Abstract
The cell–substrate interface plays a key role in the regulation of cell behaviour. Defining the properties of this interface is particularly important for human embryonic stem (hES) cell culture, because changes in this environment can regulate hES cell differentiation. It has been established that fibronectin-coated surfaces can promote the attachment, growth and maintenance of the undifferentiated phenotype of hES cells. We investigated the influence of the surface density of adsorbed fibronectin on hES cell behaviour in defined serum-free culture conditions and demonstrated that only 25 per cent surface saturation was required to maintain attachment, growth and maintenance of the undifferentiated phenotype. The influence of surface-adsorbed fibronectin fragments was compared with whole fibronectin, and it was demonstrated that the 120 kDa fragment central binding domain alone was able to sustain hES cells in an undifferentiated phenotype in a similar fashion to fibronectin. Furthermore, hES cell attachment to both fibronectin and the 120 kDa fragment was mediated by integrin α5β1. However, although a substrate-attached synthetic arginine–glycine–aspartic acid (RGD) peptide alone was able to promote the attachment and spreading of fibroblasts, it was inactive for hES cells, indicating that stem cells have different requirements in order to attach and spread on the central fibronectin RGD–cell-binding domain. This study provides further information on the characteristics of the cell–substrate interface required to control hES cell behaviour in clearly defined serum-free conditions, which are needed for the development of therapeutic applications of hES cells.
Keywords: human embryonic stem cells, fibronectin, biointeractions, cell adhesion, extracellular matrix
1. Introduction
The behaviour of cells is determined by their surroundings, and within tissues, each cell requires a certain environment to maintain homeostasis. Understanding the key features of these environments is important in the design of substrates to control cellular responses in medical devices and tissue engineering. It is well known that the surface properties of synthetic substrates influence the biointeractions that occur. As soon as a material is placed in a biological environment, there is a thermodynamic driving force that influences the adsorption of proteins onto the surface, and it is this layer that interacts with the cell via the cell membrane. Thus, the characteristics of the adsorbed protein layer in terms of the chemical, topographical or mechanical cues presented to the cells can have a controlling influence on the cell response [1–3].
Anchorage-dependent cells have specific mechanisms for binding to their surroundings and this controls the behaviour of the cells. When it comes to designing synthetic substrates to use for in vitro or in vivo growth of cells, it is critical to understand how the properties of the substrate influence the interface between the material and the cell. It is well known that when a synthetic substrate is exposed to the in vivo or cell culture environments, which contain salts and macromolecules, then proteins from that environment will adsorb onto the surface rapidly. Furthermore, the surface properties of the substrate influence the characteristics of the adsorbed protein layer [4–6]. Subsequently, the cells will interact with the adsorbed protein layer and produce a unique response depending on the type and properties of the protein layer [6].
Fibronectin is a protein that is known to be particularly important for many cell types providing specific sites that promote attachment to surfaces [7]. These sites contain a tripeptide sequence, arginine–glycine–aspartic acid (RGD), which allows a specific interaction with integrins in the cell membrane [7,8]. Many studies have demonstrated that if fibronectin adsorbs onto a surface such that its conformation is changed, and thus the RGD tripeptide sequence is not available to the cells, then some cell types will be unable to bind to the surface or their binding will be significantly reduced [8]. Furthermore, many studies have demonstrated that the RGD sequence, or slightly longer amino acid sequences containing the RGD tripeptide, can be attached to surfaces and promote cell attachment and spreading [8,9]. It has been important to determine certain characteristics of the RGD profile, for example, the concentration of the peptide motif, their spacing, their mobility and the ability to stop them being masked by non-specific protein adsorption from the cell culture media. So although the RGD sequence alone has been demonstrated to be effective in encouraging cell attachment and spreading in certain circumstances, it is not the only requirement in many cases [9].
Plasma fibronectin is a soluble dimer of two 220 kDa monomers linked together by disulfide bonds and each monomer has three types of repeating units [10] (figure 1). Specific binding sites for a range of extracellular molecules exist within the monomers so that fibronectin is also found as an abundant extracellular matrix (ECM) solid-state protein linked to other matrix components [11,12]. Each monomer consists of three different types of protein modules or repeats; namely type I, II and III repeats (figure 1). Each repeat has a specific cell-binding region such as the N-terminal 70 kDa heparin binding region followed by the 120 kDa central cell-binding domain followed by the C-terminal domain which consists of a weak heparin binding region [11,13]. Many studies have demonstrated that integrin-mediated cell adhesion to fibronectin occurs via the RGD sequence located in the type II domain [7,8]. The conformation of the RGD sequence within the tertiary structure of fibronectin and its accessibility via chain mobility within the quaternary structure are important for its effective engagement with integrins [8,11,12].
Figure 1.

Schematic of primary sequence of fibronectin monomer representing various fragments used in the current study (adapted from [11]).
Human embryonic stem (hES) cells, similar to all cell types, require a specific micro environment in which cell surface receptors interact with surrounding ECM molecules to control their behaviour [14]. In addition to soluble growth factors, ECM proteins such as laminin [15], fibronectin [16] and vitronectin [17–19] adsorbed onto tissue culture substrates have been employed to mimic this micro environment for propagation of hES cells. Several attempts have been made and are in progress to mimic this environment to grow hES cells in defined conditions to exploit their therapeutic potential. Baxter et al. [16] showed that fibronectin-coated tissue culture dishes can be used to culture hES cells over several passages while maintaining the undifferentiated phenotype in a completely defined culture medium, and demonstrated that attachment to fibronectin was dependent on the integrin β1 subunit, and at least partially via the α5 subunit, but was independent of αv. Similarly, Braam et al. [17] demonstrated that adsorbed vitronectin maintained hES cells in an undifferentiated state, and further that the cell interaction was mediated via integrin subunit αv and β3 in this case. Both of these studies show that hES cells use specific integrin/ECM protein-binding interactions to maintain their self-renewal capacity. This information is helpful in the design of substrates that could be used to promote propagation of hES cells in their undifferentiated state for therapeutic applications.
The aim of this study was, firstly, to identify the minimum amount of adsorbed fibronectin required for maintaining the undifferentiated phenotype of hES cells and, secondly, to identify which parts of the fibronectin molecule were critical to this interaction. This study demonstrated that hES cells were maintained in their undifferentiated phenotype by a 25 per cent surface coverage of adsorbed fibronectin. Furthermore, the adsorption of the 120 kDa central cell-binding domain of fibronectin, which contains multiple cell-binding sites along with the RGD sequence, supported the attachment and maintenance of the undifferentiated phenotype of hES cells, and that this interaction was mediated via the α5β1 integrin receptor. The study demonstrated that the adsorption of all other fibronectin fragments was not sufficient to promote maintenance of the undifferentiated phenotype and neither was the chemical attachment of short peptide sequences containing the RGD tripeptide, in serum-free cultures. These results help to define the interaction between hES cells and adsorbed fibronectin in serum-free cultures and have the potential to lead to the design of a synthetic surface that contains the necessary functional groups and conformation to promote hES cell propagation for therapeutic applications.
2. Material and methods
The substrates used for experiments were tissue culture polystyrene (TCPS; Nunc). The sources for the human plasma fibronectin and the fibronectin proteolytic and recombinant fragments are presented in table 1 and their locations within the fibronectin molecule are shown in figure 1. Fibronectin fragments used in this study are N-terminal 70 kDa amino acids sequence, this fragment is further divided into N-terminal heparin binding 30 kDa fragment which includes 1–5 type I repeats, 45 kDa collagen-binding fragment which includes 6–9 type I repeats, F2 fragment which includes 1–7 type III repeats, 120 kDa fragments which include 1–10 type III repeats, whereas F3 includes 7–14 type III repeats and F4 represents C-terminal fragment of fibronectin (figure 1).
Table 1.
Details of sources and coating concentrations of fibronectin and its fragments used during this study.
| protein | source | concentrations (µg ml−1) |
|---|---|---|
| human plasma fibronectin | Millipore, Watford, UK | 0–400 |
| 120 kDa proteolytic fragment | Millipore, Watford, UK | 0–12.5 |
| 70 kDa proteolytic fragment | Sigma Aldrich, Dorset, UK | 0–160 |
| 45 kDa proteolytic fragment | Sigma Aldrich, Dorset, UK | 0–160 |
| 30 kDa proteolytic fragment | Sigma Aldrich, Dorset, UK | 0–160 |
| F2 | R&D Systems, Abingdon, UK | 0–160 |
| F3 | R&D Systems, Abingdon, UK | 0–12.5 |
| F4 | R&D systems, Abingdon, UK | 0–160 |
2.1. Fibronectin adsorption and quantification
Human plasma fibronectin (Millipore, UK) was fluorescently labelled using the FluoReporter tetramethylrhodamine (TMR) protein labelling kit (Molecular Probes, UK) following the manufacturer's protocol. The mean ratio of labelling was 5 mol of dye per mol of fibronectin. A degree of labelling above 10 was found to be critical for fluorescence quenching and biological activity of fibronectin [20]. To demonstrate that the fluorescence intensity measured was a true reflection of the amount of fibronectin present, the fluorescently labelled fibronectin (TMR-Fn) was mixed with unlabelled fibronectin in equal amounts. The resulting fluorescence intensity was half the original value. Fibronectin adsorption on TCPS (Nunc 48 well plates) was studied using TMR-Fn solution concentrations between 0 and 500 µg ml–1 (namely, 1.56, 3.12, 6.25, 12.50, 25, 50, 100, 200, 400 and 500 µg ml−1) in phosphate-buffered saline (PBS). Forty-eight well plates were incubated with 100 µl of TMR-Fn for 1 h at 37°C. Protein solution was aspirated and samples were washed three times with PBS without drying. Quantification of adsorbed TMR-Fn was carried out using a previously published procedure with a few modifications [21]. Briefly, adsorbed TMR-Fn was digested using 400 μl 2.5 per cent porcine trypsin solution made in 0.9 per cent sodium chloride solution (Sigma Aldrich, UK) for 24 h at 37°C. The intensity of desorbed TMR-Fn was measured with CytoFluor Series 4000 Fluorescence Multi-Well Plate Reader with excitation and emission wavelengths set at 555 and 580 nm, respectively. Fluorescence intensity values were converted into adsorbed fibronectin using a standard curve prepared from known concentrations of TMR-Fn in trypsin solutions from 0 to 500 μg ml−1.
2.2. Cell culture
HUES7 and HUES1 cells (obtained from Harvard University, HUES cells facility, Melton Laboratory, MA, USA) were cultured in feeder cell-free/serum-free conditions on fibronectin-coated surfaces as previously described [16]. Fully defined serum-free cell culture medium was 50 : 50 F12 : DMEM (Dulbecco's modified Eagle medium, Lonza, Nottingham, UK) supplemented with 2 mM l-glutamine, 1% (v/v) non-essential amino acids, 0.1 mM β-mercaptoethanol, 1% (v/v) N2 supplement, 1% (v/v) B27 supplement, 40 ng ml−1 FGF2 (all six from Invitrogen, UK), 10 ng ml−1 activin-A and 4 ng ml−1 neutrophin-4 (Preprotech, UK) and 0.1 per cent bovine serum albumin (BSA; Sigma, UK). Cells were maintained in these conditions by passaging in 1 : 3 ratio between 3 and 4 days of culture using trypsin on 3.5 or 6 cm tissue culture dishes (Nunc) pre-coated with 50 µg ml−1 fibronectin solution in PBS for 1 h at 37°C. Human purified vitronectin (Millipore, Watford, UK) (10 µg ml−1 in PBS) was adsorbed to culture substrates by incubation for 1 h at 37°C prior to cell seeding.
Murine embryonic fibroblasts (MEFs) [16] were cultured in DMEM (Lonza, Nottingham, UK) supplemented with 10% (v/v) foetal bovine serum, 2 mM l-glutamine and 1 per cent penicillin/streptomycin (Invitrogen, UK). Cells were sub-cultured every 3–4 days. For use in serum-free conditions, cells were washed twice with PBS after trypsinization before culture in the serum-free media used for hES cells.
2.3. Cell adhesion and morphology assays
Unless otherwise stated 20 000 cells cm−2 were seeded onto TCPS 48 well plates (Nunc) pre-coated with fibronectin or fragments diluted in PBS for 1 h at 37°C. After 2 h incubation at 37°C, the unattached cells were removed by gently aspirating the media. The attached cells were counted in three random fields of view in triplicate wells by phase contrast microscopy. The mean single cell area was calculated to assess the extent of cell spreading using Image J software analysis of digitally captured images from the phase contrast microscopy. The mean cell area was calculated from randomly captured cells (n = 50).
2.4. Immunostaining
Cells were fixed for 5 min in a 4% (w/v) paraformaldehyde solution in PBS and, after washing, the cells were incubated at room temperature with primary antibody in 1% (w/v) BSA in PBS for 2 h. Primary antibodies were used at working concentrations of 2.5 μg ml−1 for Oct4 (Insight Biotech Ltd, Wembley, UK) and 2 μg ml−1 for Nanog (R&D Systems, Abingdon, UK). Cells were washed three times with PBS. Later, cells were incubated for 1 h with 4 μg ml−1 of the relevant secondary (Alexafluor488 or Alexafluor594-conjugated) antibodies (Life Tech, Paisley, UK). Samples were washed three times with PBS and mounted in Vectashield hardset mounting medium with DAPI (Vector Laboratories Ltd, Peterborough, UK).
2.5. Antibody and peptide inhibition
HUES7 cells were trypsinized, counted and incubated for 30 min in the presence or the absence of integrin-blocking antibodies α5, β1 and αvβ3 (Abcam, Cambridge, UK) from 1 to 10 µg ml−1 in cell culture medium at 37°C. For peptide-blocking experiments, Gly-Arg-Gly-Asp-Ser-Pro (GRGDSP) peptide and Gly-Arg-Gly-Glu-Ser-Pro (GRGESP) control peptide (Protein Peptide Research Fareham, UK) were used. HUES7 cells were pre-incubated with the peptide in cell culture medium for 15 min at 37°C. The cells (treated with either the blocking antibodies or the blocking peptides) were then seeded at a density of 20 000 cells cm−2 on 48 well plates pre-coated with fibronectin (10 μg ml−1), the 70 kDa (160 μg ml−1) fragment or 120 kDa (10 μg ml−1) fragment. The number of attached cells was counted after 3 h for the antibody blocking experiment or 2 h for the peptide-blocking experiment. Cell adhesion was calculated by counting the number of attached cells in three different random fields of view in triplicate cultures.
2.6. Cell growth assay
Cells were harvested after different culture times with trypsin and counted using a haemocytometer. At least triplicate samples were used in each of three separate experiments. Proliferation was also evaluated by measuring the uptake of [3H] thymidine. HUES7 or HUES1 cells were cultured in 48 well flat bottomed plates (Nunc) coated with fibronectin, the 70 kDa fibronectin fragment, or the 120 kDa fibronectin fragment at 10 000 cells per well in a final volume of 300 ml. Cells were incubated over a time course of 24, 48, 72 and 96 h in a humidified incubator at 37°C with 5 per cent CO2. Triplicate cultures were pulsed with 1 mCi [3H] thymidine for the final 4 h of culture at each time point. Cells were harvested onto glass fibre filter paper and uptake of radioactivity was assessed by liquid scintillation spectroscopy.
2.7. Long-term maintenance of undifferentiated phenotype
HUES7 cells were grown on fibronectin, the 70 kDa fibronectin fragment and the 120 kDa fibronectin fragment-coated dishes and passaged every 3–4 days of culture onto fresh coated dishes using trypsin. The cells were stained for Oct3/4 and Nanog after five passages.
2.8. Surface functionalization of glass coverslips with peptides
Thirteen-millimetre borosilicate glass coverslips (VWR International) were silanized with polyvinylsilane to provide carboxyl groups on the glass surface as reported previously [22]. Briefly, glass coverslips were cleaned with 100 per cent isopropanol in a sonicator and dried. Trimethoxy(vinyl)silane C5H12O3Si (TVS) (Aldrich) was prepared by adding 500 ml of 100 per cent isopropanol + 2 ml TVS + 2 ml double-distilled H2O in a beaker. The solution was gradually heated to 70°C while being stirred with a magnetic stirrer. Once at temperature the samples were immersed in the solution for 30 min while maintaining the temperature. The coverslips were then washed with isopropanol and sonicated for 10 min. To obtain the –COOH surface functionality, the vinyl surfaces obtained by organosilanization were further treated with an aqueous solution of 0.5 mM KMnO4, 9.5 mM NaIO4 and 1.8 mM K2CO3 with gentle stirring on a rocking platform for 24 h. After rinsing with 0.3 M NaHSO3, 0.25 M HCl, double-distilled H2O and 100% (v/v) ethanol, the surfaces were dried for 10 min at 80°C and stored in a desiccator until use.
The glass coverlips modified with carboxyl groups were activated using a standard N-hydroxysuccinimide (NHS)/dicyclohexylcarbodiimide (DCC) coupling procedure [23]. Briefly the samples were incubated in an activated solution of DCC (0.2 M) and NHS (0.05 M) for 2 h, rinsed with N,N-dimethylformamide and dried with nitrogen. For binding of the pentapeptides GRGDSP and GRGESP (custom synthesized by Protein Peptide Research, Fareham, UK), the activated surfaces were incubated in a solution of 100 µM of peptide in 1 ml PBS at 4°C overnight, allowing the reaction of the primary amine group of GRGDSP/GRGESP with the activated carboxylic acid to take place, and rinsed afterwards with double-distilled water several times. The samples were dried in nitrogen and stored in desiccators until use.
2.9. The effect of peptide-modified surfaces on human embryonic stem cells
The activity of RGD-modified samples was tested using MEF and HUES7 cells in serum-free media. GRGDSP/GRGESP-modified glass coverslips were placed in 24 well plates and were sterilized using 70 per cent ethanol followed by three washes with PBS. Both cell types were seeded at a density of 40 000 cm−2. After 2 h incubation at 37°C unattached cells were removed by gently aspirating the media. The attached cells were later counted in three random fields of view of triplicate coverslips for each sample using phase contrast microscopy.
2.10. Statistical analysis
All error bars on data are expressed as standard error of the mean. The statistical significance was determined at a 95 per cent level by Student's t-test, where p values are represented by *p < 0.05, **p < 0.01 and ***p < 0.001.
3. Results
The amount of fibronectin adsorbed to tissue culture plastic substrates was calculated from the fluorescence intensity of the fibronectin solution collected following digestion from the surfaces and compared with the calibration curve. It assumes that the fluorescently labelled fibronectin behaves exactly the same as unlabelled fibronectin and that the digestion procedure collects all the adsorbed fibronectin [21]. The amount of fibronectin adsorbed to tissue culture plastic substrates increased with the concentration of fibronectin-coating solution until it reached a plateau of 345 ± 3 ng cm−2 adsorbed from a solution of concentration of 200 µg ml−1 (figure 2a) and this value was used as 100 per cent surface saturation. To calculate the surface saturation levels, the amount adsorbed from each solution was divided by the value at 100 per cent (345 ng cm−2) and presented as a percentage (figure 2b). From this, it was calculated that 25 per cent surface saturation was achieved from a 10 µg ml−1 solution, which was used for all further experiments. There was no significant difference in the amount of fibronectin adsorbed from solution concentrations of 200, 400 and 500 µg ml−1 (p = 0.104). This suggests that the attachment of HUES7 cells to fibronectin-coated TCPS demonstrated that adsorbed fibronectin levels significantly lower than saturation were sufficient for maximum cell attachment (figure 2b). The maximum percentage cell attachment and spreading was achieved with 25 per cent saturation of surface-adsorbed fibronectin, which is equivalent to fibronectin adsorption levels of 80 ± 9 ng cm−2 (figure 2a). Typical spread cell morphology was observed at both 25 and 70 per cent fibronectin surface saturation (70% surface saturation was chosen as this was the coverage produced from a 50 µg ml−1 solution [16]; figure 2c). After 48 h culture, there was no significant difference in the number of cells attached to substrates with fibronectin surface saturation levels of 25 and 70 per cent (figure 3a), and these cells remained undifferentiated as evidenced qualitatively by expression of the pluripotency markers Oct4 (red) and Nanog (green) in the cell nuclei (figure 3b).
Figure 2.
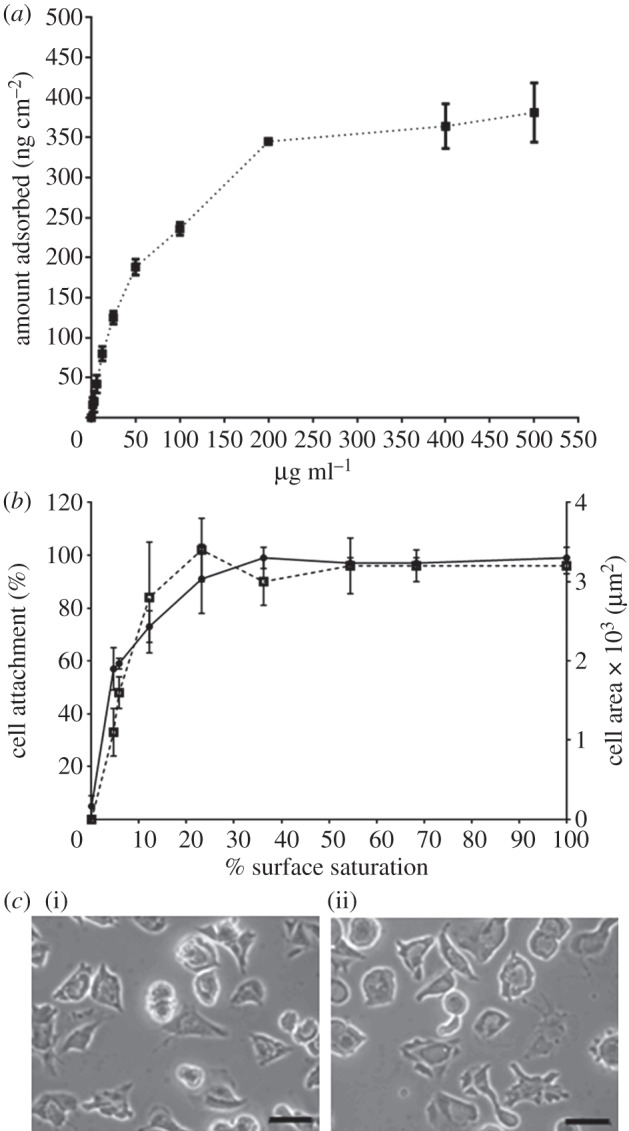
(a) Fibronectin adsorption to TCPS determined by quantification of adsorbed fluorescently labelled fibronectin on TCPS, after 1 h of incubation at 37°C, the amount of adsorbed fibronectin recovered by trypsinization being quantified using a standard curve as described in §2. (b) The effect of fibronectin surface saturation on initial HUES7 cell attachment (circles with solid line, where n = 3), and spreading after 2 h of culture, where cell spreading is represented in terms of mean cell area (squares with dotted line, where n = 50). Percentage surface saturation was calculated using 345 ± 3 ng cm−2 as 100% surface saturation. (c) The effect of fibronectin surface saturation at (i) 25 and (ii) 70% on cell morphology after 2 h of culture. Scale bar, 50 μm.
Figure 3.
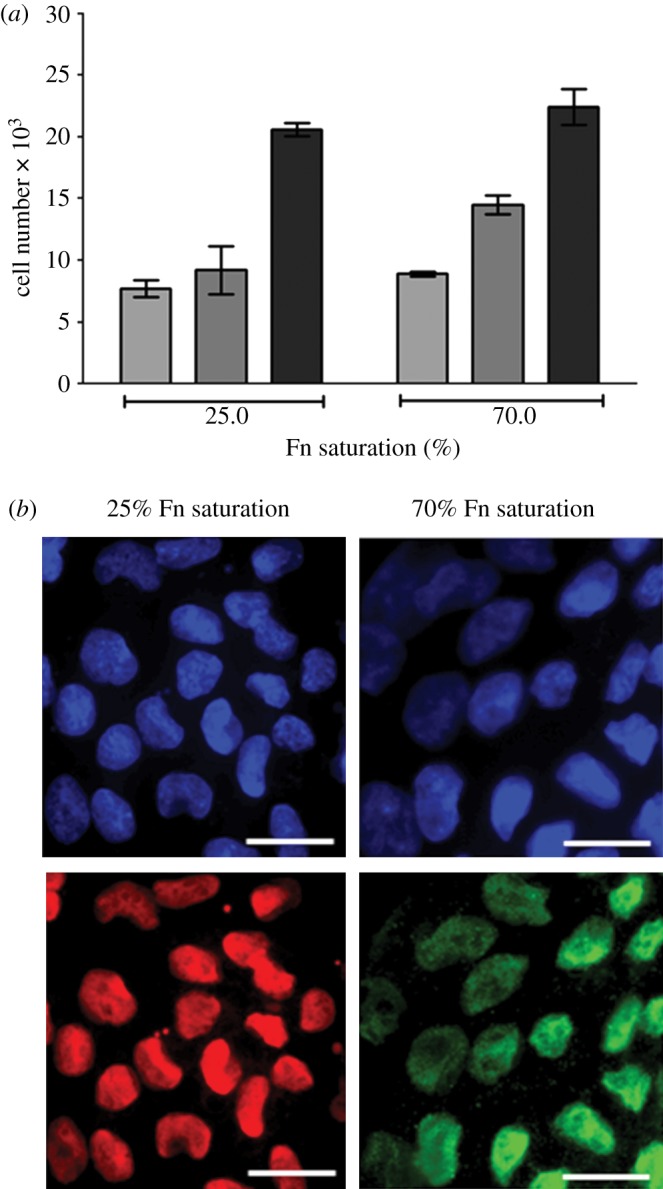
(a) The effect of fibronectin surface saturation at 25 and 70% on HUES7 cell growth (error bars are standard errors from the mean, where n = 3; light grey bars, 2 h; dark grey bars, 24 h; black bars, 48 h). There is no significant difference in cell number on the samples (p > 0.05). (b) Immunostaining of HUES7 cells for pluripotency markers Oct3/4 (in red) and Nanog (in green), nuclei stained in blue with DAPI, after 4 days of culture. Scale bar, 25 μm.
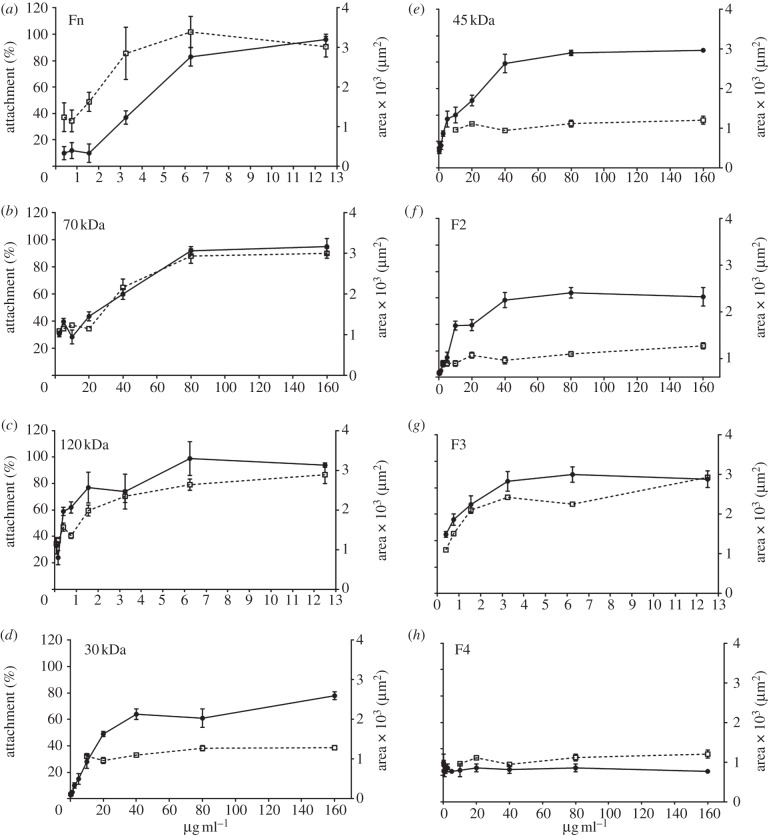
Using fibronectin adsorption isotherms as a control (figure 4a), the influence of adsorbed fibronectin fragments on HUES7 cell attachment and spreading was evaluated after 2 h. These data are plotted as the percentage attachment, in relation to those cells attached to the fibronectin adsorbed from a 10 µg ml−1 solution, against the solution concentration of the particular fibronectin fragment. The solution concentrations of the fragments varied as presented in table 1. Both the 70 and 120 kDa fragments supported HUES7 cell attachment and spreading in a similar fashion to the fibronectin-coated surface (figure 4b,c). The 30 and 45 kDa fragments are components of the 70 kDa fragment and were also evaluated. HUES7 cell attachment was observed on both these fragment-coated surfaces; on the 45 kDa fragment the percentage of cell attachment was similar to fibronectin and the 70 kDa fragment, but on the 30 kDa fragment the percentage of cell attachment was lower than for the fibronectin and the 70 kDa fragment (figure 4d,e). On both these surfaces, cell spreading was lower than on the fibronectin or the 70 kDa fragment. Fragment F2 allowed some cell attachment, although less than on fibronectin; the cell spreading was also low (figure 4f). Fibronectin fragment F3 allowed HUES7 cell attachment and spreading similar to fibronectin (figure 4g). Fragment F4-coated surface was not conducive to HUES7 cell attachment and those cells that did attach failed to spread (figure 4h).
Figure 4.
(a–h) The relation between cell attachment (circles with solid line, where n = 3) and spreading (squares with dotted line, where n = 50) for different coating concentration of fibronectin and its proteolytic fragments. The cell attachment on fibronectin (10 µg ml−1) was taken as 100% to compare effectiveness of individual fragments. Cell spreading is presented in terms of average cell area (μm2). Error bars are standard errors from the mean.
After 2 h, HUES7 cell attachment and spreading was observed on fibronectin and the 70, 120, 40 kDa and F3 fragments while the other fragments all demonstrated sub-optimal attachment and spreading. It was important, however, to evaluate if early attachment translated into longer term growth of the cell cultures. Over the 96 h time period, cells on fibronectin proliferated exponentially as expected; those on the 120 and 70 kDa fragments proliferated, although exponentially, at a slower rate. On all other substrates the cells failed to grow (figure 5a). A similar proliferation study using HUES1 cells demonstrated that they only proliferated on surfaces coated with fibronectin and the 120 kDa fragment and not on the surfaces coated with the 70 kDa fragment (figure 5b). Proliferation of both HUES7 and HUES1 cells on fibronectin and the 120 and the 70 kDa fragment-coated surfaces was also measured using the uptake of [3H] thymidine as an alternative methodology. This study demonstrated the same results providing confidence that both hES cell lines could proliferate on the fibronectin and the 120 kDa fragment but that some differences were observed in relation to the 70 kDa fragment-coated surfaces for the two cell lines (figure 6). Immunostaining of Oct4 and Nanog was maintained in all HUES7 cells cultured for 96 h on 70 and 120 kDa fragments similar to fibronectin (figure 7). Furthermore, Oct 4 and Nanog staining of HUES7 cells was maintained after growth of the cells on the fibronectin and the 120 and the 70 kDa fragment-coated surfaces after five passages (figure 8).
Figure 5.
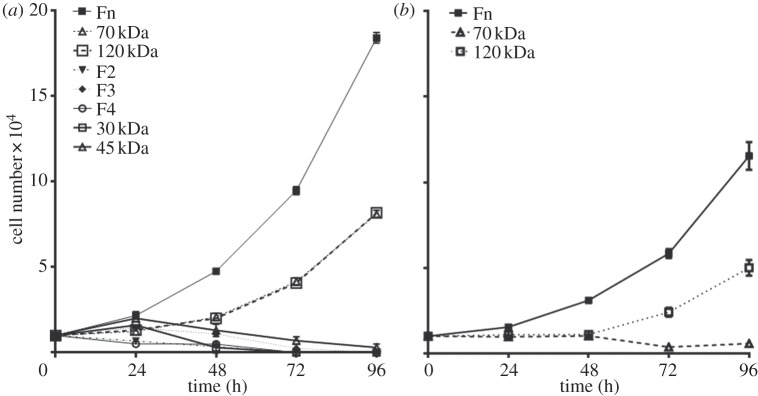
The effect of fibronectin and its fragments on (a) HUES7 and (b) HUES1 cell growth in terms of increase in cell number, when cultured over a period of 96 h. Error bars are standard errors from the mean, where n = 3, at least triplicate samples were used in each of three separate experiments. Fibronectin, 120 kDa and F3 were used at a coating concentration of 10 µg ml−1, whereas all other fragments were used at 160 µg ml−1. HUES1 cell growth was only tested on fibronectin, 120 and 70 kDa fragments, following results from (a).
Figure 6.
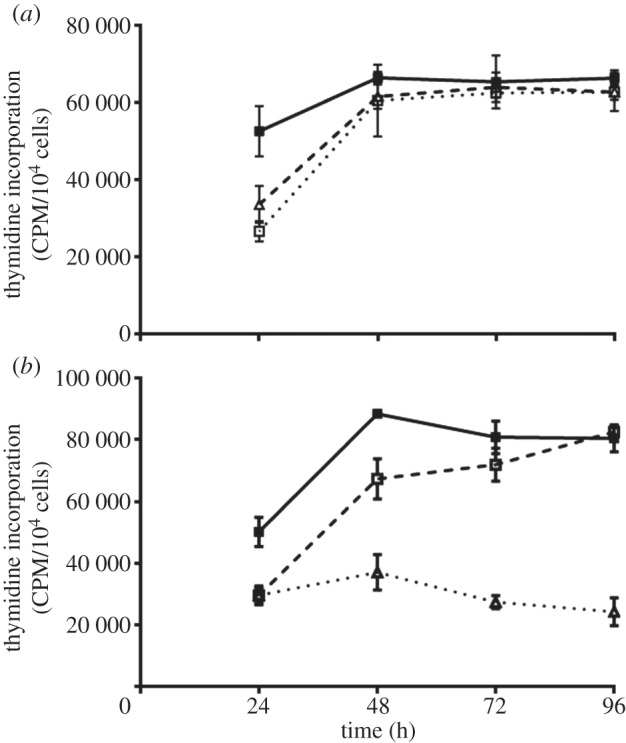
Proliferation of (a) HUES7 and (b) HUES1 cells on fibronectin (filled squares), the 120 (open squares) and the 70 kDa (triangles) fragments measured using the uptake of [3H] thymidine. The cells were incubated over a time course of 24, 48, 72 and 96 h and pulsed with 1 mCi [3H] thymidine for the final 4 h of culture at each time point. Triplicate experiments were run.
Figure 7.
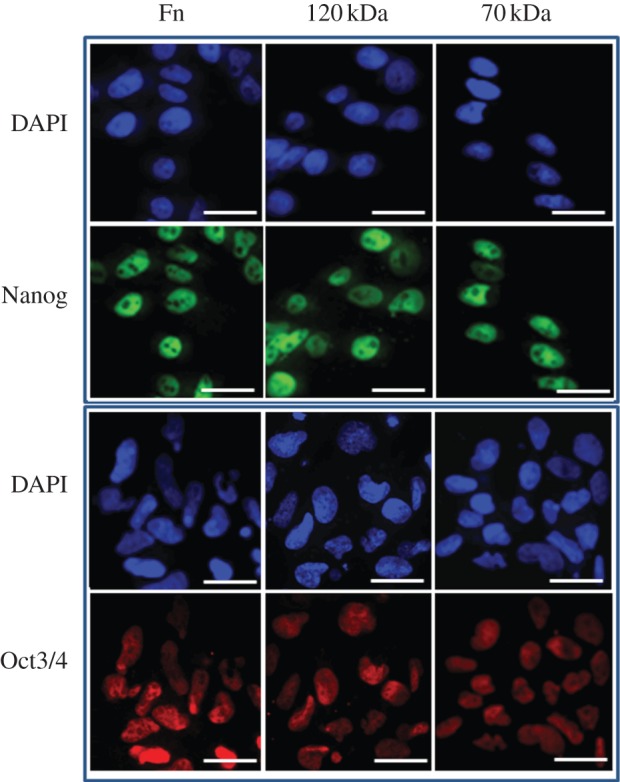
Immunostaining of HUES7 cells for pluripotency markers Oct3/4 (in red) and Nanog (in green) on TCPS dishes coated with fibronectin and its fragments, 70 kDa (160 µg ml−1) and 120 kDa (10 µg ml−1), after 4 days of culture. Nuclei are stained in blue to show co-localization with Oct3/4 or Nanog. Scale bar, 25 μm.
Figure 8.
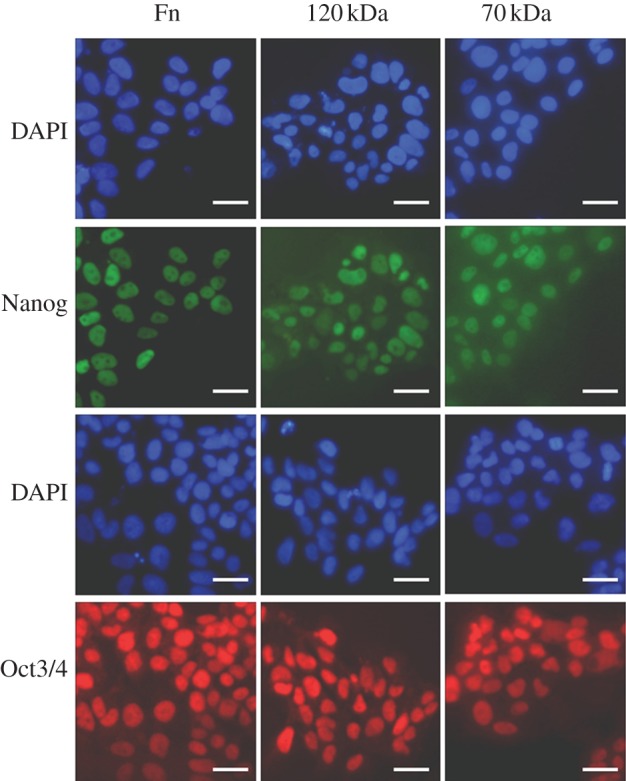
Immunostaining of HUES7 cells for pluripotency markers Oct3/4 (in red) and Nanog (in green) after five passages on dishes coated with fibronectin and its fragments: 70 kDa (160 mg ml−1) and 120 kDa (10 mg ml−1). Nuclei are stained in blue to show co-localization of Oct3/4 or Nanog. Scale bar, 25 µm.
In order to determine the role of specific integrins in HUES7 cell attachment to fibronectin and the 70 and 120 kDa fragments, peptide-blocking and antibody inhibition studies were undertaken. Addition of GRGDSP-based peptide to the culture resulted in a dose-dependent decrease in cell attachment (figure 9). Integrin β1 and α5 blocking resulted in a dose-dependent decrease in cell attachment on fibronectin and the 70 and 120 kDa fragments (figure 10a,b), although complete blocking of the cell attachment was not observed. Vitronectin is recognized by αvβ3 integrin receptor [17] and fibronectin recognized by α5β1 integrin receptor [16], and thus vitronectin-coated plates were used as a control to assess specificity of blocking antibodies to fibronectin-coated plates. Thus, when integrin-blocking antibody to αvβ3 was used, cell attachment to fibronectin and the 70 and 120 kDa fragments remained unaffected (figure 10c), whereas it reduced, but did not completely block, cell attachment to vitronectin, suggesting specificity of cell interactions to this protein. This suggests that the HUES7 cell interaction with fibronectin and both the 70 and 120 kDa fragments was substantially mediated via integrin α5β1.
Figure 9.
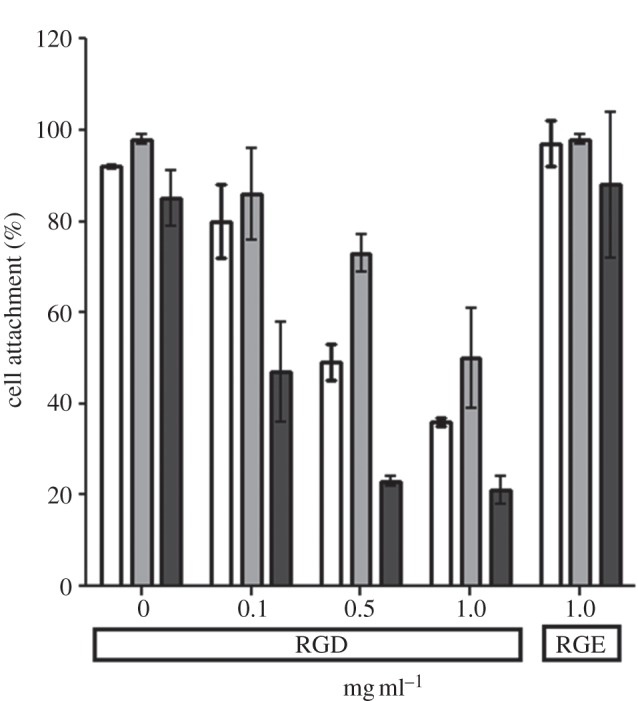
The effect of integrin-blocking peptide GRGDSP on HUES7 cell attachment to fibronectin (white bars, 10 µg ml–1) and its fragments: 120 kDa (light grey bars, 10 µg ml−1) and 70 kDa (dark grey bars, 160 µg ml−1). Control peptide GRGESP was used at a concentration of 1 mg ml−1. Error bars are standard errors from the mean, where n = 3.
Figure 10.
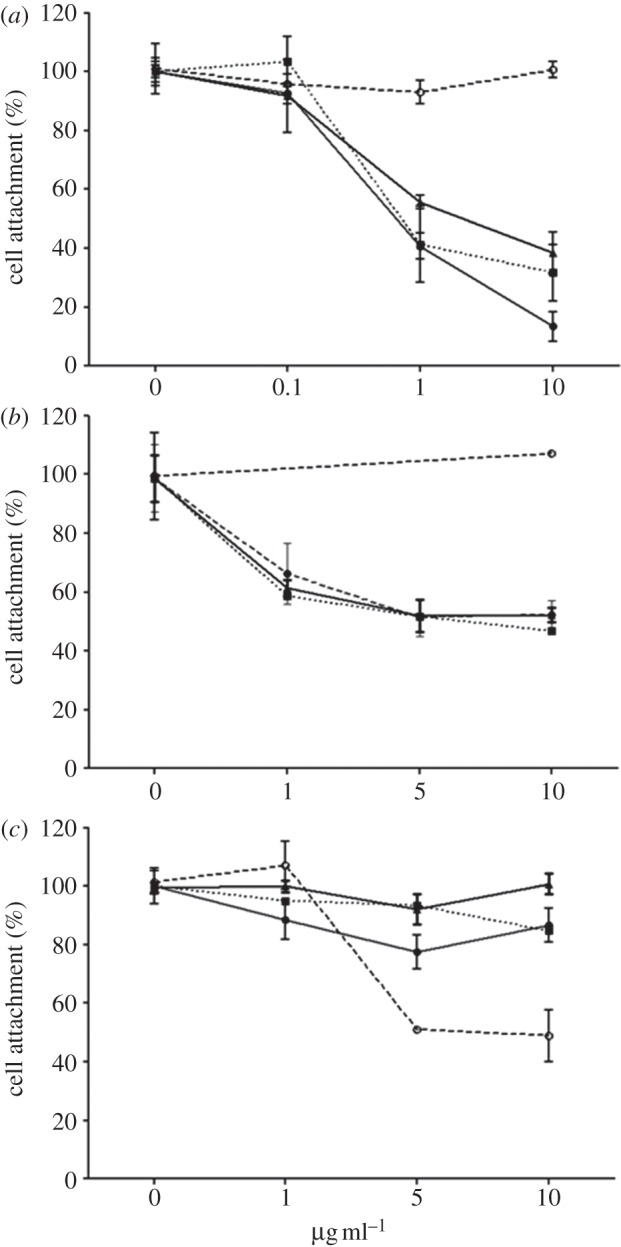
The effect of integrin β1 blocking antibody (a), integrin α5 blocking antibody (b) and integrin αvβ3 blocking antibody (c) on HUES7 attachment to fibronectin (10 µg ml−1) and its fragments: 120 kDa (10 µg ml−1) and 70 kDa (160 µg ml−1). Error bars are standard errors from the mean, where n = 3.
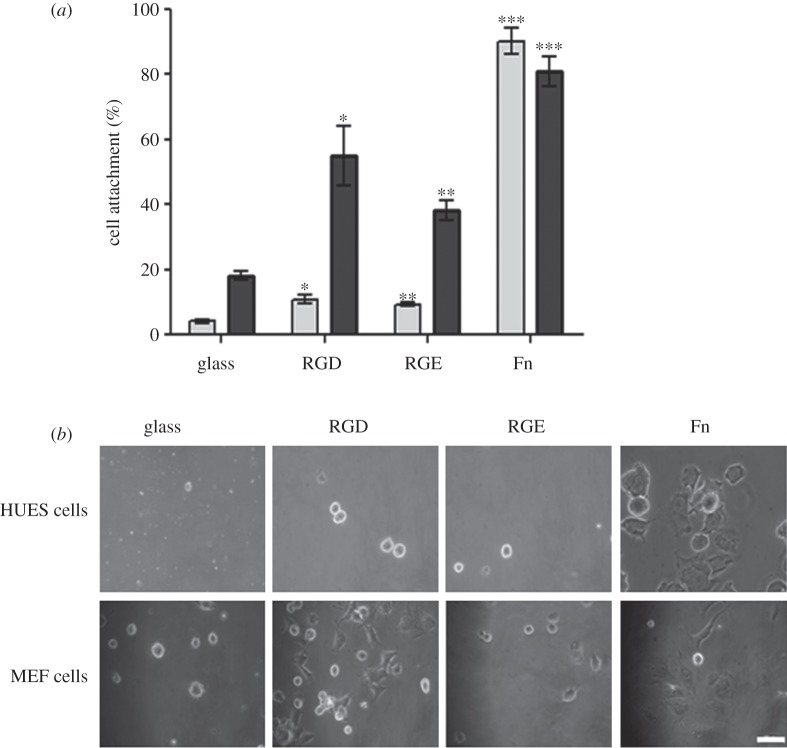
The pentapeptide GRGDS was attached to the glass substrates and its activity was demonstrated by the attachment and spreading of MEF cells although fewer cells were shown to attach to the GRGDS surface than to a fibronectin-coated glass surface (figure 11a,b). When HUES7 cells were cultured on these surfaces, however, the level of attachment was very low with little or no spreading (figure 11a,b).
Figure 11.
(a) The effect of RGD peptide-modified glass coverslip on HUES7 cell attachment after 2 h in serum-free conditions; cells seeded on fibronectin were used as a positive control (light grey bars, HUES cells; dark grey bars, MEF cells). Error bars are standard error from the mean, where n = 3. Statistical significance is reported with respect to cell attachment on unmodified glass for respective cell type. (b) The typical cell morphology of HUES7 and MEF cells after 2 h for the samples shown in (a). Scale bar, 50 μm.
4. Discussion
The aim of this study was to identify the characteristics of an adsorbed fibronectin layer on a synthetic substrate that would provide the necessary interface with hES cells to encourage them to attach, spread and proliferate while maintaining their pluripotent characteristics. The literature has a number of examples where ECM-based components were used to culture hES cells [24–26]. ECM proteins such as laminin [15], fibronectin [16] and vitronectin [17,18] adsorbed onto surfaces have been used for long-term propagation of hES cells. Abraham et al. [27] studied the role of heparin sulfate protyoglycan (HPSG) with various ECM proteins and showed that HPSG along with fibronectin was enough to maintain hES cells in long-term culture further demonstrating that fibronectin has a unique role in the maintenance of hES cells. In an attempt to design synthetic surfaces to culture hES cells in defined conditions, Derda et al. [28] screened an array of short peptides derived from fibronectin or laminin immobilized on synthetic surfaces for short-term hES cell cultures. In their study, the effect of the surface density of the immobilized peptide on hES cell pluripotency was established but not quantified. In another study, a cyclic RGD peptide was covalently bound to tissue culture plastic and was successfully used to maintain hES cells in long-term culture with an estimated surface density of 10–30 fmol cm−2 [29]; however, Hs27 conditioned media was used to maintain the cells for 10 passages. Shorter term 5 day cultures grown in mTESR1 media were also maintained on these cyclic RGD-modified substrates. Another commercially available culture media StemPro was used to maintain HUES9 and HUES1 cells for five passages on the synthetic polymer poly(methylvinylether-alt-maleic anhydride) [30]. The use of synthetic polymers with variable wettability and rigidity has been evaluated to support hES cell expansion in conditioned medium in short-term cultures but long-term propagations on these surfaces failed in defined, serum-free medium [31]. The use of ECM components or undefined culture media, for example conditioned media, makes it difficult to determine how the defined ECM components alone are influencing the hES cell response. Also commercial media although defined may have undisclosed confidential components.
The fibronectin culture system developed by Baxter et al. [16] employed defined, serum-free conditions and demonstrated the role of fibronectin in maintaining the long-term undifferentiated phenotype of hES cells. In order to define these conditions further, the present study investigated the minimum amount of fibronectin required to maintain the undifferentiated phenotype and evaluated the role of various domains of fibronectin on cell attachment, growth, proliferation and maintenance of the undifferentiated phenotype in short-term culture. The Baxter et al. [16] fibronectin culture system used a fibronectin concentration of 50 μg ml−1 to coat tissue culture dishes. Based on the experiments we present here determining the binding isotherm of fibronectin to culture substrates, this would be expected to have resulted in the adsorption of approximately 200 ng cm−2 fibronectin, and approximately 60 per cent surface saturation, whereas the present study demonstrated that a 25 per cent surface coverage of fibronectin was enough to promote maintenance of the undifferentiated phenotype of the HUES7 cells. This fibronectin coverage can be achieved from a solution concentration of nearer 10 μg ml−1. No difference is HUES7 cell behaviour was observed between a 25 and a 70 per cent saturated fibronectin layer suggesting that it is not necessary for the entire surface to be covered by fibronectin molecules. It may be speculated that this difference between fibronectin-coating saturation and maximal cell response to fibronectin is a reflection either of maximum stimulation of cellular response with only partial occupancy of the cell's fibronectin receptors, or alternatively that the distribution of those receptors on the cell membrane is uneven, being spaced similarly to the fibronectin molecules on the substrate. The orientation and/or conformation of the fibronectin molecule are also important for cell receptor binding and, although this study did not evaluate the fibronectin molecule conformation on the surface, it may be speculated that since the tissue culture plastic surfaces are hydrophilic then the conformation of the molecule will be conserved [4]. Furthermore, at the low surface saturation levels, it is likely that the fibronectin molecules will have space to re-orient to make optimal attachment as driven by the thermodynamics of the interface.
It would also be useful to know whether particular parts of the fibronectin molecule could on their own support the maintenance of the undifferentiated phenotype of hES cells. Specific fibronectin fragments corresponding to segments of the whole of the fibronectin molecule were adsorbed onto the substrate to demonstrate, as expected, that HUES7 cell attachment and spreading was different on the different fragments. Only the 120 and the 70 kDa fragments were able to maintain HUES7 cells for 96 h in a similar fashion to fibronectin. The 120 kDa fragment was the largest of the fragments tested; it contained the RGD integrin-binding motif and other amino acid sequences around this motif are likely to be involved in stabilizing the integrin binding. By contrast, fragment F4 promoted very little attachment and those cells that did attach did not spread. This fragment is short and represents the C-terminal section of fibronectin. The 30 and 45 kDa fragments promoted cell attachment but these cells were not able to spread well, whereas when the 70 kDa fragment, which comprises both the 30 and 45 kDa fragments, was adsorbed to the surface the cells attached and spread. Similar to the 70 kDa fragment, fragment F3 allowed attachment and spreading but not longer term growth. Fragment F3 contains the RGD sequence but lacks surrounding amino acid sequences as in the 120 kDa fragment. This is in agreement with the study by Doran et al. [32] where they increased the concentration of the F3 domain (represented as rFN in their study) by chemical coupling a layer-by-layer surface of self assembled molecules on the surface of hyaluronic acid and chitosan. Higher levels of HUES7 cell attachment were observed on F3 similar to those of the cells on fibronectin. However, this study did not show longer term results. Even though the 120 and 70 kDa fragments were able to maintain cell attachment, spreading and maintenance of the undifferentiated phenotype, the growth of the cells was much lower compared with on the complete fibronectin molecule. Furthermore, when compared for their individual coating concentration and their effect on cells, the 70 kDa fragment required an 8–10 times higher coating concentration compared with both the 120 kDa fragment and fibronectin. This could be due to its low attachment to the tissue culture plastic, the orientation of the adsorbed fragment or that the cells need a higher ligand density of the 70 kDa fragment to influence the cell behaviour. These results suggest that the 120 kDa fragment is most likely to be the part of the fibronectin molecule used by HUES7 cells during their surface interactions. Using only HUES1 cells, the fibronectin and the 120 kDa fragment-coated surfaces were able to maintain cell proliferation over the 96 h time frame, demonstrating the specificity of different hES cells in terms of surface interactions and providing further evidence of the importance of the 120 kDa fragment. Long-term cultures of HUES7 cells on fibronectin, the 120 and the 70 kDa fragment-coated surfaces have maintained the cells in their undifferentiated phenotype over five passages.
Cell surface integrin receptors play an important role in mediating cell attachment and long-term survival on ECM proteins, such as fibronectin [7,11,12]. hES cell interaction with intact fibronectin has been shown previously to be mediated via α5β1 integrin receptors [16]. Peptide- and integrin-blocking experiments in the present study also suggested α5β1 integrin-mediated attachment to the 120 and 70 kDa fragments similar to intact fibronectin, consistent with the same binding sites being used for all these ECM components. However, in no case was the cell attachment completely blocked, suggesting that the cells could also use other integrin receptors when necessary.
Since the use of soluble GRGDSP peptide blocked cell attachment to intact fibronectin and the 120 kDa fragment in a dose-dependant fashion, this suggested that the cells were using this peptide sequence for their interaction with fibronectin. To test this hypothesis and evaluate the specificity of the RGD peptide in cell attachment, HUES7 cells were seeded on surface bound GRGDSP peptide in serum-free conditions. MEF cells, which were used to test surface modification, showed an increase in cell attachment and spreading on RGD-modified samples, which is consistent with previously published data with fibroblast cell types on RGD-modified synthetic surfaces [33–36]. However, HUES7 cells failed to attach on RGD peptide-modified glass surfaces. The inability of HUES7 cell attachment to RGD-coupled glass surface either suggests that RGD coupled on to the surface lacked appropriate concentration, conformation or spacing, or HUES7 cells needed peptides other than or in conjunction with RGD to induce their attachment and spreading with subsequent maintenance of the undifferentiated phenotype. In a recent study, the effect of RGD peptide was shown on hES cell attachment after 1 h of cell seeding [28]. Even though no information is available on the amount of coupled RGD peptide on the surface, electronic supplementary material in that study shows that hES cells required a higher density of RGD peptide to achieve high levels of cell attachment than other cell types. This agrees with our observation, where only MEF cell attachment and spreading was observed on RGD-modified glass coverslips. In addition, short-term culture of hES cells has been investigated on RGD-modified hydrogel [37]. However, since protein-containing media were used in this study, the influence of other proteins adsorbed on the RGD-modified hydrogel cannot be ignored.
In this study, the maintenance of the undifferentiated phenotype of the HUES7 cells was assessed qualitatively by staining for Oct4 and Nanog. Further analysis of hES cell behaviour on these substrates by quantifying the expression levels of these and other pluripotency markers in comparison with hES cells cultured on whole fibronectin would provide further evidence of the usefulness of these protein fragments in the expansion of hES cells and in determining the mechanism of their interaction with specific substrates.
In summary, this study demonstrates that the interface provided by the adsorbed fibronectin that promotes hES cell interactions is quite specific. It also suggests that although the density of binding sites in the whole fibronectin molecule that is needed to support the HUES7 cells is low, there are specific regions of the molecule that are required. Certain regions of the fibronectin molecule do not support HUES7 cell attachment on their own; however, this does not necessarily mean they are not involved in the interaction of the hES cells with their substrate. If the adsorbed fibronectin layer needed to sustain hES cell cultures could be characterized such that it could be reproduced synthetically, this could enhance the scale up of hES cells for therapeutic application. It would appear from this study that the characteristics of the interface required for HUES7 cells may be more specific than for other cell types, and thus it may be more difficult to model synthetically with enough chemical and architectural structure to be sufficiently robust.
Acknowledgements
The authors gratefully acknowledge the financial support from a project grant awarded by the BBSRC (BB/D014638/1).
References
- 1.Lewandowska K, Balachander N, Sukenik CN, Culp LA. 1989. Modulation of fibronectin adhesive functions for fibroblast and neural cells by chemically derivatised substrata. J. Cell. Physiol. 141, 334–345 10.1002/jcp.1041410215 (doi:10.1002/jcp.1041410215) [DOI] [PubMed] [Google Scholar]
- 2.Lord MS, Cousins BG, Doherty PJ, Whitelock JM, Simmons A, Williams RL, Milthorpe BK. 2006. The effect of silica nanoparticulate coatings on serum protein adsorption and cellular response. Biomaterials 27, 4856–4862 10.1016/j.biomaterials.2006.05.037 (doi:10.1016/j.biomaterials.2006.05.037) [DOI] [PubMed] [Google Scholar]
- 3.Ventre M, Causa F, Netti PA. 2012. Determinants of cell–material crosstalk at the interface: towards engineering of cell instructive materials. J. R. Soc. Interface 9, 2017–2032 10.1098/rsif.2012.0308 (doi:10.1098/rsif.2012.0308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grinnell F, Feld MK. 1982. Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J. Biol. Chem. 257, 4888–4893 [PubMed] [Google Scholar]
- 5.Keselowsky BG, Collard DM, Garcia AJ. 2003. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion . J. Biomed. Mater. Res. A 66A, 247–259 10.1002/jbm.a.10537 (doi:10.1002/jbm.a.10537) [DOI] [PubMed] [Google Scholar]
- 6.Lewandowska K, Pergament E, Sukenik CN, Culp LA. 1992. Cell-type-specific adhesion mechanism mediated by fibronectin adsorbed to chemically derivatized substrata . J. Biomed. Mater. Res. 26, 1343–1363 10.1002/jbm.820261007 (doi:10.1002/jbm.820261007) [DOI] [PubMed] [Google Scholar]
- 7.Garcia AJ, Boettiger D. 1999. Integrin–fibronectin interactions at the cell–material interface: initial integrin binding and signaling. Biomaterials 20, 2427–2433 10.1016/S0142-9612(99)00170-2 (doi:10.1016/S0142-9612(99)00170-2) [DOI] [PubMed] [Google Scholar]
- 8.Hersel U, Dahmen C, Kessler H. 2003. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24, 4385–4415 10.1016/S0142-9612(03)00343-0 (doi:10.1016/S0142-9612(03)00343-0) [DOI] [PubMed] [Google Scholar]
- 9.Mardilovich A, Craig JA, Mccammon MQ, Garg A, Kokkoli E. 2006. Design of a novel fibronectin-mimetic peptide-amphiphile for functionalized biomaterials . Langmuir 22, 3259–3264 10.1021/la052756n (doi:10.1021/la052756n) [DOI] [PubMed] [Google Scholar]
- 10.Yamada KM. 1991. Fibronectin and other cell interactive glycoproteins. In Cell biology of extracellular matrix (ed. Hay ED.), pp. 111–145, 2nd edn New York, NY: Plenum Press [Google Scholar]
- 11.Potts JR, Campbell ID. 1996. Structure and function of fibronectin modules. Matrix Biol. 15, 313–320 10.1016/S0945-053X(96)90133-X (doi:10.1016/S0945-053X(96)90133-X) [DOI] [PubMed] [Google Scholar]
- 12.Johansson S, Svineng G, Wennerberg K, Armulik A, Lohikangas L. 1997. Fibronectin–intergrin interactions. Front. Biosci. 2, 126–146 [DOI] [PubMed] [Google Scholar]
- 13.Mould AP, Humphries MJ. 1991. Identification of a novel recognition sequence for the integrin alpha-4-beta-1 in the COOH-terminal heparin-binding domain of fibronectin. Embo J. 10, 4089–4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilic D. 2006. Culture of human embryonic stem cells and the extracellular matrix micro environment. Regen. Med. 1, 95–101 10.2217/17460751.1.1.95 (doi:10.2217/17460751.1.1.95) [DOI] [PubMed] [Google Scholar]
- 15.Rodin S, Domogatskaya A, Strom S, Hansson EM, Chien KR, Inzunza J, Hovatta O, Tryggvason K. 2010. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 28, 611–615 10.1038/nbt.1620 (doi:10.1038/nbt.1620) [DOI] [PubMed] [Google Scholar]
- 16.Baxter MA, Camarasa MV, Bates N, Small F, Murray P, Edgar D, Kimber SJ. 2009. Analysis of the distinct functions of growth factors and tissue culture substrates necessary for the long-term self-renewal of human embryonic stem cell lines. Stem Cell Res. 3, 28–38 10.1016/j.scr.2009.03.002 (doi:10.1016/j.scr.2009.03.002) [DOI] [PubMed] [Google Scholar]
- 17.Braam SR, et al. 2008. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alpha v beta 5 integrin. Stem Cells 26, 2257–2265 (doi:10.1634/stemcells.2008–0291) [DOI] [PubMed] [Google Scholar]
- 18.Li J, Bardy JA, Yap LYW, Chen A, Nurcombe V, Cool SM, Oh SKW, Birch WR. 2010. Impact of vitronectin concentration and surface properties on the stable propagation of human embryonic stem cells. Biointerphases 5, FA132–FA142 10.1116/1.3525804 (doi:10.1116/1.3525804) [DOI] [PubMed] [Google Scholar]
- 19.Prowse ABJ, Chong F, Gray PP, Munro TP. 2011. Stem cell integrins: implications for ex-vivo culture and cellular therapies. Stem Cell Res. 6, 1–12 10.1016/j.scr.2010.09.005 (doi:10.1016/j.scr.2010.09.005) [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann C, Leroy-Dudal J, Patel S, Gallet O, Pauthe E. 2008. Fluorescein isothiocyanate-labeled human plasma fibronectin in extracellular matrix remodeling. Anal. Biochem. 372, 62–71 10.1016/j.ab.2007.07.027 (doi:10.1016/j.ab.2007.07.027) [DOI] [PubMed] [Google Scholar]
- 21.Velzenberger E, El Kirat K, Legeay G, Nagel MD, Pezron I. 2009. Characterization of biomaterials polar interactions in physiological conditions using liquid–liquid contact angle measurements: relation to fibronectin adsorption. Colloids Surf. B 68, 238–244 10.1016/j.colsurfb.2008.10.022 (doi:10.1016/j.colsurfb.2008.10.022) [DOI] [PubMed] [Google Scholar]
- 22.Cousins BG, Zekonyte J, Doherty PJ, Garvey MJ, Williams RL. 2008. Manufacturing a nanometre scale surface topography with varying surface chemistry to assess the combined effect on cell behaviour. Int. J. Nano Biomater. 1, 320–338 10.1504/ijnbm.2008.016878 (doi:10.1504/ijnbm.2008.016878) [DOI] [Google Scholar]
- 23.Knerr R, Weiser B, Drotleff S, Steinem C, Göpferich A. 2006. Measuring cell adhesion on RGD-modified, self-assembled PEG monolayers using the quartz crystal microbalance technique. Macromol. Biosci. 6, 827–838 10.1002/mabi.200600106 (doi:10.1002/mabi.200600106) [DOI] [PubMed] [Google Scholar]
- 24.Dellatore SM, Garcia AS, Miller WM. 2008. Mimicking stem cell niches to increase stem cell expansion. Curr. Opin. Biotechnol. 19, 534–540 10.1016/j.copbio.2008.07.010 (doi:10.1016/j.copbio.2008.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chase LG, Firpo MT. 2007. Development of serum-free culture systems for human embryonic stem cells. Curr. Opin. Chem. Biol. 11, 367–372 10.1016/j.cbpa.2007.06.421 (doi:10.1016/j.cbpa.2007.06.421) [DOI] [PubMed] [Google Scholar]
- 26.Prowse ABJ, et al. 2010. Long term culture of human embryonic stem cells on recombinant vitronectin in ascorbate free media. Biomaterials 31, 8281–8288 10.1016/j.biomaterials.2010.07.037 (doi:10.1016/j.biomaterials.2010.07.037) [DOI] [PubMed] [Google Scholar]
- 27.Abraham S, Riggs MJ, Nelson K, Lee V, Rao RR. 2010. Characterization of human fibroblast-derived extracellular matrix components for human pluripotent stem cell propagation. Acta Biomater. 6, 4622–4633 10.1016/j.actbio.2010.07.029 (doi:10.1016/j.actbio.2010.07.029) [DOI] [PubMed] [Google Scholar]
- 28.Derda R, Li LY, Orner BP, Lewis RL, Thomson JA, Kiessling LL. 2007. Defined substrates for human embryonic stem cell growth identified from surface arrays. ACS Chem. Biol. 2, 347–355 10.1021/cb700032u (doi:10.1021/cb700032u) [DOI] [PubMed] [Google Scholar]
- 29.Kolhar P, Kotamraju VR, Hikita ST, Clegg DO, Ruoslahti E. 2010. Synthetic surfaces for human embryonic stem cell culture. J. Biotechnol. 146, 143–146 10.1016/j.jbiotec.2010.01.016 (doi:10.1016/j.jbiotec.2010.01.016) [DOI] [PubMed] [Google Scholar]
- 30.Brafman DA, Chang CW, Fernandez A, Willert K, Varghese S, Chien S. 2010. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials 31, 9135–9144 10.1016/j.biomaterials.2010.08.007 (doi:10.1016/j.biomaterials.2010.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villa-Diaz LG, Nandivada H, Ding J, Nogueira-De-Souza NC, Krebsbach PH, O'Shea KS, Lahann J, Smith GD. 2010. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotechnol. 28, 581–583 10.1038/nbt.1631 (doi:10.1038/nbt.1631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doran MR, Frith JE, Prowse ABJ, Fitzpatrick J, Wolvetang EJ, Munro TP, Gray PP, Cooper-White JJ. 2010. Defined high protein content surfaces for stem cell culture. Biomaterials 31, 5137–5142 10.1016/j.biomaterials.2010.03.015 (doi:10.1016/j.biomaterials.2010.03.015) [DOI] [PubMed] [Google Scholar]
- 33.Pierschbacher MD, Ruoslahti E. 1984. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30–33 10.1038/309030a0 (doi:10.1038/309030a0) [DOI] [PubMed] [Google Scholar]
- 34.Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. 2000. Cell adhesion and motility depend on nanoscale RGD clustering . J. Cell Sci. 113, 1677–1686 [DOI] [PubMed] [Google Scholar]
- 35.Massia SP, Hubbell JA. 1990. Covalent surface immobilization of arg-gly-asp-containing and tyr-ile-gly-ser-arg-containing peptides to obtain well-defined cell-adhesive substrates. Anal. Biochem. 187, 292–301 (doi:10.1016/0003–2697(90)90459-m) [DOI] [PubMed] [Google Scholar]
- 36.Hirano Y, Okuno M, Hayashi T, Goto K, Nakajima A. 1993. Cell-attachment activities of surface immobilized oligopeptides RGD, RGDS, RGDY, RGDT, and YIGSR toward five cell lines. J. Biomater. Sci. Polym. Ed. 4, 235–243 10.1163/156856293X00546 (doi:10.1163/156856293X00546) [DOI] [PubMed] [Google Scholar]
- 37.Li YJ, Chung EH, Rodriguez RT, Firpo MT, Healy KE. 2006. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J. Biomed. Mater. Res A 79A, 1–5 10.1002/jbm.a.30732 (doi:10.1002/jbm.a.30732) [DOI] [PubMed] [Google Scholar]