Abstract
The effects of chronic moderate sleep restriction and exercise training on carcinogenesis were examined in adenomatous polyposis coli multiple intestinal neoplasma (APC Min+/-) mice, a genetic strain which is predisposed to developing adenomatous polyposis. The mice were randomized to one of four 11 week treatments in a 2×2 design involving sleep restriction (by 4 h/day) vs. normal sleep and exercise training (1 h/day) vs. sedentary control. Wild-type control mice underwent identical experimental treatments. Compared with the wild-type mice, APC Min+/- mice had disrupted hematology and enhanced pro-inflammatory cytokine production from peritoneal exudate cells. Among the APC Min+/- mice, consistent interactions of sleep loss and exercise were found for measures of polyp formation, inflammation, and hematology. Sleep loss had little effect on these variables under sedentary conditions, but sleep loss had clear detrimental effects under exercise conditions. Exercise training resulted in improvements in these measures under normal sleep conditions, but exercise tended to elicit no effect or to exacerbate the effects of sleep restriction. Significant correlations of inflammation with polyp burden were observed. Among wild-type mice, similar, but less consistent interactions of sleep restriction and exercise were found. These data suggest that the benefits of exercise on carcinogenesis and immune function were impaired by chronic moderate sleep restriction, and that harmful effects of sleep restriction were generally realized only in the presence of exercise.
Keywords: sleep deprivation, sleep restriction, exercise, cancer, inflammation, cytokines
Introduction
In animals, prolonged sleep deprivation causes mortality (Rechtschaffen and Bergmann, 1995), and milder acute reductions in sleep can elicit altered immune function, increases in inflammatory substances, including cytokines, and multiple pathologies (Zielinski and Krueger, 2011; Besedovsky et al., 2012). These findings are consistent with human epidemiologic studies showing that short sleep (< 6 h) is associated with mortality, including cancer-related mortality (Heslop et al., 2002; Hublin et al., 2007; Kripke et al., 1979). Short sleep is also associated with many morbidities, many of which are associated with chronic inflammation, such as colorectal adenoma (Thompson et al., 2011), type 2 diabetes (Cappuccio et al., 2010), hypertension (Gangwisch et al., 2006), cardiovascular disease (Meisinger et al., 2007), and obesity (Cappuccio et al., 2008).
However, limitations of experimental studies of sleep restriction in animals, as well as in humans, have been that they have been restricted to short term and/or profound manipulations of sleep duration. There is a need to examine the effects of chronic moderate sleep loss, which is likely more common in humans. Indeed, many scientists have posited causal associations of declines in sleep duration in recent decades with modern societal epidemics of obesity, diabetes, and certain cancers (Spiegel et al., 2009). However, available evidence from experimental studies of chronic sleep loss (> 30 days) in humans has not indicated ill effects for many variables (Youngstedt et al., 2009; Zielinski et al., 2008).
In humans, regular physical activity is associated with a reduction in the incidence, progression, and reoccurrence of colorectal cancer (Wei et al., 2010). Moreover, exercise has consistently resulted in improvements in fatigue and sleep in cancer patients (Payne et al., 2008). Hence, moderate exercise training is a commonly prescribed adjunct treatment of cancer (de Nijis et al., 2008).
In mice, moderate exercise training has also been found to attenuate carcinogenesis (Zielinski et al., 2004; Mehl et al., 2005; Colbert et al., 2000), and to attenuate cancer-associated inflammation and pro-inflammatory cytokines. However, to our knowledge, the influence of exercise on carcinogenesis under conditions of sleep loss has never been investigated.
Conceivably, exercise could attenuate the effects of sleep loss on cancer and inflammation, analogous to its protective effects on other consequences of sleep loss, such as impairments in glucose tolerance (VanHelder et al., 1993). On the other hand, it is also plausible that exercise could exacerbate the effects of sleep loss, for example via enhanced fatigue or inflammation, which can also occur following exercise under some conditions (Walsh et al., 2011).
The adenomatous polyposis coli multiple intestinal neoplasma (APC Min+/-) mouse is a commonly used model for examining interventions that could influence cancer development. APC Min+/- mice are genetically identical to C57BL/6 mice but have a leucine codon truncated APC gene in codon 850 originating from an ethynitrosourea mutation, which causes polyps to form in the small intestines and colon (Shoemaker et al., 1997). The APC gene mutation is similar to that found in people with familial adenomatous polyposis who develop sporadic colon cancer. Colorectal cancer development is associated with an APC mutation which activates the nuclear localization of the β-catenin and the Wnt pathway to activate growth factors such as proinflammatory cytokines to induce polyp formation (Terzic et al, 2010).
The aim of this study was to determine the effects of chronic moderate sleep restriction and exercise training on colorectal cancer development, hematological markers of immunity, and pro-inflammatory cytokines in APC Min+/- mice. A group of C57BL/6 wild-type mice were also assessed to compare changes in markers of inflammation and immunity in control mice not experiencing cancer development.
Methods
Animals and Care
Seventy-four male APC Min+/- mice and 40 male C57BL/6J wild-type mice were obtained from breeding colonies purchased from Jackson Laboratory (Bar Harbor, ME). Mice were group housed 2-5 per cage by strain and maintained on a 12:12 h lightdark cycle (lights on 0700 h) with ad libitum access to food and water. The Institutional Animal Care and Usage Committee of the University of South Carolina approved all experiments.
Treatment Randomization and Acclimation
At 4 weeks of age, APC Min+/- mice were randomized to one of four 11-wk treatments: normal sleep (i.e., ad libitum sleep) + sedentary (n = 18), normal sleep + exercise training (NS+EX, n = 18), sleep restriction + sedentary (SR+SED, n = 19), and sleep restriction + exercise training (SR+EX, n = 19) (described below). Also at 4 weeks of age, 40 wild-type mice were randomized into one of these four treatments (n = 10 mice per treatment).
Sleep Restriction
The sleep restriction intervention was accomplished by placing the mice on a slowly-rotating drum (30 cm, 1 rev/min) surrounded by water. Mice were allowed to move freely on the drum. This sleep restriction apparatus had an electric filtration/regulation system for the water. The animals were enclosed by plexiglass with an opening on the top where food and drinking water was accessible. Mice were acclimated to the sleep restriction apparatus by placement of the mice on the drum for 20 min at 3 days before the sleep restriction intervention began.
For the experimental intervention, mice were placed on this drum for 12 h/day during the dark period. The timing of the daily sleep restriction during the dark period was chosen in order to elicit a modest degree of sleep loss in these nocturnal animals. Mice were transferred to their home cages and allowed ad libitum sleep during the light period. In our studies, this sleep restriction protocol attenuated polysomnographically-assessed sleep by approximately 4 h/day and exercise had no significant effect on 24-hr sleep of mice (data not shown).
Mice in the normal sleeping treatments were placed on the same apparatus during the same time period as mice in the sleep restriction treatments, but the disk was locked, allowing the mice ad libitum sleep during the dark period as well as the light period.
Exercise training
Mice in the exercise training treatments were acclimated to the treadmill the day prior to the treatments. The acclimation consisted of a single day of running on a motorized treadmill at a speed of 18-21 m/min at 5% grade for 20 minutes.
The exercise portion of the 11-week treatment consisted of running on a treadmill for 6 days/week (18-21 m/min and 5% grade) for 60 min/day at the beginning of the dark-period, a protocol that resulted in attenuated polyp development in APC Min+/- mice (Mehl et al., 2005) and a time in which spontaneous activity is generally the highest in mice. Exercise at this time is more ecologically valid and less likely to hinder sleep or alter the circadian system. The mice did not, however, exercise on the day prior to tissue collection (see below). The moderate level of exercise intensity required very limited gentle hand-prodding to make the mice run.
Mice in the sedentatry treatments were exposed to the treadmill room environment at the same time and picked up from their cages briefly in a similar manner as mice in the exercise treatments. However, mice in the sedentary control treatments did not run.
Plasma Corticosterone and Hematologic Measures
Submandibular bleeds were performed with a lancet immediately after the conclusion of the 11-week treatments. Approximately 50 μL of blood was collected in cappilary tubes containing heparin and then centrifuged. Plasma was obtained and stored at −80°C for later analysis of corticosterone using a radio-immunoassay kit (Diagnostics Products, Los Angelos, CA).
A second blood sample was also collected immediately after the 11-week treatments and used for hematological analysis with VetScan (Abaxis, Union City, CA). The following hemotological analytes associated with cancer development were measured according to the manufacturers' instructions: leukocyte numbers, lymphocyte numbers, monocyte numbers, granulocyte numbers, red blood cell (RBC) numbers, hematocrit levels (HCT), hemoglobin (HB), and platelet numbers (PLT).
Post-Treatment Tissue Collection
Immediately following collection of the blood samples, the mice were euthanized by isoflurane inhalation. Peritoneal exudate cells were obtained by lavage of the peritoneal cavity with 5 ml of Roswell Park Memorial Institute (RPMI)-1640 media (GIBCO, BRL) and placed on ice until cell processing. For both wild-type and APC Min+/- mice, spleens were dissected and flash frozen in liquid nitrogen and weighed later.
Peritoneal Exudate Cell Cytokine Collection and Stimulation
Peritoneal exudate cells were centrifuged and washed in RPMI-1640, and any remaining red blood cells were lysed with tris-(hydroxymethyl)-aminomethane-ammonium chloride, pH 7.2. Cells were quantified using a hemocytometer under a microscope, re-suspended (100 μl of a cell solution, 2 × 106 cells/ml), and seeded in 96-well flat-bottom microtiter plates. Next, the cells were either stimulated with 100 μl of lipopolysaccharide (LPS) (2 μg/ml) or unstimulated with 100 μl of RPMI-1640 and 10% fetal bovine serum. The cell culture plates were maintained at 37°C and 5% CO2 for 24 h allowing macrophages to adhere to the plate. Thereafter, supernatants were collected from each well and frozen at −70°C until cytokine [tumor necrosis factor-alpha (TNF), inteleukin-6 (IL6), interleukin-1beta (IL1)] protein production levels were analyzed using an enzyme-linked immunosorbent assay (ELISA) kit (R & D Systems, MN).
Colon and Intestine Extraction in APCMin
For the APC Min+/- mice, the colons and small intestines were also taken for analysis. The small intestines were dissected distally to the stomach and proximal to the cecum, and the large intestine was removed from the distal end of the cecum to the anus. Mesentary tissue was removed and the small intestine was cut into four equal sections. All intestinal sections were flushed with phosphate-buffered-saline (PBS), opened longitudinally, and flattened with a cotton swab. All intestine sections were fixed in 10% buffered formalin for 24 h and then preserved in 70% ethyl alcohol until polyp quantificatication analysis.
Adenomatous Polyp Quantification
Intestines previously fixed in formalin were rinsed in deionized water and briefly stained in 0.1% methylene blue. Intestinal polyps were counted under a dissecting microscope (2.5 × magnification) by an investigator blinded to the treatments. In addition, polyps were categorized based upon location (i.e., small intestine or colon) and size [i.e., < 1 mm (small), 1 mm to 2 mm (medium), or > 2 mm (large)]. The degree of adenoma prevalence (i.e. tumor burden) was ascertained by summing the intestinal polyps based on the three-size diameter classification of each individual mouse. Due to the circular composition of polyps, the area of each polyp was calculated using the equation area = πr2 (r = 0.5 × diameter) and added to determine tumor burden.
Statistical Analysis
Among the APC Min+/- mice, a 2 SLEEP (sleep restriction vs. normal sleep) × 2 ACTIVITY (sedentary vs. exercise training) analysis of variance (ANOVA) with Tukey post hoc comparisons was used to compare polyp number and burden between treatments. Likewise, separately for each strain 2 × 2 SLEEP by ACTIVITY ANOVAs were used to compare levels of cytokines, hematological measures and corticosterone levels between treatments. Differences in these measures between APC and wild type micer were assessed via ANOVA. Associations of cytokine changes with polyps were assessed with Pearson correlations. All statistical analyses are reported in Mean ± SE, with significances set at p < 0.05.
Results
Adenomatous Polyp Quantity and Burden
In the APC Min+- mice, a SLEEP-by-ACTIVITY interaction was observed [F(1, 70) = 19.367, p < 0.001] for intestinal polyps. Sleep restriction had little effect on intestinal polyp number under sedentary conditions. However, sleep restriction elicited a dramatic increase in intestinal polyps under exercise conditions (p < 0.001), which resulted in a main SLEEP effect [F(1, 70) = 23.202, p < 0.001] (Table 1). Moreover, whereas exercise elicited a modest increase in intestinal poly number under sleep restriction conditions, exercise elicited a large decrease in intestinal polyps under normal sleep conditions (p < 0.001) resulting in a main ACTIVITY effect [F(1, 70) = 4.956, p = 0.029].
Table 1.
Intestinal polyp number, size, and burden.
| Experimental group | Small polyps (number) | Medium polyps (number) | Large polyps (number) | Colon polyps (number) | Intestinal polyps (number) | Polyp burden (mm2) |
|---|---|---|---|---|---|---|
| NS+SED | 18.56 ± 1.55 | 49.22 ± 2.15 | 14.50 ± 1.69 | 3.22 ± 0.36 | 82.28 ± 2.40 | 147.03 ± 4.84 |
| NS+EX | 22.50 ± 1.97 | 32.17 ± 2.19* | 7.72 ± 1.34* | 2.00 ± 0.33 | 62.39 ± 2.73* | 98.72 ± 4.61* |
| SR+SED | 17.74 ± 1.81 | 37.32 ± 3.11* | 28.47 ± 2.36* | 3.00 ± 0.34 | 83.53 ± 3.72 | 169.24 ± 9.37 |
| SR+EX | 25.42 ± 1.86* | 47.42 ± 2.34 | 17.21 ± 1.28 | 3.32 ± 0.40 | 90.05 ± 2.90 | 157.75 ± 5.30 |
Mean ± SE for intestinal polyp number, size, and burden following 11-week treatments in APC Min+/- mice. Significant differences between controls and each treatment group (*) were set at p < 0.05.
Very few polyps were found in the colon (Table 1). A SLEEP-by-ACTIVITY interaction was found for the number of colon polyps [F(1, 70) = 4.576, p = 0.036]. Sleep restriction had little effect on colon polyp numbers under sedentary or exercise conditions but elicited a nonsignificant increase in colon polyps when combined with exercise. Exercise elicited a reduction in colon polyps under normal sleep conditions, but elicited an increase under sleep restricted conditions. Neither main SLEEP nor main ACTIVITY effects were found for polyp numbers in the colon.
More small sized polyps were found following exercise compared with the sedentary treatment [ACTIVITY: F(1, 70) = 10.359, p = 0.002] (Table 1). This effect was observed both under normal sleep (p = 0.044) and sleep restriction conditions (p = 0.017). No significant SLEEP or SLEEP-by-ACTIVITY interactions were seen for small polyp numbers.
A SLEEP-by-ACTIVITY interaction was found for medium sized polyps [F(1, 70) = 29.594, p < 0.001]. Sleep restriction elicited a significant decrease in medium polyps under sedentary conditions (p = 0.007), but a non-significant increase under exercise conditions. Exercise elicited a significant decrease in medium polyps under normal sleeping conditions (p < 0.001), but a moderate non-significant increase under sleep restriction conditions. No significant main SLEEP or ACTIVITY effects were found for medium sized polyps.
No significant SLEEP-by-ACTIVITY interaction was found for large polyps (Table 1). Sleep restriction resulted in more large polyps than normal sleep [SLEEP: F(1, 70) = 45.827, p < 0.001], and this was apparent under both sedentary and exercise conditions (ps < 0.001). A significant main ACTIVITY effect was found for large polyp formation [ACTIVITY: F(1, 70) = 27.096, p < 0.001]. Under both normal sleep and sleep restriction conditions, exercise training resulted in fewer large polyps compared with sedentary activity (p = 0.039; p < 0.001, respectively).
A significant SLEEP-by-ACTIVITY interaction was observed for total tumor burden [F (1, 70) = 16.976, p < 0.001]. Sleep restriction resulted in a modest increase in tumor burden under sedentary and exercise conditions, which resulted in a significant main SLEEP effect [SLEEP: F (1, 70) = 7.020, p < 0.001]. Exercise resulted in a dramatic reduction (48.31 mm2) in total tumor burden in normal sleep conditions (p < 0.001), but a small increase in tumor burden under sleep restriction conditions. However, no significant main ACTIVITY effect was found for tumor burden.
Hematological Measures
Hematological data are presented in Table 2. APC Min+/- mice had a disrupted hematologic immune profile compared to wild-type mice. APC Min+/- mice exhibited significantly greater leukocytes, lymphocytes, monocytes, granulocytes, and platelets than wild-types. [STRAIN: F(1, 84) = 16.410, p < 0.001; F(1, 84) = 12.547, p = 0.001; F(1, 84) = 4.981, p = 0.028; F(1, 84) = 8.576, p = 0.004, F(1, 84) = 8.022, p = 0.006, respectively]. Furthermore, APC Min+/- mice had significantly less red blood cells, hematocrits, and hemoglobin levels than wild-types [STRAIN: F(1, 84) = 31.451, p < 0.001; F(1, 84) = 30.652, p < 0.001; F(1, 84) = 33.904, p < 0.001, respectively].
Table 2.
Hematological measures.
| Wild-type | NS+SED | NS+EX | SR+SED | SR+EX |
|---|---|---|---|---|
| Leukocyte (m/mm3) | 5.95 ± 0.49 | 5.54 ± 0.40 | 7.13 ± 0.71 | 6.50 ± 0.46 |
| Lymphocyte (m/mm3) | 3.89 ± 0.29 | 3.60 ± 0.26 | 4.12 ± 0.50 | 3.90 ± 0.29 |
| Monocyte (m/mm3) | 0.33 ± 0.02 | 0.33 ± 0.02 | 0.30 ± 0.04 | 0.35 ± 0.03 |
| Granulocyte (m/mm3) | 1.73 ± 0.20 | 1.61 ± 0.17 | 2.72 ± 0.32* | 2.25 ± 0.24 |
| Erythrocyte (m/mm3) | 9.96 ± 0.21 | 9.83 ± 0.27 | 9.89 ± 0.12 | 9.65 ± 0.30 |
| HCT (%) | 51.42 ± 1.33 | 51.72 ± 1.70 | 52.28 ± 1.03 | 50.87 ± 1.56 |
| HB (g/dl) | 16.95 ± 0.38 | 16.95 ± 0.48 | 17.00 ± 0.26 | 16.61 ± 0.56 |
| PLT (m/mm3) | 955.00 ± 72.84 | 943.80 ± 43.96 | 1097.60 ± 35.16 | 1061.20 ± 63.43 |
|
| ||||
| APC MIN+/- | NS+SED | NS+EX | SR+SED | SR+EX |
|
| ||||
| Leukocyte (m/mm3) | 6.79 ± 0.38 | 10.07 ± 0.93 | 15.46 ± 2.89* | 9.33 ± 0.71 |
| Lymphocyte (m/mm3) | 4.32 ± 0.33 | 6.77 ± 0.90 | 11.38 ± 2.91* | 6.64 ± 0.68 |
| Monocyte (m/mm3) | 0.31 ± 0.03 | 0.39 ± 0.03 | 0.37 ± 0.03 | 0.44 ± 0.04 |
| Granulocyte (m/mm3) | 2.16 ± 0.16 | 2.91 ± 0.31 | 3.72 ± 0.54* | 2.25 ± 0.17# |
| Erythrocyte (m/mm3) | 8.65 ± 0.62 | 7.81 ± 0.62 | 7.20 ± 0.68 | 7.96 ± 0.51 |
| HCT (%) | 46.82 ± 2.90 | 42.71 ± 2.81 | 38.72 ± 3.06 | 41.59 ± 2.43 |
| HB (g/dl) | 14.45 ± 1.06 | 13.54 ± 0.98 | 12.13 ± 1.15 | 13.73 ± 0.95 |
| PLT (m/mm3) | 1135.64 ± 80.13 | 1178.91 ± 102.96 | 1134.38 ± 100.33 | 1251.55 ± 85.99 |
Mean ± SE for hematological measures following 11-week treatments. Significant differences between controls for each treatment group (*) and between SR+SED and SR+EX (#) were set at p < 0.05.
In wild-type mice, sleep-restriction resulted in a significant elevations in granulocytes, platelets, and granulocytes [SLEEP: F(1, 36) = 11.530, p = 0.002; F(1, 36) = 5.408, p = 0.026, respectively]. A similar SLEEP pattern was evident for all the other hematologic variables, except monocytes, but the main SLEEP effect was not significant for any of these measures. Exercise resulted in a slight, non-significant attenuation of most of these measures compared with the sedentary treatment. However, no significant ACTIVITY or SLEEP-by-ACTIVITY interaction effects were found for these hematological variables.
In APC Min+/- mice, significant SLEEP-by-ACTIVITY interactions were observed for leukocyte, granulocyte, and lymphocyte numbers [F(1, 42) = 7.214, p = 0.010; F(1, 42) = 9.552, p = 0.004; F(1, 42) = 4.216, p = 0.046, respectively]. Sleep restriction elicited significant and large increases in leukocytes, lymphocytes, and granulocytes under sedentary conditions (p = 0.05, p = 0.028, p = 0.016, respectively). The elevation in leukocytes produced main SLEEP effect [SLEEP: F(1, 42) = 5.124, p = 0.029], although no significant main SLEEP effect was found for the other hematological measures. Whereas exercise elicited nonsignificant increases in these variables under normal sleep conditions, exercise greatly attenuated the increases that occurred with sleep restriction. Exercise trained APC Min+/- mice had significantly enhanced monocytes compared to the sedentary mice [ACTIVITY: F(1, 42) = 5.103, p = 0.029], but no main exercise effect was found for the other hematological measures. No significant SLEEP-by-ACTIVITY interaction effects were found for the other hematological measures for APC Min+/- mice.
Peritoneal Exudate Cytokine Protein Levels
Peritoneal exudate cytokine protein production levels are presented in Figures 1, 2, and 3. In wild-type mice, unstimulated peritoneal exudates cell production levels of IL1, TNF, or IL6 were not detectable; therefore comparisons between strains could not be made. However, compared to wild-type mice, APC Min+/- mice exhibited significantly enhance LPS-stimulated peritoneal exudate cell protein levels of IL1, TNF, and IL6 [F(1, 61) = 27.696, p < 0.001; F(1, 61) = 34.819, p < 0.001; F(1, 61) = 127.927, p < 0.001, respectively].
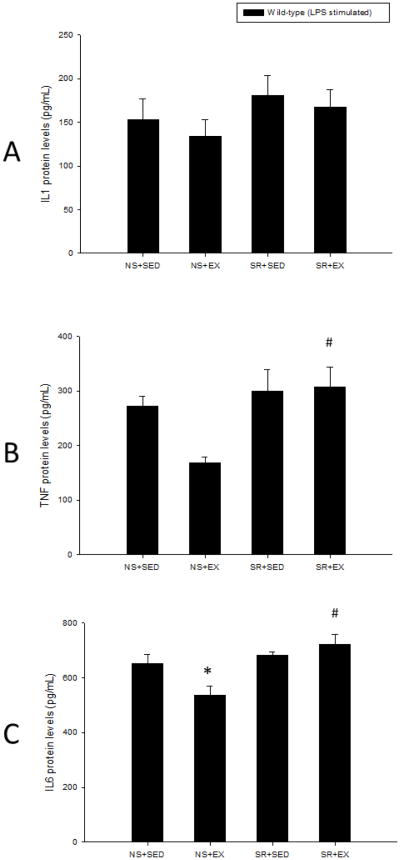
Figure 1.
Unstimulated peritoneal exudate cell IL1, TNF, and IL6 protein levels were not detectable in wild-type mice. Wild-type mice that were sleep restricted, exercised, or received both treatments did not have significantly different LPS-stimulated peritoneal exudate cell IL1 (A) or TNF (B) protein levels than controls. Compared to controls, wild-type mice that exercised under normal sleeping conditions exhibited significantly attenuated LPS-stimulated peritoneal exudate cell IL6 (C) protein levels. Sleep restriction under sedentary or exercise conditions did not significantly alter LPS-stimulated peritoneal exudate cell IL6 (C) protein levels compared to controls in wild-type mice. Significant differences between the treatment groups and the NS+SED control group treatment group (*) and between NS+EX and SR+EX (#) were set at p < 0.05
Figure 2.
APC Min+/- mice that were sleep restricted, exercised, or received both treatments did not have significantly different unstimulated peritoneal exudate cell IL1 (A) or TNF (B) protein levels than controls. Exercise under normal sleeping conditions but not sleep restricted conditions significantly attenuated unstimlated and LPS-stimulated peritoneal exudate cell IL6 (C) protein levels. Exercise under normal sleep conditions but not sleep-restricted conditions attenuated LPS-stimulated peritoneal exudate cell IL1 (A), TNF (B), and IL6 (C) protein levels compared to controls. Sleep restriction did not significantly alter LPS-stimulated peritoneal exudate cell IL1 (A), TNF (B), or IL6 (C) protein levels compared to controls. Significant differences between the treatment groups and the NS+SED control group treatment group (*) and between NS+EX and SR+EX (#) were set at p < 0.05.
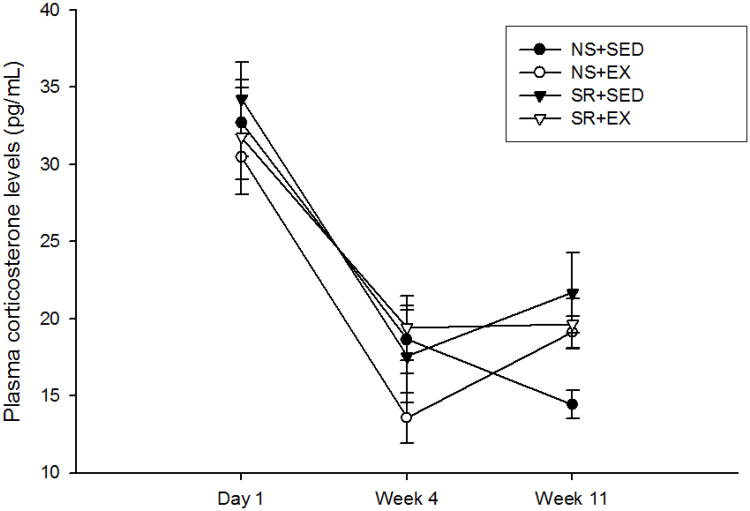
Figure 3.
Plasma corticosterone levels (pg/mL) were not significantly different with sleep restriction or exercise treatments. However, plasma corticosterone levels were reduced following 4 weeks of experimental treatments compared to day 1 post-treatments including the control treatments.
In wild-type mice, significant SLEEP-by-ACTIVITY interactions were observed for LPS stimulated IL6 levels [F(1, 27) = 6.903, p = 0.014]. Sleep restriction resulted in a negligible increase in IL6 under sedentary conditions, but relatively large increase in IL6 and TNF levels under exercise conditions, which resulted in significant SLEEP main effects for IL6 (F(1, 27) = 13.127, p = 0.001) and TNF (F(1, 27) = 8.567, p = 0.007). Exercise training elicited a reduction in IL6 levels (p = 0.044) and near significant reductions in TNF levels (p = 0.063) under normal sleep conditions, but exercise had little effect on these variables under sleep restriction conditions. However, no significant main ACTIVITY effect was found for these variables. No significant SLEEP, ACTIVITY, or SLEEP-by-ACTIVITY interaction was found for LPS stimulated IL1 levels in wild-type mice.
In APC Min+/- mice, a SLEEP-by-ACTIVITY interaction was seen for unstimulated IL6 levels [F(1, 28) = 18.880, p < 0.001]. Sleep restriction resulted in non-significant increases in IL6 levels under sedentary and exercise conditions compared to normal sleeping conditions, which resulted in a significant main SLEEP effect [SLEEP: F(1, 28) = 17.411, p < 0.001]. A significant main ACTIVITY effect was found [ACTIVITY: F(1, 28) = 6.386, p = 0.017]. Compared to sedentary conditions, exercise resulted in a significant decrease in IL6 levels under normal sleep conditions (p < 0.001). However, mice that exercised under the normal sleep condition had significantly lower IL6 levels than mice that exercise in sleep restricted condition (p < 0.001). No significant SLEEP, ACTIVITY or SLEEP-by-ACTIVITY interaction effects were observed for unstimulated peritoneal exudates cell IL1 and TNF levels.
LPS stimulated IL1, TNF, and IL6 levels were significantly positively correlated with tumor burden values (r = 0.364, p = 0.04; r = 0.351, p = 0.49; r = 0.551, p = 0.001, respectively). In APC Min+/- mice, a significant SLEEP-by-ACTIVITY interaction was observed for LPS stimulated IL6 levels [F(1, 28) = 9.395, p = 0.005]. Sleep restriction resulted in no change in IL6 levels under sedentary conditions, but a significant increase in IL6 levels under exercise conditions resulting in a main SLEEP effect [F(1, 28) = 10.979, p < 0.001]. Exercise training did not significantly alter IL6 levels under sleep restriction conditions, but exercise training resulted in a significant decrease in IL6 levels under normal sleep conditions (p = 0.001), resulting in a significant main ACTIVITY effect [ACTIVITY: F(1, 28) = 9.878, p = 0.004].
No significant SLEEP-by-ACTIVITY interaction effects were found for LPS stimulated IL1 or TNF levels. Sleep restricted mice had significantly greater LPS stimulated IL1 and TNF levels than normal sleeping mice [SLEEP: F(1, 28) = 8.630, p = 0.007; F(1, 28) = 9.984, p = 0.004]. However, the normal sleeping exercised group had significantly lower LPS stimulated IL1 and TNF levels than the normal sleeping sedentary group (p = 0.046, p = 0.001, respectively). Exercise-trained mice had significantly lower LPS stimulated TNF levels than sedentary mice [ACTIVITY: F(1, 28) = 8.250, p = 0.008]. Further, under exercised conditions the sleep restriction group had significantly greater LPS stimulated IL1 and TNF levels than the normal sleeping group (p = 0.009, p = 0.011, respectively). No significant main ACTIVITY effect was found for LPS stimulated IL1 levels.
Plasma Corticosterone
Plasma corticosterone data are shown in Figure 3. Plasma corticosterone levels did not differ between wild-type and APC Min+/- strains. A decrease in corticosterone levels was observed over TIME in wild-type and APC Min+/- mice [F(1, 31) = 57.455, p < 0.001; F(1, 70) = 7.845, p = 0.008], respectively]. No significant SLEEP, ACTIVITY, SLEEP-by-ACTIVITY interactions were found for plasma corticosterone levels in wild-type or APC Min+/- mice.
Spleen Weight
APC Min+/- mice had heavier spleens than wild-type mice [F(1, 72) = 1310.771, p < 0.001] (Table 3). However, no significant SLEEP, ACTIVITY, SLEEP-by-ACTIVITY interactions were found for spleen weight for either strain.
Table 3.
Spleen weights.
| Wild-type | Spleen weight (mg) | APC Min+/- | Spleen weight (mg) |
|---|---|---|---|
| NS+SED | 79.68 ± 1.33 | NS+SED | 203.27 ± 4.46 |
| NS+EX | 78.08 ± 1.05 | NS+EX | 213.37 ± 4.63 |
| SR+SED | 81.00 ± 1.58 | SR+SE | 211.26 ± 6.05 |
| SR+EX | 79.28 ± 1.32 | SR+EX | 213.32 ± 5.65 |
Mean ± SE for spleen weights following 11-week treatments. No significant differences in spleen weights were found between controls and treatment groups. Significance was set at p < 0.05.
Discussion
Interactions between sleep restriction and exercise training were found for polyp developmet and peritoneal inflammatory cytokines. Sleep restriction had few effects under sedentary conditions, but numerous negative effects under exercise conditions. Whereas exercise elicited consistent reductions in inflammation and polyp formation under normal sleep conditions, these effects were attenuated or reversed under sleep restricted conditions. The effects of sleep restriction and exercise on inflammation were much more evident in APC Min+/- mice than wild-type controls.
Consistent with previous reports (Baltgalvis et al., 2008), in the present study the production of pro-inflammatory cytokines IL1, TNF, and IL6 from peritoneal exudate cells were elevated in APC Min+/- mice compared with wild-type mice. Moreover, compared with the wild type mice, the APC Min+/- mice had a markedly disrupted blood profile, as evidenced by elevations in leukocytes, monocytes, granulocytes, and platelets, and reductions in red blood cells, hematocrits, splenomegaly, and hemoglobin levels. The reduced hemoglobin levels are indicative of anemia, which is common in individuals with colon cancer and associated with enhanced inflammation (Lee et al., 2006). In the present study, the elevations in inflammation were correlated with polyp formation in the APC Min+/- mice; however, it is unknown the extent to which these correlations are consequences of cancer development or part of the progression of cancer.
The effects of sleep restriction on polyp numbers under sedentary conditions in the present study are not consistent with the results from other animal models which have suggested that prior sleep deprivation has protective effects against the development of tumors following tumor inoculation (Bergmann et al., 1996; Zielinski et al., 2007). Other models of sleep loss prior to immune challenge, such as influenza in mice and in bacterial challenge in Drosophila have also found a similar protective effect of sleep loss (Renegar et al., 2001; Williams et al., 2007). Differences between these results and results of the present study might be attributed to the models of sleep deprivation and cancer. It is plausible that an immune priming effect occurred in these previous models resulting in a protective effect against a single infection bolus, whereas the current study employed a genetic mouse model of cancer that induces chronic inflammation that likely interacts differently with immune responses to sleep loss.
The attenuated polyp development that was found with exercise under normal sleep conditions in the present study is consistent with data from numerous other rodent studies involving moderate intensity exercise training (Zielinski et al., 2004; Mehl et al., 2005; Colbert et al., 2000). In addition, these data are in agreement with epidemiologic data indicating moderate exercise reduces colon cancer occurrence and reoccurrence (Wei et al., 2010). Mechanisms by which exercise training might attenuate or prevent cancer development include reduced inflammation, adiposity, body weight, insulin-like growth factors, and pro-inflammatory cytokines.
The protocol of forced treadmill exercise used in this study has been found in other studies to attenuate polyp development in APC Min+/- mice (Mehl et al., 2005; Baltgalvis et al., 2008). Other studies indicate that voluntary wheel running exercise also can attenuate polyp development (Colbert et al., 2006). However, it has been reported that voluntary wheel running does not alter intestinal polyp number or size in APC Min+/- mice (Mehl et al., 2005). Both forced treadmill exercise and voluntary wheel running exercise are effective in attenuating certain cancers (Na & Oliynyk, 2011). Nevertheless, it is likely that these modes of exercise regulate immuno-physiological functions differently. Thus, the interaction of chronic sleep loss and voluntary exercise warrants investigation.
However, moderate sleep loss attenuated or reversed the anti-carcinogenic and antiinflammatory effects of exercise. These findings might have implications for prescribing exercise for cancer patients, for whom exercise might need to be reduced or avoided if their sleep loss is even more severe than is commonly associated with cancers. Contraindications for exercise in individuals with pathogenesis have been noted including uncontrolled diabetes, thrombophlebitis, active pericarditis and myocarditis, and severe aortic stenosis (Myers, 2008). Another potential implication is that cancer patients undergoing an exercise regimen might take caution to avoid extra sleep loss, for example, waking up early in order to exercise. However, further research in human cancer patients is needed to justify recommendations regarding exercise.
Insofar as modest population declines in sleep duration in recent decades may be indicative of chronic sleep restriction, there are other potential interactions with exercise which could have health implications. Further experimental research of this issue is warranted.
Our findings of enhanced granulocyte numbers following sleep restriction under sedentary conditions are consistent with those from human studies showing that acute sleep deprivation enhances the number of circulating leukocytes and granulocytes (Dinges et al, 1994; Boudjetia et al, 2008; Ruiz et al., 2011). Interestingly, in the present study exercise attenuated the sleep restriction-induced increase in circulating granulocyte numbers in wild-type and APC Min+/- mice and lymphocyte numbers in APC Min+/- mice, which contrasts with the interactions of sleep restriction and exercise noted for the polyp data and the inflammatory data. The mechanisms and pathological consequences of enhanced granulocytes following sleep restriction in animals without cancer are yet-to-be identified. Nevertheless, granulocytes and lymphocytes produce pro- and anti-inflammatory cytokines that could potentially serve to modulate carcinogenesis (Mantovani et al., 2011).
The pro-inflammatory cytokine data more closely resembled the polyp data. In contrast with human studies indicating enhanced plasma levels of pro-inflammatory cytokines following prolonged profound sleep restriction (Shearer et al., 2001; Meier-Ewert et al., 2004; Haack et al., 2007; Chennaoui et al., 2011) and following just a single night of partial sleep loss (Irwin et al., 2010), the present study revealed no significant effect of chronic moderate sleep restriction on peritoneal IL1, TNF, and IL6 production levels under sedentary conditions in either wild-type or APC Min+/- mice.
In the wild-type mice, exercise training elicited a significant, but modest, reduction in peritoneal IL6 production levels, but no significant changes in the other inflammatory markers, perhaps due to floor effects. However, consistent with previous research (Mehl et al., 2005), under normal sleeping conditions, exercise greatly attenuated peritoneal exudate cell IL1, TNF, and IL6 production levels in APC Min+/- mice. These data suggest that the anti-inflammatory effects of exercise are more discernible in chronic inflammatory conditions, as has been shown in other chronic inflammatory disease models, such as in Zucker diabetic fatty rats (Teixeira et al., 2009). The mechanisms by which exercise reduced cytokine-mediated inflammation are not well-understood (Timmerman et al., 2007).
As with the polyp data, the pro-inflammatory cytokine data suggest negative effects of combining moderate sleep restriction with exercise. Sleep loss and exercise both alter many inflammatory pathways, including nuclear factor-kappa-B, cyclooxygenase, and mitogen activated protein kinase pathway, that alter pro-inflammatory cytokine activation (Zielinski & Krueger, 2011; Walsh et al., 2011). Exactly how exercise and sleep loss interact to alter these pathways remains unknown. Chronic sleep restriction might inhibit the attenuation of these responses which occur with exercise training under normal sleep conditions. Alternatively, during chronic sleep restriction, even moderate exercise could potentiate inflammatory pathways.
Our method of exercise and sleep restriction did not alter plasma corticosterone levels or body weights (data not shown) in either strain, suggesting that little stress was induced with these treatments and that the results cannot be attributed to stress effects. Our findings are consistent with those from studies of rats that found that even a more severe amount of sleep restriction (i.e., allowing only 6 h of sleep per day) did not elicit an increase in plasma corticosterone levels compared with ad libitum sleep conditions (Zager et al., 2007). Further, sleep restriction in humans also has failed to alter cortisol levels (Martins et al., 2010). Notwithstanding these findings, sleep loss does induce activation of the HPA-axis that might manifest effects in other ways besides plasma corticosterone levels (Anderson et al., 2004). Other stress-related measures, such as corticotropin-releasing hormone, adrenocorticotropic hormone, or norepinephrine levels, might be altered with chronic sleep restriction. On the other hand, in some chronic stress conditions, corticosterone levels can be attenuated or show no elevation. Thus, in the present study, familiarity with the sleep restriction protocol might have resulted in a desensitized stress response (Cyr et al., 2007; Marin et al., 2007).
The relatively greater effects of sleep restriction in the APC Min+/- mice compared with the wild-type mice might be explained, in part, by a relatively greater need for sleep in the APC Min+/- mice, and this need might be mediated by enhanced peritoneal inflammation. Interestingly, pro-inflammatory cytokines IL1 and TNF applied within the peritoneal cavity induce sleep by stimulating the vagal afferent nerves or through the circulation via leaky areas of the blood-brain barrier (Zielinski and Krueger, 2011).
In conclusion, sleep restriction had little effect on carcinogenesis or pro-inflammatory cytokine levels under sedentary conditions. Exercise had anti-carcinogenic effects and antiinflammatory effects under normal sleep conditions. However, sleep restriction combined with exercise produced harmful interaction effects on these variables. These findings suggest that the anti-cancer benefits of exercise should be examined more closely in relation to sleep and might have clinical relevance for exercise prescription or for protection of sleep in cancer patients.
Highlights.
Sleep restriction attenuates or eliminates many of the benefits exercise has on reducing inflammation and carcinogenesis.
Acknowledgments
This research was supported by the National Institutes of Health (HL 71560 to SDY). We thank Matthew Davis, David Elliott, J. Larry Durstine, Marj Pena, and Frank Berger for their contributions to this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen M, Bignotto M, Machado RB, Tufik S. Different stress modalities result in distinct steroid hormone responses by male rats. Braz J Med Biol Res. 2004;37:791–797. doi: 10.1590/s0100-879x2004000600003. [DOI] [PubMed] [Google Scholar]
- Baltgalvis KA, Berger FG, Pena MM, Davis JM, Carson JA. Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. Journal of Applied Physiology. 2008;104:1137–1143. doi: 10.1152/japplphysiol.00955.2007. [DOI] [PubMed] [Google Scholar]
- Bergmann BM, Rechtschaffen A, Gilliland MA, Quintans J. Effect of extended sleep deprivation on tumor growth in rats. Am J Physiol. 1996;271:R1460–1464. doi: 10.1152/ajpregu.1996.271.5.R1460. [DOI] [PubMed] [Google Scholar]
- Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children, adolescents, and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, Gourby B, Bourrihon C, Florence G, Gomez-Merino D. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-α) levels in healthy men. Cytokine. 2011;56:318–324. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Colbert LH, Davis JM, Essig DA, Ghaffar A, Mayer EP. Exercise and tumor development in a mouse predisposed to multiple intestinal adenomas. Med Sci Sports Exerc. 2000;32:1704–1708. doi: 10.1097/00005768-200010000-00007. [DOI] [PubMed] [Google Scholar]
- Cyr NE, Michael Romero L. Chronic stress in free-living European starlings reduces corticosterone conentrations and reproductive success. Gen Comp Endocrinol. 2007;151:82–89. doi: 10.1016/j.ygcen.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Douglas SD, Zaugg L, Campbell DE, McMann JM, Whitehouse WG, Orne EC, Kapoor SC, Icaza E, Orne MT. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. Journal of Clinical Investigation. 1994;93:1930–1939. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction anre associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: The effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Med. 2002;3:304–314. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Hublin C, Partinen M, Koskenvuo M, Kaprio J. Sleep and mortality: a population-based 22-year follow-up study. Sleep. 2007;30:1245–1253. doi: 10.1093/sleep/30.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Carillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills Is increased mortality associated? Arh Gen Psychiatry. 1979;36:103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- Lee RA, Kim HA, Kang BY, Kim KH. Hemoglobin induces colon cancer cell proliferation by release of reactive oxygen species. World Journal of Gastroenterology. 2006;12:5644–5650. doi: 10.3748/wjg.v12.i35.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Martins PJ, Marques MS, Tufik S, D'Almeida V. Orexin activation precedes increased NPY expression, hyperphagia, and metabolic changes in response to sleep deprivation. Am J Physiol Endocrinol Metab. 2010;298:E726–E734. doi: 10.1152/ajpendo.00660.2009. [DOI] [PubMed] [Google Scholar]
- Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol. 2005;98:2219–2225. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- Meisinger C, Heiger M, Lowell H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg Cohort Study. Sleep. 2007;30:1121–1127. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. Principles of exercise prescription for patients with chronic heart failure. Heart Fail Rev. 2008;13:61–68. doi: 10.1007/s10741-007-9051-0. [DOI] [PubMed] [Google Scholar]
- Na HK, Oliynyk S. Effects of physical activity on cancer prevention. Ann N Y Acad Sci. 2011;1229:176–183. doi: 10.1111/j.1749-6632.2011.06105.x. [DOI] [PubMed] [Google Scholar]
- Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum. 2008;35:635–642. doi: 10.1188/08.ONF.635-642. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behavioural Brain Research. 1995;69:55–63. doi: 10.1016/0166-4328(95)00020-t. [DOI] [PubMed] [Google Scholar]
- Renegar KB, Crouse D, Floyd RA, Krueger J. Progression of influenza viral infection through the murine respiratory tract: the protective role of sleep deprivation. Sleep. 2001;23:859–863. [PubMed] [Google Scholar]
- Ruiz FS, Andersen ML, Martins RC, Zager A, Lopes JD, Tufik S. Immune alterations after selective rapid eye movement or total sleep deprivation in healthy male volunteers. Innate Immun. 2010;18:44–54. doi: 10.1177/1753425910385962. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Reuben JM, Mullington JM, Price NJ, Lee BN, Smith EO, Szuba MP, Van Dongen HP, Dinges DF. Soluble TNF-alpha receptor 1 and IL-6 plasma levels in humans subjected to the sleep deprivation model of spaceflight. J Allergy Clin Immunol. 2001;107:165–170. doi: 10.1067/mai.2001.112270. [DOI] [PubMed] [Google Scholar]
- Shoemaker AR, Gould KA, Luongo C, Moser AR, Dove WF. Studies of neoplasia in the Min mouse. Biochim Biophys Acta. 1997;1332:F25–48. doi: 10.1016/s0304-419x(96)00041-8. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5:253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira de Lemos F, Reis F, Baptista S, Pinto R, Sepodes B, Vala H, Rocha-Pereira P, Correia da Silva G, Teixeira N, Silva AS, Carvalho L, Teixeira F, Das UN. Exercise training decreases proinflammatory profile in Zucker diabetic (type 2) fatty rats. Nutrition. 2009;25:330–339. doi: 10.1016/j.nut.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Larkin EK, Patel S, Berger NA, Redline S, Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer. 2011;117:841–847. doi: 10.1002/cncr.25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman KL, Flynn MG, Coen PM, Markofski MM, Pence BD. Exercise training-induced lowering of inflammatory (CD14+CD16+) monocytes: a role in the anti-inflammatory influence of exercise? Journal of Leukocyte Biology. 2008;84:1271–1278. doi: 10.1189/jlb.0408244. [DOI] [PubMed] [Google Scholar]
- VanHelder T, Symons JD, Radomski MW. Effects of sleep deprivation and exercise on glucose tolerance. Aviat Space Environ Med. 1993;64:487–492. [PubMed] [Google Scholar]
- Walsh NP, Gleeson M, Shephard RJ, Geeson M, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- Wei EK, Wolin KY, Colditz GA. Time course of risk factors in cancer etiology and progression. J Clin Oncol. 2010;28:4052–4057. doi: 10.1200/JCO.2009.26.9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Sathvanarayanan S, Hendricks JC, Sehgal A. Interaction between sleep and the immune response in Drosophila: a role of the NFkappaB relish. Sleep. 2007;30:389–400. doi: 10.1093/sleep/30.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstedt SD, Kline CE, Zielinski MR, Kripke DF, Devlin TM, Bogan RK, Wilcox S, Hardin JW. Tolerance of chronic 90-minute time-in-bed restriction in older long sleepers. Sleep. 2009;32:1467–1479. doi: 10.1093/sleep/32.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager A, Andersen ML, Ruiz FS, Antunes IB, Tufik S. Effects of acute chronic sleep loss on immune modulation of rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R504–R509. doi: 10.1152/ajpregu.00105.2007. [DOI] [PubMed] [Google Scholar]
- Zielinski MR, Kline CE, Kripke DF, Bogan RK, Youngstedt SD. No effect of 8-week time in bed restriction on glucose tolerance in older long sleepers. J Sleep Res. 2008;17:412–419. doi: 10.1111/j.1365-2869.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MR, Krueger JM. Sleep and innate immunity. Front Biosci (Schol Ed) 2011;3:632–642. doi: 10.2741/s176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MR, Muenchow M, Wallig MA, Horn PL, Woods JA. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J Appl Physiol. 2004;96:2249–2256. doi: 10.1152/japplphysiol.01210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MR, Davis JM, Wyatt WC, Montagu DL, Youngstedt SD. Effects of chronic sleep restriction and exercise training on metastasis. Med Sci Sports Exerc. 2007;39:62. [Google Scholar]