Abstract
Many animals extract, synthesize and refine chemicals for colour display, where a range of compounds and structures can produce a diverse colour palette. Feather colours, for example, span the visible spectrum and mostly result from pigments in five chemical classes (carotenoids, melanins, porphyrins, psittacofulvins and metal oxides). However, the pigment that generates the yellow colour of penguin feathers appears to represent a sixth, poorly characterized class of feather pigments. This pigment class, here termed ‘spheniscin’, is displayed by half of the living penguin genera; the larger and richer colour displays of the pigment are highly attractive. Using Raman and mid-infrared spectroscopies, we analysed yellow feathers from two penguin species (king penguin, Aptenodytes patagonicus; macaroni penguin, Eudyptes chrysolophus) to further characterize spheniscin pigments. The Raman spectrum of spheniscin is distinct from spectra of other feather pigments and exhibits 17 distinctive spectral bands between 300 and 1700 cm−1. Spectral bands from the yellow pigment are assigned to aromatically bound carbon atoms, and to skeletal modes in an aromatic, heterocyclic ring. It has been suggested that the penguin pigment is a pterin compound; Raman spectra from yellow penguin feathers are broadly consistent with previously reported pterin spectra, although we have not matched it to any known compound. Raman spectroscopy can provide a rapid and non-destructive method for surveying the distribution of different classes of feather pigments in the avian family tree, and for correlating the chemistry of spheniscin with compounds analysed elsewhere. We suggest that the sixth class of feather pigments may have evolved in a stem-lineage penguin and endowed modern penguins with a costly plumage trait that appears to be chemically unique among birds.
Keywords: plumage, Raman, spectroscopy, Sphenisciformes, spheniscin
1. Introduction
Biologists have long been fascinated with the variation in and vibrancy and evolution of avian plumage colours. Birds achieve much of this plumage-colour diversity using nanostructural mechanisms, as in the case of blues and iridescent colour [1], as well as with five different classes of chemical pigment: indole polymers (melanins: black, brown, grey, rufous and buff), tetraterpenoids (carotenoids: yellow, orange, red and purple), linear polyenals (psittacofulvins: yellow, orange and red), porphyrins (red, brown and green) and metal oxides (rust-red) [2–9]. However, the colour-producing pigmentary mechanisms for the vast majority of bird species have not yet been analysed [10] and have only been inferred from common ancestry or shared reflectance characteristics [11].
Though many classes of feather colourants are thought to be widespread in birds (e.g. melanin across Aves, carotenoids across Passeriformes [12,13]), others appear to be quite rare. Parrots are believed to be the only organisms in the world that produce their unique class of feather pigments, psittacofulvins [14]. Moreover, turacos (Cuculiformes; Musophagidae) are considered to be the lone group to produce their red and green copper-rich plumage compounds, turacin and turacoverdin, respectively [15]. A recent study of yellow feathers from penguins (Sphenisciformes) revealed a sixth class of pigment that fluoresces in ultraviolet light [16]. The solubility properties of the penguin pigment distinguish it from other yellow feather colourants, and chromatography and light-elemental analyses suggest the novel compound is chemically similar to yellow and red pterin pigments [17], which are common in amphibians, reptiles and butterflies [18–20] but only previously found in the irides of birds [21].
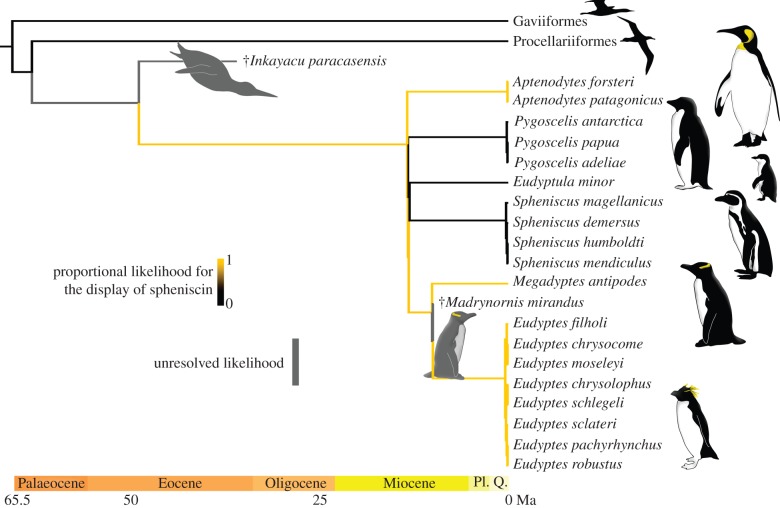
The yellow feather pigment in penguins is potentially unique to Sphenisciformes, and based on ancestral-state reconstructions may have evolved de novo in a stem-penguin lineage (figure 1). Furthermore, yellow plumage pigmentation is a condition-dependent trait [28–30] and is important to the sexual selection criteria of half the living penguin genera [31–34]. Thus, further characterization of the chemical identity of this pigment could provide clues into the costs, benefits and evolution of these display traits.
Figure 1.
Emperor and king penguins (Aptenodytes spp.), crested penguins (Eudyptes spp.) and the yellow-eyed penguin (Megadyptes antipodes) are the only living animals known to display spheniscin pigments. If we consider losses and gains of spheniscin to be equally likely evolutionarily, then the most recent common ancestor shared by all living penguins did not have yellow feathers. Alternatively, if we consider a single evolution of spheniscin to be more likely than multiple appearances (i.e. an asymmetric model), then spheniscin probably evolved between 63 and 13 million years ago in an ancient penguin lineage. Both models suggest that the extinct penguin, Madrynornis mirandus, had a spheniscin-pigmented ancestor. Results have been interpreted from maximum-likelihood ancestral-state reconstructions performed in Mesquite v. 2.75 [22] (Mk1, equal rates model; Asymm. 2. param., asymmetric model); Mesquite output file in the electronic supplementary material. Figure shows results from asymmetric model. Not all gaviiform or procellariform taxa are shown. Sphenisciformes branch lengths are from [23,24], in units of geological time; Gaviiformes and Procellariiformes branch lengths are from [25] and have been scaled to geological time. The relationship between Gaviiformes, Procellariiformes and Sphenisciformes follows [26] as adapted by [25]. Inkayacu paracasensis included to represent stem-lineage penguins, silhouette from [27]. Pl., Pliocene; Q., Quaternary. (Online version in colour.)
We sought additional biochemical information on yellow plumage pigments in penguins (hereafter named spheniscins) using vibrational spectroscopic techniques. Such techniques include Raman and mid-infrared spectroscopies, which describe atomic interactions within molecules and minerals. Raman and mid-infrared spectroscopies are versatile methods for analysing chemical composition and molecular structure. Notably, Veronelli et al. [35] determined chemical characteristics of psittacofulvins from a Raman spectroscopic study comparing parrot feather pigments and carotenoids, and Mendes-Pinto recently used Raman spectral information to link feather coloration with carotenoid-contortion [36]. Previous studies of spheniscin pigments have identified chromatic, chromatographic retention-time and solubility properties [17]. Vibrational spectroscopic techniques were used here to study functional groups within spheniscin, which could potentially be elaborated into a chemical structure for the penguin pigment.
2. Material and methods
2.1. Selected feathers
Raman and mid-infrared spectra were collected from feather barbs and barbules of several species, including two penguin species and several reference species in which the pigmentary basis of coloration is known. Studied specimens included three yellow crest feathers from a macaroni penguin (National Museum of Natural History catalogue no. USNM 533533) and three yellow-orange auricular and three yellow-orange breast feathers from a king penguin (USNM 59243). The sex of each penguin is unknown. The macaroni penguin crest feather was entirely yellow, whereas the king penguin feather had a white proximal end and became yellow-orange towards the distal end. Permission for removing feathers from study skin specimens (USNM 533533 and USNM 59243) was granted by the Division of Birds, National Museum of Natural History, Smithsonian Institution. Chromatographic evidence from an earlier study [17] had revealed a second, related pigment in the yellow feathers of yellow-eyed (Megadyptes antipodes), rockhopper (Eudyptes chrysocome) and Snares-crested (Eudyptes robustus) penguins [17]. Feathers from these other penguin species were not plucked for analysis in the current study to reduce the impact of our work on the study skin collection.
A comparative library of pigment spectra was constructed from feathers of other birds for which feather colour had previously been established (summarized in [12,13,37]). A library is useful for determining whether the spectrum of a compound with an unknown structure (e.g. spheniscin) is distinct from spectra of structurally resolved compounds. Spectral libraries may also be useful for identifying functional groups in a studied compound. Feather colours analysed in the current study included the red on a single remige from a female Livingstone's turaco (Tauraco corythaix livingstonii; USNM 636319); the turquoise from a remige of a male lilac-breasted roller (Coracias caudata; USNM 634477); rust coloration on the wing covert from a female sandhill crane (Grus canadensis; USNM 641570); a white wing covert from a male greater black-backed gull (Larus marinus; USNM 638661); yellow on the rectrix of a female rose-ringed parakeet (Psittacula krameri; USNM 643550); yellow on the rectrix of a female bokmakierie (Telophorus zeylonus; USNM 642574); a rufous breast contour feather from a female American robin (Turdus migratorius; USNM 623483). Additional black and brown feathers were also analysed, including brown-orange feathers from short-tailed albatross (Phoebastria albatrus; see the electronic supplementary material).
2.2. Spectroscopy
Raman spectra were measured using excitation wavelengths 780 nm (dispersive) and 1064 nm (Fourier transform, FT). Dispersive Raman spectra were collected using a Nicolet Almega XR spectrometer (Thermo Electron Corporation, Madison, WI, USA) equipped with a 150 mW diode laser and a Peltier-cooled CCD detector. Spectra were collected through a 50× or 100× Mplan apochromatic objective lens (Olympus, Melville, NY, USA) and 50 or 100 μm pinhole aperture, in a BX51 confocal microscope (Olympus). Each spectrum was a co-addition of 32 scans across 200–3400 cm−1 (2.6–4.9 cm−1 resolution), collected at 1, 10 or 100 per cent laser power. Magnification, aperture and laser power were optimized between analyses to minimize spectral noise and to maximize spectral signal from samples with a range of energy tolerances. FT-Raman spectra were collected using an NXR FT-Raman module coupled to a 6700 FTIR spectrometer (Thermo Electron Corporation). The Raman module is equipped with a Nd : YVO4 laser, a CaF2 beam splitter and a Peltier-cooled InGaAs detector. Spectra were collected using the 1 mm laser spot of a microstage. Each spectrum was a co-addition of 1024 scans across 100–3700 cm−1 (4 cm−1 resolution), with laser power set at 0.2, 1 or 1.4 W depending on the energy tolerance of the feather barbs. Note that laser power at the sample surface was not measured. Instrument control and data collection were managed by OMNIC 7.2 (FT) and OMNIC 8.2 (dispersive) (Thermo Fisher Scientific, Waltham, MA, USA). Raman instruments are housed in the Museum Conservation Institute, Smithsonian Institution, Suitland MD, USA.
Fourier transform infrared (FTIR) spectra of feathers were collected using a Perkin Elmer Spotlight 400 IR imaging system with an attenuated total reflectance (ATR) imaging attachment (Perkin Elmer, Waltham, MA, USA). A 100 × 100 µm area was imaged using a 6.25 µm pixel; the spectrum of each pixel was the co-addition of 32 scans recorded with 4 cm−1 resolution from 750 to 4000 cm−1. Ten spectra recorded from feather barbule regions within the 100 × 100 μm area were averaged for each sample. The infrared spectrometer is housed in the Department of Bioengineering, Temple University, Philadelphia, PA, USA.
2.3. Pigment extractions and pH tests
A single, yellow crest feather from a macaroni penguin (USNM 533533) was available for destructive analysis. The feather was soaked in 1 ml of 0.5 M NaOH for 30 min in a 2.5 ml Eppendorf tube following a method adapted from McGraw et al. [17]. The solution turned yellow and the feather remained yellow. Note that the yellow pigment was very weakly soluble in 0.5, 5 and 13 M NH4OH, and insoluble in acetone, acetonitrile, ethyl acetate, methanol, toluene and a 50 : 50 mixture of acetonitrile and methanol, and that the insolubility in volatile solutions and hydrolysis of keratin in strong basic solutions has complicated our efforts to collect a mass spectrum. The feather was removed from the tube, and 1 M HCl was added dropwise to the feather extract solution. A white precipitate formed at neutral pH (confirmed with pH indicator strips), which was concentrated by centrifugation, separated from the supernatant and evaporated on a glass slide. Raman spectra were collected from the residue according the method outlined above. Infrared absorption spectra were collected using a Nicolet 6700 FTIR spectrometer (Thermo Electron Corporation) with an ATR accessory (Golden Gate, Specac Ltd, Orpington, UK). The residue was compressed against a 1 mm2 diamond window for data collection. Each spectrum was a co-addition of 64 scans across 100–3700 cm−1 at 4 cm−1 resolution. The infrared spectrometer is housed in the Museum Conservation Institute, Smithsonian Institution, Suitland, MD, USA.
The effect of pH on the Raman spectrum of the yellow pigment was studied by immersing barbs from a king penguin feather (USNM 59243) in water or in aqueous acid. The pH of 100 ml distilled water was adjusted with 0.5 M HCl and 0.1 M NaOH; pH was monitored with an Accumet AB15 pH meter (Fisher Scientific, Pittsburgh, PA, USA). The pH meter was calibrated using tris (hydroxymethyl) amino methane (pH = 10.4) and potassium biphthalate (pH = 4). A single aliquot (0.5 ml) was removed from the stock volume at pH 7, 5, 3, 2 and 1. One feather barb was immersed in each aliquot for 30 min and removed immediately in order to collect Raman spectra with the dispersive instrument (100% laser power, 16 scans, 50× objective, 100 μm aperture).
3. Results
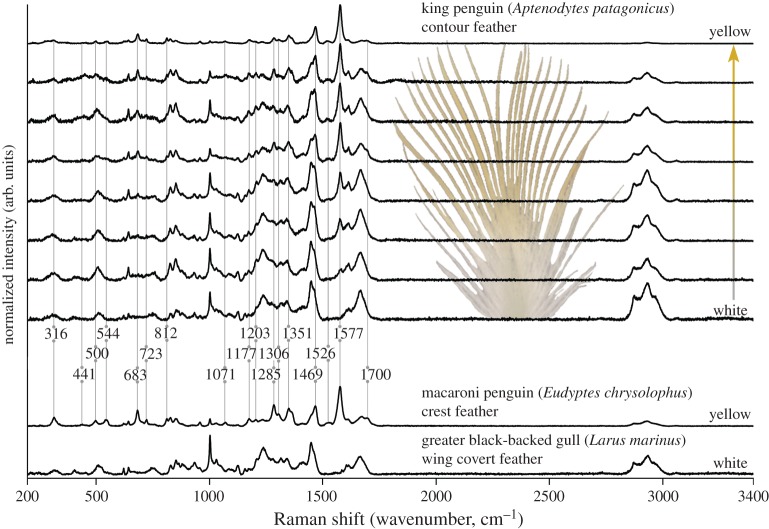
Raman spectra support the chemical disparity between known feather pigments and the yellow pigment in penguin feathers (figure 2). Raman spectra from the yellow feather barbs of macaroni and king penguins contained identical bands, in addition to β-keratin constituents, that were distinct from the Raman spectra of other feather pigments (figure 3). We observed 17 distinct bands in the spheniscin spectrum; the five most intense bands occurred at 1577 (vs), 1285 (s), 683 (m), 1469 (m) and 1351 (m) cm−1. The band with the highest wavenumber position occurred at 1700 cm−1. The feather extracts fluoresced at 780 and 1064 nm excitation and did not produce informative Raman spectra. In contrast, spectra from the turaco, bokmakierie and parrot feathers were characteristic of a porphyrin, carotenoid and psittacofulvin, respectively [35,38]. Rufous-brown barbs in the robin feather fluoresced at both 780 and 1064 nm excitation; 780 nm excitation induced a sinusoidal disturbance of the baseline between 200 and approximately 1600 nm for all brown feather pigments, i.e. a spectral artefact (see the electronic supplementary material). Similarly, the turquoise and black feather regions from the roller feather intensely fluoresced under both wavelengths (including spectral artefact at 780 nm excitation). Spectral consistency between the eumelanin-bearing colour regions of the same feather, and comparison with other black feather barbs and barbules (see the electronic supplementary material), confirm this is a diagnostic fluorescent response for eumelanin. Spectra from the white gull feather showed only bands from β-keratin [39].
Figure 2.
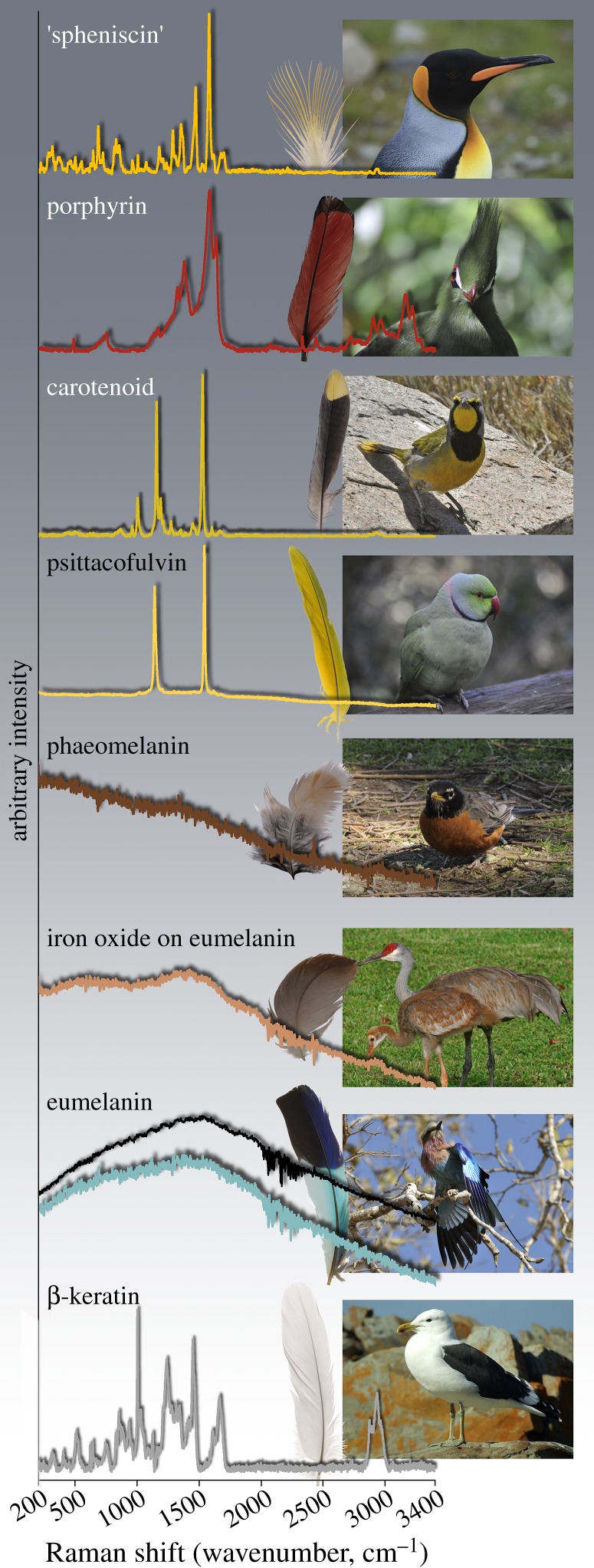
Raman spectra from feather pigments and an unpigmented feather. Five chemical classes have previously been recognized as feather pigments: porphyrins (Livingstone's turaco), tetraterpenoids (carotenoid, bokmakierie), linear polyenals (psittacofulvin, rose-ringed parakeet), metal oxides (iron oxide, sandhill crane) and indole polymers (eumelanin, lilac-breasted roller; phaeomelanin, American robin). Spectra from known pigments and unpigmented β-keratin (greater black-backed gull) differ from the spectrum recovered from yellow penguin feathers (‘spheniscin’), which represents a sixth class of feather pigment. Images of a Knysna turaco and kelp gull are shown; other bird images match the respective feathers. The porphyrin spectrum is from extracted pigment; all other analyses were in situ. Spheniscin, porphyrin, carotenoid, psittacofulvin and β-keratin spectra were measured at 780 nm; melanin-containing spectra were measured at 1064 nm. ‘Spheniscin’, carotenoid and β-keratin spectra have been baseline corrected. Unmanipulated spectra are available in the electronic supplementary material. King penguin photo by Liam Quinn and sandhill crane photo by Tom Friedel. Other photos by D.B.T. (Online version in colour.)
Figure 3.
Raman spectra of the yellow penguin pigment and β-keratin. Penguin pigment spectral bands become more intense towards the distal tips of king penguin feather barbs (i.e. with yellow saturation). Changes in relative intensities allowed bands in the penguin pigment spectrum to be differentiated from β-keratin spectral bands. The macaroni penguin feather is yellow throughout and mostly gives a ‘spheniscin’ spectrum (i.e. dominated by the penguin pigment), with a minor contribution from β-keratin. The greater black-backed gull feather was unpigmented and does not contain the 17 bands identified in the spheniscin spectrum. Spectra were collected at 780 nm excitation and have been baseline corrected. Unmanipulated spectra are available in the electronic supplementary material. (Online version in colour.)
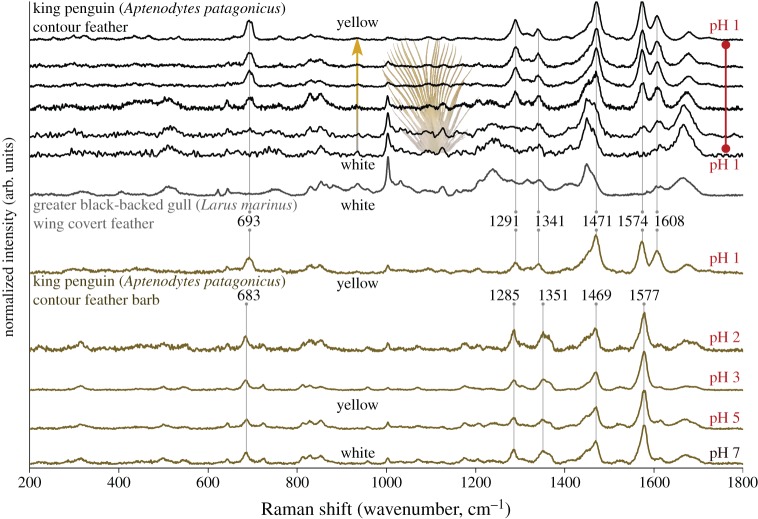
Barbs from yellow penguin feathers that were exposed to pH 7, 5, 3 and 2 for 30 min retained the spheniscin spectrum (figure 4), though the barb exposed to pH 1 for 30 min yielded a different spectrum. In this case, a new band appeared at 1608 cm−1, and the prominent 1577 cm−1 band lost relative intensity and shifted to 1574 cm−1. Bands at 1469, 1351, 1285 and 683 cm−1 shifted to 1471, 1341, 1291 and 693 cm−1, respectively. Prominent pigment bands were associated with yellow colour saturation (figure 4). Bands attributed to β-keratin did not shift after exposure to pH 1.
Figure 4.
Raman spectra of king penguin feather barbs exposed to different pH levels. Band positions in the yellow penguin pigment shifted between pH 1 and pH 2, and a new band appeared at 1608 cm−1. Raman spectral bands of the pH-altered pigment became more intense towards the distal tips of king penguin feather barbs (i.e. with yellow saturation). β-keratin bands did not shift position in the pH-altered feather (when compared with an untreated, white gull feather). Spectra were collected at 780 nm excitation and have been baseline corrected. Unmanipulated spectra are available in the electronic supplementary material. (Online version in colour.)
FTIR-ATR spectra of each feather were dominated by bands attributed to β-keratin [40] and do not inform about pigments (see the electronic supplementary material). The FTIR-ATR spectra of the feather extracts were identical to β-keratin, which suggests that dissolution of the pigment in NaOH co-occurs with hydrolysis of keratin [41] and will require a subsequent separation step for isolating the pigment in future studies.
4. Discussion
The distinct Raman spectrum measured in yellow feathers from both king and macaroni penguins differentiates this colour from the five known classes of avian plumage pigmentation. Spheniscin spectral bands were most intense in the yellow feather tips, and absent in the white, calamus-proximal regions of the barbs. Indeed, the Raman spectrum of the white feather barbs only contained bands attributed to β-keratin, and the relative intensity of the spheniscin bands increased with yellow coloration. The relationship between colour saturation and band intensities shows the ‘spheniscin’ spectrum to be the spectral response from the yellow colourant and that the colour is due to a pigment and not a structural manipulation of keratin: the β-keratin spectral bands lost relative intensity and did not shift band position, as the spheniscin bands became stronger. Each spheniscin spectrum collected from the king and macaroni penguin feathers showed the same bands at the same relative intensities, which indicates a single compound or a strictly conserved ratio between multiple compounds.
4.1. Functional groups
Raman data provide indications of the functional groups present within spheniscin, and permit comparison of its spectrum to that of known compounds. The most prominent band in the spheniscin spectrum occurs at 1577 cm−1. Very strong bands between 1500 and 1650 cm−1 have been attributed elsewhere to carbon atoms bound with double bonds (C=C) in aromatic (i.e. graphene) or conjugated (i.e. polyacetylene) systems [35,42]. The relative intensities of bands attributed to C=C stretching are stronger in polymers (i.e. pyrrole < tetrapyrrole < polypyrrole) [43–45] or molecules capable of Fermi resonance [46], suggesting strong conjugation or aromaticity in spheniscin. Note that the ν3 band typical of conjugated chains (C–C stretch, approx. 1155 cm−1) is absent from the spheniscin spectrum, and the single band between 1515 and 1540 cm−1 is weak instead of dominant (i.e. conjugated ν1(C=C stretch), approx. 1520 cm−1) [35]: hence, aromatic C=C stretching is a more likely assignment for the 1577 cm−1 band than carbon stretching in a conjugated chain. Furthermore, any aliphatic C–H stretching bands that would typically occur around 2900 cm−1 are very weak (i.e. obscured by β-keratin bands) or absent from spheniscin. Weak aliphatic C–H stretching bands can also signify a molecule structured around an aromatic ring [44,47].
Several possible assignments are available for the second, fourth and fifth strongest bands (1285, 1469 and 1351 cm−1, respectively). Assignment options include C–H bending (1288 cm−1), cyclic amide stretching (lactam; 1466 cm−1) and C–C stretching (1351 cm−1) [48,49], all of which are common functionalities in yellow pigments (e.g. bilirubin in vertebrates, riboflavin in plants and bacteria, xanthopterin in micro-organisms, insects and vertebrates [50–52]). Bands between 1285 and 1469 cm−1 have also been attributed to C–N stretching, Fermi resonance in a heterocyclic aromatic ring and aromatic ring breathing [50,53,54]. The absence of a spheniscin band around 1670 cm−1 suggests that assignments for 1351 and 1469 cm−1 are unlikely to be amide II or III stretches (β-keratin amide I occurs at 1672 cm−1) [55]. The third strongest band (683 cm−1) occurs in the fingerprint region. Such low wavenumber bands typically indicate stretching between heavier atoms (e.g. Si–O) or skeletal modes of organic systems, such as pyrimidine ring breathing [56,57]. Indeed, a strong band around 683 cm−1 is found in many pterins [49].
4.2. Pterin hypothesis
McGraw et al. [17] noted that the penguin pigment shared solubility, light-absorption and retention-time properties with pterin compounds, which can be produced endogenously in animals (discussed in [37]). Pterins have not been reported from feathers, although they are abundant in nature; pterins occur in the eyes of some birds and fruit flies, in the integuments of butterflies, amphibians and reptiles [18–21], and perform physiological roles within many animals (e.g. folic acid, an essential vitamin that many animals obtain from their diet [58]). Pterins are heterocyclic compounds that tautomerize such that each pterin has several structural isomers. The general structure of a pterin includes two six-membered rings and keto-, imine, amine, hydroxyl functional groups (dependent on the tautomer; see the electronic supplementary material). A ‘pterin-specific’ arrangement of functional groups should provide a ‘pterin-diagnostic’ Raman spectrum.
We tested the hypothesis that the yellow feather pigment is a pterin by comparing the spheniscin spectrum to published Raman spectra for pterins, which are summarized as follows. Moore et al. [49] studied three pterin tautomers that varied by pyrazine oxidation state. The tallest band in biopterin, 7,8 dihydrobiopterin and 5,6,7,8 tetrahydrobiopterin spectra was a pteridine skeletal mode, which is a pyrimidine ring out of plane deformation (694.2, 705.7 and 690.9 cm−1, respectively). Other low wavenumber bands attributed to skeletal modes included pyrimidine out of plane bending (539.5, 538.2 and 535.5 cm−1, respectively) and pyrazine in plane deformation (509.3, 513.9 and 515.2 cm−1, respectively). Lower wavenumber bands linked to skeletal modes exhibited a range of relative intensities and band positions, particularly pyrazine ring quadrant stretching (1480.9, 1472.0, 1473.8 cm−1, respectively) and pyrimidine ring quadrant stretching (1582.2, 1589.8 and 1574.4 cm−1, respectively). In contrast to these spectrally variable tautomers, surface-enhanced Raman spectra of two related pterins with different pyrazine oxidation states showed identical band positions with only slight differences in relative intensities [59]. Both 6-acetyl-7,7-dimethyl-7,8-dihydropterin and 6-acetyl-7,7-dimethyl-7,8-dihydropterin featured strong bands around 1479 and 1567 cm−1. Vibrational modes were not assigned, but they may be analogous to the quadrant stretches described by Moore et al. [49], which would indicate a strong alkyl group influence on pteridine skeletal modes. Indeed, the Raman spectra of folic acid [60] and xanthopterin [51] differ substantially between 1470–1480 cm−1 and 1570–1590 cm−1, both from one another and from the previously discussed pterins.
We sought unambiguous pterin marker bands for comparison with spheniscin; the band positions and relative intensities of high wavenumber pteridine skeletal modes may be too variable for this purpose, but the low wavenumber skeletal modes might be useful. For example, pyrimidine and pyrazine deformations produce an intense band between 640 and 710 cm−1. For biopterins, the pyrimidine skeletal mode is constrained between 690 and 710 cm−1 and the pyrazine skeletal mode occurs between 640 and 670 cm−1 [49]. Other pterins, such as folic acid [60] and xanthopterin [51], have skeletal modes between these two regions. Hence, pterins appear to have a strong band between 640 and 710 cm−1 that is due to deformation in the pteridine skeleton. Here, we interpret the 683 cm−1 band in the spheniscin spectrum as deformation in an aromatic, nitrogenous heterocyclic ring, which is consistent with a pteridine skeleton (following similar assignments in [49,51,60]). Bands between 500 and 540 cm−1 have also been attributed to skeletal modes in pterins [49], and the presence of these bands in the spheniscin spectrum is further evidence for an aromatic, heterocyclic ring. The spheniscin spectrum does not match any published spectrum, however. Aromatic and nitrogen-bearing heterocyclic rings are features of several chemical classes, including pterins, tetrapyrroles and flavins, for example. Hence we cannot unambiguously describe spheniscin as a pterin.
4.3. pH effect
The most intense band in the Raman spectrum of a pterin molecule is rarely attributed to C=C stretching, but band positions and intensities shift with tautomerization; different pH environments can alter the ratio of tautomers [49,51,52,60,61]. King penguin feather barbs were exposed to dilute hydrochloric acid to observe any changes in Raman spectra (figure 4). We observed a shift from the spheniscin spectrum at pH 2, to a new spectrum at pH 1, including major changes in the C=C stretch region. An interpretation of the shifted spectrum could involve protonation of nitrogen atoms in an aromatic heterocyclic ring, which would affect ring conjugation and thus change the C=C stretching environment. Here, the pH influence on the spheniscin spectrum allows us to confidently assign the 1577 cm−1 band as aromatic C=C stretching. A similar pH effect has been observed with pterins [49,62,63] and other aromatic compounds such as flavins and imidazoles; [47,64]), all of which contain nitrogen-bearing heterocyclic rings. While this effect is not diagnostic for pterins, it does indicate aromatic functionality and excludes spheniscin from being a completely aliphatic molecule like palmitic acid; note that aliphatic C–H stretching around 2900 cm−1 was not observed in spheniscin.
4.4. Other compounds
We considered whether the spheniscin spectrum could be better matched to other yellow compounds in nature. Most of the compounds we examined were spectrally inconsistent with spheniscin. Flavone derivatives, such as quercitin, tend to have a medium to strong band at 1000±10 cm−1, that has been identified as C–C stretching in each of the three benzinoid rings, and a strong band at 1600±10 cm−1, owing to stretching within the phenyl ring [65]. Curcuminoids (e.g. turmeric) and xanthonoids (e.g. saffron) also have a very strong band at 1600±10 cm−1 [66,67]. Anthraquinones, including carminic acid, have a single prominent band between 450 and 490 cm−1 attributed to a skeletal vibration [66,68–70]. Carotenoids and psittacofulvins have two very strong bands, where the most intense bands fall between 1500 and 1540 cm−1 (C=C stretching) and the second, slightly less intense band falls between 1140 and 1160 cm−1 (C–C stretching) [35]. Fatty acids such as bees wax have medium to strong bands around 1130±5 and 1300±5 cm−1, and very strong bands between 2800 and 3000 cm−1 [66,71]. Tetrapyrroles, such as bilirubin, exhibit a prominent band between 1610 and 1620 cm−1 attributed to C=C stretching [72,73].
In contrast to the above listed compounds, many of the band positions in the spheniscin spectrum exactly match the porphyrin marker bands in cytochrome c’ (i.e. haem C), albeit with different relative intensities [74–76]. Furthermore, porphyrin marker band positions and intensities shift under different pH environments [74]. Like pterin compounds, haem C contains nitrogenous heterocyclic aromatic rings, which give distinct skeletal stretching modes. Detailed comparisons are currently limited, however, because most Raman spectra of cytochrome c’ and Fe-porphyrins have been reported from experiments using ultraviolet excitation wavelengths, which gave resonance Raman spectra [75,77–79]. Relative intensities can vary between resonant and non-resonant spectra from the same compound. Haem-group porphyrins should be considered potential candidates in future investigations of the yellow penguin pigment, especially considering the precedence of porphyrins as feather colourants [37]. Both pterin and porphyrin pigments are endogenously synthesized by birds [37], which is consistent with the display of yellow pigments by both captive and wild birds that have taxonomically disparate diets (wild diets summarized by [80]). Furthermore, we collected X-ray fluorescence spectra from a king penguin feather (yellow, spheniscin) and a turaco feather (red, copper porphyrin). X-ray spectral evidence for copper was readily apparent in the turaco feather, but a metal centre could not be identified in the spectrum from the penguin feather. Further research could evaluate whether spheniscin is a free-base porphyrin (i.e. without a metal centre).
5. Conclusions
Much of the interest to date in penguin plumage colours has either been on black-and-white countershading [81] or on the communication role of yellow plumage [29,31,33,34,82,83]. For example, the yellow feathers in great (Aptenodytes), crested (Eudyptes) and yellow-eyed (Megadyptes) penguins are important to both sexes for mate selection and can reveal individual quality [17,29,30,84]. The colour-based sexual selection strategy probably evolved once in a stem-penguin lineage and was retained by members of the penguin crown group, including extinct Madrynornis mirandus. The display of spheniscin feathers has likely been abandoned at least twice by crown-clade penguins (Pygoscelis and Eudyptula + Spheniscus), with the loss of an ancient colour-based selection strategy suggesting that the pigment only confers an advantage under specific, currently unknown conditions. Resolving the chemistry of spheniscin will help us to understand the selective advantage of displaying the penguin pigment.
Previous chemical analyses described fluorescence, chromatographic, solubility and light element properties [17]. Here, we provide further chemical insights into the origin of these brilliant colours. We have shown that Raman spectroscopy can provide a unique spectral fingerprint for the pigment in situ and without feather destruction, and can describe functional groups within the molecule. The prominent bands in a Raman spectrum of the pigment occur at 1577 (vs), 1285 (s), 683 (m), 1469 (m) and 1351 (m) cm−1. We assign 1577 cm−1 to C=C stretching in an aromatic molecule and 683 cm−1 to a skeletal mode in a heterocyclic aromatic ring. The pigment undergoes structural changes at low pH, which is consistent with a molecule that has different tautomers. Each of the observations made here are consistent with a pterin or porphyrin compound, but in combination do not match any previously described molecule. We have eliminated several classes of candidate molecules, including many yellow pigments found in avian food items: indeed, given the range of diets among wild and captive penguins that display spheniscin, we anticipate that the pigment is synthesized endogenously. Further evidence about the chemistry of the pigment may be gained from resonance Raman spectroscopy, pyrolysis mass spectrometry or nuclear magnetic resonance. Raman spectroscopy has brought us closer to the chemical identity of spheniscin. Most importantly, Raman spectroscopy has proved useful for studying feather pigments and could be readily applied to many of the mysteries within avian coloration.
Acknowledgements
We gratefully acknowledge Christopher Milensky (Division of Birds, NMNH) for help with specimen access, Matthew Carrano (Paleobiology Department), Daniel Ksepka (North Carolina State University), Jennifer Giaccai and Molly McGath (Museum Conservation Institute) for technical assistance and helpful comment, and Peter Buck for generously funding postdoctoral research. We are also grateful for the comments and improvements from Dr Tim Holt and four anonymous referees. D.B.T. is funded by a Peter Buck Fellowship administered by the Smithsonian Institution.
References
- 1.Prum RO. 2006. Anatomy, physics, and evolution of avian structural colors. In Bird coloration, vol. 1. Mechanisms and measurements (eds Hill GE, McGraw KJ.), pp. 295–353 Cambridge, MA: Harvard University Press [Google Scholar]
- 2.Church AH. 1869. Researches on turacin, an animal pigment containing copper. Phil. Trans. R. Soc. Lond. 159, 637–636 10.1098/rstl.1869.0025 (doi:10.1098/rstl.1869.0025) [DOI] [Google Scholar]
- 3.Krukenberg CFW. 1881. Die Farbstoffe der Federn. Vergleich. Physiol. Studien 5, 72–99 [Google Scholar]
- 4.Krukenberg CFW. 1882. Die Federfarbstoffe der Psittaciden. Vergleich. Physiol. Studien 2, 29–36 [Google Scholar]
- 5.Strong RM. 1902. The development of color in the definitive feather. Bull. Mus. Comp. Zool. Harvard 40, 147–188 [Google Scholar]
- 6.Grinnell J. 1910. Birds of the 1908 Alexander Alaska Expedition with a note on the avifaunal relationships’ of the Prince William Sound district. Univ. Calif. Pub. Zool. 5, 361–428 [Google Scholar]
- 7.Brockmann H, Völker O. 1934. Der gelbe Federfarbstoff des Kanarienvogels (Serinus canaria canaria [L.]) und das Vorkommen von Carotinoiden bei Vögeln. Z. Physiol. Chem. 224, 193–215 10.1515/bchm2.1934.224.5-6.193 (doi:10.1515/bchm2.1934.224.5-6.193) [DOI] [Google Scholar]
- 8.Nicholas REH, Rimington C. 1951. Isolation of unequivocal uroporphyrin III. Biochem. J. 50, 194–201 [PMC free article] [PubMed] [Google Scholar]
- 9.Stradi R, Pini E, Celetano G. 2001. The chemical structure of the pigments in Ara macao plumage. Comp. Biochem. Physiol. B 130, 57–63 10.1016/S1096-4959(01)00402-X (doi:10.1016/S1096-4959(01)00402-X) [DOI] [PubMed] [Google Scholar]
- 10.Hill GE, McGraw KJ. 2006. Bird coloration, vol. 1, mechanisms and measurements. Cambridge, MA: Harvard University Press [Google Scholar]
- 11.Stoddard MC, Prum RO. 2011. How colorful are birds? Evolution of the avian plumage color gamut. Behav. Ecol. 22, 1042–1052 10.1093/beheco/arr088 (doi:10.1093/beheco/arr088) [DOI] [Google Scholar]
- 12.McGraw KJ. 2006. The mechanics of carotenoid coloration. In Bird coloration, vol. 1. Mechanisms and measurements (eds Hill GE, McGraw KJ.), pp. 177–242 Cambridge, MA: Harvard University Press [Google Scholar]
- 13.McGraw KJ. 2006. The mechanics of melanin coloration. In Bird coloration, vol. 1. Mechanisms and measurements (eds Hill GE, McGraw KJ.), pp. 243–294 Cambridge, MA: Harvard University Press [Google Scholar]
- 14.McGraw KJ, Nogare MC. 2004. Carotenoid pigments and the selectivity of psittacofulvin-based coloration systems in parrots. Comp. Biochem. Physiol. B 138, 229–233 10.1016/j.cbpc.2004.03.011 (doi:10.1016/j.cbpc.2004.03.011) [DOI] [PubMed] [Google Scholar]
- 15.Dyck J. 1992. Reflectance spectra of plumage areas colored by green feather pigments. Auk 109, 293–301 10.2307/4088197 (doi:10.2307/4088197) [DOI] [Google Scholar]
- 16.McGraw KJ, et al. 2004. You can't judge a pigment by its color: carotenoid and melanin content of yellow and brown feathers in swallows, bluebirds, penguins, and domestic chickens. Condor 106, 390–395 10.1650/7384 (doi:10.1650/7384) [DOI] [Google Scholar]
- 17.McGraw KJ, Toomey MB, Nolan PM, Morehouse NI, Massaro M, Jouventin P. 2007. A description of unique fluorescent yellow pigments in penguin feathers. Pigment Cell Res. 20, 301–304 10.1111/j.1600-0749.2007.00386.x (doi:10.1111/j.1600-0749.2007.00386.x) [DOI] [PubMed] [Google Scholar]
- 18.Hopkins FG. 1942. A contribution to the chemistry of pterins. Proc. R. Soc. Lond. B 130, 359–379 10.1098/rspb.1942.0006 (doi:10.1098/rspb.1942.0006) [DOI] [Google Scholar]
- 19.Ephrussi B, Herold JL. 1944. Studies of the eye pigments of Drosophila. I. Methods of extraction and quantitative estimation of the pigment component. Genetics 29, 148–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steffen JE, McGraw KJ. 2007. Contributions of pterin and carotenoid pigments to dewlap coloration in two anole species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 146, 42–46 10.1016/j.cbpb.2006.08.017 (doi:10.1016/j.cbpb.2006.08.017) [DOI] [PubMed] [Google Scholar]
- 21.Oliphant LW. 1987. Pteridines and purines as major pigments of the avian iris. Pigment Cell Res. 1, 129–131 10.1111/j.1600-0749.1987.tb00401.x (doi:10.1111/j.1600-0749.1987.tb00401.x) [DOI] [PubMed] [Google Scholar]
- 22.Maddison WP, Maddison DR. 2011. Mesquite: a modular system for evolutionary analysis, v. 2.75. See http://mesquiteproject.org
- 23.Ksepka DT, Thomas DB. 2012. Multiple Cenozoic invasions of Africa by penguins (Aves, Sphenisciformes). Proc. R. Soc. B 279, 1027–1032 10.1098/rspb.2011.1592 (doi:10.1098/rspb.2011.1592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ksepka DT, Fordyce RE, Ando T, Jones CM. 2012. New fossil penguins (Aves, Sphenisciformes) from the Oligocene of New Zealand reveal the skeletal plan of stem penguins. J. Vert. Paleontol. 32, 235–254 10.1080/02724634.2012.652051 (doi:10.1080/02724634.2012.652051) [DOI] [Google Scholar]
- 25.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448 10.1038/nature11631 (doi:10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 26.Hackett SJ, et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763–1768 10.1126/science.1157704 (doi:10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 27.Clarke JA, Ksepka DT, Salas-Gismondi R, Altamirano AJ, Shawkey MD, D'Alba L, Vinther J, DeVries TJ, Baby P. 2010. Fossil evidence for evolution of the shape and color of penguin feathers. Science 330, 954–957 10.1126/science.1193604 (doi:10.1126/science.1193604) [DOI] [PubMed] [Google Scholar]
- 28.Nolan PM, Dobson FS, Dresp B, Jouventin P. 2006. Immunocompetence is signalled by ornamental color in king penguins, Aptenodytes patagonicus. Evol. Ecol. Res. 8, 1325–1332 [Google Scholar]
- 29.Nicolaus M, Le Bohec C, Nolan PM, Gauthier-Clerc M, Le Maho Y, Komdeur J, Jouventin P. 2007. Ornamental colors reveal age in the king penguin. Polar Biol. 31, 53–61 10.1007/s00300-007-0332-9 (doi:10.1007/s00300-007-0332-9) [DOI] [Google Scholar]
- 30.McGraw KJ, Massaro M, Rivers TJ, Mattern T. 2009. Annual, sexual, size- and condition-related variaion in the colour and fluorescent pigment content of yellow crest-feathers in Snares penguins (Eudyptes robustus). Emu 109, 93–99 10.1071/MU08034 (doi:10.1071/MU08034) [DOI] [Google Scholar]
- 31.Jouventin P. 1982. Visual and vocal signals in penguins, their evolution and adaptive characters. Berlin, Germany: Paul Parey [Google Scholar]
- 32.Jouventin P, Nolan PM, Dobson FS, Nicolaus M. 2008. Coloured patches influence pairing in king penguins. Ibis 150, 193–196 10.1111/j.1474-919X.2007.00749.x (doi:10.1111/j.1474-919X.2007.00749.x) [DOI] [Google Scholar]
- 33.Nolan PM, Dobson FS, Nicolaus M, Karels TJ, McGraw KJ. 2010. Mutual mate choice for colorful traits in king penguins. Ethology 116, 635–644 [Google Scholar]
- 34.Dobson FS, Couchoux C, Jouventin P. 2011. Sexual selection on a coloured ornament in king penguins. Ethology 117, 872–879 10.1111/j.1439-0310.2011.01940.x (doi:10.1111/j.1439-0310.2011.01940.x) [DOI] [Google Scholar]
- 35.Veronelli M, Zerbi G, Stradi R. 1995. In situ resonance Raman spectra of carotenoids in bird's feathers. J. Raman Spectrosc. 26, 683–692 10.1002/jrs.1250260815 (doi:10.1002/jrs.1250260815) [DOI] [Google Scholar]
- 36.Mendes-Pinto MM, LaFountain AM, Caswell Stoddard M, Prum RO, Frank HA, Robert B. 2012. Variation in carotenoid protein interaction in bird feathers produces novel plumage coloration. J. R. Soc. Interface 9, 3338–3350 10.1098/rsif.2012.0471 (doi:10.1098/rsif.2012.0471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGraw KJ. 2006. Mechanics of uncommon colours: pterins, porphyrins, and psittacofulvins. In Bird coloration, vol. 1. Mechanisms and measurements (eds Hill GE, McGraw KJ.), pp. 354–398 Cambridge, MA: Harvard University Press [Google Scholar]
- 38.Czernuszewicz RS, Macor KA, Li JX-Y, Kincaid JR, Spiro TG. 1989. Resonance Raman spectroscopy reveals a1u versus a2u character and pseudo-Jahn–Teller distortion in radical cations of NiII, CuII, and ClFeIII octaethyl- and tetraphenylporphyrins. J. Am. Chem. Soc. 111, 3860–3869 10.1021/ja00193a017 (doi:10.1021/ja00193a017) [DOI] [Google Scholar]
- 39.Hsu SL, Moore WH, Krimm S. 1976. Vibrational spectrum of the unordered polypeptide chain: a Raman study of feather keratin. Biopolymers 15, 1513–1528 10.1002/bip.1976.360150807 (doi:10.1002/bip.1976.360150807) [DOI] [PubMed] [Google Scholar]
- 40.Ambrose EJ, Elliott A. 1951. Infra-red spectra and structure of fibrous proteins. Proc. R. Soc. Lond. A 206, 206–219 10.1098/rspa.1951.0065 (doi:10.1098/rspa.1951.0065) [DOI] [Google Scholar]
- 41.Kim WK, Lorenz ES, Patterson PH. 2002. Effect of enzymatic and chemical treatments on feather solubility and digestibility. Poult. Sci. 81, 95–98 [DOI] [PubMed] [Google Scholar]
- 42.Ni Z, Wang Y, Yi T, Shen Z. 2008. Raman spectroscopy and imaging of graphene. Nano Res. 1, 273–291 10.1007/s12274-008-8036-1 (doi:10.1007/s12274-008-8036-1) [DOI] [Google Scholar]
- 43.Kneip C, Hildebrandt P, Németh K, Mark F, Schaffner K. 1999. Interpretation of the resonance Raman spectra of linear tetrapyrroles based on DFT calculations. Chem. Phys. Lett. 311, 479–484 10.1016/S0009-2614(99)00868-4 (doi:10.1016/S0009-2614(99)00868-4) [DOI] [Google Scholar]
- 44.Geidel E, Billes F. 2000. Vibrational spectroscopic study of pyrrole and its deuterated derivatives: comparison of the quality of the applicability of the DFT/Becke3P86 and the DFT/Becke3LYP functionals. J. Mol. Struct. 507, 75–87 10.1016/S0166-1280(99)00352-8 (doi:10.1016/S0166-1280(99)00352-8) [DOI] [Google Scholar]
- 45.Arjomandi J, Ali Shah A, Bilal S, Van Hoang H, Holze R. 2011. In situ Raman and UV-vis spectroscopic studies of polypyrrole and poly(pyrrole-2,6-dimethyl-β-cyclodextrin). Spec. Acta A 78, 1–6 10.1016/j.saa.2009.12.026 (doi:10.1016/j.saa.2009.12.026) [DOI] [PubMed] [Google Scholar]
- 46.Asundi RK, Padhye ME. 1949. Fermi resonance in benzene. Nature 163, 638. 10.1038/163638a0 (doi:10.1038/163638a0) [DOI] [Google Scholar]
- 47.Güllüoğlu MT, Erdogdu Y, Karpagam J, Sundaraganesan N, Yurdakul Ş. 2011. DFT, FT-Raman, FT-IR and FT-NMR studies of 4-phenylimidazole. J. Mol. Struct. 990, 14–20 10.1016/j.molstruc.2011.01.001 (doi:10.1016/j.molstruc.2011.01.001) [DOI] [Google Scholar]
- 48.Yang B, Morris MD, Xie M, Lightner DA. 1991. Resonance Raman spectroscopy of bilirubins: band assignments and application to bilirubin/lipid complexation. Biochemistry 30, 688–694 10.1021/bi00217a015 (doi:10.1021/bi00217a015) [DOI] [PubMed] [Google Scholar]
- 49.Moore J, Wood JM, Schallreuter KU. 1999. Evidence for specific complex formation between R-melanocyte stimulating hormone and 6(R)-L-erythro-5,6,7,8-tetrahydrobiopterin using near infrared Fourier transform Raman spectroscopy. Biochemistry 38, 15 317–15 324 10.1021/bi991448j (doi:10.1021/bi991448j) [DOI] [PubMed] [Google Scholar]
- 50.Benecky M, Yu T-J, Watters KL, McFarland JT. 1980. Metal-flavin complexation. A resonance Raman investigation. Biochim. Biophys. Acta 626, 197–207 10.1016/0005-2795(80)90211-1 (doi:10.1016/0005-2795(80)90211-1) [DOI] [PubMed] [Google Scholar]
- 51.Feng Z, Liang C, Li M, Chen J, Li C. 2001. Surface-enhanced Raman scattering of xanthopterin adsorbed on colloidal silver. J. Raman Spectrosc. 32, 1004–1007 10.1002/jrs.789 (doi:10.1002/jrs.789) [DOI] [Google Scholar]
- 52.Smyth CA, Mehigan S, Rakovich YP, Bell SEJ, McCabe EM. 2011. Pterin detection using surface-enhanced Raman spectroscopy incorporating a straightforward silver colloid-based synthesis technique. J. Biomed. Opt. 16, 077007. 10.1117/1.3600658 (doi:10.1117/1.3600658) [DOI] [PubMed] [Google Scholar]
- 53.Harada I, Miura T, Takeuchi H. 1986. Origin of the doublet at 1360 and 1340 cm−1 in the Raman spectra of tryptophan and related compounds. Spec. Acta A 42, 307–312 10.1016/0584-8539(86)80193-3 (doi:10.1016/0584-8539(86)80193-3) [DOI] [Google Scholar]
- 54.Edwards HGM, Munshi T, Anstis M. 2005. Raman spectroscopic characterisations and analytical discrimination between caffeine and demethylated analogues of pharmaceutical relevance. Spec. Acta. A 61, 1453–1459 10.1016/j.saa.2004.10.022 (doi:10.1016/j.saa.2004.10.022) [DOI] [PubMed] [Google Scholar]
- 55.Benaki DC, Aggeli A, Chryssikos GD, Yiannopoulos YD, Kamitsos EI, Brumley E, Case ST, Boden N, Hamodrakas SJ. 1998. Laser-Raman and FT-IR spectroscopic studies of peptide-analogues of silkmoth chorion protein segments. Int. J. Biol. Macromol. 23, 49–59 10.1016/S0141-8130(98)00032-4 (doi:10.1016/S0141-8130(98)00032-4) [DOI] [PubMed] [Google Scholar]
- 56.Lord RC, Marston AL, Miller FA. 1957. Infra-red and Raman spectra of the diazines. Spec. Acta 9, 113–125 10.1016/0371-1951(57)80205-7 (doi:10.1016/0371-1951(57)80205-7) [DOI] [Google Scholar]
- 57.Huang E, Chen CH, Huang T, Lin EH, Xu J-A. 2000. Raman spectroscopic characteristics of Mg-Fe-Ca pyroxenes. Am. Mineral. 85, 473–479 [Google Scholar]
- 58.Hanson AD, Gregory JF. 2002. Synthesis and turnover of folates in plants. Curr. Opin. Plant. Biol 5, 244–249 10.1016/S1369-5266(02)00249-2 (doi:10.1016/S1369-5266(02)00249-2) [DOI] [PubMed] [Google Scholar]
- 59.Stevenson R, Stokes RJ, MacMillan D, Armstrong D, Faulds K, Wadsworth R, Kunuthur S, Suckling CJ, Graham D. 2009. In situ detection of pterins by SERS. Analyst 134, 1561–1564 10.1039/b905562b (doi:10.1039/b905562b) [DOI] [PubMed] [Google Scholar]
- 60.Stokes RJ, McBride E, Wilson CG, Girkin JM, Smith WE, Graham D. 2008. Surface-enhanced Raman scattering spectroscopy as a sensitive and selective technique for the detection of folic acid in water and human serum. App. Spectrosc. 62, 372–376 10.1366/000370208784046812 (doi:10.1366/000370208784046812) [DOI] [PubMed] [Google Scholar]
- 61.Deng H, Callender R, Dale GE. 2000. A vibrational structure of 7,8-dihydrobiopterin bound to dihydroneopterin aldolase. J. Biol. Chem. 275, 30 139–30 143 10.1074/jbc.M004464200 (doi:10.1074/jbc.M004464200) [DOI] [PubMed] [Google Scholar]
- 62.Chen Y-Q, Kraut J, Blakely RL, Callender R. 1994. Determination by Raman spectroscopy of the pKa of N5 dihydrofolate bound to dihydrofolate reductase: mechanistic implications. Biochemistry 33, 7021–7026 10.1021/bi00189a001 (doi:10.1021/bi00189a001) [DOI] [PubMed] [Google Scholar]
- 63.Soniat M, Martin CB. 2008. Theoretical study on the relative energies of neutral pterin tautomers. Pteridines 19, 120–124 [Google Scholar]
- 64.Faria PA, Chen X, Lombardi JR, Birke RL. 2000. A surface-enhanced Raman and ab initio study of spectra of lumazine molecules. Langmuir 16, 3984–3992 10.1021/la991351y (doi:10.1021/la991351y) [DOI] [Google Scholar]
- 65.Teslova T, Corredor C, Livingstone R, Spataru T, Birke RL, Lombardi JR, Cañamares MV, Leona M. 2007. Raman and surface-enhanced Raman spectra of flavone and several hydroxy derivatives. J. Raman Spectrosc. 38, 802–818 10.1002/jrs.1695 (doi:10.1002/jrs.1695) [DOI] [Google Scholar]
- 66.Burgio L, Clark RJH. 2001. Library of FT-Raman spectra of pigments, minerals, pigment media and varnishes, and supplement to existing library of Raman spectra of pigments with visible excitation. Spec. Acta A 57, 1491–1521 10.1016/S1386-1425(00)00495-9 (doi:10.1016/S1386-1425(00)00495-9) [DOI] [PubMed] [Google Scholar]
- 67.Senthil K, Iyandurai N, Sarojini R. 2009. Curcumin-nucleotide interaction by FT-Raman spectroscopy. Asian J. Chem. 21, 4237–4240 [Google Scholar]
- 68.Cañamares MV, Garcia-Ramos JV, Domingo C, Sanchez-Cortes S. 2004. Surface-enhanced Raman scattering study of the adsorption of the anthraquinone pigment alizarin on Ag nanoparticles. J. Raman Spectrosc. 35, 921–927 10.1002/jrs.1228 (doi:10.1002/jrs.1228) [DOI] [Google Scholar]
- 69.Lee CJ, Kang JS, Park Y-T, Rezaul KM, Lee MS. 2004. Study of substitution effect of anthraquinone by SERS spectroscopy. Bull. Korean Chem. Soc. 25, 1779–1783 10.5012/bkcs.2004.25.12.1779 (doi:10.5012/bkcs.2004.25.12.1779) [DOI] [Google Scholar]
- 70.Chang J, Cañamares MV, Aydin M, Vetter W, Schreiner M, Xu W, Lombardi JR. 2009. Surface-enhanced Raman spectroscopy of indanthrone and flavanthrone. J. Raman. Spectrosc. 40, 1557–1563 10.1002/jrs.2298 (doi:10.1002/jrs.2298) [DOI] [Google Scholar]
- 71.De Gelder J, De Gussem K, Vandenabeele P, Moens L. 2007. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 38, 1133–1147 10.1002/jrs.1734 (doi:10.1002/jrs.1734) [DOI] [Google Scholar]
- 72.Hsieh Y-Z, Lee N-S, Sheng R-S, Morris MD. 1987. Surface-enhanced Raman spectroscopy of free and complexed bilirubin. Spectrochim. Acta Part A 3, 1141–1146 [Google Scholar]
- 73.Chen J, Hu J-M, Xu Z-S, Sheng R-S. 1994. Surface-enhanced Raman spectroscopy of free bilirubin and bilirubin complexes with transition metals iron(II), nickel(II) and cobalt(II). Spec. Acta 50A, 929–936 10.1016/0584-8539(94)80141-X (doi:10.1016/0584-8539(94)80141-X) [DOI] [Google Scholar]
- 74.Hobbs JD, Larsen RW, Meyer TE, Hazzard JH, Cusanovish MA, Ondrias MR. 1990. Resonance Raman characterization of Chromatium vinosum cytochrome c’. Effect of pH and comparison of equilibrium and photolyzed carbon monoxide species. Biochemistry 29, 4166–4174 10.1021/bi00469a020 (doi:10.1021/bi00469a020) [DOI] [PubMed] [Google Scholar]
- 75.Andrew CR, Green EL, Lawson DM, Eady RR. 2001. Resonance Raman studies of cytochrome c‘ support the binding of NO and CO to opposite sides of the heme: implications for ligand discrimination in heme-based sensors. Biochemistry 40, 4115–4122 10.1021/bi0023652 (doi:10.1021/bi0023652) [DOI] [PubMed] [Google Scholar]
- 76.Andrew CR, et al. 2005. Accessibility of the distal heme face, rather than Fe-His bond strength, determines the heme-nitrosyl coordination number of cytochromes c’: evidence from spectroscopic studies. Biochemistry 44, 8664–8672 10.1021/bi050428g (doi:10.1021/bi050428g) [DOI] [PubMed] [Google Scholar]
- 77.Spiro TG, Strekas TC. 1972. Resonance Raman spectra of hemoglobin and cytochrome c: inverse polarization and vibronic scattering. Proc. Natl Acad. Sci. USA 69, 2622–2626 10.1073/pnas.69.9.2622 (doi:10.1073/pnas.69.9.2622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Callahan P, Babcock GT. 1981. Insights into heme structure from soret excitation Raman spectroscopy. Biochemistry 20, 952–958 10.1021/bi00507a048 (doi:10.1021/bi00507a048) [DOI] [PubMed] [Google Scholar]
- 79.Hu S, Morris IK, Singh JP, Smith KM, Spiro TG. 1993. Complete assignment of cytochrome c resonance Raman spectra via enzymatic reconstruction with isotopically labeled hemes. J. Am. Chem. Soc. 115, 12 446–12 458 10.1021/ja00079a028 (doi:10.1021/ja00079a028) [DOI] [Google Scholar]
- 80.Thomas DB, Fordyce RE, Gordon K. 2013. Evidence for a krill-rich diet from non-destructive analyses of penguin bone. J. Avian Biol. 44, 203–207 10.1111/j.1600-048X.2012.00095.x (doi:10.1111/j.1600-048X.2012.00095.x) [DOI] [Google Scholar]
- 81.Rowland HM. 2009. From Abbott Thayer to the present day: what have we learned about the function of countershading? Phil. Trans. R. Soc. B 364, 519–527 10.1098/rstb.2008.0261 (doi:10.1098/rstb.2008.0261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Viera VM, Nolan PM, Côté SD, Jouventin P, Groscolas R. 2008. Is territory defence related to plumage ornaments in the king penguin Aptenodytes patagonicus? Ethology 114, 146–153 10.1111/j.1439-0310.2007.01454.x (doi:10.1111/j.1439-0310.2007.01454.x) [DOI] [Google Scholar]
- 83.Pincemy G, Dobson FS, Jouventin P. 2009. Experiments on colour ornaments and mate choice in king penguins. Anim. Behav. 78, 1247–1253 10.1016/j.anbehav.2009.07.041 (doi:10.1016/j.anbehav.2009.07.041) [DOI] [Google Scholar]
- 84.Massaro M, Davis LS, Darby JT. 2003. Carotenoid-derived ornaments reflect parental quality in male and female yellow-eyed penguins (Megadyptes antipodes). Behav. Ecol. Sociobiol. 55, 169–175 10.1007/s00265-003-0683-3 (doi:10.1007/s00265-003-0683-3) [DOI] [Google Scholar]