Abstract
Background
Rotator cuff tears are common injuries that are often treated with surgical repair. Because of the high concentration of growth factors within platelets, platelet-rich plasma (PRP) has the potential to enhance healing in rotator cuff repairs.
Hypothesis
Platelet-rich plasma would alter the biomechanical and histologic properties of rotator cuff repair during an acute injury response.
Study Design
Controlled laboratory study.
Methods
Platelet-rich plasma was produced from inbred donor rats. A tendon-from-bone supraspinatus tear was created surgically and an immediate transosseous repair performed. The control group underwent repair only. The PRP group underwent a repair with PRP augmentation. Rats in each group were sacrificed at 7, 14, and 21 days. The surgically repaired tendons underwent biomechanical testing, including failure load, stiffness, failure strain, and stress relaxation characteristics. Histological analysis evaluated the cellular characteristics of the repair tissue.
Results
At 7- and 21-day periods, augmentation with PRP showed statistically significant effects on the biomechanical properties of the repaired rat supraspinatus tear, but failure load was not increased at the 7-, 14-, or 21-day periods (P = .688, .209, and .477, respectively). The control group had significantly higher stiffness at 21 days (P = .006). The control group had higher failure strain at 7 days (P = .02), whereas the PRP group had higher failure strain at 21 days (P = .008). Histologically, the PRP group showed increased fibroblastic response and vascular proliferation at each time point. At 21 days, the collagen fibers in the PRP group were oriented in a more linear fashion toward the tendon footprint.
Conclusion
In this controlled, rat model study, PRP altered the tissue properties of the supraspinatus tendon without affecting the construct’s failure load.
Clinical Relevance
The decreased tendon tissue stiffness acutely and failure to enhance tendon-to-bone healing of repairs should be considered before augmenting rotator cuff repairs with PRP. Further studies will be necessary to determine the role of PRP in clinical practice.
Keywords: platelet-rich plasma, rotator cuff tear, biologic augmentation, tendon healing
Symptomatic rotator cuff tears are often surgically repaired when symptoms have failed to resolve with non-operative treatment. Surgical repair has long-term favorable outcomes with 94% survivorship at 5 years and 83% survivorship at 10 years.27 However, 85% of surgical repair failures occur during the first 6 months of recovery, with 74% occurring in the first 3 months.20 Two years after revision arthroscopic rotator cuff repair, 70% of supraspinatus repairs and only 27% of supraspinatus with infraspinatus repairs were intact.22 Meta-analysis of rotator cuff outcome studies suggests significant differences in outcomes of healed versus nonhealed rotator cuff repairs38 such as decreased strength of external rotation and forward flexion with increasing size of the rotator cuff defect.12 The poor outcomes of failed rotator cuff repairs indicate that improvement in immediate rotator cuff healing has implications for long-term outcomes.20
Local administration of growth factors can potentially aid in tissue healing. Platelets contain alpha granules that hold growth factors, including platelet-derived growth factor (PDGF), transforming growth factor (TGF), platelet-derived epidermal growth factor (PDEGF), and insulin growth factor (IGF).1,40 TGF-β1 controls cellular growth, proliferation, differentiation, and apoptosis19 while playing an important role in controlling the immune system and inflammatory response. Increased concentrations of platelet-derived growth factors upregulate osteocalcin, which assists in bone formation and type I collagen formation.40
To take advantage of growth factors’ potential to aid healing, a concentrated preparation of autogenous platelets was developed as platelet-rich plasma (PRP). Platelet-rich plasma provides 3 to 5 times the concentration of platelets when compared with the normal concentration of platelets in blood.25 The platelet concentration in PRP is typically more than 1 million platelets per microliter.24,28 With this high concentration of platelets and concurrently increased growth factors, PRP has the potential to improve healing of arthroscopically repaired rotator cuff tears.
Clinical studies have tested the ability of PRP to enhance tendon-to-bone repairs. Randelli et al34 reported initial improvement in visual analog scale for pain (VAS), University of California at Los Angeles (UCLA), and Constant scores with no adverse outcomes when PRP was used clinically to augment arthroscopic rotator cuff repair. A study at 2 years showed decreased pain and improved outcome scores during the first 3 postoperative months.35 However, a recently published randomized controlled clinical trial using PRP as augmentation of arthroscopic rotator cuff repair failed to show a difference in healing or postoperative course.7
The lack of consensus in clinical literature, combined with possible important sequelae of PRP augmentation, warrants further analysis on the effect of PRP on rotator cuff repairs. In this investigation, we used a previously developed rat rotator cuff injury model to create supraspinatus tendon insertion injuries in rats; there is a close structural relationship between the rat and human shoulder.3,6,14,39 The purpose of the current investigation was to determine if the addition of PRP to repaired supraspinatus tears in rats would improve healing as evaluated by both biomechanical and histologic testing. The hypothesis was that PRP would alter the biomechanical and histologic properties of rotator cuff repair during an acute injury response.
METHODS
Platelet-Rich Plasma Production
Although abundant research on PRP has been completed, there is no standardized preparation or activation of PRP. In this study, Kajikawa et al’s technique18 of making PRP was used because it is nonproprietary (thus reducing bias), has a technique similar to clinically available techniques, and is easily tested and reproduced. Three donor rats for each time period were euthanized and a complete body blood collection was performed via decapitation. An average 7- to 8-mL blood sample was retrieved per rat, and 3.8% sodium citrate (Sigma, St Louis, Missouri) was immediately added to the blood collection as an anticoagulant. The blood was processed and stored at 4°C. The whole blood was centrifuged at 1500 rpm for 10 minutes. The platelet-rich layer including the supernatant was separated from the red blood cell layer. This platelet-rich layer was centrifuged at 3000 rpm for 10 minutes to create a cell pellet within the supernatant. Most of the supernatant was removed, leaving the cell pellet. The pellet was then resuspended in the residual supernatant. Using an automated blood cell counting device (Hemavet 950; Drew Scientific, Waterbury, Connecticut), the platelet count was confirmed. Additional supernatant was added as needed to achieve the desired platelet concentration of 3 to 5 times the whole-blood platelet concentration: 7-day specimens received 4.3×, 14-day specimens received 5.8×, and 21-day specimens received 3.0× for an average of 4.4× the normal whole-blood platelet concentration. The PRP was divided into 20-μL aliquots and stored overnight at 4°C. At the time of use, the 20-μL PRP was mixed with 3.33 μL 10% CaCl (Sigma) and 3.33 μL 300 IU thrombin (Sigma) for activation, and the PRP was immediately pipetted onto the rotator cuff repair site.
Rat Surgical Model and Procedure
Before initiation of this project, the study was approved by the Institutional Animal Care and Use Committee. An a priori power analysis using load to failure data from the most similar rat rotator cuff experiment available was performed. This resulted in a sample size of 12 for a power of 80% with a P value of .05 determining significance.26
Unilateral right shoulder surgery was performed on 105 inbred Fischer 344 adult male rats (Harlan Laboratories, Indianapolis, Indiana), weighing 200 to 250 g. Inbred rats were used for isogeneic blood utilization without immunogenic response or rejection. Rats were randomly assigned to 1 of 6 groups: repair only (control group) and repair augmented with PRP (PRP group) at 7-, 14-, and 21-day periods. These periods represent the acute healing response before plateau based on previously published rat tendon healing rates.26 Each group consisted of 15 shoulders. Six rats served as sham-surgery controls. Nine rats served as blood donors for the creation of PRP. Three developed postoperative infections and were excluded, leaving 102 rats for inclusion in this study.
The surgical procedure (Figure 1) was based on previously published rat rotator cuff repair models.3,14 The rats were anesthetized with an intraperitoneal injection of xylazine (10 kg/mg) and ketamine (90 mg/kg). A preoperative dose of buprenex (0.02 mg/kg) and gentamycin (8 mg/kg) was administered. Anesthesia was maintained with isofluorane (9% O2 with isofluorane) via nasal cone inhalation. A shaved, sterile right upper quarter surgical field was then created. A craniolateral incision was made over the right shoulder. The vein over the lateral acromial border was cauterized. The acromioclavicular joint was sharply dissected and the deltoid was split from its origin on the acromion. The supraspinatus tendon was identified (Figure 1A); secured with a 4-0 braided, absorbable suture using a modified Mason-Allen technique (Figure 1B); and then sharply transected from its humeral footprint (Figure 1C). The footprint was sharply debrided of soft tissues. A 25-gauge needle made a hole through the humerus 2 mm lateral to the articular surface and 2 mm distal to the supraspinatus footprint (Figure 1D). The suture was passed through the humeral hole and tied to the humerus (Figure 1E). This reduced the tendon back onto its native footprint. In the PRP group, the PRP was pipetted using a sterile technique onto the site of the tendon-bone approximation. A layered closure of the soft tissues and skin was performed, and 2.5 mL of saline resuscitation was given via subcutaneous injection. Postoperative pain was managed with buprenex (0.02 mg/kg, subcutaneously [SC]) for 48 hours. The sham surgery group underwent an identical exposure of the right supraspinatus followed by layer closure.
Figure 1.
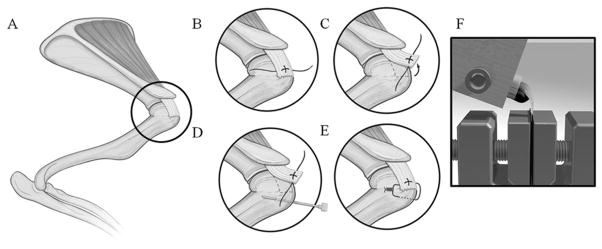
(A) Supraspinatus identified under acromion. (B) Modified Mason-Allen suture placed within intact supraspinatus. (C) Supraspinatus tendon sharply dissected from footprint. (D) A 25-gauge needle through the proximal humerus near insertion of supraspinatus. (E) Suture passed through bone tunnel, reducing and maintaining tendon to its original insertion. (F) The 3-mL syringe was placed into a custom metal block, allowing the supraspinatus tendon to hang vertically at a 90° angle to the long axis of the humerus.
After 7, 14, or 21 days, the rats were humanely euthanized. Their right scapula and humerus were dissected en bloc. These specimens were frozen at −20°C in saline-soaked gauze. Two specimens in each group had the supraspinatus tendon and humeral head harvested, then preserved in formalin for histologic analysis.
Biomechanical Testing
After thawing, the humerus and supraspinatus tendon specimen was dissected free of surrounding tissue. The humerus was potted into a polymethylmethacrylate base in a 3-mL syringe. The supraspinatus tendon was protected in saline solution–soaked gauze at room temperature, thus preventing thermal necrosis and dehydration during the exothermic cement-curing process. The suture used to repair the tendon to its footprint was divided and loosened in all specimens, taking care not to disturb surrounding healing tissue. This allowed testing to evaluate only the tendon-to-bone healing. The 3-mL syringe was placed into a custom metal block, allowing the supraspinatus tendon to hang vertically at a 90° angle to the long axis of the humerus (Figure 1F). This positioning facilitated biomechanical testing in the anatomic orientation of the tendon fibers. Superglue and sandpaper were used to secure the tendon in a clamp. An Instron materials testing machine was used to complete the biomechanical testing (Model 8841; Instron, Norwood, Massachusetts). Each specimen testing was video recorded using a high-definition recording device (Logitech Orbitz, Fremont, California).
The biomechanical testing protocol was previously established by Galatz et al.14 The tendons were subjected to a preload of 0.2 N, 5 preconditioning cycles to 0.38 mm displacement, 300-second stress-relaxation test at 0.38 mm displacement, and 300 seconds of recovery and were subsequently pulled to failure at a rate of 0.1 mm/s. The 300-second time point of the stress-relaxation test determined equilibrium load. Failure load was recorded for each specimen. Machine cross-head was used to control the relaxation test. The maximum slope of the load versus extension curve determined the stiffness. To determine failure strain at the repair site, displacement at the tendon-to-bone repair interface was measured with a digital caliper, after preconditioning and at the time of failure, using high-definition video recording. Each measurement was repeated 3 times by the primary author.
For comparison, biomechanical testing of intact supraspinatus tendons was completed (n = 4) per the above protocol. Intact tendon failure occurred intrasubstance of the tendon instead of at the repair site where the control and experimental groups failed. The intact group failed at a mean ± SD of 26.2 ± 4.73 N (±4.63 N) of force with an average stiffness of 26.3 ± 6.38 N/mm (±6.24 N/mm). Because the intact tendons failed intrasubstance, strain of intact tendon failure was not completed because of lack of comparability to a tendon-to-bone failure.
Histological Analysis
After resection, the supraspinatus-humerus units were placed in 10% buffered formalin and allowed to fix for at least 24 hours before routine processing. The specimens were carefully embedded in paraffin so that the humeral head and supraspinatus tendon would be cut in a sagittal plane, thereby optimizing the orientation of the supraspinatus fibers and humeral head attachment for histologic examination. Each paraffin block was placed in Surgipath Decalcifier II (Surgipath Medical Industries, Inc, Richmond, Illinois) immediately before sectioning surface decalcification. Sections were cut at a thickness of 4 μm and then stained with hematoxylin and eosin, toluidine blue, and trichrome. An independent pathologist experienced in musculoskeletal pathology reviewed the slides and evaluated each for cellular response, inflammatory cells, matrix quality, vascularity, healing, and collagen orientation.
Representative slides from the control and experimental groups at each time point were randomized and blinded for review by an independent pathologist. After blinded review, the primary author and pathologist reexamined the slides in an unblinded fashion for comparison. Because of a lack of accepted histologic grading scheme for tendon-to-bone healing, the pathologist qualitatively evaluated the slides.
Statistical Analysis
Before the data collection, a statistical analysis for load to failure determined that a sample size of 12 would achieve a power of 80% with significance set to P <.05.26 Statistical analysis was performed on the −PRP repair and +PRP repair groups only. They were not compared with intact tendons. Failure load, failure strain, and stiffness were the primary outcomes in each group at each time point (7, 14, and 21 days). Stress-relaxation tests yielded relaxation forces at 1, 5, 10, 20, 30, 50, 100, and 300 seconds, which were compared at each time point. For each of these outcome parameters, a t test for equality of means was used to compare the control versus experimental group at each time point. Data sets were evaluated by an independent statistician for statistical outliers defined as greater than 2 standard deviations from the mean, and none were found that would affect the results of the statistics.
RESULTS
General Observations
The rats in the present study showed no adverse effects related to surgery or PRP administration. Postoperatively, all but 3 rats exhibited normal gait patterns and had full use of the involved forelimb within 3 to 4 days. After the initial recovery period, all but 3 animals displayed preoperative levels of activity and food intake. The 3 rats demonstrating postoperative recovery differences consisted of 1 rat in the PRP 14-day group and 2 rats in the PRP 21-day group that developed infections at the operative site. This was confirmed by Gram stain in the 14-day group. There were no failures of tendon surgical repair at any time point.
Biomechanical Effects of PRP on Supraspinatus Tendon Repair
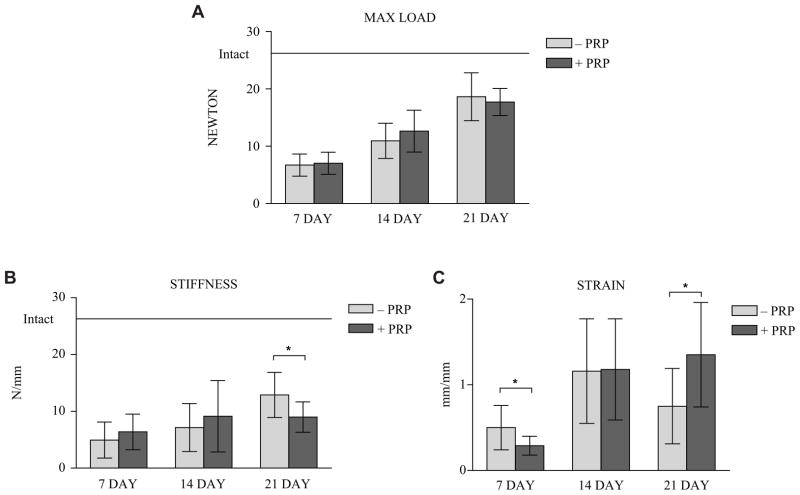
The effects of PRP treatment on biomechanical aspects of tendon healing were examined at 7, 14, and 21 days after repair surgery. Three parameters—failure load, stiffness, and strain—were examined. They are reported as mean ± 1 SD of the control group versus PRP group, P value, and 95% CI. At the 7-day point (Figure 2), both the failure load (6.71 ± 1.94 N vs 7.03 ± 1.93 N; P = .688; CI, −1.96 to 1.31) and stiffness (4.94 ± 3.17 N/mm vs 6.38 ± 3.14 N/mm; P = .274; CI, −4.11 to 1.22) did not differ significantly between the control and PRP groups, but the control group showed increased failure strain (0.50 ± 0.26 mm/mm vs 0.29 ± 0.12 mm/mm; P = .02; CI, 0.038 to 0.39). The stress-relaxation characteristics showed the PRP group had a higher percentage of relaxation at 1 second only (0.86 ± 0.04 vs 0.89 ± 0.03; P = .046). The percentage of relaxation force was not significantly different at 5, 10, 30, 50, 100, or 300 seconds (Figure 3). The equilibrium load at 300 seconds showed no statistical difference between groups.
Figure 2.
Maximum load (A), stiffness (B), and strain (C) data at 7-, 12-, and 21-day points for comparison. Error bars are 1 standard deviation. *Significant value (P <.05). Solid horizontal bar indicates intact tendon values. Because of intrasubstance failure of the intact tendons, strain data were not considered comparable and therefore not completed. PRP, platelet-rich plasma.
Figure 3.
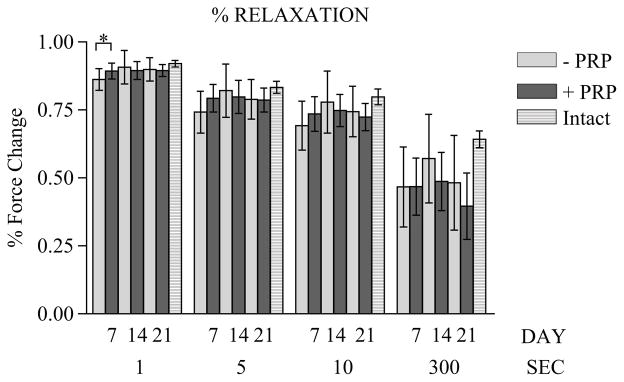
Relaxation force as percentage change in force at 7-, 14-, and 21-day points. *Significant value (P <.05). Striped bars indicate intact tendon values for comparison. PRP, platelet-rich plasma.
At the 14-day point (Figure 2), the failure load (10.92 ± 3.07 N vs 12.63 ± 3.65 N; P = .21; CI, −4.44 to 1.03), stiffness (7.14 ± 4.22 N/mm vs 9.13 ± 6.29 N/mm; P = .35; CI, −6.36 to 2.38), and failure strain (1.16 ± 0.61 vs 1.18 ± 0.59 mm/mm; P = .949; CI, −0.51 to 0.482) were not statistically different between the control group and the PRP group. The percentage of relaxation force was not significant between the 2 groups at 1, 5, 10, 30, 50, 100, or 300 seconds (Figure 3).
At the 21-day point (Figure 2), the failure load (18.64 ± 4.18 N vs 17.71 ± 2.37 N; P = .48; CI, −1.83 to 3.7) was not significantly different, but the control group showed increased stiffness (12.89 ± 3.98 N/mm vs 8.99 ± 2.67 N/mm; P = .006; CI, 1.22 to 6.56) and the PRP group showed increased failure strain (0.75 ± 0.43 mm/mm vs 1.35 ± 0.61 mm/mm; P = .008; CI, −1.03 to −0.17). The percentage of relaxation force was not significantly different at 1, 5, 10, 30, 50, 100, or 300 seconds (Figure 3).
Histological Examination of the Effects of PRP on Tendon Healing
Sections of the injured and repaired tendon site for each group showed a reactive fibroblastic response involving the entire length of the tendon with varying degrees of inflammation. This inflammation was not seen in the sham, intact tendon group. At day 7, the increased cellular response was most prominent (Figure 4), and the PRP group also showed increased focal fibrinoid necrosis within the tendon tissue.
Figure 4.
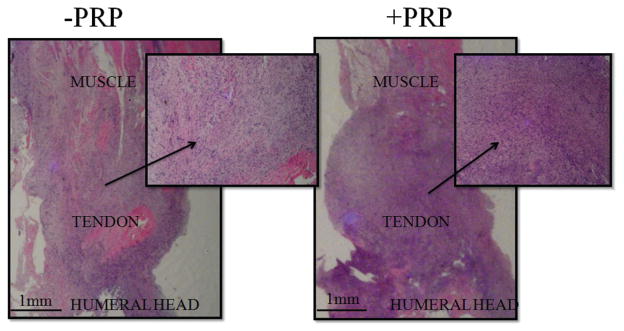
Day 7: increased acute cellular inflammation in the platelet-rich plasma (PRP) group (right) when compared with the control group (left). (Hematoxylin and eosin, 8×; insets, 32×.)
At days 7 and 14, the PRP group showed striking changes in the articular cartilage adjacent to the repair site, characterized by hypertrophic chondrocytes with a marked basophilic matrix best seen with toluidine blue stain (Figure 5). These findings were not seen in the control group or in the PRP group at day 21. The marked basophilic matrix in the cartilage most likely represents increased glycosaminoglycans produced by the hypertrophied chondrocytes.
Figure 5.
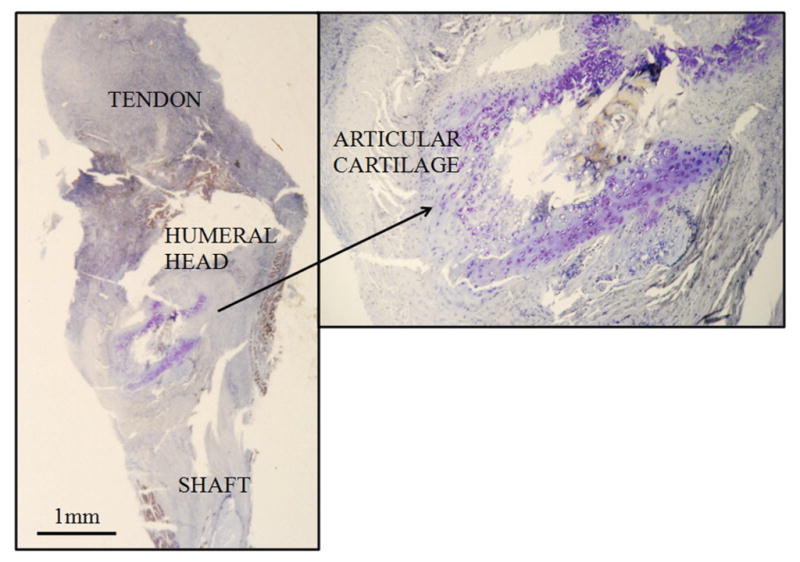
Articular cartilage with increased matrix basophilia in the platelet-rich plasma (PRP) group indicating increased glycosaminoglycans. Seven-day point shown. (Toluidine blue, 8×; inset, 40×.)
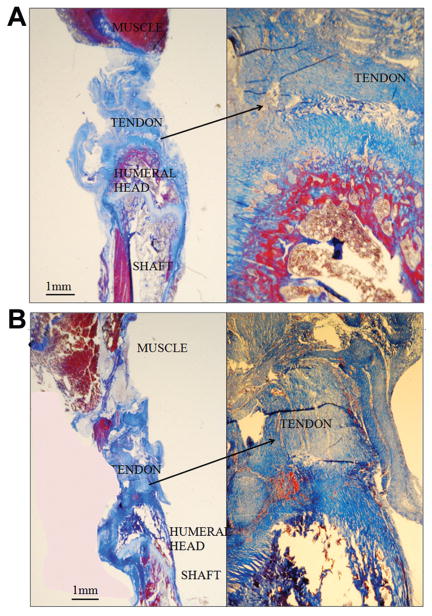
At day 21, the collagen fibers of the PRP group were directed toward the native tendon footprint in a more linear and organized fashion than the control group (Figure 6). Although the control group had some organization and orientation, the collagen fibers were smaller in caliber, and qualitatively the organization of the fibers was less advanced when compared with the organization seen in the experimental group.
Figure 6.
More advanced organization and alignment of the collagen fibers of the healing tendon toward the footprint in the platelet-rich plasma (PRP) group at 21 days (B) when compared with the control group (A). (Trichrome, 8×; insets, 32×.)
DISCUSSION
In this study, we sought to determine the effects of PRP treatment on the healing of a repaired supraspinatus rotator cuff tendon construct using a rodent model. Our data demonstrated that the failure load of rotator cuff repairs was not altered by PRP augmentation.
The combined histologic and biomechanical data suggest that the 7-day PRP group demonstrated a decrease in tissue strain due to the cellular infiltration, increased areas of fibrinoid necrosis, and lack of collagen orientation. The decrease in tissue strain at 7 days may transmit force more directly to the suture portion of the repair, thus stressing the repair. These changes were normalized by the 14-day time point where no significant differences were found between the control and PRP groups.
Of undetermined clinical importance, the tendon strain in response to force was improved in the PRP group in comparison with the control group with increased healing time. By 21 days, the PRP group had increased failure strain, which may reduce the forces transmitted to the suture fixation site. In a human model, this may have clinical implications and should be further investigated.
Basic science experiments using animal models of injury have suggested that PRP may provide a benefit to the healing of soft tissue injuries.2,4,13,23,29–31 Most of these studies describing a positive effect of PRP have been performed in tendon-to-tendon healing models in contrast to our tendon-to-bone healing model.2,4,23,30 In these previous animal studies, biomechanical analysis demonstrated an increase in stiffness and tendon callus strength by 30% in the first 3 weeks of intratendinous Achilles tendon repair in rats,2 an increase in failure load and stiffness in New Zealand White rabbit patellar tendon defects at 14 days returning to normal strength at 28 days,23 and increased linear stiffness in PRP-augmented anterior cruciate ligament (ACL) repairs.13,30 The first 2 studies concluded that PRP had an effect on early tendon healing. In contrast to these studies, we found no significant effect on tissue stiffness or failure load at our early time points, 7 and 14 days. At the 21-day point, the stiffness was increased in the control group instead of the PRP group. The differences in the biology of tendon-to-tendon healing versus tendon-to-bone healing may account for these differences. The data from these previous studies combined with this current study’s data indicate that PRP may be more appropriately used in tendon-to-tendon healing and not tendon-to-bone healing.
Unlike the biomechanical differences noted above, our histologic examination of tissue-specific consequences of PRP administration demonstrates similarities to studies performed on intrasubstance patellar tendon and flexor tendon repairs. At 24 weeks, Bosch et al4 found increased collagen and glycosaminoglycans in PRP-treated equine superficial digital flexor tendons with improved collagen organization. Also, in patellar tendon repairs augmented with PRP,23 specimens from the 3-week time point had more mature and dense collagen. Both of these studies complement the findings that we described by concluding that PRP improves the orientation and density of collagen and increases the glycosaminoglycan content of tissues.
Although clinical trials on PRP including lateral epicon-dylitis,16,33 acute and chronic Achilles tendinopathy,9–11,15,37 patellar tendinopathy,21 and ACL reconstructions8,32,41 have shown potential uses for PRP, randomized controlled clinical studies of PRP on arthroscopically repaired tendon-to-bone rotator cuff tears have not shown improvement in healing or patients’ symptoms.7 Rodeo et al36 and Buford5 showed no significant change in healing of the rotator cuff repair with PRP augmentation in comparison with controls. Within our 3-week study, the rotator cuff repairs achieved 70% of the intact tendon strength without showing any improvements or detriments to failure load with the application of PRP to the repair. Our data confirm the rapid healing of rat tendon injuries.14 We hypothesize that this amount of healing corresponds to the first 6 to 12 weeks of human rotator cuff healing, although a direct human biomechanical comparison cannot be performed to confirm this hypothesis. With this significant recovery of preinjury strength, the data obtained, and prior research showing that the effects of PRP tend to occur early in the healing process, it is unlikely that later time points would have shown any significant difference in failure load constructs treated with PRP.
Additional clinical and basic science is needed before any clinical guidelines or recommendations can be made on the use of PRP. Initially, a standardized method of PRP preparation and administration needs to be developed. This includes testing current and proposed methods of PRP preparation and administration to determine which, if any, has the best effect. Next, a detailed mechanism of action should be described as to how and when PRP creates its effect. With this information, conclusions could be compared across studies to determine the appropriate application and utilization of PRP. Different injury causes (ie, acute vs chronic, degenerative vs traumatic) and types (soft tissue vs bone vs both) need to be thoroughly investigated to determine the clinical applicability and efficacy of PRP.
The strengths of the current study are its reproducibility and minimization of confounding variables, enabling an accurate assessment of the effect of the PRP on the repair tissue. The relative simplicity of the surgical procedure and preparation of the PRP make it highly reproducible. We attempted to standardize our model by using one PRP preparation for each time point. Single-batch preparation prevented inconsistencies in preparation and any errors that may have occurred due to repeated production of PRP samples. There was great variation in the concentration of PRP used in previous studies. To maximize clinical relevance of our study, we used PRP concentrations comparable with levels used in humans. Using the rat model allowed us to perform biomechanical and histologic testing of the repair, which is not possible with human subjects.
There are limitations to the current study. It is an animal study, with all the inherent limitations of animal studies. We used an inbred strain of rat to create allogenic PRP in attempts to standardize the PRP. This allogenic PRP may have exacerbated the inflammatory response in the host rat. In the rat model, we cannot prevent the rats from ambulating on and using their forelimbs for feeding and grooming. This may place unusually high or unique stresses on the supraspinatus repair that is not seen in the usual human postoperative protocol. The model used is an immediate repair model of an acutely created tear, and most rotator cuff tears are degenerative in nature.17 However, a reproducible degenerative tear rat model has not been standardized to re-create this lesion. Histologic tendon-to-bone healing is also lacking a standardized method or grading system, forcing our histologic results to be qualitative in nature. In addition, we could not confirm that the PRP stayed in the desired location after surgical closure; however, our data show there is a clear effect produced in the PRP group compared with the control group, indicating that the PRP was present in sufficient quantity at the repair site to produce an effect.
In conclusion, our data demonstrate that the administration of PRP during a tendon-to-bone rotator cuff repair in rats did not affect the failure load of the repair. In addition, the risk of decreased tissue strain acutely, increased acute inflammation, and lack of improvement in tendon-to-bone failure load demonstrated in this current study should be considered before augmenting rotator cuff repairs with PRP. Further research elucidating the mechanism of action of PRP will help guide appropriate clinical use of this treatment modality.
Acknowledgments
The authors thank Dr Wasyl Fedoriw for assistance with protocol design and training; Rachel Nauer and Dr Kristen Lauing for general laboratory assistance; Dr Ryan Sullivan, Dr Kirstyn Brownson, and James Verner, BS, for assistance with protocol completion; Karen Rychlik for statistical support; Dr Avinash Patwardhan for biomechanical support; and Lu Leach for assistance with histology preparation and analysis.
Footnotes
For reprints and permission queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav
One or more of the authors has declared the following potential conflict of interest or source of funding: This project was aided by a grant from the Orthopedic Research and Education Foundation. The Walgreen Foundation also funded this study through an unrestricted institutional grant. This foundation funds research through the Department of Orthopedic Surgery but did not have any direct role in this investigation.
References
- 1.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery. J Bone Joint Surg Br. 2009;91(8):987–996. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 2.Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93–99. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 3.Bedi A, Kovacevic D, Hettrich D, et al. The effect of matrix metalloproteinase inhibition on tendon-to-bone healing in a rotator cuff repair model. J Shoulder Elbow Surg. 2010;19:384–391. doi: 10.1016/j.jse.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Bosch G, van Schie HT, de Groot MW, et al. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: a placebo-controlled experimental study. J Orthop Res. 2010;28(2):211–217. doi: 10.1002/jor.20980. [DOI] [PubMed] [Google Scholar]
- 5.Buford DA. Rotator cuff healing with and without platelet rich plasma application. Arthroscopy. 2011;27(5S):e37–e38. [Google Scholar]
- 6.Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg. 1998;7:599–605. doi: 10.1016/s1058-2746(98)90007-6. [DOI] [PubMed] [Google Scholar]
- 7.Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. Am J Sports Med. 2011;39(2):258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 8.Cervellin M, de Girolamo L, Bait C, Denti M, Volpi P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: a randomized, controlled clinical study. Knee Surg Sports Traumatol Arthrosc. 2012;20(1):114–120. doi: 10.1007/s00167-011-1570-5. [DOI] [PubMed] [Google Scholar]
- 9.De Jonge S, de Vos RJ, van Schie HT, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy; a double-blind randomized placebo-controlled trial. Am J Sports Med. 2011;39(8):1623–1629. doi: 10.1177/0363546511404877. [DOI] [PubMed] [Google Scholar]
- 10.De Vos RJ, Weir A, Tol JL, Verhaar JA, Weinans H, van Schie HT. No effects of PRP on ultrasonographic tendon structure and neovascularization in chronic midportion Achilles tendinopathy. Br J Sports Med. 2011;45(5):387–392. doi: 10.1136/bjsm.2010.076398. [DOI] [PubMed] [Google Scholar]
- 11.De Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 12.Dodson CC, Kitay A, Verma NN, et al. The long-term outcome of recurrent defects after rotator cuff repairs. Am J Sports Med. 2010;38(1):35–39. doi: 10.1177/0363546509341654. [DOI] [PubMed] [Google Scholar]
- 13.Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galatz LM, Sandell LJ, Rothermich SY, et al. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24(3):541–550. doi: 10.1002/jor.20067. [DOI] [PubMed] [Google Scholar]
- 15.Gaweda K, Tarczynska M, Krzyzanowski W. Treatment of Achilles tendinopathy with platelet-rich plasma. Int J Sports Med. 2010;31(8):577–583. doi: 10.1055/s-0030-1255028. [DOI] [PubMed] [Google Scholar]
- 16.Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200–1208. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto T, Nobuhara K, Hamada T. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res. 2003;415:111–120. doi: 10.1097/01.blo.0000092974.12414.22. [DOI] [PubMed] [Google Scholar]
- 18.Kajikawa Y, Motihara T, Sakamoto H, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J Cell Physiol. 2008;215:837–845. doi: 10.1002/jcp.21368. [DOI] [PubMed] [Google Scholar]
- 19.Kashiwagi K, Mochizuki Y, Yasunaga Y, et al. Effects of transforming growth factor–beta 1 on the early stages of healing of the Achilles tendon in a rat model. Scand J Plast Reconstr Hand Surg. 2004;38:193–197. doi: 10.1080/02844310410029110. [DOI] [PubMed] [Google Scholar]
- 20.Kluger R, Bock P, Mittlbock M, Krampla W, Engel A. Long-term survivorship of rotator cuff repairs using ultrasound and magnetic resonance imaging analysis. Am J Sports Med. 2011;39(10):2071–2081. doi: 10.1177/0363546511406395. [DOI] [PubMed] [Google Scholar]
- 21.Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: a new clinical application: a pilot study for treatment of jumpers knee. Injury. 2009;40(6):598–603. doi: 10.1016/j.injury.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Kowalsky MS, Keener JD. Revision arthroscopic rotator cuff repair: repair integrity and clinical outcome. J Bone Joint Surg Am. 2011;93(suppl 1):62–74. doi: 10.2106/JBJS.J.01173. [DOI] [PubMed] [Google Scholar]
- 23.Lyras DN, Kazakos K, Verettas D, et al. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch Orthop Trauma Surg. 2009;129:1577–1582. doi: 10.1007/s00402-009-0935-4. [DOI] [PubMed] [Google Scholar]
- 24.Marx R. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10:225–228. doi: 10.1097/00008505-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Mikolyzk DK, Wei AS, Tonino P, et al. Effect of corticosteroids on the biomechanical strength of rat rotator cuff tendon. J Bone Joint Surg Am. 2009;91:1172–1180. doi: 10.2106/JBJS.H.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Millet PJ, Horan MP, Maland KE, Hawkins RJ. Long-term survivor-ship and outcomes after surgical repair of full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2011;20:591–597. doi: 10.1016/j.jse.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Mishra A, Woodall J, Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28:113–125. doi: 10.1016/j.csm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Morizaki Y, Zhao C, An KN, Amadio PC. The effects of platelet-rich plasma on bone marrow stromal cell transplants for tendon healing in vitro. J Hand Surg Am. 2010;35:1833–1841. doi: 10.1016/j.jhsa.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray MM, Palmer M, Abreu E, Spindler KP, Zurakowski D, Fleming BC. Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res. 2009;27(5):639–645. doi: 10.1002/jor.20796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 32.Nin JR, Gasgue GM, Azcarate AV, Beola JD, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009;25(11):1206–1213. doi: 10.1016/j.arthro.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1 year follow-up. Am J Sports Med. 2010;38(2):255–262. doi: 10.1177/0363546509355445. [DOI] [PubMed] [Google Scholar]
- 34.Randelli PS, Arrigoni P, Cabitza P, Volpi P, Maffulli N. Autogenous platelet rich plasma for arthroscopic rotator cuff repair: a pilot study. Disabil Rehabil. 2008;30(20–22):1584–1589. doi: 10.1080/09638280801906081. [DOI] [PubMed] [Google Scholar]
- 35.Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20:518–528. doi: 10.1016/j.jse.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 36.Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. Am J Sports Med. 2012;40:1234–1241. doi: 10.1177/0363546512442924. [DOI] [PubMed] [Google Scholar]
- 37.Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human Achilles tendon ruptures: a randomized single blinded study. Am J Sports Med. 2011;39(1):38–47. doi: 10.1177/0363546510383515. [DOI] [PubMed] [Google Scholar]
- 38.Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographicaly healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393–403. doi: 10.1016/j.arthro.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 40.Van Den Dolder J, Mooren R, Vloon AP, Stoelinga PJ, Jansen JA. Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng. 2006;12(11):3067–3073. doi: 10.1089/ten.2006.12.3067. [DOI] [PubMed] [Google Scholar]
- 41.Vogrin M, Rupreht M, Dinevski D, et al. Effect of a platelet gel on early graft revascularization after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind clinical trial. Eur Surg Res. 2010;45(2):77–85. doi: 10.1159/000318597. [DOI] [PubMed] [Google Scholar]