.0 D can significantly reduce intermediate VA and contrast sensitivity (CS).19,35 Furthermore, monocular blur is known to significantly degrade stereoacuity, or depth perception.19,22,25,33,36–38
Binocular summation is known to decrease as the interocular difference in retinal image quality increases.35,39–41 Binocular summation refers to the ratio of binocular to monocular CS. In a seminal study, Campbell and Green observed a binocular summation factor of √2 at each spatial frequency (using 2.8-mm pupils and no anisometropia).42 Pardhan and Gilchrist found that binocular summation at distance decreased as anisometropia in monovision increased.35 Interestingly, they found that when anisometropia was between 2.0 and 2.5 D, CS at 6 cyc/deg is poorer with binocular viewing than monocular viewing with the distance eye—that is, the binocular summation factor was below unity. Moreover, Jiménez et al. found that interocular inequality of higher-order aberrations also has a significant impact on binocular summation.39,40
Therefore, while traditional monovision can improve VA for object distances from far to near (i.e., through-focus), binocular visual functions (e.g., summation and stereoacuity) tend to suffer. To overcome the limitations to traditional monovision, recently, Reinstein et al. have proposed the use of nonlinear aspheric ablation profiles in refractive surgery aimed at leaving the monovision patient with residual SA, intended to increase each eye's DoF.28,29,43 Due to the nature of SA induction in LASIK,44 this surgical technique leaves hyperopic patients with residual negative SA,43 and myopic patients with residual positive SA.45 Although both positive and negative SAs increase DoF, they have different effects on through-focus retinal image quality.46
It was the purpose of this study to examine the impact of positive and negative SA with different magnitudes in modified monovision on through-focus visual performance. It is proposed that by extending the DoF of the nondominant eye, through-focus retinal image quality improves as well as the congruence of the image of the two eyes, leading to an improvement in binocular summation.35
Methods
Subjects
This research was approved by the University of Rochester Research Review board and informed consent was obtained from all subjects before their participation. All procedures involving human subjects were in accordance with the tenets of the Declaration of Helsinki. Three normal emmetropic subjects were recruited for this study (age: 34 ± 11 years). All subjects' pupils were dilated and accommodation was paralyzed with cyclopentolate hydrochloride (1%). Although the subjects were younger than the typical presbyope, their accommodative ability was fully impaired due to the cycloplegia.
Binocular AO Vision Simulator
The binocular AO vision simulator consisted of two identical monocular AO vision simulators operating simultaneously (one for each eye). Intersubject variability of interpupillary distance (IPD) was corrected using translational stages which can accommodate IPDs ranging from 50 to 80 mm, in accordance with the population statistics.47 The binocular AO vision simulator is described in detail elsewhere.48 Each monocular AO system was comprised of a custom-made Shack-Hartmann wavefront sensor; a large stroke deformable mirror (Mirao-52; Imagine Eyes, Orsay, France); a Badal optometer; an artificial pupil; and a visual stimulus display. The deformable mirror was used in closed-loop (12 Hz) to manipulate the subjects' wavefront aberrations in real-time. The wavefront sensor laser beacons were produced by a super-luminescent diode with center wavelength of 840 nm and a bandwidth of 40 nm. Badal optometers were used to control object distance for through-focus vision testing. Vision testing occurred in white light with the subject viewing through a 4-mm artificial pupil. To aid in maintaining fusion, artificial apertures conjugate to the subject's retinal planes were used as peripheral fusion locks. The fusion locks subtended 2° of visual field and were centered about the visual performance stimuli (1°). During binocular testing, subjects reported single vision. A dental-impression bite bar mounted to a translational stage was used to stabilize head movements. Subjects' pupil alignment was maintained continuously with a camera focused at the pupil planes.
Testing Conditions
For eye-assignment in monovision, ocular dominance is typically assessed using sighting tests, after which the dominant eye is corrected for distance vision and nondominant eye for near vision.20 This study used the “hole-in-card” sighting test49 to determine ocular dominance in all subjects. In this test, the subject held with both hands a rectangular white card (8.5 × 11 inches) at arm's length. The card had a central aperture (0.5-inch diameter) through which the subject was directed to view (with both eyes open) a distant target of a cross. The subject then had each eye occluded. The eye which retained a clear view of the target was determined to be the dominant eye.
Subjects' lower and higher order native aberrations of both eyes were fully corrected in all monovision conditions in this study with adaptive optics. Due to the significant longitudinal chromatic aberration between the visible spectrum and the wavefront sensing light source (840 nm), subjects found their far point (0 D) by adjusting defocus with the Badal optometer to optimize the image quality of a 20/40 Snellen letter “E” viewed through a 4-mm pupil with astigmatism and higher order aberrations corrected with AO. This process was repeated for both eyes separately.
All monovision conditions included 1.5 D of anisometropia in the nondominant eye (induced with the Badal optometer), as previous literature has suggested this level of anisometropia to be favorable.33 Four modified monovision conditions were tested, and consisted of spherical aberration induction in the nondominant eye of ±0.2 and ±0.4 μm (for a 4-mm pupil). Traditional monovision was also measured for comparison, in which both eyes had zero spherical aberration.
Theoretical Modeling of Through-Focus Retinal Image Quality
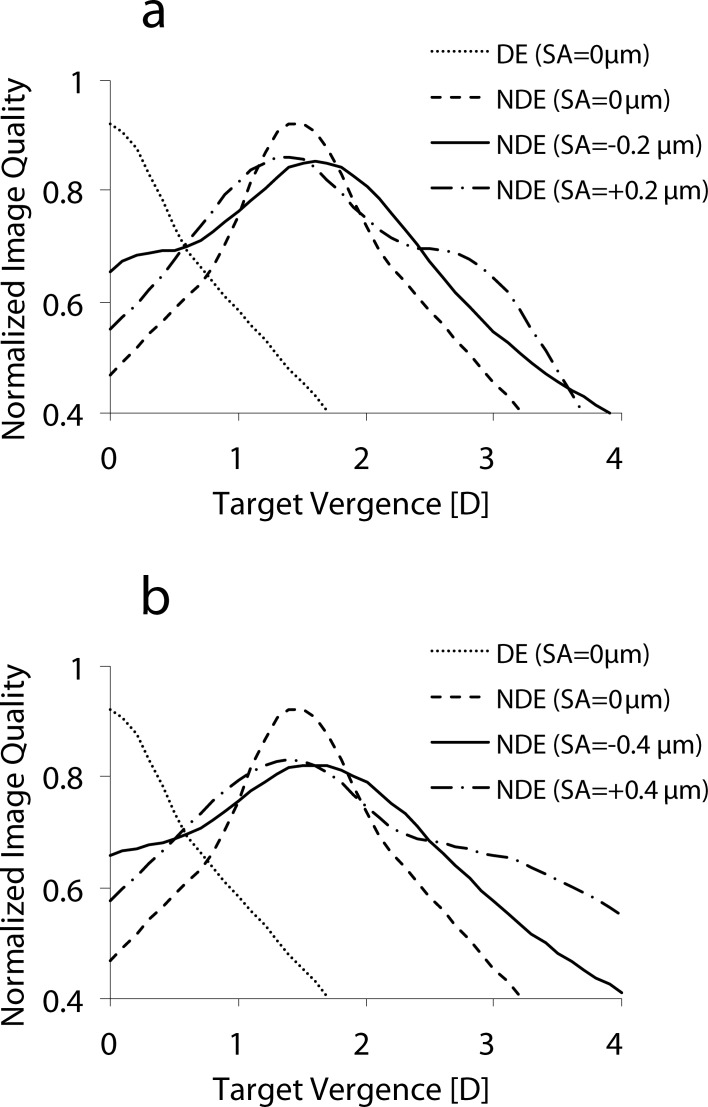
Theoretically calculated monocular through-focus retinal image quality for the dominant and nondominant eyes in traditional and modified monovision conditions is illustrated in Figure 1. Through-focus image quality was simulated by using a custom-developed computing program (MATLAB; MathWorks, Natick, MA) to calculate an image convolution based retinal image quality metric adapted from that described by Watson and Ahumada.50 The metric proposed here differed in that it was calculated for polychromatic light, incorporated the Stiles-Crawford effect, and did not take into account neural factors of the visual system. To compute the metric, at each through-focus position, the ocular polychromatic point spread function (405–695 nm wavelength, weighted by the photopic spectral sensitivity function Vλ) is calculated from the wavefront aberration. Using values published by Applegate and Lakshminarayan,51 the Stiles-Crawford effect is incorporated in the point spread function by apodizing the pupil's amplitude. The point spread function is then convolved with a reference image to yield a simulated image. Finally, the correlation coefficient of the convolved (aberrated) and reference (unaberrated) images is calculated to give to the final image quality metric value. In this study, a tumbling letter “E” chart was used as the reference image. The tumbling “E” letter chart included letter sizes ranging from −0.3 to +0.3 log units of minimum angle of resolution in arc minutes (logMAR) to ensure a broad spatial frequency spectrum.
Figure 1.
(a) Theoretical simulation of through-focus image quality for the dominant eye (DE) and nondominant eye (NDE) with SA = 0, −0.2, and +0.2 μm. (b) Theoretical simulation of through-focus image quality for the DE and NDE with SA = 0, −0.4, and +0.4 μm. The DE was aberration-free. In the case of SA in the NDE, defocus was added to bring peak image quality to 1.5 D of anisometropia.
To obtain a single-value retinal image quality metric to represent binocular visual performance, interocular blur suppression was incorporated into the model. At each through-focus position, the better of the two eyes' image quality metric value was taken to represent a single binocular metric value.
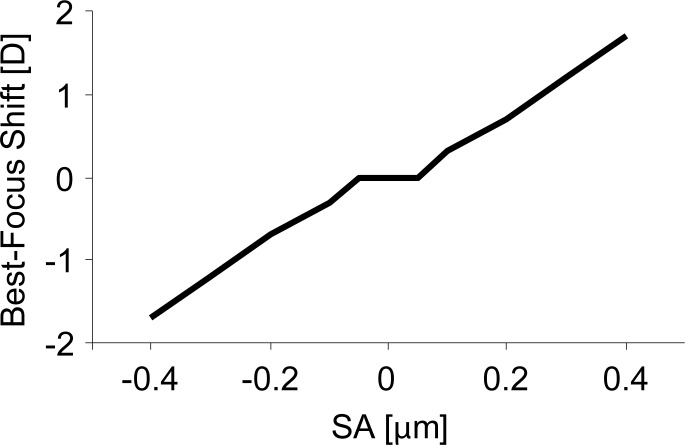
Introduction of positive Zernike primary SA into a diffraction limited eye causes a hyperopic shift of best focus,16,17,52,53 requiring the addition of positive power to optimize image quality at distance (0 D). Alternatively, negative Zernike primary SA causes a myopic shift of best focus, requiring the addition of negative power to optimize image quality at distance (0 D). The defocus required to correct for the shift in peak image quality caused by Zernike primary SA is illustrated in Figure 2.
Figure 2.
The defocus required to correct for the shift in peak image quality caused by Zernike primary SA for a 4-mm pupil.
Through-Focus Visual Performance Testing
Visual performance was assessed both binocularly and monocularly with high-contrast VA and CS. During monocular measurements, an eye patch occluded the eye not being measured. VA and CS were measured over a range of target vergences (i.e., through-focus), spanning from distance (0 D) to near (positive diopters).
High-contrast VA was measured with a black tumbling letter “E” on a white background using a four-alternate forced-choice method. A digital light projector (Sharp PG-M20X; Sharp Corporation, Abeno-ku, Osaka, Japan), conjugate to the retinal plane for each eye, was used to present the visual stimulus with retinal illuminance of 70 cd/m2 for a 4-mm pupil. A psychometric function based on 30 trials was obtained using the QUEST54 algorithm where VA was defined as the letter size for which 62.5% of responses were correct. Three acuity measurements were averaged for each optical condition and are represented in units of logMAR.
Binocular through-focus VA was measured for all modified and traditional monovision conditions, listed above. Monocular through-focus VA was measured with three magnitudes of induced SA in the nondominant eye: 0, +0.2, and +0.4 μm. DoF was defined as the dioptric range from distance (0 D) to near (positive diopters) for which VA was better than 0.18 logMAR (20/30 Snellen acuity).
CS was measured with Gabor stimuli at 10 cyc/deg using a two-alternate forced choice method. A cathode-ray tube monitor (NEC MultiSync FP950; NEC, Itasca, IL) was used to display the visual stimulus with retinal illuminance of 5 cd/m2 for a 4-mm pupil. A psychometric function based on 40 trials was obtained using the QUEST54 algorithm. The threshold was defined as the contrast for which 75% of responses were correct. Three threshold measurements were averaged for each optical condition.
Binocular through-focus CS was measured for modified monovision conditions with ±0.2 μm of SA in the nondominant eye and traditional monovision. Monocular through-focus CS was measured with three magnitudes of SA in the nondominant eye: 0 and ±0.2 μm. Monocular through-focus CS was measured in the dominant eye with full aberration correction. The binocular summation factor was defined as the ratio of the binocular CS to the monocular CS of the better eye.55
Statistical Analysis
We employed a mixed ANOVA to look at the association of visual performance as a function of defocus and monovision condition and interaction of these effects; the models included random intercepts to account for potential within-subject dependencies. Our interest resided in the difference between modified and traditional monovision conditions at each object distance, and these comparisons were carried out as Wald tests subsequent to the overall analysis. Using a Bonferroni correction to adjust for multiple comparisons, statistical significance was indicated with P values less than or equal to 0.0016 and 0.0031 for VA and CS, respectively. All analyses were carried out with commercial software (SAS 9.3; SAS Institute Inc., Cary, NC) on a Windows 7 platform (Microsoft, Redmond, WA).
Results
Through-Focus Visual Acuity
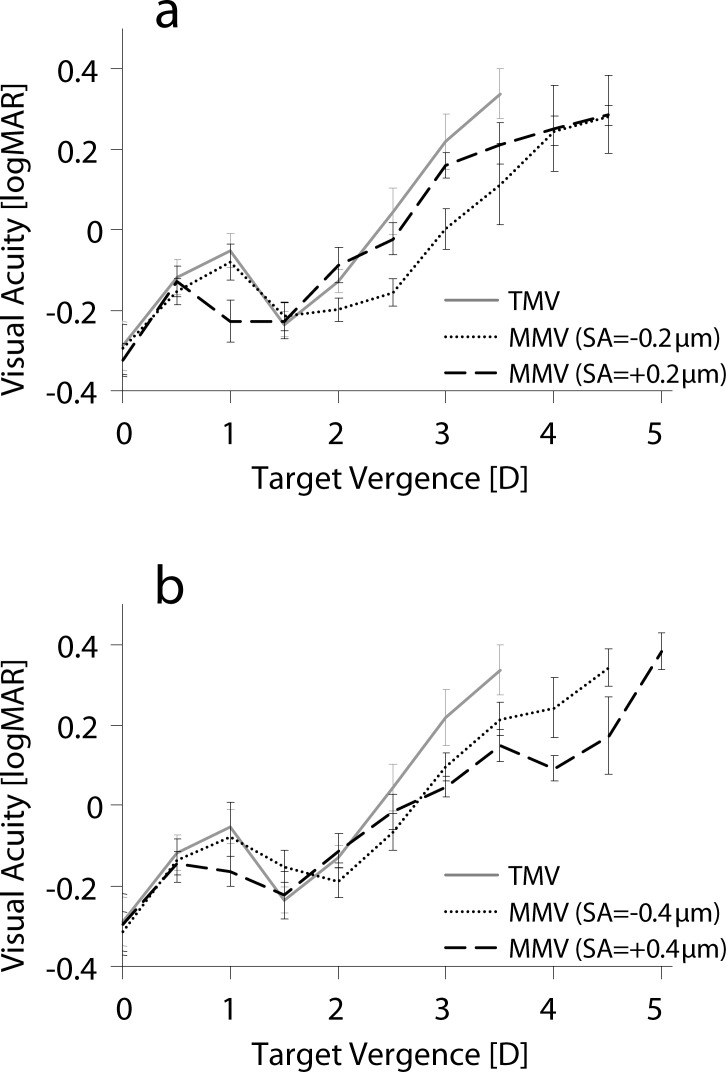
The main effects of defocus, and monovision condition and the interaction of defocus and monovision condition on through-focus VA were significant, as shown in Table 1. Figure 3 summarizes the binocular through-focus VA measurements for traditional and modified monovision conditions. VA is plotted in logMAR as a function of defocus in diopters, where increasing values indicate nearer objects. The error bars represent the standard deviation of three subjects.
Table 1. .
ANOVA Results for Through-Focus VA
|
Statistical Analysis for Visual Acuity | ||||
|
Effect |
Numerator DF |
Denominator DF |
F Value |
Pr > F |
| Defocus | 7 | 76 | 393.13 | <0.0001 |
| Monovision condition | 4 | 76 | 25.30 | <0.0001 |
| Defocus × condition | 28 | 76 | 8.10 | <0.0001 |
Figure 3.
Through-focus VA for traditional (TMV) and modified monovision (MMV) with (a) ±0.2 μm and (b) ±0.4 μm of SA.
Distance VA (0 and 0.5 D) with traditional monovision was −0.29 ± 0.06 and −0.12 ± 0.04 logMAR, respectively, and was not significantly different for the modified monovision conditions (P > 0.11). At the intermediate object distance of 1 D, traditional monovision resulted in VA −0.05 ± 0.04 logMAR, whereas modified monovision with positive SA of 0.2 and 0.4 μm significantly improved VA (−0.23 ± 0.02 and −0.17 ± 0.01 logMAR, P < 0.0001, respectively). Negative SA in the nondominant eye also improved VA at 1.0 D as compared with traditional monovision; however, this improvement was not statistically significant.
At the anisometropic point of 1.5 D, VA with traditional monovision was −0.23 ± 0.03 logMAR. Inducing ±0.2 μm of SA in the nondominant eye did not have a significant impact on VA at 1.5 D; however, with −0.4 μm of SA, a reduction of 0.08 logMAR was observed at 1.5 D (P = 0.0023).
Near VA (beyond 2.0 D) was significantly improved with all modified monovision conditions as compared with traditional monovision. However, modified monovision with SA = −0.2 μm had the greatest benefit in VA at object distances of 2.0 to 3.5 D, whereas the SA = +0.4 μm condition had the greatest improvement beyond 3.5 D. Binocular DoF in modified monovision (nondominant eye SA = −0.4, −0.2, +0.2, +0.4 μm) was 3.2 ± 0.3, 3.5 ± 0.0, 3.2 ± 0.8, and 4.2 ± 0.3 D, respectively, as compared with 2.7 ± 0.3 D found in traditional monovision.
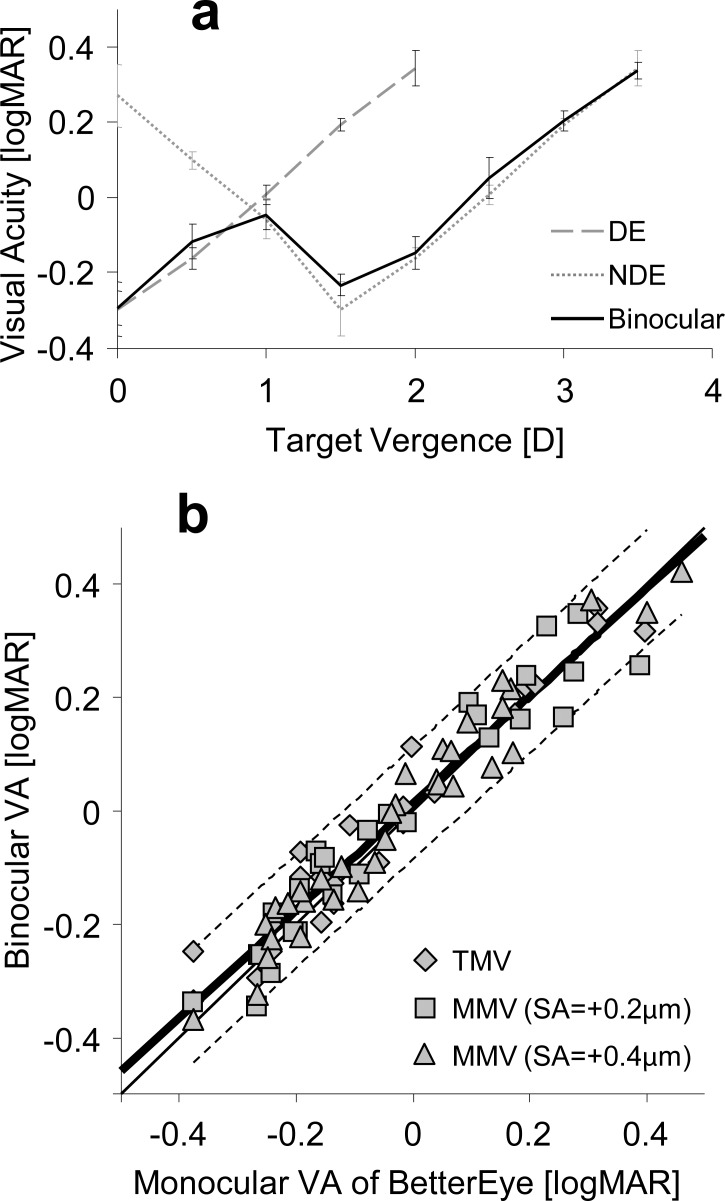
Binocular VA was not significantly different from the monocular VA of the better eye at each through-focus position for traditional monovision (Fig. 4a) and modified monovision. The correlation of binocular VA to the better monocular VA at each target vergence for traditional and modified monovision (r = 0.97) is shown in Figure 4b.
Figure 4.
(a) Binocular and monocular (DE, NDE) through-focus VA for both eyes in traditional monovision. (b) Binocular VA plotted as a function of the monocular VA of the better eye for each object distance for traditional monovision and modified monovision with positive SA. The thick solid line represents the linear fit. The dashed lines represent the 95% confidence intervals. The thin solid line represents the equal-acuity line.
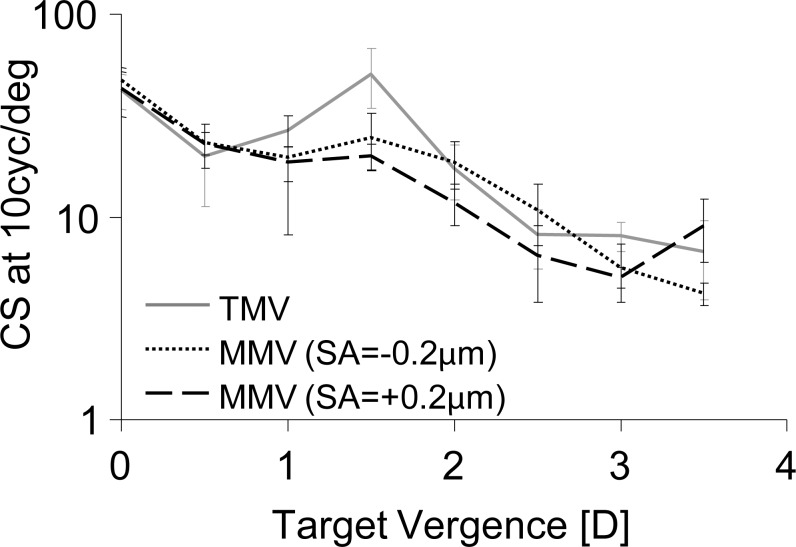
Through-Focus Contrast Sensitivity
The main effects of defocus, monovision condition, and the interaction of defocus and monovision condition on through-focus CS were significant, as shown in Table 2. Through-focus binocular CS for traditional and modified monovision with ±0.2 μm of SA induced in the nondominant eye is shown in Figure 5. Distance and intermediate CS for 10 cyc/deg (0–1.0 D) was not significantly affected in modified monovision. However, at 1.5 D, induction of SA in the nondominant eye in modified monovision significantly degraded CS with respect to traditional monovision (P < 0.0003). Binocular CS at 1.5 D degraded by factors of 2.1 and 2.5, for −0.2 and +0.2 μm of SA, respectively. Modified monovision did not significantly impact CS with respect to traditional monovision at near object distances (2.0–3.5 D, P > 0.02). However, modified monovision with +0.2 μm of SA had worse binocular CS than with −0.2 μm of SA at 2.0 and 2.5 D object distances.
Table 2. .
ANOVA Results for Through-Focus Contrast Sensitivity
|
Statistical Analysis for Contrast Sensitivity | ||||
|
Effect |
Numerator DF |
Denominator DF |
F Value |
Pr > F |
| Defocus | 7 | 48 | 88.68 | <0.0001 |
| Monovision condition | 2 | 48 | 6.08 | 0.0044 |
| Defocus × condition | 14 | 48 | 3.89 | 0.0002 |
Figure 5.
Through-focus binocular CS at 10 cyc/deg for traditional and modified monovision with ±0.2 μm SA.
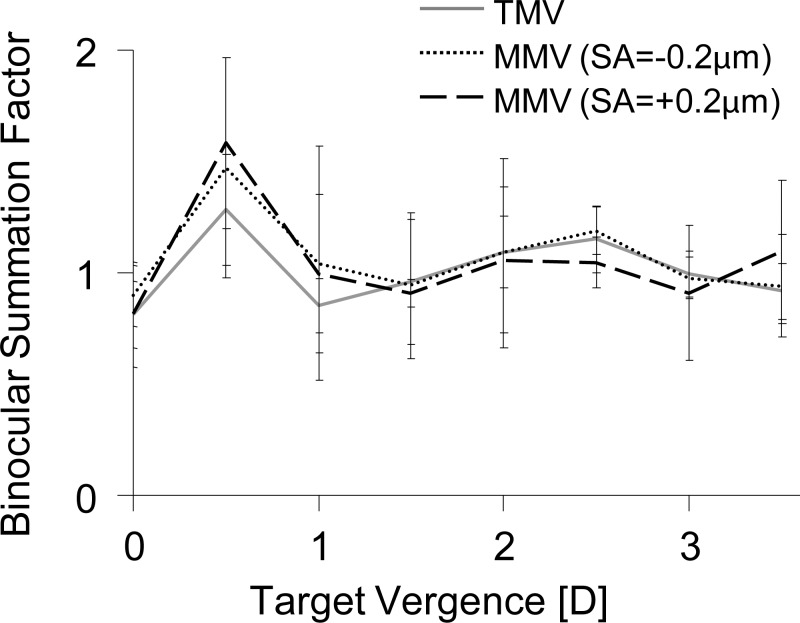
Through-focus binocular summation is shown in Figure 6. At distance (0 D), the binocular summation factor was close to unity for all three monovision conditions. In traditional monovision, binocular summation increased at the 0.5 D position to 1.28 ± 0.25. For modified monovision with −0.2 and +0.2 μm of SA, the average binocular summation factor at 0.5 D was 1.47 ± 0.49 and 1.58 ± 0.38, respectively; however, this improvement was not statistically significant. Beyond 0.5 D, the average binocular summation factor for traditional and modified monovision conditions were approximately unity.
Figure 6.
Through-focus binocular summation factor at 10 cyc/deg for traditional and modified monovision with ±0.2 μm SA.
Image Convolution Based Retinal Image Quality Metric
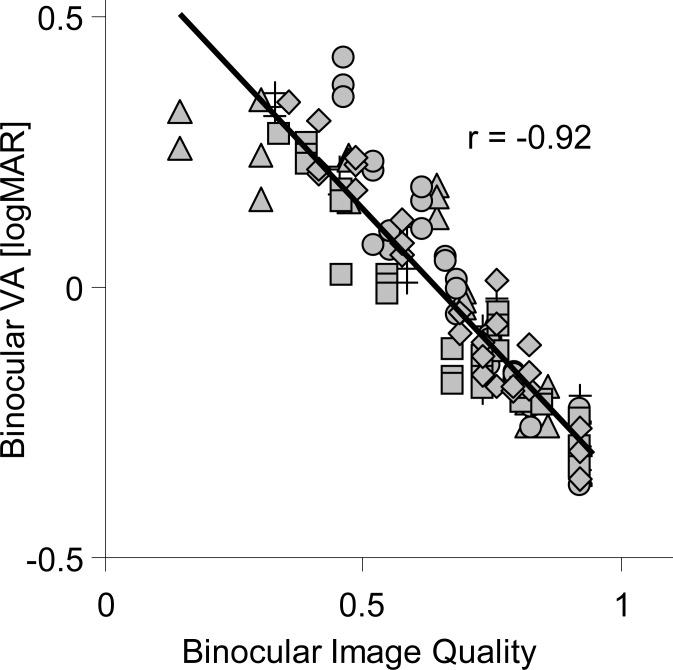
Figure 7 shows the correlation of the binocular visual performance metric with measured binocular through-focus VA of traditional and modified monovision paradigms. A high correlation (r = −0.92) was found between the image convolution-based image quality metric and binocular through-focus VA. For comparison, the same dataset was applied to other image quality metrics,56 such as the log of the visual Strehl ratio (VSOTF), the log of the area under the modulation transfer function (aMTF), and the log of the Strehl ratio. The correlations of binocular through-focus VA with all monovision paradigms and log(VSOTF), log(aMTF), and log(Strehl) were r = −0.86, −0.85, and −0.79, respectively.
Figure 7.
Binocular VA versus binocular image quality, as calculated with the image convolution based image quality metric. Cross: TMV. Diamond: MMV (SA = −0.4 μm). Square: MMV (SA = −0.2 μm). Triangle: MMV (SA = +0.2 μm). Circle: MMV (SA = +0.4 μm).
Discussion
The presbyopic-correction strategy of monovision is clinically established and has been routinely practiced for decades. However, relatively few studies have investigated the relative contribution of each eye's optical quality to through-focus binocular visual performance in monovision. In this study, we used a binocular AO vision simulator to investigate the impact of modifying monovision by extending the DoF of the nondominant eye with SA. We found that modified monovision led to a substantial benefit in through-focus VA and binocular DoF as compared with traditional monovision. However, this improvement came at the cost of a reduction in CS at the anisometropic point of 1.5 D. Despite the degradation in CS for intermediate object distances, through-focus interocular image quality became more similar in modified monovision, leading to an improvement in binocular summation, particularly at 0.5 D. In addition, we found that binocular through-focus high-contrast VA in modified and traditional monovision was determined by the monocular performance of the better eye at each dioptric position. This result can provide a useful guideline for theoretical models of binocular through-focus visual performance.
The VA benefit of modified monovision was dependent on the sign and magnitude of SA induced in the nondominant eye. The sign of SA determined the defocus points of VA benefit within the vicinity of the anisometropic point. Positive SA (+0.2 μm) resulted in a significant benefit in VA at intermediate object distance (1.0 D), whereas negative SA (−0.2 μm) resulted in a significant benefit at near (2.0 D). The largest DoF was observed with modified monovision with +0.4 μm of SA. The benefit to DoF with +0.4 μm SA may be in part explained by the influence of phase transfer function on the retinal image.57,58 As explained by Ravikumar et al.,58 when defocus and SA are of the same sign (i.e., myopic defocus and positive SA), the phase-reversed regions of the modulation transfer function have relatively little energy and therefore a small contribution to the retinal image. Therefore, the influence of the sign of SA on through-focus image quality is determined by both the amplitude and phase of spatial frequencies in the retinal image.
The laser blended vision approach described by Reinstein et al. also takes advantage of refractive surgery–induced SA to extend monocular DoF in monovision. They found that subjects with positive postoperative SA were more likely to have near VA of 0.18 logMAR or better, as compared with those with negative postoperative SA.43 Similarly, our study found the large magnitude of positive SA (+0.4 μm) resulted in greater benefit for near VA than negative SA. Moreover, their studies28,29,43 support our finding that extending monocular DoF with SA in monovision provides significant benefits over traditional monovision.
In addition to near VA, extending monocular DoF in modified monovision offers the further benefit of reducing the difference in interocular retinal image quality. Ocular DoF may be extended by means of a small-aperture corneal inlay,6–8 multifocal optical designs,13,14 and certain combinations of Zernike primary and secondary SAs.16,17,52 These techniques (diffraction, multifocality, higher-order aberrations) are viable options for reducing the difference in through-focus interocular retinal image quality in modified monovision.
Interocular difference in retinal image quality has been shown to compromise aspects of visual function which rely on the neural combination of two monocular channels into a single binocular percept, such as binocular summation34,35,39,40 and stereopsis.22,25,33,36–39 Pardhan and Gilchrist35 described a reduction in binocular summation as anisometropia increased. Their study found that in the absence of anisometropia, the binocular summation at 6 cyc/deg was approximately 40%, similar to Campbell and Green's finding of 41%.42 However, binocular summation degraded with anisometropia, reaching unity (no summation) at 1.5 D anisometropia, beyond which summation was <1, indicating binocular inhibition. Similarly, Loshin et al. found that binocular summation to be absent in monovision with add powers of 1.5 D and higher for mid to high spatial frequencies.59 The results presented in this study also show distance binocular summation (at 10 cyc/deg) to be absent (near unity) with 1.5 D of anisometropia (for both traditional and modified monovision). It is important to note that the benefits in binocular summation with modified monovision may vary at additional spatial frequencies. By examining the monocular retinal image quality curves in Figure 1b, it is clear that the interocular difference in retinal image quality reached a minimum in the neighborhood of 0.5 D. Therefore, it was not surprising to observe a peak in binocular summation at 0.5 D for traditional and modified monovision. At 0.5 D, the binocular summation improved by 19 ± 6% for the two modified monovisions as compared with traditional monovision. However, at intermediate and near object distances, no summation was observed.
In an investigation of the effect of anisometropia on distance VA, Collins et al. found that binocular acuity at distance was approximately equal to the monocular acuity of the distance-corrected eye.23 This can be attributed to what Schor et al. described as interocular suppression of anisometropic blur.21 The current study extends this finding from distance performance to include intermediate and near object positions. As shown in Figure 4b, a strong correlation (r = 0.97) between binocular and monocular (of the superior eye) through-focus VA was observed for both traditional and modified monovision. This finding was applied to the theoretical model that produced the monocular through-focus image quality curves of Figure 1. The model produced a single-value retinal image quality metric for predicting through-focus binocular VA (r = −0.92) in modified and traditional monovision. It should be noted that such a model may not be directly applicable to the early presbyope who may still have some degree of active accommodation. Also, the application of a single-value metric to the prediction of CS may be confounded by factors such as differences in spatial frequency content of the metric and the stimulus58,60,61 and the neural contrast sensitivity function.62–65 While some variability in the metric predictability among monovision conditions was found, the image convolution metric provides a robust estimate of binocular visual acuity in the presence of large amount of blur and in the case when the two eyes have significantly different image quality. A reliable metric that enables the prediction of binocular through-focus visual performance is important for the design and optimization modified monovision variables.
At the anisometropic point (1.5 D), the induction of ±0.2 μm of SA did not have a significant impact on VA. In the aberration-free case of traditional monovision, binocular VA at 1.5 D was −0.23 logMAR as compared with −0.20 ± 0.04 logMAR, the average of all modified monovision paradigms. However, CS at 1.5 D was significantly reduced in modified monovision. Modified monovision with ±0.2 μm of SA resulted in a CS reduction at 1.5 D by a factor of 2.3 ± 0.3, as compared with traditional monovision. This can be explained by examining the modulation transfer function, which shows an approximately factor of 3 decrease in contrast of a 10 cyc/deg sinusoidal grating due to +0.2 μm of SA. At best focus, positive and negative SAs have the same effect on the modulation transfer function. This result was in agreement with a study by Piers et al., in which they confirmed that CS is maximized when ocular SA is fully corrected.66
A limitation of this study was that the experimental protocol used cyclopentolate to dilate the pupils and arrest accommodation. In natural viewing conditions, accommodative effort for viewing near objects is accompanied by pupil miosis, thereby extending ocular DoF due to diffraction. However, pupil miosis also reduces the magnitude of SA (i.e., wavefront multifocality) and can lead to changes in refraction.67 It is important for the topic of pupil and wavefront interaction and the consequences on through-focus image quality to be addressed; however, this was not the goal of the current investigation. Furthermore, it will be important to study the effectiveness of modified monovision in relation to practical factors such as native higher order aberrations, interocular scatter, and other age-related deficits in vision.
For future work, it will be interesting to investigate the role of neural adaptation to traditional monovision and modified monovision. Collins et al. found that during the first 8 weeks of monovision contact lens wear, patients subjectively observed an improvement in some aspects of visual performance, such as walking confidence and hand-eye coordination.68 Interestingly, this trend was not observed in objective measures (VA, stereoacuity, and blur suppression). In addition, depth perception, a key component to binocular vision, was not examined in this study. It will be interesting to investigate the impact of modified monovision on stereoacuity. It is well-known that anisometropia leads to a reduction in stereoacuity.19,22,25,33,36–38 However, by extending the DoF of one or both eyes in modified monovision, the interocular difference in retinal image quality is reduced and may lead to an improvement in stereoacuity.24
Conclusions
It was found that modified monovision in which SA is induced in the nondominant eye improved through-focus VA and DoF. Binocular summation also increased as the interocular optical quality became more similar. Therefore, significant visual benefit from extending the DoF by inducing SA to one or both eyes can be a promising binocular approach to overcoming presbyopia.
Acknowledgments
Supported by NIH UL1 TR000042, NIH/NEI EY014999, RPB, and NIH Training Grant T32 EY007125. The authors alone are responsible for the content and writing of the paper.
Disclosure: L. Zheleznyak, None; R. Sabesan, None; J.-S. Oh, None; S. MacRae, None; G. Yoon, None
References
- 1. Duane A. Normal values of the accommodation at all ages. JAMA. 1912; 59: 1010 [Google Scholar]
- 2. Croft MA, Glasser A, Kaufman PL. Accommodation and presbyopia. Int Ophthalmol Clin. 2001; 41: 33–46 [DOI] [PubMed] [Google Scholar]
- 3. Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye—aging of the anterior segment. Vision Res. 1989; 29: 1685–1692 [DOI] [PubMed] [Google Scholar]
- 4. Findl O, Leydolt C. Meta-analysis of accommodating intraocular lenses. J Cataract Refract Surg. 2007; 33: 522–527 [DOI] [PubMed] [Google Scholar]
- 5. Koeppl C, Findl O, Menapace R, et al. Pilocarpine-induced shift of an accommodating intraocular lens: AT-45 Crystalens. J Cataract Refract Surg. 2005; 31: 1290–1297 [DOI] [PubMed] [Google Scholar]
- 6. Seyeddain O, Riha W, Hohensinn M, Nix G, Dexl AK, Grabner G. Refractive surgical correction of presbyopia with the AcuFocus small aperture corneal inlay: two-year follow-up. J Refract Surg. 2010; 26: 707–715 [DOI] [PubMed] [Google Scholar]
- 7. Waring G IV. Correction of presbyopia with a small aperture corneal inlay. J Refractive Surg. 2011; 27: 842 [DOI] [PubMed] [Google Scholar]
- 8. Tabernero J, Schwarz C, Fernández EJ, Artal P. Binocular visual simulation of a corneal inlay to increase depth of focus. Invest Ophthalmol Vis Sci. 2011; 52: 5273–5277 [DOI] [PubMed] [Google Scholar]
- 9. Atwood JD. Presbyopic contact lenses. Current Opin Ophthalmol. 2000; 11: 296–298 [DOI] [PubMed] [Google Scholar]
- 10. Martin JA, Roorda A. Predicting and assessing visual performance with multizone bifocal contact lenses. Optom Vis Sci. 2003; 80: 812–819 [DOI] [PubMed] [Google Scholar]
- 11. Alió JL, Chaubard JJ, Caliz A, Sala E, Patel S. Correction of presbyopia by technovision central multifocal LASIK (presbyLASIK). J Refract Surg. 2006; 22: 453–460 [DOI] [PubMed] [Google Scholar]
- 12. Epstein RL, Gurgos MA. Presbyopia treatment by monocular peripheral presbyLASIK. J Refract Surg. 2009; 25: 516–523 [DOI] [PubMed] [Google Scholar]
- 13. Davison JA, Simpson MJ. History and development of the apodized diffractive intraocular lens. J Cataract Refract Surg. 2006; 32: 849–858 [DOI] [PubMed] [Google Scholar]
- 14. Kim MJ, Zheleznyak L, MacRae S, Tchah H, Yoon G. Objective evaluation of through-focus optical performance of presbyopia-correcting intraocular lenses using an optical bench system. J Cataract Refract Surg. 2011; 37: 1305–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bellucci R. Multifocal intraocular lenses. Current Opin Ophthalmol. 2005; 16: 33–37 [DOI] [PubMed] [Google Scholar]
- 16. Yi F, Robert Iskander D, Collins M. Depth of focus and visual acuity with primary and secondary spherical aberration. Vision Res. 2011; 15; 51: 1648–1658 [DOI] [PubMed] [Google Scholar]
- 17. Benard Y, Lopez-Gil N, Legras R. Subjective depth of field in presence of 4th-order and 6th-order Zernike spherical aberration using adaptive optics technology. J Cataract Refract Surg. 2010; 36: 2129–2138 [DOI] [PubMed] [Google Scholar]
- 18. Jain S, Arora I, Azar DT. Success of monovision in presbyopes: Review of the literature and potential applications to refractive surgery. Surv Ophthalmol. 1996; 40: 491–499 [DOI] [PubMed] [Google Scholar]
- 19. Johannsdottir KR, Stelmach LB. Monovision: a review of the scientific literature. Optom Vis Sci. 2001; 78: 646 [DOI] [PubMed] [Google Scholar]
- 20. Evans BJW. Monovision: a review. Ophthalmic Physiol Opt. 2007; 27: 417–439 [DOI] [PubMed] [Google Scholar]
- 21. Schor C, Landsman L, Erickson P. Ocular dominance and the interocular suppression of blur in monovision. Am J Optom Physiol Opt. 1987; 64: 723 [DOI] [PubMed] [Google Scholar]
- 22. Collins MJ, Bruce AS. Factors influencing performance with monovision. Cont Lens Anterior Eye. 1994; 17: 83–89 [Google Scholar]
- 23. Collins M, Goode A, Brown B. Distance visual acuity and monovision. Optom Vis Sci. 1993; 70: 723 [DOI] [PubMed] [Google Scholar]
- 24. Fisher K. Presbyopic visual performance with modified monovision using multifocal soft contact lenses. Int Contact Lens Clin. 1997; 24: 91–100 [Google Scholar]
- 25. McGill E, Erickson P. Stereopsis in presbyopes wearing monovision and simultaneous vision bifocal contact lenses. Am J Optom Physiol Opt. 1988; 65: 619 [DOI] [PubMed] [Google Scholar]
- 26. Goldberg DB. Laser in situ keratomileusis monovision 1. J Cataract Refract Surg. 2001; 27: 1449–1455 [DOI] [PubMed] [Google Scholar]
- 27. Braun EHP, Lee J, Steinert RF. Monovision in LASIK. Ophthalmology. 2008; 115: 1196–1202 [DOI] [PubMed] [Google Scholar]
- 28. Reinstein DZ, Archer TJ, Gobbe M. LASIK for myopic astigmatism and presbyopia using non-linear aspheric micro-monovision with the Carl Zeiss Meditec MEL 80 platform. J Refract Surg. 2011; 27: 23 [DOI] [PubMed] [Google Scholar]
- 29. Reinstein DZ, Couch DG, Archer TJ. LASIK for hyperopic astigmatism and presbyopia using micro-monovision with the Carl Zeiss Meditec MEL80 platform. J Refract Surg. 2009; 25: 37 [DOI] [PubMed] [Google Scholar]
- 30. Greenbaum S. Monovision pseudophakia. J Cataract Refract Surg. 2002; 28: 1439–1443 [DOI] [PubMed] [Google Scholar]
- 31. Finkelman YM, Ng JQ, Barrett GD. Patient satisfaction and visual function after pseudophakic monovision. J Cataract Refract Surg. 2009; 35: 998–1002 [DOI] [PubMed] [Google Scholar]
- 32. Ito M, Shimizu K, Amano R, Handa T. Assessment of visual performance in pseudophakic monovision. J Cataract Refract Surg. 2009; 35: 710–714 [DOI] [PubMed] [Google Scholar]
- 33. Hayashi K, Yoshida M, Manabe SI, Hayashi H. Optimal amount of anisometropia for pseudophakic monovision. J Refract Surg. 2011; 27: 332–338 [DOI] [PubMed] [Google Scholar]
- 34. Legras R, Hornain V, Monot A, Chateau N. Effect of induced anisometropia on binocular through-focus contrast sensitivity. Optom Vis Sci. 2001; 78: 503 [DOI] [PubMed] [Google Scholar]
- 35. Pardhan S, Gilchrist J. The effect of monocular defocus on binocular contrast sensitivity. Ophthalmic Physiol Opt. 1990; 10: 33–36 [PubMed] [Google Scholar]
- 36. Kirschen DG, Hung CC, Nakano TR. Comparison of suppression, stereoacuity, and interocular differences in visual acuity in monovision and acuvue bifocal contact lenses. Optom Vis Sci. 1999; 76: 832 [DOI] [PubMed] [Google Scholar]
- 37. Lovasik JV, Szymkiw M. Effects of aniseikonia, anisometropia, accommodation, retinal illuminance, and pupil size on stereopsis. Invest Ophthalmol Vis Sci. 1985; 26: 741 [PubMed] [Google Scholar]
- 38. Harris MG, Sheedy JE, Gan CM. Vision and task performance with monovision and diffractive bifocal contact lenses. Optom Vis Sci. 1992; 69: 609 [DOI] [PubMed] [Google Scholar]
- 39. Jiménez JR, Castro JJ, Jiménez R, Hita E. Interocular differences in higher-order aberrations on binocular visual performance. Optom Vis Sci. 2008; 85: 174 [DOI] [PubMed] [Google Scholar]
- 40. Anera RG, Jiménez R, Salas C. Impact of interocular differences in corneal asphericity on binocular summation. Am J Ophthalmol. 2003; 135: 279–284 [DOI] [PubMed] [Google Scholar]
- 41. Castro JJ, Jiménez JR, Hita E, Ortiz C. Influence of interocular differences in the Strehl ratio on binocular summation. Ophthalmic Physiol Opt. 2009; 29: 370–374 [DOI] [PubMed] [Google Scholar]
- 42. Campbell FW, Green DG. Monocular versus binocular visual acuity. Nature. 1965; 208: 191–192 [DOI] [PubMed] [Google Scholar]
- 43. Reinstein DZ. Aspheric ablation profile for presbyopic corneal treatment using the MEL80 and CRS Master Laser Blended Vision module. J Emmetropia. 2011; 2: 161–175 [Google Scholar]
- 44. Yoon G, MacRae S, Williams DR, Cox IG. Causes of spherical aberration induced by laser refractive surgery. J Cataract Refract Surg. 2005; 31: 127–135 [DOI] [PubMed] [Google Scholar]
- 45. Moreno-Barriuso E, Lloves JM, Marcos S, Navarro R, Llorente L, Barbero S. Ocular aberrations before and after myopic corneal refractive surgery: LASIK-induced changes measured with laser ray tracing. Invest Ophthalmol Vis Sci. 2001; 42: 1396–1403 [PubMed] [Google Scholar]
- 46. Bakaraju RC, Ehrmann K, Papas EB, Ho A. Depth-of-focus and its association with the spherical aberration sign. A ray-tracing analysis. J Optom. 2010; 3: 51–59 [Google Scholar]
- 47. Dodgson NA. Variation and extrema of human interpupillary distance. Proc Soc Photo Opt Instrum Eng. 2004; 5291: 36–46 [Google Scholar]
- 48. Sabesan R, Zheleznyak L, Yoon G. Binocular visual performance and summation after correcting higher order aberrations. Biomed Opt Express. 2012; 3: 3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seijas O, Gómez de Liaño P, Gómez de Liaño R, Roberts CJ, Piedrahita E, Diaz E. Ocular dominance diagnosis and its influence in monovision. Am J Ophthalmol. 2007; 144: 209–216 e201 [DOI] [PubMed] [Google Scholar]
- 50. Watson AB, Ahumada AJ Jr. Predicting visual acuity from wavefront aberrations. J Vis. 2008; 8: 1–19 [DOI] [PubMed] [Google Scholar]
- 51. Applegate RA, Lakshminarayanan V. Parametric representation of Stiles-Crawford functions: normal variation of peak location and directionality. J Opt Soc Am A. 1993; 10: 1611–1623 [DOI] [PubMed] [Google Scholar]
- 52. Rocha KM, Vabre L, Chateau N, Krueger RR. Expanding depth of focus by modifying higher-order aberrations induced by an adaptive optics visual simulator. J Cataract Refract Surg. 2009; 35: 1885–1892 [DOI] [PubMed] [Google Scholar]
- 53. Cheng X, Bradley A, Ravikumar S, Thibos LN. The visual impact of Zernike and Seidel forms of monochromatic aberrations. Optom Vis Sci. 2010; 87: 300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983; 33: 113–120 [DOI] [PubMed] [Google Scholar]
- 55. Pardhan S. A comparison of binocular summation in young and older patients. Current Eye Res. 1996; 15: 315–319 [DOI] [PubMed] [Google Scholar]
- 56. Cheng X, Bradley A, Thibos LN. Predicting subjective judgment of best focus with objective image quality metrics. J Vis. 2004; 4: 310–321 [DOI] [PubMed] [Google Scholar]
- 57. Applegate RA, Thibos LN, Hilmantel G. Optics of aberroscopy and super vision. J Cataract Refract Surg. 2001; 27: 1093–1107 [DOI] [PubMed] [Google Scholar]
- 58. Ravikumar S, Bradley A, Thibos L. Phase changes induced by optical aberrations degrade letter and face acuity. J Vis. 2010; 10: 18 [DOI] [PubMed] [Google Scholar]
- 59. Loshin D, Loshin M, Comer G. Binocular summations with monovision contact lens correction for presbyopic patients. Int Contact Lens Clin. 1982; 9: 161–165 [Google Scholar]
- 60. Fredericksen R, Bex PJ, Verstraten FAJ. How big is a Gabor patch, and why should we care? J Opt Soc Am A. 1997; 14: 1–12 [DOI] [PubMed] [Google Scholar]
- 61. Gabor D. Theory of communication. Part 1: The analysis of information. IEEE Radio Comm. 1946; 93: 429–441 [Google Scholar]
- 62. Williams DR. Visibility of interference fringes near the resolution limit. J Opt Soc Am A. 1985; 2: 1087–1093 [DOI] [PubMed] [Google Scholar]
- 63. Campbell FW, Green DG. Optical and retinal factors affecting visual resolution. J Physiol. 1965; 181: 576–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yoon GY, Williams DR. Visual performance after correcting the monochromatic and chromatic aberrations of the eye. J Opt Soc Am A. 2002; 19: 266–275 [DOI] [PubMed] [Google Scholar]
- 65. de Gracia P, Marcos S, Mathur A, Atchison DA. Contrast sensitivity benefit of adaptive optics correction of ocular aberrations. J Vis. 2011; 11: 1–10 [DOI] [PubMed] [Google Scholar]
- 66. Piers PA, Manzanera S, Prieto PM, Gorceix N, Artal P. Use of adaptive optics to determine the optimal ocular spherical aberration. J Cataract Refract Surg. 2007; 33: 1721–1726 [DOI] [PubMed] [Google Scholar]
- 67. Amigo A, Bonaque S, López-Gil N, Thibos L. Simulated effect of corneal asphericity increase (Q-factor) as a refractive therapy for presbyopia. J Refract Surg. 2012; 28: 413 [DOI] [PubMed] [Google Scholar]
- 68. Collins M, Bruce A, Thompson B. Adaptation to monovision. Int Contact Lens Clin. 1994; 21: 218–224 [Google Scholar]