Abstract
BACKGROUND
Middle aged and older American men and women have almost twice the rate of diabetes of men and women in England. This differential was not explained by conventional risk factors including age, smoking, social position, and BMI.
METHODS
We use large and representative samples of non-minority adults ages 52 to 85 years old taken from the 1999–2006 American NHANES and the 2004 English Longitudinal Study of Aging. Our surveys contain self-reported and objective biological disease markers of diabetes as well as indicators of major risk factors for diabetes including anthropometric measures of BMI, height and waist circumference.
RESULTS
The older American population has much higher rates of diabetes than the English population—a differential not yet explained. But we show that this population also has higher waist circumference at each level of BMI than does the equivalent group in England. We find that by controlling for such waist circumference differences and allowing for different effects of waist on diabetes in each country, we can explain about three-quarters of the country differences for women and 38% among men.
CONCLUSIONS
Higher rates of diabetes in the US old-age population than in England were largely accounted for by raised waist circumference and not BMI differences, especially among women. In addition, elevated diabetes risk associated with higher waist circumference in the US as opposed to England could arise as a result of a number of different mechanisms. Investigation of the relative importance of such mechanisms is an important topic for further study.
Diabetes prevalence is about twice as high in the US compared to England for both sexes.[1] This difference was not due to differential disease self-reporting by country since the differential exists using either self-reports of doctor diagnosis or objective markers of diabetes. Conventional models of diabetes prevalence that included standard risk factors (smoking, age, social position) indicated that little of this international difference was explained by country differences in these risk.[1] These differences remained substantial when body mass index (BMI) indicators of over-weight or obese were included in the analyses indicating other factors were responsible.[1]
Recent data suggested that other aspects of body shape including waist circumference were different across countries.[2]. Increasing waist circumference was associated with increasing risk of subsequent diabetes and more predictive than BMI.[3–4] The association of increased diabetes or its risk factors with shorter height has been described in many populations.[5–7]
Earlier analyses examining international differences in diabetes did not examine the role of waist circumference.[1] We study its importance in the English Longitudinal Study of Ageing (ELSA) and NHANES which are nationally representative cohorts with both BMI and waist circumference measurements. Our aim was to expand these models to consider the simultaneous risks of two BMI and waist circumference and to assess how much of differences in diabetes prevalence between England and America was explained by these body shape risks.
METHODS
Data
We used data from eight waves of the American National Health and Nutrition Examination Survey (NHANES), fielded between 1999 and 2006,[8] and the 2004 second wave of the English Longitudinal Survey of Aging (ELSA). We combined NHANES waves from before and after ELSA 2004 to have comparable sample sizes. There was no statistically significant trend in diabetes prevalence over NHANES years 1999–2006 so NHANES was time comparable to ELSA. NHANES included data obtained through personal interviews, physical and lab exams for people two months and older. For age comparability, we restricted our NHANES sample to respondents between 52 and 85 years old—age 52 was the minimum age in ELSA and age 85 was the maximum age in NHANES. To ensure that country differences were not due to high rates of diabetes among African-Americans or Hispanics in America or Asian and Black immigrants in England, our analysis was restricted to Non-Hispanic whites. There were 4,570 Non-Hispanic white NHANES respondents in this age range.
ELSA contained respondents recruited from three separate years of the Health Survey for England providing representative English samples aged 50 and over in 2002. Health data were supplemented by collection of social and economic data.[9] ELSA protocols included interview visits followed by a nurse visits where saliva and blood samples were drawn. Blood samples were analyzed for glycosylated hemoglobin, also available in NHANES. There were 6,888 respondents in our ELSA analytical sample.
NHANES is a nationally representative survey of Americans less than 85 years old with a high response rate. Baseline response rate in 2005–2006 was 80% and 77% for those receiving medical exams. Its sample characteristics closely match the Current Population Surveys. NHANES is a repeated cross-section survey so it was unaffected by sample attrition. http://www.cdc.gov/nchs/nhanes/response_rates_CPS_.htm
We examined impacts of differential response bias and sample attrition in ELSA on estimates of disease prevalence including diabetes by comparing estimates to those from the Health Survey for England.[9] Differences in diabetes prevalence between the two countries were equally large in cross-sectional national surveys indicating that disease prevalence rates were not significantly biased due to differential attrition or response rates.
Measures of Diabetes Prevalence
Both surveys collected data on individual diabetes self-reports of the form ‘Did a doctor ever tell you that you had diabetes…’ as well as glycosylated hemoglobin (HbA1c), a measure of percent of hemoglobin molecules bound to glucose. Although not usually a screener for diabetes, HbA1c is highly correlated with fasting plasma glucose levels.[10–11] While there is no strict diagnosis threshold value, we took values greater than or equal to 6.5% as indicating clinical diabetes (an international threshold recently approved).[12] Our results were insensitive to specific thresholds chosen. Respondents were classified as having diabetes if either they answered affirmatively to the self-reported diabetes question or their HbA1c levels ≥ 6.5%. Thus, our measures were unaffected by country differences in undiagnosed diabetes.
Measures of BMI and Waist Risk
Height and weight measurements were taken during nurse conducted physical examinations so objective BMI measures were computed free of errors in self-reports.[13] We experimented with two BMI measures—categorical indicators of whether respondents were obese (BMI ≥ equal to 30) or overweight (BMI ≥ 25 and < 30) and continuous BMI measures (kg/m2) entered as quadratics. Since height may have an independent effect,[14] height in meters was entered as a quadratic.
BMI provides a crude index of adiposity by not considering central fat mass which is more strongly associated with disease risk. Our data contained both BMI and central fat mass—objectively measured waist circumference in centimeters.[15] Similar to BMI, we experimented with two measures of waist risk. The first categorized respondents into three waist risk groups (low, medium, and high) where centimeter cut off points differed by gender.[16–17] The male groups were low (<94 cm), moderate (94–101 cm) and high risk (≥102 cm), while for women cutoff points were low (<80 cm); moderate (80–88cm) and high risk (≥88cm). Our second measure was waist in centimeters, entered as a quadratic.
Other Risk Factors
American education was separated into high school or less (0–12 years), more than high school but not a college graduate (13–15 years), and college or more (16 years or greater). The English three-way education division was qualified to a level lower than “O-level” or equivalent (typically 0–11 years), qualified to a level lower than “A-level” or equivalent (typically 12–13 years of schooling), and a higher qualification (more than 13 years). Our surveys collected several health-related behaviors including smoking status (ever smoked) and marital status (single or married).
Statistical Methods
We estimated six models of diabetes prevalence separately for men and women. We began with a baseline model pooled across countries with no covariates except an indicator of country—our “unadjusted” model or country differences in diabetes prevalence we tried to explain. The subsequent five models labeled 1 through 5 all included quadratics in age and in height, and indicator variables described above for education, marital status, and smoking.
We were particularly interested in the role of BMI and waist circumference combined with how they were measured. Models 1 through 5 differed in how we specified them. Model 1 used conventional BMI categorical indicators of obesity and overweight; Model 2 had the same BMI categorical indicators and added waist categorical indicators of moderate and high waist risk; Model 3 had continuous BMI entered as a quadratic; Model 4 had continuous BMI and continuous waist both as quadratics; Model 5 had the same model as model 4 but in addition allowed effects of continuous BMI and continuous waist to differ between America and England.
We relied on Ordinary Least Squares regression (OLS) models of prevalence, estimated using STATA statistical software. Since our models contained interaction terms, logistic regressions do not provide a straightforward interpretation.[18] Nevertheless our conclusions from the non-interactive models were unchanged if multiple logistic or multivariate probit models were used.
RESULTS
Table 1 lists means and standard deviations of variables and displays results of statistical tests for country differences. American male prevalence was 16% compared to 11% for English men and 14% for American women compared to 7% for English women. Both country differences in diabetes prevalence were statistically significant at the one percent level.
Table 1.
Means and Standard Deviations
| English Men | English Women | American Men | American Women | |
|---|---|---|---|---|
|
| ||||
| Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | |
| Diabetes prevalence | 0.11 (0.32) | 0.07 (0.25) | 0.16** (0.36) | 0.14** (0.34) |
| BMI | 27.84 (4.14) | 28.04 (5.29) | 28.12* (5.00) | 28.01 (6.17) |
| Waist (centimeters) | 101.59 (11.49) | 90.99 (12.59) | 104.44** (13.17) | 95.94** (14.40) |
| Height (meters) | 1.73 (0.07) | 1.59 (0.07) | 1.74 (0.07) | 1.60 (0.07) |
| Age | 65.54 (8.68) | 65.81 (8.98) | 68.87** (10.68) | 68.97** (10.44) |
| Single | 0.20 (0.40) | 0.36 (0.48) | 0.24 (0.43) | 0.48** (0.50) |
| Ever Smoked | 0.72 (0.45) | 0.56 (0.50) | 0.68 (0.47) | 0.44** (0.50) |
| Medium education | 0.27 (0.44) | 0.33 (0.47) | 0.25 (0.43) | 0.32 (0.47) |
| Low Education | 0.42 (0.49) | 0.42 (0.49) | 0.23** (0.42) | 0.32** (0.47) |
Statistically significant at 0.05;
statistically significant at 0.01 difference between American and English.
Note: Non-Hispanic whites aged 52–85 from the 2004 ELSA and 1999–2006 NHANES samples respectively.
Conventional diabetes risk factors were similar in American and English populations examined. On average Americans are only a little taller, have slightly higher BMIs and about three years older. The English were less educated and more likely to have smoked, risk factors that normally increase diabetes.[3, 19–21] The only attribute offering some explanatory promise was waist circumference. American men have almost three centimeters larger waists than English men, and waists of American women are five centimeters bigger than English women (p<0.01).
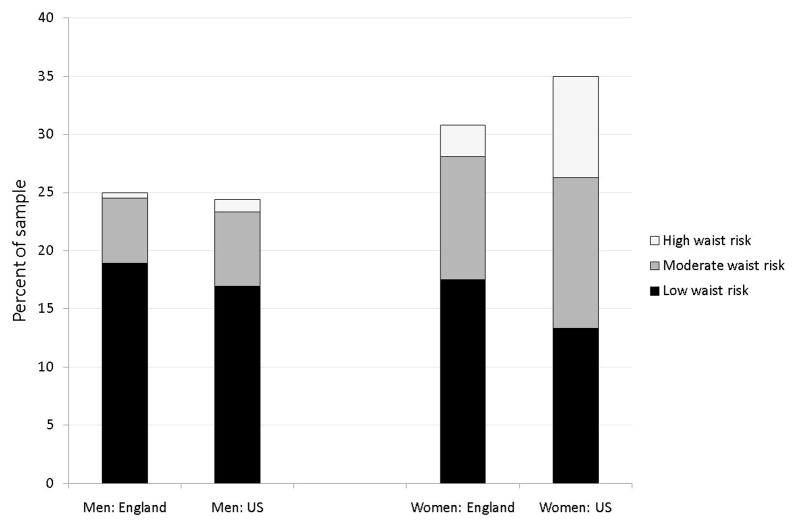
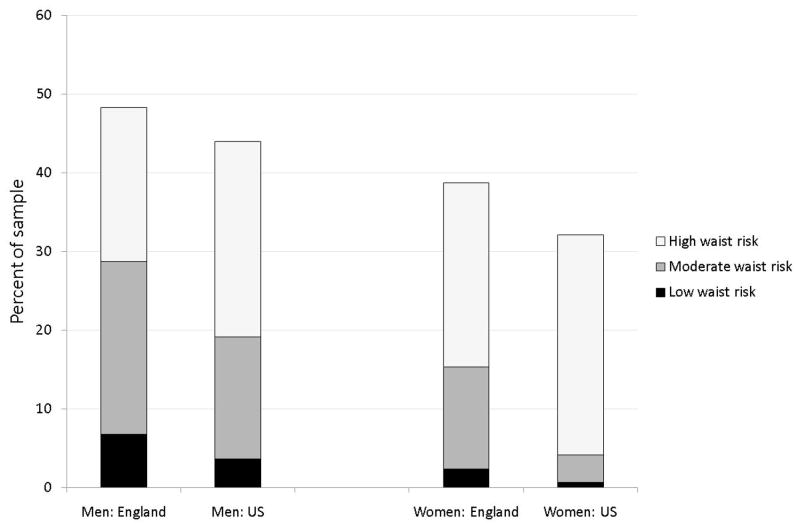
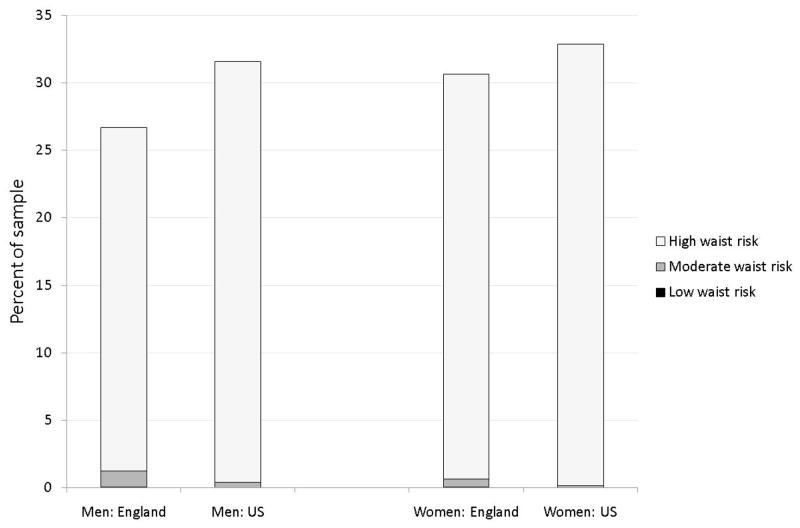
Figures 1, 2, and 3 present age standardized distribution of waist risk (low, moderate, and high) among those whose BMI is below 25, between 25 and 30 and 30 and over separately by gender in both countries. Age standardisation eliminated impacts of country differences in age distributions. Using age groups 50–59, 60–69, 70–79, 80 and over, age weighted BMI and waist risks were calculated. To capture risk variation, individuals were placed into three BMI groups and three waist risk groups defined above. Appendix Table A, available on line, includes statistical tests of differences in waist and BMI between countries.
Figure 1.
Distribution of waist risk for those with BMI below 25
Notes:
1. Non-hispanic whites aged 52–85 from the 2004 ELSA and 1999–2006 NHANES samples respectively.
2. Low waist risk defined as < 94 cm (M) < 80 cm (W); Moderate waist risk defined as 94–102 cm (M), 80–88 cm (W); High waist risk defined as ≥102cm (M), ≥88cm (W).
3. Statistics are age-standardised and weighted.
4. The height of the bars are the fraction whose BMI is in this category.
Figure 2.
Distribution of waist risk for those with BMI between 25 and 30
Note: See Notes to Figure 1.
Figure 3.
Distribution of waist risk for those with BMI 30 or over
Note: See Notes to Figure 1.
American men were more obese than English men (31.6% compared to 26.7%) but less overweight (44.0% compared to 48.3%) with no difference in normal weight. Female obesity differences were even smaller (32.9% of Americans compared to 30.6% among English women), a disparity not statistically significant and not large enough to explain a two-to-one diabetes prevalence differential between countries. Compared to English women, American women were less overweight (32.1% compared to 38.7%) and more of normal weight. (35.0% compared to 30.8%). Given these BMI distributions, it is unsurprising that BMI alone cannot explain higher diabetes rates among Americans.
Marginal distributions of waist risk within each BMI group were more to the disadvantage of American men than BMI differences and female disparities are striking—56.0% of English women have high waist risk whereas the comparable fraction among Americans was 69.4%—a statistically significant difference. These differences are in accord with recent evidence from smaller more specialist studies in which body-shape was measured using three-dimensional body-scanning equipment in both countries.
If there was a one-to-one correspondence between BMI and waist risk categories, there would be little reason to prefer one. But this is not true. Among the overweight in Figure 2, 56% of American men have high waist risk compared to 41% of English men, while 87% of American women have high waist risk compared to 61% of English women. Even among those with normal weight, the fraction with raised waist risk was not trivial for US women—40.6% of Americans who were neither overweight nor obese were categorized as high waist risk compared to 8.9% among equivalent English women. The only BMI category with little additional information obtained from waist were the obese (Figure 3). At least 95% of respondents labeled obese were simultaneously labeled high waist risk.
Figure 4 summarizes the ability of factors to explain country differences in diabetes prevalence using our six models. For each model, the solid bar indicates our estimate of ‘unexplained’ prevalence rate differences between America and England while the lines represent 95% confidence intervals around that estimate. The unadjusted specification reported a baseline model pooled across countries with just the country dummy (=1 indicates US). This model highlighted unadjusted country differences in diabetes—4.8% for men and 7.3% for women.
Figure 4. Estimated diabetes prevalence difference between US and England.
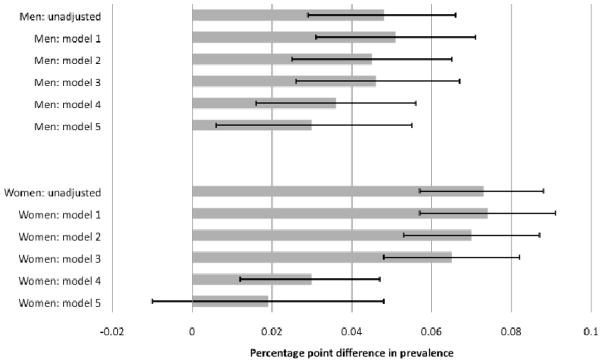
Notes to figure:
1. Solid bars indicate estimated prevalence differences, lines indicate 95% confidence interval around the estimates.
2. Positive values of prevalence indicate greater prevalence in the US.
3. Models 2–5 all include the following risk factors: age and age2, height and height2 and indicators of education, marital status and past smoking behavior.
4. BMI and waist adjustments in models 1–4 are as follows. Model 1: categorical BMI indicators; Model 2: categorical BMI indicators and categorical waist risk indicators (medium, high); Model 3: BMI (kg/m2) and BMI2; Model 4: BMI and BMI2, waist (cm) and waist2.
5. Model 5 contains the same adjustments as model 4, but with the effects of BMI and waist being allowed to differ in the two countries.
Subsequent models in the next five rows in Figure 4 sequentially expand the set of covariates while maintaining the assumption that effects of covariates are the same in both countries. All models below unadjusted included quadratic controls for age and height, indicator variables for education groups, single, and smoking. Models 1 to 4 varied the way in which BMI and waist circumference were included in models.
Model 1 estimates in Figure 4 represented the traditional approach to measuring BMI—two indicators for whether one was overweight or obese. This model did not explain any between-country prevalence difference—some factors (age, BMI) predict higher US diabetes prevalence, other factors (education, height) predict the opposite with combined effects largely offsetting. Model 2 highlights impacts of adding categorical variables to capture waist risk—these effects are quite modest in reducing unexplained country prevalence differences.
Model 3 was similar to model 1 except continuous quadratic BMI replaced standard obese and overweight categories controls. Estimated country prevalence differences were unchanged. Model 4 shows effects of including continuous quadratic measures of BMI and waist risk. With both shape variables measured continuously instead of as categories, we explained one quarter of the original male difference in prevalence and about 60% of the female difference. Continuous BMI by itself does not help explain country differences (another illustration of BMI deficiency), but a combination of continuous measures of BMI and waist significantly reduced country differences in ‘unexplained’ diabetes prevalence.
Models 1–4 did not allow effects of BMI or waist on predicting diabetes to vary by country. Our preferred model 5 allowed estimated impacts of both body shape measures to vary between countries. The remaining unexplained male country difference was 3 percentage points and 1.9 percentage points for women. As indicated by 95% confidence intervals in Figure 4, female country difference in diabetes was no longer statistically significant. Control variables in this model explained 38% of country difference in male prevalence and 74% of female differences.
The regression models underlying model 5 in Figure 4 are presented in Table 2. Estimates for non-anthropometric variables were as expected.[22] The probability of diabetes rose with age at a decreasing rate, increased height is associated with lower diabetes risk, and smoking and marital status did not matter.[23–24]
Table 2.
Models of Diabetes Prevalence
| B (se) Men | B (se) Women | |
|---|---|---|
| USA | 0.0302* (0.013) | 0.0191 (0.015) |
| Age | 0.0114** (0.002) | 0.0049** (0.002) |
| Age squared | −0.0003** (0.000) | −0.0000* (0.000) |
| Height | −0.3168** (0.079) | −0.2958** (0.065) |
| Height squared | −0.0040 (0.604) | −0.2081 (0.578) |
| Single | 0.0160 (0.015) | 0.0045 (0.008) |
| Ever smoke | 0.0050 (0.010) | −0.0073 (0.008) |
| Med education | −0.0041 (0.012) | 0.0090 (0.009) |
| Low education | −0.0150 (0.011) | 0.0267** (0.009) |
| BMI | 0.0016 (0.004) | −0.0028 (0.003) |
| BMI squared | −0.0005 (0.000) | −0.0003 (0.000) |
| USA BMI | −0.0014 (0.006) | −0.0033 (0.004) |
| USA BMI squared | 0.0009* (0.000) | 0.0000 (0.000) |
| Waist | 0.0037* (0.002) | 0.0023 (0.001) |
| Waist squared | 0.0001* (0.000) | 0.0002** (0.000) |
| USA waist | 0.0013* (0.002) | 0.0024* (0.002) |
| USA waist squared | −0.0001 (0.000) | −0.0000 (0.000) |
| Constant | −0.0424* (0.020) | −0.0414** (0.016) |
Statistically significant at 0.05;
statistically significant at 0.01.
After controlling for waist, we found no statistically significant effect of BMI for American or English men or women. In contrast to weak BMI effects, waist risk was strongly associated with diabetes, with risk increasing in waist circumference. Based on a likelihood ratio test, quadratic waist terms were statistically significant for American men and women implying that consequences of raised waist are higher for them compared to the English.
DISCUSSION
We found significant roles for height and waist on diabetes prevalence. The mechanisms by which height reduces diabetes risk are unclear. Adult stature reflects childhood growth patterns and an association of short stature with type 2 diabetes indicates that impaired childhood growth leads to adult insulin resistance and diabetes.[25] Mechanisms include poor nutrition in childhood.[26] There may be reverse causation at older ages, where co-morbidity with diabetes is associated with height reduction, but we found no significant age interactions indicating these influences are unlikely to vary by country. While height was related to diabetes risk, height cannot explain country differences since average height was approximately the same in both countries.
In contrast, waist circumference explained a substantial proportion of higher diabetes in America for men and virtually all differences for women, but continuous measures were required to capture differences in diabetes risks between the two populations.
Evidence cites increasing prevalence of obesity with increased burden of disease[22] but little was explained here by obesity categorized by BMI index.[1] BMI provides a crude index of adiposity by not considering central fat mass which is more strongly associated with disease risk. Acknowledging this is important when making health comparisons across different populations. Our data suggested that in each BMI category a greater proportion of Americans were at increased disease risk and that waist circumference played the key role.
There are several potential mechanisms, including different rates of physical activity through exercise or activities of daily life, diet differences, or a more adverse psychosocial environment in America; all associated with central adiposity and type 2 diabetes.[18, 27–29] Adverse stressful’ environments may be associated with development of central obesity.[30]
Evidence between central fat accumulation and diabetes risk is striking.[3–4] Compared to waist hip ratio, waist circumference is a better marker of visceral fat.[31] Fat cells located in the viscera display distinct cellular features compared to fat cells elsewhere and a specific dysfunction of these cells may be the patho-physiological basis for negative consequences of abdominal obesity. Central fat cells have a higher turnover rate of triglycerides, produce more pro-inflammatory and metabolic markers than fat cells from other depots. They are involved in the mobilization of free fatty acids into portal circulation, impaired liver function leading to insulin resistance and diabetes.
Our results were the same if we limited analyses to those whose diabetes onset occurred after age 34 so our conclusions are not driven by country differences in type 1 diabetes. Thus, our results apply most directly to type 2 diabetes.
ELSA and NHANES allowed international comparisons in large representative cohorts, a key strength of this paper, but some limitations exist. Several covariates that might influence diabetes risk were unavailable- equivalent collection of physical activity or dietary information and medication intakes. Evidence suggests that while more recently developed antihypertensive medication are not diabetogenic, older treatments increase diabetes risk.[32] We cannot examine antihypertensive medication in detail, but speculate that, while physical activity and dietary intake may play a role, antihypertensive medication prescribing practices are similar in America and England and unlikely to affect these international differences.
Two powerful conclusions follow from this research. Higher American rates of diabetes compared to England were largely due to a combination of high waist among Americans and a higher impact of waist risk among Americans. The reasons may be related to measures that were not available such as objective measures of physical activity and dietary intake.
This combination of higher waist and higher risk of high waist in America explained virtually all excess diabetes of American women and almost half for American men. BMI alone failed to explain any of these differences. Besides this key substantive conclusion, our results showed that modeling diabetes successfully requires good measures of waist risk, particularly in populations which might differ in distributions of body fat like the US and English. Similar issues should be investigated for other cardiovascular disease and countries.
What is already known on this subject
Middle aged and older Americans have twice the rate of diabetes prevalence compared to England
This differential not explained by conventional risk factors including age, smoking, social position, and BMI
What this study adds
Middle aged and older American men and particularly American women have much higher waist circumference compared to the English
High waist circumference measured continuously was far more important than BMI in predicting diabetes prevalence
Higher diabetes in America than in England was largely accounted for by higher American waist circumference and larger impacts of high waist on diabetes in America.
Acknowledgments
Support received from by a grant from the NIA to RAND and from the NIA and the Economic and Social Research Council to the Institute for Fiscal Studies.
Contributor Information
James Banks, Department of Economics, University College London and Institute for Fiscal Studies, London, England.
Meena Kumari, Department of Epidemiology, University College London, London, England.
James P. Smith, The RAND Corporation, Santa Monica, California, USA
Paola Zaninotto, Department of Epidemiology, University College London, London, England.
References
- 1.Banks J, Marmot M, Oldfield Z, et al. Disease and disadvantage in the United States and in England. JAMA. 2006;295:2037–45. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- 2.Wells JCK, Cole TJ, Bruner D, et al. Body shape in American and British adults: between country and inter-ethnic comparisons. Int J Obesity. 2008;32:152–59. doi: 10.1038/sj.ijo.0803685. [DOI] [PubMed] [Google Scholar]
- 3.Perry IJ, Wannamethee SG, Walker MK, et al. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ. 1995;310:560–64. doi: 10.1136/bmj.310.6979.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulze MB, Heidemann C, Schienkiewitz A, et al. Comparison of anthropometric characteristics in predicting the incidence of type 2 diabetes in the EPIC-Potsdam study. Diabetes Care. 2006;29:1921–23. doi: 10.2337/dc06-0895. [DOI] [PubMed] [Google Scholar]
- 5.Brown DC, Byrne CD, Clark PMS, et al. Height and glucose tolerance in adult subjects. Diabetologia. 1991;34:531–33. doi: 10.1007/BF00403292. [DOI] [PubMed] [Google Scholar]
- 6.Kousta E, Lawrence NJ, Penny A, et al. Women with a history of gestational diabetes of European and South Asian origin are shorter than women with normal glucose tolerance in pregnancy. Diabet Med. 2000;17:792–97. doi: 10.1046/j.1464-5491.2000.00393.x. [DOI] [PubMed] [Google Scholar]
- 7.Kumari M, Chandola T, Brunner E, et al. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II Study. J Clin Endocrinol Metab. doi: 10.1210/jc.2009–2105. Published Online first: 30 June 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention, National Center for Health Statistics. [accessed 9 January 2006];National Health and Nutrition Examination Survey data sets and related documentation. Available at http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm.
- 9.Banks J, Muriel A, Smith JP. Disease prevalence, disease incidence, and mortality in the United States and in England. Forthcoming in. Demography. 2010 Oct; doi: 10.1353/dem.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson M, Schriger D, Peters A, et al. Relationship between fasting plasma glucose and glycosylated hemoglobin. Potential for false positive diagnoses of type 2 diabetes using new diagnostic criteria. JAMA. 1999;281:1203–10. doi: 10.1001/jama.281.13.1203. [DOI] [PubMed] [Google Scholar]
- 11.Goldman N, Lin I, Weinstein M, et al. Evaluating the quality of self-reports of hypertension and diabetes. Journal of Clinical Epidemiology. 2003;56:148–54. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- 12.International Expert Committee. The International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunnell D, Berney L, Holland P, et al. How accurately are height, weight and leg length reported by the elderly, and how closely are they related to measurements recorded in childhood? Int J Epidemiol. 2000;29:456–64. [PubMed] [Google Scholar]
- 14.Han TS, Feskens EJ, Lean ME, et al. Associations of body composition with type 2 diabetes mellitus. Diabet Med. 1998;15:129–35. doi: 10.1002/(SICI)1096-9136(199802)15:2<129::AID-DIA535>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Turcato E, Bosello O, Di Francesco V, et al. Waist circumference and abdominal sagittal diameter as surrogates of body fat distribution in the elderly: their relation with cardiovascular risk factors. Int J Obes Relat Metab Disord. 2000;24:1005–10. doi: 10.1038/sj.ijo.0801352. [DOI] [PubMed] [Google Scholar]
- 16.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. Br Med J. 1995;311:158–61. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flegal KM. Waist circumference of healthy men and women in the United States. Int J Obes. 2007;31:1134–39. doi: 10.1038/sj.ijo.0803566. [DOI] [PubMed] [Google Scholar]
- 18.Knol JK, van der Tweel J, Grobbee DE, et al. Estimating interaction effects on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36:1111–18. doi: 10.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- 19.Holbrook TL, Barrett-Connor E, Wingard DL. A prospective population-based study of alcohol use and non-insulin-dependent diabetes mellitus. Am J Epidemiol. 1990;132:902–09. doi: 10.1093/oxfordjournals.aje.a115733. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Chan J, Stampfer MJ, et al. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ. 1995;310:555–59. doi: 10.1136/bmj.310.6979.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk to type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 22.Smith JP. Nature and causes of male diabetes trends, undiagnosed diabetes, and the SES health gradient. P Natl Acad Sci USA. 2007;104:13,225–31. doi: 10.1073/pnas.0611234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowie CC, Eberhardt MS. National Diabetes Data Group, editor. Diabetes in America. 2. Bethesda, MD: National Diabetes Data Group, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1995. Sociodemographic characteristics of persons with diabetes. NIH publication 95–1468. [PubMed] [Google Scholar]
- 24.Kumari M, Head J, Marmot M. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med. 2004;164:1873–80. doi: 10.1001/archinte.164.17.1873. [DOI] [PubMed] [Google Scholar]
- 25.Eriksson JG, Forsen T, Tuomilehto J, et al. Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia. 2003;46:190–94. doi: 10.1007/s00125-002-1012-5. [DOI] [PubMed] [Google Scholar]
- 26.Wadsworth M, Hardy R, Paul A, et al. Leg and trunk length at 43 years in relation to childhood health, diet and family circumstances: Evidence from the 1946 national birth cohort. Int J Epidemiol. 2002;31:383–90. [PubMed] [Google Scholar]
- 27.Folsam AR, Kushi LH, Hong CP. Physical activity and incident diabetes mellitus in postmenopausal women. Am J Public Health. 2000;90:134–38. doi: 10.2105/ajph.90.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tammelin T, Laitinen J, Nayha S. Change in the level of physical activity from adolescence into adulthood and obesity at the age of 31 years. Int J Obes. 2004;28:775–82. doi: 10.1038/sj.ijo.0802622. [DOI] [PubMed] [Google Scholar]
- 29.Brunner EJ, Mosdøl A, Witte DR, et al. Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am J Clin Nutr. 2008;87:1414–21. doi: 10.1093/ajcn/87.5.1414. [DOI] [PubMed] [Google Scholar]
- 30.Epel ES, McEwen B, Seeman T, et al. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62:623–32. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Han TS, McNeill G, Seidell JC, et al. Predicting intra-abdominal fatness from anthropometric measures: the influence of stature. Int J Obes Relat Metab Disord. 1997;21:587–53. doi: 10.1038/sj.ijo.0800446. [DOI] [PubMed] [Google Scholar]
- 32.Mancia G, Grassi G, Zanchetti A. New-onset diabetes and antihypertensive drugs. J Hypertens. 2006;24:3–10. doi: 10.1097/01.hjh.0000194119.42722.21. [DOI] [PubMed] [Google Scholar]