Abstract
Cobalt-doped magnetite (CoxFe3 −xO4) nanoparticles have been produced through the microbial reduction of cobalt–iron oxyhydroxide by the bacterium Geobacter sulfurreducens. The materials produced, as measured by superconducting quantum interference device magnetometry, X-ray magnetic circular dichroism, Mössbauer spectroscopy, etc., show dramatic increases in coercivity with increasing cobalt content without a major decrease in overall saturation magnetization. Structural and magnetization analyses reveal a reduction in particle size to less than 4 nm at the highest Co content, combined with an increase in the effective anisotropy of the magnetic nanoparticles. The potential use of these biogenic nanoparticles in aqueous suspensions for magnetic hyperthermia applications is demonstrated. Further analysis of the distribution of cations within the ferrite spinel indicates that the cobalt is predominantly incorporated in octahedral coordination, achieved by the substitution of Fe2+ site with Co2+, with up to 17 per cent Co substituted into tetrahedral sites.
Keywords: coercivity, anisotropy, hyperthermia, Geobacter, Fe(III) reduction, X-ray magnetic circular dichroism
1. Introduction
Research into magnetic nanoparticles (MNPs) of the form MxFe3 −xO4 (M = Co, Zn, Ni, Cr, etc.) has developed into an area of widespread activity in recent years in response to a broad range of potential applications, such as remediation of contaminated land and water [1–3], catalysis [4,5], targeted drug delivery [6], magnetic data storage [7] and magnetic thermotherapy targeting cancerous tumours [8–10].
Targeted cancer therapies include magnetic hyperthermia in which a heating effect is induced by MNPs (localized within a tumour) under the influence of an oscillating magnetic field. Using this effect, once the temperature at the localized region reaches 40–45°C [10], cancerous cells will be destroyed without causing extensive damage to healthy tissue (cancerous cells are more sensitive to heat than normal cells [11]). The heating effect is dominated either by the physical rotation of MNPs within solution (Brownian relaxation), or by the magnetization reversal within the particles (e.g. Néel relaxation or magnetic hysteresis losses). Both processes lead to a release of heat; however, the effect is dependent on the size and anisotropy of the MNPs [12]. The overall heating produced by the MNPs under the influence of an AC field is defined by the specific loss power (SLP) [13].
Several methods have been developed for producing MNPs, including chemical and mechanical methods such as co-precipitation and ball milling [14]. Many of these processes can be expensive and environmentally damaging owing to the use of high temperatures and toxic materials. An alternative way to produce MNPs at ambient temperatures is via the use of subsurface Fe(III)-reducing bacteria such as Geobacter sulfurreducens or Shewanella oneidensis, which can conserve energy through the oxidation of an electron donor such as organic matter or hydrogen, coupled with the reduction of Fe(III) [15,16]. The reduction leads to the release of soluble Fe(II) which is able to recrystallize with solid phase Fe(III) to produce magnetite (Fe3O4) and potentially other mineral phases, including siderite, goethite, viviantite, haematite and green rust depending on the conditions of formation (i.e. pH and temperature) [17–19].
Magnetite has a cubic spinel lattice structure with a unit cell containing 32 oxygen ions, eight Fe3+ ions in tetrahedral (A) sites, eight Fe2+ and eight Fe3+ ions in octahedral [B] sites, i.e. Fe3+(A)Fe2+[B]Fe3+[B]O4. The magnetism exhibited by magnetite MNPs is a result of the superexchange interaction between the (A) and [B] site cations, which yields antiparallel magnetic moments between the two sublattices. The distribution of iron within the spinel lattice is such that the magnetic moments of Fe3+ (approx. 5 μB per atom) on both lattice sites effectively cancel each other, resulting in a net magnetization owing to Fe2+ (approx. 4 μB per atom). The substitution of Fe2+ or Fe3+ cations with transition metal dopants such as cobalt, zinc, nickel or chromium in the magnetite serves as a method to change the magnetic properties of the MNPs [20,21].
The aim of this work is to further the understanding of the effect of cobalt substitution into the structure of biogenic magnetite and to demonstrate the potential use of such biologically synthesized cobalt ferrites for magnetic hyperthermia applications. Several techniques have been applied in order to fully characterize the material that was produced through the reduction of Co(II)–Fe(III)-oxyhydroxide starting material by G. sulfurreducens.
2. Results and discussion
2.1. Physical characteristics and crystal structure
Inductively coupled plasma (ICP) spectrometer analyses show that the cobalt content in the starting materials (at%CoICP) is approximately 30 per cent less than the designed starting Co fraction at the beginning of the synthesis process (table 1). This ‘loss’ occurs during the process of producing the cobalt–iron precursor and is considered to be due to incomplete precipitation of cobalt into solid phase oxyhydroxide at pH 7 [22]. Electron probe microanalysis (EPMA) used to determine the relative ratios of cobalt to iron (xEPMA) in the nanoparticles shows that the amount of cobalt incorporated into the nanoparticles closely matches the amount that is available in the starting gels. As EPMA analyses a volume of 2 µm3, it represents the average nanoparticle composition and gives compositions to a high degree of accuracy (error < ±0.5%); from the %Co results, the xEPMA, according to the formula CoxFe3 −xO4, was determined.
Table 1.
The composition of the starting materials and nanoparticles. The at% cobalt concentrations in the starting material were measured using ICP-AES (%CoICP), and the nanoparticles by EPMA and calculated from the XAS spectra. Values of x in the formula CoxFe3 −xO4 were also calculated from both the EMPA (xEPMA) and XAS (xXAS).
| sample | designed starting material %Co | ICP-AES starting material %CoICP | EPMA final material %Co | xEPMA | XAS final material %Co | xXAS |
|---|---|---|---|---|---|---|
| Co0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Co5 | 5 | 4.2 | 4.3 | 0.13 | 5.6 | 0.17 |
| Co15 | 15 | 12.0 | 12.7 | 0.38 | 11.7 | 0.35 |
| Co20 | 20 | 14.4 | 14.0 | 0.42 | 14.5 | 0.44 |
| Co33 | 33 | 24.9 | 23.7 | 0.71 | 20.0 | 0.60 |
| Co50 | 50 | 34.3 | 33.7 | 1.01 | 29.8 | 0.89 |
Powder X-ray diffraction (XRD) was used to characterize the phases that were produced by the microbial reduction in the Co(II)Fe(III)-oxyhydroxide gel (figure 1). The first three samples (Co0, Co5, Co15) clearly show (220), (311), (400), (511) and (440) reflections characteristic of magnetite, with the Co20 sample displaying only (311) and (440) peaks. Samples with the highest cobalt concentrations (Co33 and Co50) do not exhibit any peaks corresponding to magnetite, which is attributed to low particle sizes, resulting in reflections that are indistinguishable from the background noise. At the highest cobalt concentration (Co50), it is possible to see the emergence of a peak at 2θ = 11° consistent with the main reflection for green rust that appears to be produced as an additional mineral phase.
Figure 1.
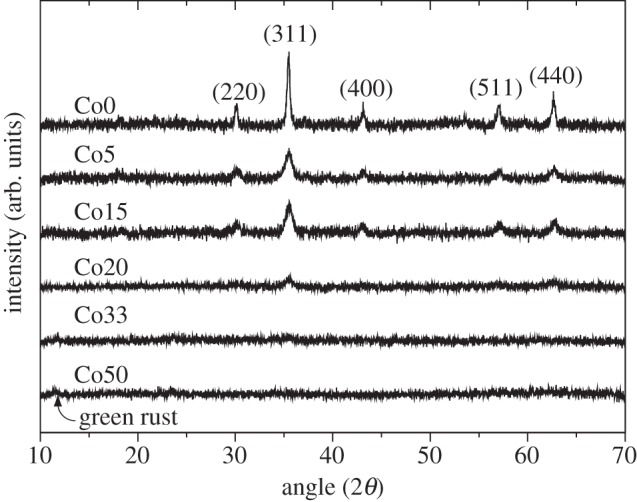
X-ray diffraction with 10° ≤ 2θ ≤ 70°. Reflections for magnetite are labelled on the Co0 trace (top). Low and intermediate cobalt concentrations exhibit reflections corresponding to magnetite. No magnetite peaks are visible for Co33 and Co50 samples; however, a small green rust peak is observable at 2θ = 11° for sample Co50.
Analysis of the magnetite (311) reflection enabled the determination of mean crystal diameter for samples Co0–Co20. The incorporation of cobalt has a significant effect on the particle size, with an immediate drop in mean crystallite size from 36.7 (Co0) to 11.3 nm with the addition of just approximately 5 per cent cobalt (Co5). There is little change in particle size for cobalt concentrations higher than this with values of 13.7 and 9.5 nm determined for Co15 and Co20 samples, respectively. Cobalt has a comparable ionic radius to iron (0.074 and 0.077 nm for Co(II) and Fe(II) in octahedral coordination, respectively [23]), thus the particle size change is not due directly to the substitution of the Co(II) cation.
Transmission electron microscopy (TEM) was performed on the samples to investigate morphology and size changes in the series (figure 2). The results confirm XRD measurements with size varying from 30 to 40 nm (Co0), approximately 10 nm (Co5, Co15 and Co20) and approximately 2–4 nm (Co33 and Co50). Selected area electron diffraction (SAED) indicates that the first four samples in the series (Co0–Co20) contain only cobalt-doped magnetite nanoparticles (figure 2, insets). The two samples with the higher cobalt concentrations (Co33 and Co50) contained both the small particles, identified as magnetite from SAED, as well as large plate-like and needle features. These larger particles were attributed to green rust that occurs as hexagonal plates [24], as indicated by the XRD (figure 1), although SAED measurements could not confirm this.
Figure 2.
TEM images of cobalt-doped magnetite. (a) Co0 shows large crystals of 30–40 nm. (b) Co5, (c) Co15 and (d) Co20 particles have approximately 10 nm diameter. (e) Co33 and (f) Co50 are much smaller (approx. 2–4 nm diameter) nanoparticles with needle features also visible in Co50 samples. (Online version in colour.)
Energy dispersive X-ray (EDX) spectroscopy was used to confirm differences in cobalt concentration throughout the series. The amount of cobalt in the nanoparticles was measured at 4.4 per cent, 12.8 per cent, 13.9 per cent, 18.8 per cent and 23.3 per cent for samples Co5, Co15, Co20, Co33, Co50, respectively. The values of the first four samples match very closely with EPMA measurements; however, Co33 is marginally lower, and Co50 is roughly one-third less than expected from electron probe analysis. This variation is due to a high level of Co incorporation into the plate-like green rust phase that would have formed a part of the EPMA. The EDX spectra from the large plate-like phases indicated the presence of chlorine, iron and cobalt in ratios of 3.0 per cent Cl, 42.5 per cent Fe, 54.5 per cent Co and 2.2 per cent Cl, 36.0 per cent Fe, 61.8 per cent Co for samples Co33 and Co50, respectively, which suggests the plates to be an Fe2+–Co2+ phase similar to green rust I (chloride) group [17]. High cobalt concentrations are known in green rust (general formulae (Fe2+)6Fe3+2(OH, Cl)18.4H2O), with cobalt replacing the Fe(II) [25,26].
It is not clear from the results presented why the particle size decreases so significantly with increasing cobalt concentration. Several factors could have an effect, including (i) the rate of Fe(III) reduction by G. sulfurreducens [27]; (ii) heavy metal toxicity to the micro-organism; and (iii) the initial amount of cobalt-doped ferrihydrite nanoparticles at the start of the culture experiment. This varies across the samples as the initial total Fe(III) concentration (50 mmol l−1) was constant; consequently assuming that all the cobalt–ferrihydrite particles have the same size, more particles are required to ensure an equal Fe(III) content. (iv) The size of the ferrihydrite nanoparticle starting materials might also be affected by the introduction of cobalt and lead to overall changes in the sizes of cobalt-doped magnetite nanoparticles produced. The size of magnetite nanoparticles produced by G. sulfurreducens has previously been shown to be controllable by the amount of bacteria introduced at the start of the experiment with high concentrations of microbial cells yielding smaller nanoparticles than when fewer cells are used. It would be possible to apply similar methods in the case of cobalt-doped nanoparticles and might provide a possible route through which the particle sizes could be made more uniform with increasing cobalt concentration.
2.2. Magnetic properties and hyperthermia
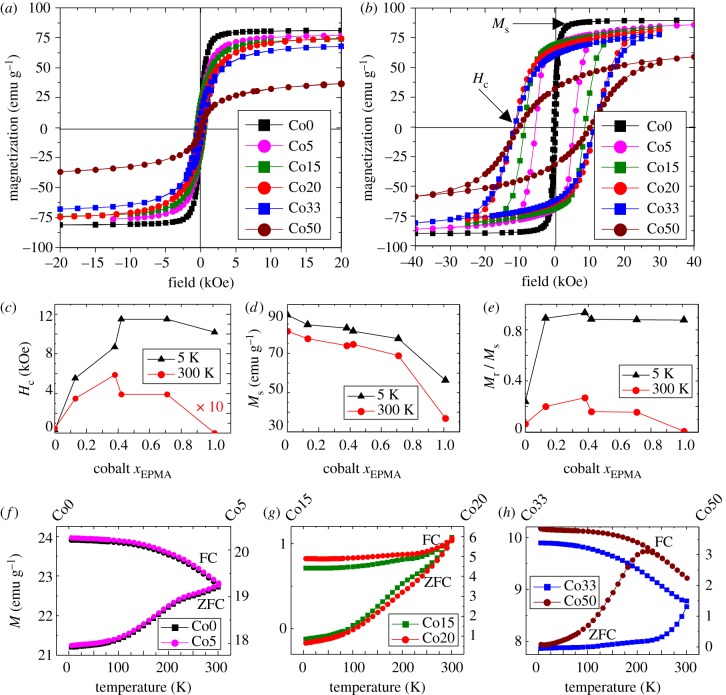
Measurements of the magnetization with respect to applied magnetic field were carried out at room temperature (RT = 300 K) and low temperature (5 K) with results for coercivity (Hc), saturation magnetization (Ms) and remanence (Mr) obtained from hysteresis loops presented in figure 3a–e. These measurements indicate a very large increase in the low-temperature coercivity (Hc) as the cobalt dopant concentration increases and, importantly, only relatively small decreases in overall saturation magnetization (Ms).
Figure 3.
Superconducting quantum interference device magnetometry results. (a) Hysteresis loops measured at T = 300 K. (b) Hysteresis loops at T = 5 K. (c) Coercivity measurements. Note room temperature coercivity measurements shown are ×10 enhanced compared with actual results to show changes more effectively on the same scale as low-temperature measurements. (d) Saturation magnetization. (e) Remanence ratio Mr/Ms. (f–h) Zero-field-cooled (ZFC) and field-cooled (FC) curves showing the changes in blocking temperature. (Online version in colour.)
The increase in Hc at 5 K reaches a maximum at Co20 (11.5 kOe), with Co33 exhibiting the same value, before a small decrease for Co50 (10.2 kOe). Ms decreases (with increasing Co doping) at both RT and low temperature, although the most significant reduction is observed for Co50. An important result that can be taken from these measurements is that at Co20 (low temperature), there is a very significant increase in coercivity compared with Co0 (37× larger), while the saturation magnetization only decreases by 6 emu g−1, corresponding to an 8 per cent decrease. Maintaining a high Ms is important in order to minimize the amount of material required to achieve the desired magnetic response for different applications, in particular those focused on medical therapies. Room temperature measurements also show increases in the coercivity of the samples as cobalt concentration increases although the maximum is reached at Co15 where Hc is 12× larger than that measured for Co0. Changes in saturation magnetization parallel those seen at 5 K although approximately 8 emu g−1 lower, excluding Co50, for which the difference is 20 emu g−1.
Additionally, figure 3e shows that Mr/Ms ratio initially increases with increasing cobalt concentration at both 5 and 300 K, with a value of zero at 300 K for Co50, corresponding to zero coercivity (i.e. superparamagnetism). This superparamagnetic state is reached through the reduction in particle size to less than 4 nm for the highest Co concentrations.
Information about the blocking temperature (TB) of the samples is obtained through examination of the field-cooled (FC) and zero-field-cooled (ZFC) magnetization collected at 100 Oe (figure 3f–h). The TB is defined as the point above which the sample exhibits superparamagnetism such that the nanoparticle magnetization is free to align in random orientations. This is best interpreted as the peak of the ZFC curve at the point where it intersects with the FC curve. The data show that at low %Co (Co0, Co5), intermediate %Co (Co15, Co20) and Co33 samples, the TB is above room temperature with no discernible peak visible below 300 K. A clear peak is visible for Co50 at 220 K at which point the gradient of the ZFC curve is zero as it joins the FC curve. This point corresponds to the TB, above which the sample becomes superparamagnetic. This is confirmed by an examination of the Hc values that indicate Co50 has zero coercivity at room temperature.
A lower limit for the anisotropy (K) of the samples can be estimated from the expression Hc = 0.958 K/Ms [28] (assuming uniaxial anisotropy), where Hc and Ms correspond to coercivity and saturation magnetization at 5 K, respectively. However, the presence of larger particles in the distribution is likely to increase the anisotropy above this threshold. The size of the anisotropy determines how strongly the particle's magnetic moment is fixed to an easy direction within the particle. It should be noted, however, that such values underestimate the true anisotropy when the samples are not fully saturated (as is the case here for samples with higher Co concentrations). The results presented in table 2 indicate that the anisotropy significantly increases by an order of magnitude through minor additions of cobalt (Co5). Thus, the inclusion of Co in the ferrite spinel leads to an increase in the magnetic anisotropy of the nanoparticles comparable to previously observed values of first-order anisotropy constants of synthetic bulk Co–ferrites (CoFe2O4; |K1| ≈ 2 × 106 erg cm−3) [29]. The effect appears to be the largest for the Co20 and Co33 samples that exhibit the highest-recorded coercivity.
Table 2.
Structural and magnetic properties of cobalt-doped magnetite nanoparticles. d, particle size; Ms, saturation magnetization; Hc, coercivity; Mr, remanence; K, anisotropy.
| sample | xEPMA | d (nm) XRD | d (nm) TEM |
Ms (emu g−1) |
Hc (kOe) |
Mr (emu g−1) |
K (erg cm−3) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 300 K | 5 K | 300 K | 5 K | 300 K | 5 K | 5 K | ||||
| Co0 | 0 | 36.7 | 30–40 | 81.3 | 89.6 | 0.05 | 0.3 | 5.8 | 19.41 | 1.4 × 105 |
| Co5 | 0.13 | 11.3 | ∼10 | 77.6 | 84.6 | 0.35 | 5.6 | 17 | 69.1 | 2.4 × 106 |
| Co15 | 0.38 | 13.7 | ∼10 | 74 | 82.9 | 0.59 | 8.7 | 22.2 | 69.1 | 3.7 × 106 |
| Co20 | 0.42 | 9.5 | ∼10 | 74.6 | 81.4 | 0.39 | 11.5 | 13 | 65.9 | 4.8 × 106 |
| Co33 | 0.71 | — | 2–4 | 68.9 | 77.6 | 0.39 | 11.5 | 12.1 | 60.5 | 4.6 × 106 |
| Co50 | 1.01 | — | 2–4 | 36.8 | 56.3 | 0 | 10.2 | 0.3 | 32.3 | 2.9 × 106 |
The potential use of the cobalt-doped magnetite nanoparticles for hyperthermia measurements was assessed from results shown in figure 4. The SLP of the nanoparticles was determined from the rate of change of temperature of a suspension containing a known concentration of cobalt–ferrite nanoparticles under the influence of an AC magnetic field, as discussed extensively elsewhere [12] (i.e.  ). A relatively low magnetic field of 205 Oe was applied at a frequency of 87 kHz for the experiments reported here. The temperature rise observed (normalized to the mass of iron in the samples measured in mg) is shown in figure 4 together with the determined SLP for the biogenic magnetite and three of the cobalt ferrite nanoparticle samples (Co20, Co33 and Co50). The results demonstrate significant heating effects at a frequency and applied field that would be suitable for clinical hyperthermia applications. Several studies have also attempted to explore the use of biogenically derived magnetite nanoparticles using magnetosomes (intracellularly produced magnetite grains) for magnetic hyperthermia [30–32] and reported high values of SLP (up to 1 kW g−1); however, it is difficult to make a direct comparison between those results and the values reported here owing to the alternative conditions applied in that study (10 kA m−1; 410 kHz). Other difficulties associated with using magnetosomes include large crystal size that probably results in magnetically blocked particles that would be difficult to control at room temperature, hence biomedical applications, owing to residual magnetization. Another advantage of using the approaches presented within this paper instead of magnetosomes is the ability to generate much larger quantities of material relatively easily.
). A relatively low magnetic field of 205 Oe was applied at a frequency of 87 kHz for the experiments reported here. The temperature rise observed (normalized to the mass of iron in the samples measured in mg) is shown in figure 4 together with the determined SLP for the biogenic magnetite and three of the cobalt ferrite nanoparticle samples (Co20, Co33 and Co50). The results demonstrate significant heating effects at a frequency and applied field that would be suitable for clinical hyperthermia applications. Several studies have also attempted to explore the use of biogenically derived magnetite nanoparticles using magnetosomes (intracellularly produced magnetite grains) for magnetic hyperthermia [30–32] and reported high values of SLP (up to 1 kW g−1); however, it is difficult to make a direct comparison between those results and the values reported here owing to the alternative conditions applied in that study (10 kA m−1; 410 kHz). Other difficulties associated with using magnetosomes include large crystal size that probably results in magnetically blocked particles that would be difficult to control at room temperature, hence biomedical applications, owing to residual magnetization. Another advantage of using the approaches presented within this paper instead of magnetosomes is the ability to generate much larger quantities of material relatively easily.
Figure 4.
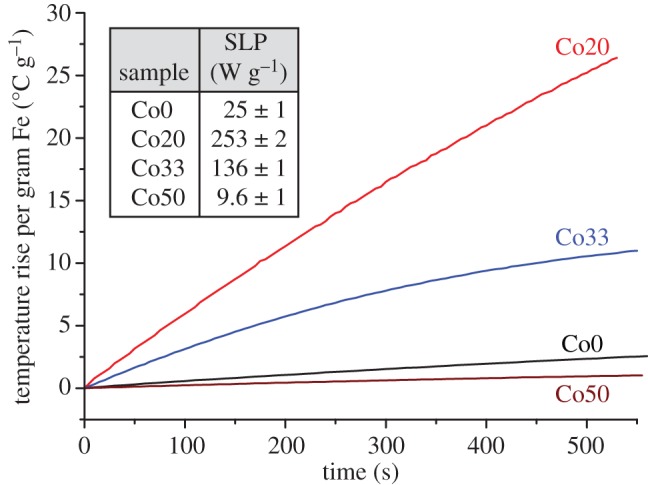
Hyperthermia experiments indicate the rate of temperature change of a solution containing MNPs Co0, Co20, Co33 and Co50 under the influence of an AC magnetic field (87 kHz). The specific loss power (SLP) describes the power achievable per gram of iron in the material. (Online version in colour.)
An enhanced heating effect can be clearly observed for the cobalt–ferrite biogenic nanoparticles in comparison with the undoped biogenic magnetite nanoparticles. For superparamagnetic particles, the SLP is highly dependent on the strength and frequency of the applied magnetic field, and the effective relaxation time (τeff) of the nanoparticles in suspension [33]. In turn, τeff depends on the intrinsic size and anisotropy of the nanoparticles, and also on their hydrodynamic size (i.e. the effective size in fluid suspension) and solution viscosity. For the nanoparticles measured here, the combination of a reduction in particle size and an increase in anisotropy will alter τeff with respect to the undoped particles. The observed enhancement in heating effect for the cobalt-doped samples could thus be caused by a better matching of τeff with the applied magnetic field frequency. However, given the very large anisotropy values generated in these particles, it is also possible that hysteresis losses could play a role. Additional heating effects could be induced for the low-cobalt-containing samples by decreasing their particle size. Simple modifications of the procedures used throughout this study could be applied to yield Fe3O4 nanoparticles with diameters of 10 nm by increasing the number of bacteria used to initiate the microbial Fe(III) reduction in ferrihydrite as previously shown [27]. It is anticipated that this modification to the technique would also yield smaller cobalt-doped magnetite. An additional benefit of applying this modification to the methods procedure is a decrease in the size distribution of the nanoparticles. The smallest Fe3O4 nanoparticles produced within that study have very narrow size distributions that are comparable to those generated via synthetic methods [34,35]. Further investigation of the hyperthermia properties of these particles is in progress and will be presented in a future publication.
2.3. Distribution of cations within the crystal structure
2.3.1. X-ray absorption spectra and X-ray magnetic circular dichroism
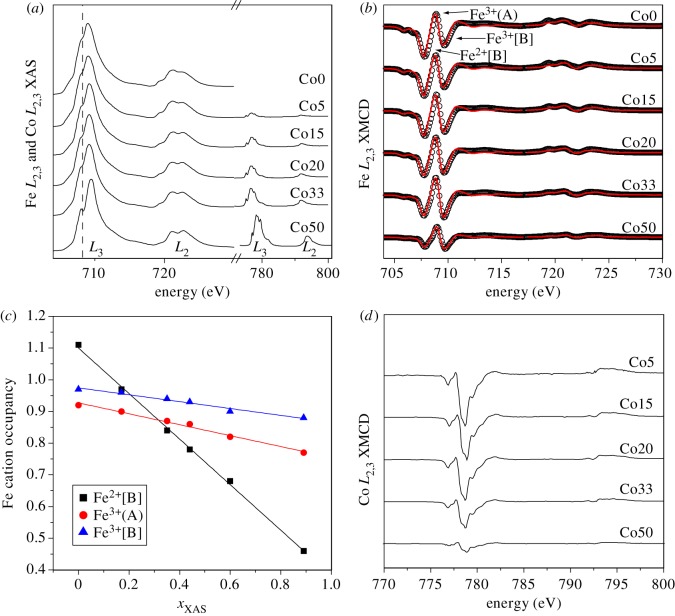
X-ray absorption spectra (XAS) and X-ray magnetic circular dichroism (XMCD) spectra were collected to understand the changes in the magnetic structure of the MNPs as cobalt is incorporated (figure 5). The XAS spectra obtained in total-electron yield (TEY) mode for both Fe and Co L2,3 edges are shown in figure 5a, with the absorption intensity of the Fe L3 maximum normalized to 1, and the Co signal scaled accordingly. The peak intensity of Co with respect to Fe provides the relative cobalt concentration in the structure of the nanoparticles. The values match closely with those found using EPMA (table 1). Examination of the leading Fe L3 peak also shows the emergence of a shoulder feature (marked by a dashed vertical line) as more cobalt is incorporated. Such a feature is characteristic of ‘oxidized’ magnetite (a composition relatively deficient in Fe2+[B]) [2,36]; a result that will serve to confirm that cobalt is entering the magnetite in place of the Fe2+ on the [B] site.
Figure 5.
XAS and XMCD measurements. (a) Both Fe L2,3 (700–730 eV) and Co L2,3 (770–810 eV) XAS, (b) Fe L2,3 XMCD, (c) change in cation occupancy with Co concentration, xXAS, determined from fitting Fe L2,3 XMCD spectra and (d) Co L2,3 XMCD. (Online version in colour.)
Figure 5b shows the XMCD spectra obtained for the Fe L2,3 edges. The spectra show a systematic variation in cation distributions within the magnetite as the cobalt concentration increases, with the leading Fe2+[B] peak clearly reducing in magnitude with respect to the peaks corresponding to Fe3+. The relative intensities of the XMCD spectra provide information on the magnetization of the samples relative to each other. It is clear that sample Co50 has the smallest XMCD amplitude in all cation sites, reflecting its lower saturation magnetization as shown in figure 3d.
The cations in the magnetite structure produce unique XMCD signatures determined by site location, valence state and magnetization direction. Atomic multiplet calculations [37,38] have been applied to the Fe L2,3 XMCD spectra and were fitted to obtain the most accurate description of the experimental data to determine the relative occupancy and oxidation state of the Fe cations in the three sites in the lattice. Stoichiometric magnetite has a total iron occupancy of 3 (i.e. Fe2+[B] : Fe3+(A) : Fe3+[B] = 1 : 1 : 1); however, this decreases as cobalt is introduced, towards a ferrite spinel with a formula of CoFe2O4 with a total iron occupancy of 2 distributed between the three sites. The sum of the Fe cations in table 3 has been normalized to account for cobalt in the spinel such that
| 2.1 |
where x = xXAS (EPMA is a bulk technique, whereas XAS and XMCD are surface measurements, and thus the XAS results are more relevant for examination of XMCD data) and where Fe2+[B], Fe3+(A) and Fe3+[B] represent the respective site occupancies in the unit formula.
Table 3.
Results of XMCD peak fitting. The Fe site occupancies normalized using equation (2.1) to include Co determined from xXAS.
| xXAS | Fe2+[B] | Fe3+(A) | Fe3+[B] | |
|---|---|---|---|---|
| Co0 | 0 | 1.11 | 0.92 | 0.97 |
| Co5 | 0.17 | 0.97 | 0.90 | 0.96 |
| Co15 | 0.35 | 0.84 | 0.87 | 0.94 |
| Co20 | 0.44 | 0.78 | 0.86 | 0.93 |
| Co33 | 0.60 | 0.68 | 0.82 | 0.90 |
| Co50 | 0.89 | 0.46 | 0.77 | 0.88 |
The results in table 3 confirm a strong decrease in Fe2+[B] site occupancy with increasing Co; however, they also point to a decrease in both Fe3+(A) and Fe3+[B], although less severely. These results show that the majority of the cobalt is incorporated into the octahedral site in place of ferrous iron; however, there is also substitution into Fe3+ tetrahedral and octahedral sites for all Co concentrations (although the results for Co5 are within the error of±0.02 from the results of Co0).
Figure 5c graphically shows the results from table 3 for the changing Fe cation occupancy as a function of Co. The plots indicate almost no deviation from linear fits as Co content increases with the slopes corresponding to the probability of Co entering a particular site. A linear fit of the data gives Fe2+[B] : Fe3+(A) : Fe3+[B] = 0.72 : 0.17 : 0.11 which would suggest that 17 per cent of the Co goes in place of Fe3+(A) which is in close agreement with Pattrick et al. [39].
Further analysis of the Co XMCD (figure 5d) is useful in determining where cobalt is entering the crystal structure. The Co L3 XMCD signal has negative sign which is indicative for the magnetic alignment of the octahedral site. The highest cobalt-containing samples (Co50) have the most intense XAS signal, corresponding to higher Co concentration; however, these also have the least intense Co XMCD, reflecting the overall reduction in Ms and also the presence of a non-magnetic phase (most likely unconverted Co(II)Fe(III)-oxyhydroxide; see Mössbauer results below) that contains Co. The Co XAS and XMCD spectra obtained for all samples show very little differences in spectral shape, indicating the same cobalt composition is present in all samples. The cobalt species can be identified through a comparison of experimental and calculated spectra [37,38]. The spectra observed most closely match that of calculated Co2+ octahedral (as identified by Coker et al. [20]), a result that confirms the incorporation of cobalt in place of ferrous iron in the octahedral site and agreeing well with results for CoFe2O4 thin films [40]. However, the complex multiplet structure of the various Co species fails to exclude a small amount of tetrahedral Co substitution. In fact, simulations by Hochepied et al. [41] would suggest that between 10 and 20 per cent Co exists as tetrahedral Co in cobalt–ferrite nanoparticles.
Using the results in figure 5c, we can speculate on the several possible scenarios to describe the incorporation of cobalt into the magnetite crystal structure. Clearly, the majority of the cobalt (72%) substitutes as Co2+ in the Fe2+[B] site, which preserves the overall neutral charge balance of the unit cell. However, also the other Fe site occupancies can be seen to change in figure 5c. It seems that part of the cobalt (11%) substitutes in Fe3+[B], or that some Fe2+[B] has changed its oxidation state to Fe3+[B]. Also, it appears that 17 per cent of the cobalt substitutes into the Fe3+(A) site. However, replacement of Fe3+ by Co2+ results in an overall negatively charged unit cell. Alternatively, 17 per cent cobalt could substitute as Co3+ into the Fe3+(A) site, conserving the charge balance. However, there is insufficient evidence to suggest that the Co2+ from the starting compound has been oxidized to Co3+ either through chemical or microbial oxidation. Moreover, the 2+ and 3+ charges of the Fe and Co cations should not be regarded as integral numbers but represent only nominal values, of which the precise value is still a matter of debate [42]. Therefore, any charge balance arguments should be considered with caution.
It is possible that the substitution of Co for Fe3+ cations in addition to the expected substitution on Fe2+(A) sites could be related to the preservation of the high saturation magnetization of the biogenic cobalt-doped magnetite nanoparticles observed here. However, further work is required to confirm this.
2.3.2. Mössbauer spectroscopy
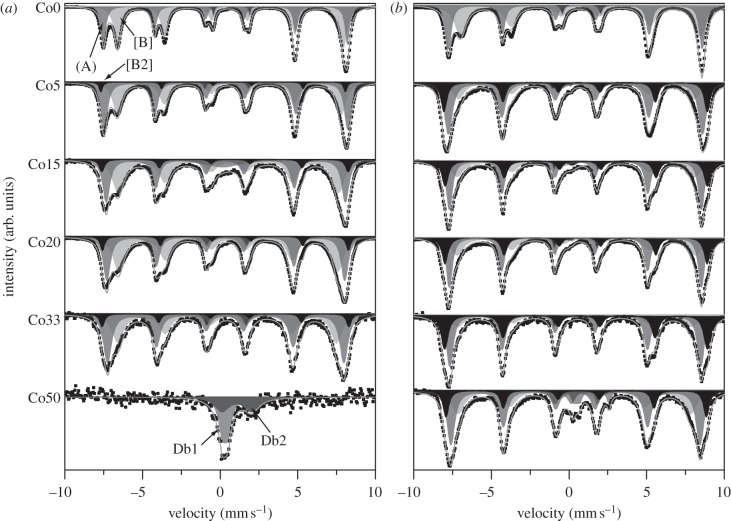
Mössbauer analysis was carried out to characterize the bulk cation properties of the materials at room temperature (RT = 300 K) and low temperature (LT = 110 K; figure 6 and table 4). The spectra were fitted to obtain the isomer shift (IS), quadrupole splitting (QS) and magnetic hyperfine field (Bhf). Almost all samples exhibit Bhf spectra except Co50 at RT that displays a paramagnetic doublet. This is superparamagnetic behaviour, the result of the TB < RT as observed using superconducting quantum interference device (SQUID) magnetometry (TB ≈ 220 K). All hyperfine field spectra were fitted with two sextets, the first corresponding to Fe3+(A) and the second to Fe2+–Fe3+[B]. Analysis of cobalt-doped samples (i.e. Co5 to Co50) showed that an additional sextet (denoted [B2]) is required to provide an accurate fit. Normally, electron hopping between octahedral Fe2+ and Fe3+ is too fast to distinguish different spectra for each cation [43,44]; however, the incorporation of Co2+ eliminates some of the Fe2+–Fe3+[B] interactions available owing to a decrease in the amount of Fe2+ present for pairing (assuming Fe2+ substitution by Co2+). It is therefore reasonable to anticipate the extra sextet arising as a result.
Figure 6.
Mössbauer spectra obtained for the cobalt-doped magnetite samples at (a) room temperature and (b) low temperature. The different types of sextet visible correspond to (A) sites (dark grey), [B] sites (light grey) and [B2] sites (black).
Table 4.
Mössbauer fitting parameters determined for cobalt-doped ferrite spinel samples at room temperature (RT) and low temperature (LT). Parameter values are given for the isomer shift (IS), quadrupole splitting (ΔEQ), hyperfine field (Bhf) and population (pop.).
| sample | temp. | site | IS (mm s−1) | ΔEQ (mm s−1) | Bhf (kOe) | pop. (%) |
|---|---|---|---|---|---|---|
| Co0 | RT | Db | 0.74 | 2.32 | — | 2.7 |
| (A) | 0.33 | 0.04 | 486.6 | 37.6 | ||
| [B] | 0.64 | 0.06 | 451.1 | 59.7 | ||
| LT | Db | 0.80 | 2.47 | — | 3.2 | |
| (A) | 0.42 | −0.02 | 506.5 | 38.7 | ||
| [B] | 0.73 | 0 | 478.8 | 58.1 | ||
| Co5 | RT | Db | 0.43 | 2.81 | — | 2.4 |
| (A) | 0.36 | 0.04 | 486.3 | 47.9 | ||
| [B] | 0.58 | 0.02 | 447.2 | 44.6 | ||
| [B2] | 0.49 | −0.60 | 488.3 | 5.1 | ||
| LT | Db1 | 0.39 | 2.84 | — | 2.5 | |
| Db2 | −0.40 | 0.99 | — | 1.3 | ||
| (A) | 0.41 | 0 | 507.2 | 50.5 | ||
| [B] | 0.61 | −0.22 | 471.9 | 24.4 | ||
| [B2] | 0.54 | −0.24 | 525.8 | 21.3 | ||
| Co15 | RT | Db | 0.18 | 0.02 | 8.7 | |
| (A) | 0.34 | 0.06 | 477.4 | 36.9 | ||
| [B] | 0.54 | −0.02 | 439.8 | 46.5 | ||
| [B2] | 0.50 | −0.36 | 492.1 | 7.9 | ||
| LT | Db1 | 0.44 | 2.79 | — | 1.9 | |
| Db2 | 0.19 | 0.24 | — | 2.0 | ||
| (A) | 0.39 | 0.04 | 503.2 | 42.8 | ||
| [B] | 0.60 | −0.28 | 474.9 | 29.7 | ||
| [B2] | 0.59 | −0.26 | 523.7 | 23.5 | ||
| Co20 | RT | Db | 0.40 | 2.53 | — | 9.4 |
| (A) | 0.34 | 0.06 | 475.0 | 29.8 | ||
| [B] | 0.54 | −0.04 | 438.2 | 39.0 | ||
| [B2] | 0.54 | −0.40 | 491.1 | 21.8 | ||
| LT | Db1 | 0.43 | 2.75 | — | 2.8 | |
| Db2 | 0.08 | 0.26 | — | 0.8 | ||
| (A) | 0.39 | 0.04 | 503.3 | 41.1 | ||
| [B] | 0.62 | −0.28 | 475.8 | 30.0 | ||
| [B2] | 0.58 | −0.26 | 523.5 | 25.4 | ||
| Co33 | RT | Db | 0.40 | 2.53 | — | 4.2 |
| (A) | 0.33 | 0.06 | 472.0 | 47.2 | ||
| [B] | 0.49 | −0.08 | 436.3 | 41.4 | ||
| [B2] | 0.49 | −0.30 | 492.9 | 7.3 | ||
| LT | Db | 0.45 | 2.80 | — | 3.2 | |
| (A) | 0.39 | 0.02 | 501.4 | 45.6 | ||
| [B] | 0.56 | −0.40 | 467.3 | 20.0 | ||
| [B2] | 0.55 | −0.20 | 524.7 | 31.3 | ||
| Co50 | RT | Db1 | 0.32 | 0.34 | — | 52.1 |
| Db2 | 1.07 | 1.92 | — | 48.0 | ||
| LT | Db1 | −0.38 | 1.06 | — | 4.8 | |
| (A) | 0.42 | 0 | 497.5 | 43.8 | ||
| [B] | 0.44 | −0.20 | 464.7 | 30.4 | ||
| [B2] | 0.54 | −0.16 | 520.1 | 15.6 | ||
| Db2 | 1.35 | 0.85 | 23.4 | 5.4 |
Room temperature measurements of Co50 indicate the presence of two doublets, which based on IS and QS correspond to an Fe2+-containing mineral determined to have similar parameters to green rust and was identified as an Fe2+–Co2+ structure from TEM results [45], and an unidentified Fe3+-containing mineral purportedly unreduced Co(II)Fe(III)-oxyhydroxide. The low-temperature spectrum is best fitted with five different components that correspond to the (A), [B] and [B2] sites, the Fe2+–Co2+ double-layered phase and non-reduced Co(II)Fe(III)-oxyhydroxide starting material.
A decrease in the LT IS can be observed in the [B] spectra from 0.73 (Co0) to 0.44 mm s−1 (Co50). No significant change is observable in IS for (A) or [B2] spectra that display values of approximately 0.40 ± 0.01 and 0.56 ± 0.02 mm s−1, respectively. The QS observed shows the general trend for the (A) and [B2] sites to remain relatively unchanged throughout the series; however, there is seen to be a linear decrease in [B] site splitting until sample Co50, for which the value more closely matches that of Co5. Only unpaired electrons contribute to Bhf. The Bhf determined for the pure magnetite sample at room temperature is calculated at 486.6 kOe at the tetrahedral (A) site, and 451.1 kOe at the octahedral [B] site, which closely matches established data [46]. Low-temperature measurements of 506.5 and 478.8 kOe for (A) and [B] sites, respectively, also match pre-existing data closely [45,46]. There is an observable overall decrease in the Bhf of all three sextets through the series that supports the interpretation of the XMCD data that suggests that some Co enters the tetrahedral site in place of Fe3+(A).
3. Conclusions
This work has explored the magnetic properties of biogenic cobalt-doped magnetite nanoparticles formed by the reduction of Co(II)Fe(III)-oxyhydroxide starting material by an Fe(III)-reducing bacterium at ambient temperature and pressure. The nature of the techniques that we have used allows studies of the surface layers of the nanoparticles to be compared against the bulk measurements, with differences from the original biogenic magnetite observable using both. The Fe XMCD and Mössbauer results suggest that the majority of Co substitution occurs into octahedral coordination in place of Fe2+[B]. From examination of the Fe XMCD, it is possible that there is also incorporation of Co in place of Fe3+(A). Further analysis based on the calculated multiplet fits for the Fe presented in figure 5c indirectly suggests that up to 17 per cent Co is incorporated into the tetrahedral site. Changes in the hyperfine field detected using Mössbauer spectroscopy also lend support to the likelihood of tetrahedral cobalt substitution.
The overall coercivity of the particles is found to increase strongly with increased cobalt doping, with a concurrent minor decrease in saturation magnetization. We have demonstrated that a biological method can be used to obtain ferrite nanoparticles with tailored magnetic anisotropy values through Co doping. For applications in magnetic hyperthermia, this ability to control anisotropy, together with the possibility of controlling nanoparticle sizes as demonstrated previously [27], could provide a method to optimize the nanoparticle relaxation time for a specific applied field frequency, thus greatly enhancing the heating effect. Large heating effects are essential to realize the therapeutic potential of the technique where the applied field amplitude and frequency are limited by practical and clinical considerations.
4. Experimental methods
Six different cobalt–iron suspensions (Co(II)Fe(III)-oxyhydroxide) were prepared through the co-precipitation of a mixture of iron(III) chloride (FeCl3) and cobalt(II) chloride (CoCl2) via the addition of sodium hydroxide (10 N) acting as the hydrolysing agent until the suspension reached a pH of 7.0 [15]. The starting concentrations of FeCl3 and CoCl2 were varied to produce six different ‘gels’ corresponding to 0 per cent, 5 per cent, 15 per cent, 20 per cent, 33 per cent and 50 per cent Co (by molar percentage) referred to as samples Co0, Co5, Co15, Co20, Co33 and Co50, respectively. After precipitation, the chloride ions were removed by centrifugation at 17 000g for 20 min and washed with de-ionized water, with this step repeated six times. The total concentration of iron was determined by Ferrozine assay [47]. ICP atomic emission spectroscopy (ICP-AES), after extraction using 3 M HNO3, was used to determine the relative amounts of cobalt and iron in the starting oxyhydroxide material.
Cell cultures were prepared in deionized water to a total volume of 9.9 ml containing an electron donor (sodium acetate; 20 mM), an electron acceptor (Co(II)Fe(III)-oxyhydroxide; 50 mmol l−1), sodium bicarbonate buffer (NaHCO3; 30 mM) and an electron shuttle, anthraquinone 2,6-disulfonate (10 μM), to accelerate Fe(III) reduction. All manipulations were carried out under a N2 : CO2 (80 : 20) gas line to ensure anaerobic conditions.
Geobacter sulfurreducens was grown in anaerobic conditions in the dark at 30°C in modified fresh water medium [48] (25 mM sodium acetate as electron donor, 40 mM fumaric acid as electron acceptor). Late log-phase cultures of G. sulfurreducens were harvested by centrifugation at 5000g and 4°C, for 20 min and washed twice in bicarbonate buffer (30 mM; pH 7) under a N2 : CO2 (80 : 20) gas line. Cells were transferred into bicarbonate buffer, forming a total volume of 30 ml bacterial suspension. Optical density at a wavelength of 600 nm (OD600) was measured using an M501 single beam scanning UV/visible spectrophotometer. The cell suspension was diluted with buffer to achieve an OD600 of 0.4 in 10 ml volume (0.2 ml cells, 9.8 ml deionized H2O). Each culture was then inoculated with 0.2 ml G. sulfurreducens suspension (0.132 mg ml−1 protein, determined by reference to a standard curve prepared with bovine serum albumin) and incubated in the dark at 30°C for one week. The end product was washed twice with deionized water to remove cells and buffer solution.
The chemical composition of the material was obtained using EPMA using a Cameca SX100 microprobe equipped with wavelength dispersive spectrometers. The operating voltage was 15 kV with a specimen current of 20 mA. Pressed pellets of nanoparticles were compared against pure metal standards.
XRD measurements were carried out using a Bruker D8 Advance with a Cu Kα1 source. Data were acquired over a 2θ range of 10–70° with a step size of 0.02°. When magnetite formation was observed in the XRD trace, the average particle size was determined by fitting a Lorentzian curve to the most intense reflection (311) in order to determine full width at half maximum and Bragg angle. These values were used to determine the particle size via input into the Scherrer equation [49,50].
TEM was carried out using a Technai F20 electron microscope equipped with a field emission gun, EDX detector (Oxford Instruments) and Gatan SC600 CCD camera. All images were obtained using an operating beam voltage of 200 kV.
Magnetic measurements were performed on polycrystalline samples restrained in eicosane using a Quantum Design MPMS-XL SQUID magnetometer equipped with a 7 T magnet. ZFC and FC magnetization curves were recorded over a 5–300 K temperature range with an applied magnetic field of 100 Oe. The diamagnetism of the sample holder and eicosane was measured and extracted from the raw magnetic data.
For hyperthermia experiments, stable aqueous suspensions of nanoparticles were prepared by coating with citric acid using a procedure similar to that described elsewhere [51]. Briefly, 25 mg of biogenic nanoparticles was added to 5 ml of dH2O and the pH lowered to less than 3 using concentrated HCl. The solution was bath sonicated for 2 min before adding dry citric acid to give a concentration in solution of 15 mg ml−1, and the pH was subsequently increased to 5.2 using concentrated ammonia. The nanoparticle plus citric acid solution was then heated to more than 80°C for 30 min. Excess citric acid was removed by magnetic decantation, and the nanoparticles washed with acetone several times. The citric-acid-coated nanoparticles were then resuspended in dH2O and subjected to several cycles of vortexing, sonication and centrifugation in order to obtain an optimum stable suspension. ICP measurements were used to obtain iron concentrations in citric-acid-coated nanoparticle suspensions, following complete digestion in high-purity nitric acid. Briefly, the nanoparticle suspensions were mixed with concentrated nitric acid (70%) and heated at 60°C for 48 h. Complete digestion of the nanoparticles was confirmed by dynamic light scattering measurements performed before and after digestion. The resulting samples were diluted with ultrapure water (giving a final 2% nitric acid concentration) and 0.2 µm filtered, prior to ICP analysis.
The solution properties of the nanoparticles were determined using a Malvern Zetasizer 3000. Following citric acid coating, the hydrodynamic cluster (particle) sizes in the aqueous suspensions were determined to be in the range of 40–75 nm for the magnetite and cobalt–ferrite nanoparticles. The measured zeta potential was −45 mV at pH 7.0 reducing to zero at pH ≈ 2.5 in agreement with previous studies [51].
Magnetic hyperthermia measurements were performed using a commercially available unit supplied by nanoTherics Ltd. The heating effects were determined in an AC magnetic field of 205 Oe at a frequency of 110 kHz. An equivalent volume of dH2O was measured to confirm that no heating occurred in the absence of nanoparticles.
XAS at the Fe and Co L2,3 edges were measured at beamline 4.0.2 of the Advanced Light Source, Lawrence Berkeley National Laboratory, CA, USA. Samples were dried and ground in an anaerobic cabinet and mounted onto carbon tape attached to the sample probe with transportation to the beamline taking place in sealed N2 containers. The sample probe was loaded into the chamber in a backflow of N2 to limit potential exposure to oxygen. Measurements were made in TEY mode with an effective probing depth of approximately 3–4 nm, where the signal decreases exponentially with increasing depth, hence most signal originates from the first few nanometres. XMCD spectra were obtained as the difference between two XAS spectra recorded with two opposite applied magnetic fields of ±0.6 T (parallel and antiparallel to the beam direction). The XMCD is dependent on the magnetic moments of the ferrite spinel sublattices in the magnetite structure (i.e. spin up and spin down), valence state (i.e. number of d electrons) and site symmetry (crystal field). The distribution of Fe cations within the magnetite structure can be determined by comparison of the XMCD with atomic multiplet calculations [37,38]. In the case of Fe3O4, each peak in the L3 edge of the spectrum corresponds primarily to a different component of the magnetite, the lowest energy negative peak corresponds to octahedral Fe2+[B], the positive peak to tetrahedral Fe3+(A) and highest energy negative peak to octahedral Fe3+[B] [39].
Mössbauer spectra were recorded with a FAST ComTek 1024-multichannel analyser system using a constant acceleration drive (RT, γ-source approx. 25 mCi 57Co/Rh matrix). Samples were sealed between two layers of Kapton tape that were glued together in an anoxic glove bag to prevent oxidation. Measurements at low temperatures were obtained using a liquid nitrogen cryostat. For line fitting, the Lagarec/Rancourt recoil fitting routine was used (Intelligent Scientific Applications Inc.). Spectra were fitted using Lorentzian line shape symmetrical doublets/sextets. IS data were calibrated with reference to metallic Fe foil recorded at RT. The absorber thickness was less than 4 mg Fe cm−2.
All data associated with this publication are available at: http://doi.pangaea.de/10.1594/pangaea.808948.
Acknowledgements
This work was carried out with the financial support of an NERC PhD studentship awarded to J.M.B. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy (under contract no. DE-AC02-05CH11231). We acknowledge NERC Envirosync II for providing support for this work. Additional thanks to Dr Michael Ward for assistance with and the provision of access to transmission electron microscope by Leeds Nanoscience and Nanotechnology Facility (LENNF). We also thank Dr Eva Cespedes for assistance with the hyperthermia measurements.
References
- 1.Cutting RS, et al. 2010. Optimizing Cr(VI) and Tc(VII) remediation through nanoscale biomineral engineering. Environ. Sci. Technol. 44, 2577–2584 10.1021/es902119u (doi:10.1021/es902119u) [DOI] [PubMed] [Google Scholar]
- 2.Telling ND, Coker VS, Cutting RS, van der Laan G, Pearce CI, Pattrick RAD, Arenholz E, Lloyd JR. 2009. Remediation of Cr(VI) by biogenic magnetic nanoparticles: an X-ray magnetic circular dichroism study. Appl. Phys. Lett. 95, 163701. 10.1063/1.3249578 (doi:10.1063/1.3249578) [DOI] [Google Scholar]
- 3.Hencl V, Mucha P, Orlikova A, Leskova D. 1995. Utilization of ferrites for water treatment. Water Res. 29, 383–385 10.1016/0043-1354(94)E0112-J (doi:10.1016/0043-1354(94)E0112-J) [DOI] [Google Scholar]
- 4.Coker VS, et al. 2010. Microbial engineering of nanoheterostructures: biological synthesis of a magnetically recoverable palladium nanocatalyst. ACS Nano 4, 2577–2584 10.1021/nn9017944 (doi:10.1021/nn9017944) [DOI] [PubMed] [Google Scholar]
- 5.Polshettiwar V, Luque R, Fihri A, Zhu H, Bouhrara M, Basset J-M. 2011. Magnetically recoverable nanocatalysts. Chem. Rev. 111, 3036–3075 10.1021/cr100230z (doi:10.1021/cr100230z) [DOI] [PubMed] [Google Scholar]
- 6.Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergemann C, Erhardt W, Wagenpfeil S, Lübbe AS. 2000. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 60, 6641–6648 [PubMed] [Google Scholar]
- 7.Zeng H, Black CT, Sandstrom RL, Rice PM, Murray CB, Sun SH. 2006. Magnetotransport of magnetite nanoparticle arrays. Phys. Rev. B 73, 020402(R). 10.1103/PhysRevB.73.020402 (doi:10.1103/PhysRevB.73.020402) [DOI] [Google Scholar]
- 8.Johannsen M, et al. 2005. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int. J. Hyperthermia 21, 637–647 10.1080/02656730500158360 (doi:10.1080/02656730500158360) [DOI] [PubMed] [Google Scholar]
- 9.Johannsen M, et al. 2007. Thermotherapy of prostate cancer using magnetic nanoparticles: feasibility, imaging, and three-dimensional temperature distribution. Eur. Urol. 52, 1653–1662 10.1016/j.eururo.2006.11.023 (doi:10.1016/j.eururo.2006.11.023) [DOI] [PubMed] [Google Scholar]
- 10.Goya GF, Grazu V, Ibarra MR. 2008. Magnetic nanoparticles for cancer therapy. Curr. Nanosci. 4, 1–16 10.2174/157341308783591861 (doi:10.2174/157341308783591861) [DOI] [Google Scholar]
- 11.Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. 2002. Hyperthermia in combined treatment of cancer. Lancet Oncol. 3, 487–497 10.1016/S1470-2045(02)00818-5 (doi:10.1016/S1470-2045(02)00818-5) [DOI] [PubMed] [Google Scholar]
- 12.Fortin J-P, Wilhelm C, Servais J, Ménager C, Bacri J-C, Gazeau F. 2007. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J. Am. Chem. Soc. 129, 2628–2635 10.1021/ja067457e (doi:10.1021/ja067457e) [DOI] [PubMed] [Google Scholar]
- 13.Piepenbrock A, Dippon U, Porsch K, Appel E, Kappler A. 2011. Dependence of microbial magnetite formation on humic substance and ferrihydrite concentrations. Geochim. Cosmochim. Acta 75, 6844–6858 10.1016/j.gca.2011.09.007 (doi:10.1016/j.gca.2011.09.007) [DOI] [Google Scholar]
- 14.Majewski PTB. 2007. Functionalized magnetite nanoparticles: synthesis, properties, and bio-applications. Crit. Rev. Solid State Mater. Sci. 32, 203–215 10.1080/10408430701776680 (doi:10.1080/10408430701776680) [DOI] [Google Scholar]
- 15.Lovley DR, Phillips EJP. 1986. Availability of ferric iron for microbial reduction in bottom sediments of the fresh-water tidal Potomac River. Appl. Environ. Microbiol. 52, 751–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coker V, Pattrick R, van der Laan G, Lloyd J. 2007. Formation of magnetic minerals by non-magnetotactic prokaryotes. In Magnetoreception and magnetosomes in bacteria (ed. Schüler D.), pp. 275–300. Heidelberg, Germany: Springer. [Google Scholar]
- 17.Cornell RM, Schwertmann U. 2003. The iron oxides: structure, properties, reactions, occurences and uses, pp. 388–401. Weinheim, Germany: Wiley-VCH. [Google Scholar]
- 18.Hansel CM, Benner SG, Fendorf S. 2005. Competing Fe(II)-induced mineralization pathways of ferrihydrite. Environ. Sci. Technol. 39, 7147–7153 10.1021/es050666z (doi:10.1021/es050666z) [DOI] [PubMed] [Google Scholar]
- 19.Hansel CM, Benner SG, Neiss J, Dohnalkova A, Kukkadapu RK, Fendorf S. 2003. Secondary mineralization pathways induced by dissimilatory iron reduction of ferrihydrite under advective flow. Geochim. Cosmochim. Acta 67, 2977–2992 10.1016/S0016-7037(03)00276-X (doi:10.1016/S0016-7037(03)00276-X) [DOI] [Google Scholar]
- 20.Coker VS, Telling ND, van der Laan G, Pattrick RAD, Pearce CI, Arenholz E, Tuna F, Winpenny REP, Lloyd JR. 2009. Harnessing the extracellular bacterial production of nanoscale cobalt ferrite with exploitable magnetic properties. ACS Nano 3, 1922–1928 10.1021/nn900293d (doi:10.1021/nn900293d) [DOI] [PubMed] [Google Scholar]
- 21.Staniland S, Williams W, Telling N, van der Laan G, Harrison A, Ward B. 2008. Controlled cobalt doping of magnetosomes in vivo. Nat. Nanotechnol. 3, 158–162 10.1038/nnano.2008.35 (doi:10.1038/nnano.2008.35) [DOI] [PubMed] [Google Scholar]
- 22.Esmadi F, Simm J. 1995. Sorption of cobalt(II) by amorphous ferric hydroxide. Colloids Surf. A 104, 265–270 10.1016/0927-7757(95)03289-4 (doi:10.1016/0927-7757(95)03289-4) [DOI] [Google Scholar]
- 23.Shannon RD, Prewitt CT. 1969. Effective ionic radii in oxides and fluorides. Acta Crystallogr. B 25, 925–946 10.1107/S0567740869003220 (doi:10.1107/S0567740869003220) [DOI] [Google Scholar]
- 24.Bernal JD, Dasgupta DR, Mackay AL. 1959. The oxides and hydroxides of iron and their structural inter-relationships. Clay Miner. Bull. 4, 15–30 10.1180/claymin.1959.004.21.02 (doi:10.1180/claymin.1959.004.21.02) [DOI] [Google Scholar]
- 25.Chaves LHG, Curry JE, Stone DA, Carducci MD, Chorover J. 2009. Nickel incorporation in Fe(II, III) hydroxysulfate green rust: effect on crystal lattice spacing and oxidation products. Rev. Bras. Cienc. Solo 33, 1115–1123 10.1590/S0100-06832009000500005 (doi:10.1590/S0100-06832009000500005) [DOI] [Google Scholar]
- 26.Refait P, Abdelmoula M, Trolard F, Génin JMR, Ehrhardt JJ, Bourrié G. 2001. Mössbauer and XAS study of a green rust mineral; the partial substitution of Fe2+ by Mg2+. Am. Miner. 86, 731–739 [Google Scholar]
- 27.Byrne JM, Telling ND, Coker VS, Pattrick RAD, van der Laan G, Arenholz E, Tuna F, Lloyd JR. 2011. Control of nanoparticle size, reactivity and magnetic properties during the bioproduction of magnetite by Geobacter sulfurreducens. Nanotechnology 22, 455709. 10.1088/0957-4484/22/45/455709 (doi:10.1088/0957-4484/22/45/455709) [DOI] [PubMed] [Google Scholar]
- 28.Craik DJ. 1975. Intrinsic and technical magnetic properties of oxides, pp. 1–96 London, UK: Wiley [Google Scholar]
- 29.López JL, Pfannes HD, Paniago R, Sinnecker JP, Novak MA. 2008. Investigation of the static and dynamic magnetic properties of CoFe2O4 nanoparticles. J. Magn. Magn. Mater. 320, e327–e330 10.1016/j.jmmm.2008.02.065 (doi:10.1016/j.jmmm.2008.02.065) [DOI] [Google Scholar]
- 30.Alphandéry E, Carvallo C, Menguy N, Chebbi I. 2011. Chains of cobalt doped magnetosomes extracted from AMB-1 magnetotactic bacteria for application in alternative magnetic field cancer therapy. J. Phys. Chem. C 115, 11 920–11 924 10.1021/jp201274g (doi:10.1021/jp201274g) [DOI] [PubMed] [Google Scholar]
- 31.Alphandéry E, Guyot F, Chebbi I. 2012. Preparation of chains of magnetosomes, isolated from Magnetospirillum magneticum strain AMB-1 magnetotactic bacteria, yielding efficient treatment of tumors using magnetic hyperthermia. Int. J. Pharm. 434, 444–452 10.1016/j.ijpharm.2012.06.015 (doi:10.1016/j.ijpharm.2012.06.015) [DOI] [PubMed] [Google Scholar]
- 32.Hergt R, Hiergeist R, Zeisberger M, Schüler D, Heyen U, Hilger I, Kaiser WA. 2005. Magnetic properties of bacterial magnetosomes as potential diagnostic and therapeutic tools. J. Magn. Magn. Mater. 293, 80–86 10.1016/j.jmmm.2005.01.047 (doi:10.1016/j.jmmm.2005.01.047) [DOI] [Google Scholar]
- 33.Gunnar G, Rudolf H, Matthias Z, Silvio D, Stefan N, Werner W. 2006. The effect of field parameters, nanoparticle properties and immobilization on the specific heating power in magnetic particle hyperthermia. J. Phys. Condens. Matter 18, S2935. 10.1088/0953-8984/18/38/S27 (doi:10.1088/0953-8984/18/38/S27) [DOI] [Google Scholar]
- 34.Liu C, Zou B, Rondinone AJ, Zhang ZJ. 2000. Chemical control of superparamagnetic properties of magnesium and cobalt spinel ferrite nanoparticles through atomic level magnetic couplings. J. Am. Chem. Soc. 122, 6263–6267 10.1021/ja000784g (doi:10.1021/ja000784g) [DOI] [Google Scholar]
- 35.Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G. 2003. Monodisperse MFe2O4 (M=Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 126, 273–279 10.1021/ja0380852 (doi:10.1021/ja0380852) [DOI] [PubMed] [Google Scholar]
- 36.Brotton SJ, Shapiro R, van der Laan G, Guo J, Glans P-A, Ajello JM. 2007. Valence state fossils in proterozoic stromatolites by L-edge X-ray absorption spectroscopy. J. Geophys. Res. 112, G03004. 10.1029/2006JG000185 (doi:10.1029/2006JG000185) [DOI] [Google Scholar]
- 37.van der Laan G, Kirkman IW. 1992. The 2p absorption spectra of 3d transition metal compounds in tetrahedral and octahedral symmetry. J. Phys. Condens. Matter 4, 4189–4204 10.1088/0953-8984/4/16/019 (doi:10.1088/0953-8984/4/16/019) [DOI] [Google Scholar]
- 38.van der laan G, Thole BT. 1991. Strong magnetic X-ray dichroism in 2p absorption spectra of 3d transition-metal ions. Phys. Rev. B 43, 13 401–13 411 10.1103/PhysRevB.43.13401 (doi:10.1103/PhysRevB.43.13401) [DOI] [PubMed] [Google Scholar]
- 39.Pattrick RAD, van der Laan G, Henderson CMB, Kuiper P, Dudzik E, Vaughan DJ. 2002. Cation site occupancy in spinel ferrites studied by X-ray magnetic circular dichroism: developing a method for mineralogists. Eur. J. Miner. 14, 1095–1102 10.1127/0935-1221/2002/0014-1095 (doi:10.1127/0935-1221/2002/0014-1095) [DOI] [Google Scholar]
- 40.Islam FS, Gault AG, Boothman C, Polya DA, Charnock JM, Chatterjee D, Lloyd JR. 2004. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 430, 68–71 10.1038/nature02638 (doi:10.1038/nature02638) [DOI] [PubMed] [Google Scholar]
- 41.Islam FS, Pederick RL, Gault AG, Adams LK, Polya DA, Charnock JM, Lloyd JR. 2005. Interactions between the Fe(III)-reducing bacterium Geobacter sulfurreducens and arsenate, and capture of the metalloid by biogenic Fe(II). Appl. Environ. Microbiol. 71, 8642–8648 10.1128/AEM.71.12.8642-8648.2005 (doi:10.1128/AEM.71.12.8642-8648.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed IAM, Benning LG, Kakonyi G, Sumoondur AD, Terrill NJ, Shaw S. 2010. Formation of green rust sulfate: a combined in situ time-resolved x-ray scattering and electrochemical study. Langmuir 26, 6593–6603 10.1021/la903935j (doi:10.1021/la903935j) [DOI] [PubMed] [Google Scholar]
- 43.Daniels JM, Rosencwaig A. 1969. Mössbauer spectroscopy of stoichiometric and non-stoichiometric magnetite. J. Phys. Chem. Solids 30, 1561–1571 10.1016/0022-3697(69)90217-0 (doi:10.1016/0022-3697(69)90217-0) [DOI] [Google Scholar]
- 44.Franke H, Rosenberg M. 1976. Mössbauer effect and charge transfer in cobalt–iron ferrites. J. Magn. Magn. Mater. 4, 186–192 10.1016/0304-8853(77)90032-4 (doi:10.1016/0304-8853(77)90032-4) [DOI] [Google Scholar]
- 45.Murad E. 1987. Mössbauer spectra of nontronites: structural implications and characterization of associated iron oxides. Z. Pflanzen. Bodenk. 150, 279–285 10.1002/jpln.19871500503 (doi:10.1002/jpln.19871500503) [DOI] [Google Scholar]
- 46.Murad E. 2010. Mössbauer spectroscopy of clays, soils and their mineral constituents. Clay Miner. 45, 413–430 10.1180/claymin.2010.045.4.413 (doi:10.1180/claymin.2010.045.4.413) [DOI] [Google Scholar]
- 47.Stookey LL. 1970. Ferrozine: a new spectrophotometric reagent for iron. Anal. Chem. 42, 779–781 10.1021/ac60289a016 (doi:10.1021/ac60289a016) [DOI] [Google Scholar]
- 48.Lovley DR, Phillips EJP. 1988. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54, 1472–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patterson AL. 1939. The Scherrer formula for X-ray particle size determination. Phys. Rev. 56, 978–982 10.1103/PhysRev.56.978 (doi:10.1103/PhysRev.56.978) [DOI] [Google Scholar]
- 50.Scherrer P. 1918. Estimation of size and internal structure of colloidal particles by means of Rontgen rays. Nachr. Ges. Wiss. Goettingen 2, 96–100 [Google Scholar]
- 51.Campelj S, Makovec D, Drofenik M. 2008. Preparation and properties of water-based magnetic fluids. J. Phys. Condens. Matter 20, 204101. 10.1088/0953-8984/20/20/204101 (doi:10.1088/0953-8984/20/20/204101) [DOI] [PubMed] [Google Scholar]