Abstract
Objectives
Clinical studies have shown alcohol to be a risk factor for traumatic orthopaedic injuries and for nonunion. Data from animal studies suggest that alcohol exposure inhibits fracture healing. This report presents a novel rodent model of impaired fracture healing caused by repeated alcohol exposure. Using this model, we examined the regenerative effects of an intravenously administered population of isolated and expanded MSCs on fracture healing.
Methods
Bone marrow-derived MSC were isolated from transgenic Green Fluorescent Protein (GFP) C57BL/6 mice and culture expanded using a lineage depletion protocol. Adult wild-type C57BL/6 mice were subjected to a two-week binge alcohol exposure paradigm, (3 days during which they received daily intraperitoneal injections of a 20% alcohol/saline solution followed by a four-day rest period and another 3 consecutive day binge cycle. At completion of the second binge cycle, mice were subjected to a midshaft tibia fracture while intoxicated. Twenty-four hours following fracture, animals were administered an intravenous (IV) transplant of GFP-labeled MSC. Two weeks following fracture, animals were euthanized and injured tibiae were collected and subjected to biomechanical, histologic, and micro-computed tomography analysis.
Results
Pre-injury binge alcohol exposure resulted in a significant impairment in biomechanical strength and decrease in callus volume. MSC transplants restored both fracture callus volume (p<0.05) and biomechanical strength (p<0.05) in animals with alcohol-impaired healing. In vivo imaging demonstrated a time-dependent MSC migration to the fracture site.
Conclusions
These data suggest that a two-week binge alcohol exposure significantly impairs fracture healing in a murine tibia fracture model. IV-administered MSC were capable of specifically homing to the fracture site and of normalizing biomechanical, histologic, and microCT parameters of healing in animals exposed to alcohol. Understanding MSC recruitment patterns and functional contributions to fracture repair may lead to their use in patients with impaired fracture healing and nonunion.
INTRODUCTION
Impaired fracture healing is a challenging problem in orthopaedic surgery, with estimates of nonunion incidence ranging between 5 and 20 percent depending on fracture site[1-3]. Nonhealing fractures are a source of substantial morbidity and are costly to the health care system and to society. Limitations exist with respect to current nonunion strategies, of which autologous bone grafting is considered the gold standard. There is harvest site morbidity associated with iliac crest graft[4, 5], as well as limited supply and varying osteoinductive potential of the graft.
Most models of nonunion in preclinical studies are based on the generation of a criticalsized bony defect[6] where the excision of a critical mass of bone produces a gap that is biologically too large to bridge. These models are not based on a systemic or local pathologic process or on a lack of biologic healing capacity. Impaired fracture healing related to alcohol exposure is a pathophysiologically relevant alternative for studying fracture repair. Preclinical studies have demonstrated that alcohol exposure impedes fracture callus composition and biomechanical strength[7] and modulates the immunoinflammatory response to blunt orthopaedic trauma[8].
In patients, acute alcohol intoxication is associated with increased risk of traumatic skeletal injuries[9-11], with binge drinking being the most common drinking pattern associated with trauma[12]. An estimated 25-40% of orthopaedic trauma patients are intoxicated at the time of admission[9, 10], and admission blood alcohol content has been shown to be correlated with a history of hazardous drinking behavior[13]. Alcohol exposure is linked to increased complications after fracture including nonunion[14-16].
While locally transplanted mesenchymal stem cell (MSC)-seeded scaffolds have shown promise in treating critical-sized defects[6], systemic MSC administration may represent a novel nonunion therapy with no surgical morbidity and with the potential for longitudinal administration over the course of the fracture healing process. Clinical studies have demonstrated systemic MSC administration is safe and well tolerated[17] and is effective in the treatment of osteogenesis imperfecta[18]. While published clinical studies on the role of systemic MSC therapy in treating nonunion are limited[19], recent evidence suggests MSC are mobilized from peripheral sites in patients with acute fractures[20] and are detectable in the circulation, implying a physiologic role of distant MSC mobilization and recruitment to the site of fracture. Animal studies have shown that infused MSC home and engraftment of into the fracture site[21] with greater efficiency than locally transplanted cells[22].
To our knowledge, there are no animal studies evaluating the regenerative capacity of MSC on fracture repair in an alcohol-induced model of impaired healing. The aims of this study were to generate clinically relevant model of impaired fracture healing, and to evaluate the functional contributions of these cells to fracture repair after intravenous administration. We hypothesized that a purified population of MSC could be isolated which were capable of specifically homing to the site of injury and of contributing to repair parameters in the setting of impaired fracture healing due to systemic alcohol exposure.
METHODS
Binge Alcohol Exposure Regimen and Mouse Tibia Fracture Injury
Male C57BL/6 mice were purchased (Jackson Laboratory®; Bar Harbor, Maine) at 6-7 weeks of age and acclimated to the animal facility. At 9 weeks of age, mice were subjected to a two-week binge alcohol exposure paradigm, during which they received daily intraperitoneal injections of a 20% alcohol/saline solution for 3 consecutive days, followed by a four-day rest period, and another 3 consecutive day binge cycle. Sterile isotonic saline in was injected to a separate group of mice (vehicle control). Saline was injected as a volume/volume control to the experimental group that received 20% alcohol. Injections for the control group were given at the same time, in the same volume dosage, and for the same frequency as the experimental group (3 days of injections, followed by a 4 day rest period, and 3 days of injections). Alcohol was given at a dose of 2 g/kg, (20% vol./vol. ethanol/saline solution) resulting in a blood alcohol content (BAC) of approximately 200 mg/dl at the time of injury. We developed the current model of 2 week binge-like alcohol administration to mimic a similar binge drinking pattern commonly associated with orthopaedic trauma patients [12] – the weekend drinker – and previous work in our lab demonstrated that this alcohol administration paradigm leads to inhibition of bone fracture repair in mice and rats [23, 24]. One hour after the final alcohol dose, the mice underwent a modified, stabilized mid-shaft tibia fracture injury as previously described[25]. Following injury, mice were resuscitated with warmed saline and returned to cages placed on heating pads for 6 hours. For pain management, mice received s.c. injections of buprenorphine postoperatively at a dose of 0.1 mg/kg every eight hours for three total doses. Animals were humanely euthanized at 14 days post injury, tibia were harvested and stored at −20 degrees C or in Fomalin for analyses detailed below.
MSC Isolation and Expansion
Bone marrow was harvested from tibia and femur as previously described[26] from 7-8 week old transgenic GFP-expressing mice (C57BL/6-Tg-UBC-GFP-30Scha/J, Jackson Labs®, Bar Harbor, Maine). Bone marrow was eluted into McCoy’s Media (Invitrogen; Carlsbad, California) containing penicillin (100 U/ml) and streptomycin (100 U/ml). Harvested cells were pelleted and a lineage depletion technique was utilized to remove contaminating hematopoietic and myeloid cells by magnetically activated cell sorting. Cell surface antigens selected for depletion included GR-1, CD3e, B220, CD11b, CD8a, CD19, CD86, Ter119, Thy1.1, CD11c (BD Bioscience; San Jose, California). Lineage negative cells were cultured on 25cm2 vented flasks, pretreated with 2μg/cm2 Poly-L-Lysine. Cells were seeded at approximately 1.0 × 105 cells/cm2. Cells were cultured until confluence and harvested for tail vein injections. To determine if cultured MSC retained differentiation potential, separate culture of cells were differentiated in osteogenic, chondrogenic or adipogenic media and determined to be capable of tri-lineage differentiation (data not shown). Cells were also shown to express MSC-specific cell surface markers such as, SSEA-1, CD29, CD31, CD44, CD51, CD105 and SCA-1 (not shown).
In Vivo Intravenous MSC administration
Intravenous MSC infusions were administered 24 hours after fracture via a catheter in the tail vein and according to the manufacturer’s recommendations (Strategic Applications Inc.; Green Oaks, Illinois). The 24-hour post-fracture time point was selected to permit normalization of the blood alcohol content to zero to assure the transplanted MSC remained alcohol-naïve.
In Vivo Fluorescence Imaging
In vivo fluorescence imaging began 24 hours after initial intravenous infusion (48 hours post-fracture) and continued at daily intervals until 14 days after injury. The number of mice imaged was 5-6 per group. Mice were anesthetized using isoflurane in a vaporizer apparatus after hair was removed from both lower legs. In vivo images were collected with a fluorescence imaging system (Xenogen IVIS 100, Caliper Life Sciences, Hopkinton, MA). Background autofluorescence was subtracted using the Living Image® Software (Caliper Life Sciences). Tissue autofluorescence was subtracted by acquiring separate images obtained from a specific GFP background excitation filter as well as from the primary GFP excitation filter. Fluorescence units in the injured tibiae region of interest (ROI) are given in efficiency (fractional ratio of fluorescently emitted photons per incident excitation photon).
Immunohistochemical (IHC) Analysis of Fracture Callus
Fractured tibiae were fixed in 4% paraformaldehyde at 4°C for 24 hours followed by decalcification in 10% EDTA for 7 days. Four-μm paraffin sections were used for antigen retrieval, followed by standard IHC staining using a primary antibody against GFP. Slides were counterstained with Harris Hematoxylin, coverslipped and positive staining was observed and photographed under light microscopy. The number of specimen appropriated for IHC analysis was 2 per group.
Histological Analysis
Fractured tibia were fixed in 10% neutral buffered formalin, and decalcified in formic acid for 10 days. The tibiae were dehydrated, paraffin-embedded, and 4-μm serial sections were cut from the central callus. The sections were stained with hematoxylin and eosin, coverslipped, and images were taken using a Leica MZ APO Stereomicroscope (Buffalo Grove, IL). The number of specimen utilized for histological analysis was 2 per group.
Micro-Computed Tomography
Four tibiae per treatment group were scanned simultaneously using an isotropic volume element (voxel) size of 31μm (μCT 40, Scanco Medical, Brüttisellen, Switzerland). For each slice, a semi-automated contouring using the system’s software defined the outer boundary of the callus and the total volume of callus plus pre-existing cortex and medulla. A threshold of 40% of the linear attenuation coefficient μ of mature cortical bone (3.2 cm−1) was utilized. The volume of newly mineralized tissue was calculated from the difference between the total bone volume (summation of volume elements with μ ≥ 1.28 cm−1) and the mature cortical bone volume (volume elements with μ ≥ 3.2 cm−1). Three-dimensional renderings were generated using the system software using the threshold of 1.28 cm−1.
Biomechanical Strength Testing
Biomechanical strength testing of fracture callus was performed using an Instron materials testing machine (Model 5544, Instron Corp., Norwood, MA) using a custom-designed 4-point bending apparatus. Force was applied at a constant crosshead displacement rate of 0.5mm/min with the fracture site placed in the middle of the inner span. Strength-to-failure of the bridging callus tissue was measured (N), and callus stiffness was computed as the slope of the linear portion of the load/displacement plot (N/mm).
Statistical Analysis
Animal numbers required for biomechanical analysis were determined using data from a previous study, which examined the effect of binge alcohol exposure alcohol on bone biomechanical strength. This data was utilized for a power analysis, which demonstrated than an N of at least 8 per treatment group was sufficient to detect significant differences in biomechanical strength in bone from saline versus alcohol-treated mice. Multiple comparisons tests between fractured and sham groups were performed by a one-way analysis of variance (ANOVA) with Tukey’s Post-hoc analysis. A probability value of p < 0.05 was considered statistically significant.
RESULTS
In-Vivo MSC Homing to Fracture Site
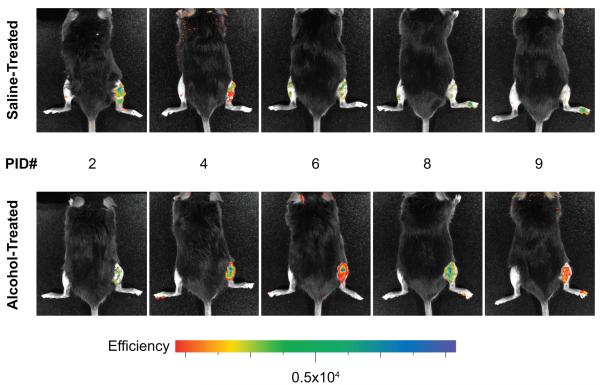
Beginning two days following injury, control saline-exposed animals receiving MSC-GFP transplant demonstrated fluorescence signal at the site of injury when compared with intact contralateral tibia (Figure 1), and this signal peaked at 2-3 days following fracture. Alcohol-exposed animals receiving MSC-GFP transplant demonstrated fluorescence signal approximately three days following injury, and this signal peaked at 3-4 days following fracture. In both treatment groups, fluorescent signal had deteriorated with time and approached background levels approximately 7 days following injury.
Figure 1. In Vivo Imaging of MSC Homing to the Fracture Site.
Beginning two days following fracture, labeled MSC were detected in the injured right lower extremity in saline-treated animals (top row, n=5), with signal persisting until approximately seven days after injury. Alcohol-treated animals (bottom row, n=6) were observed to have a relative delay in signal acquisition until 3-4 days following injury, with signal again diminishing by one week following injury.
Immunohistochemical Analysis of Fracture Callus
To further investigate the ability of transplanted labeled MSC to specifically home to the site of fracture and physically incorporate into fracture callus, immunofluorescent staining was utilized with antibody directed against GFP. The avidin-biotin-peroxidase complex detection indicated positive staining due to the biotinylated anti-GFP secondary antibody. In sections from the center of the callus near the fracture site, transplanted MSC-GFP cells were detected (Figure 2), while vehicle-only and unlabeled MSC transplants did not demonstrate the avidin-biotin-peroxidase complex reaction.
Figure 2. Immunohistochemical Analysis of Fracture Callus.
Demonstration of fracture-specific localization of GFP-expressing MSC in animals exposed to alcohol prior to injury. N=2/group. A primary antibody directed against the GFP reporter demonstrates fracture callus localization of labeled cells (arrows). Slides were counterstained with Harris Hematoxylin as per Methods. Magnification 200x. Inset, 32x, is from a H& E serial section denoting the region of interest of the zoomed immunohistochemical image (black box).
MicroCT Evaluation of Callus Size and Mineral Content
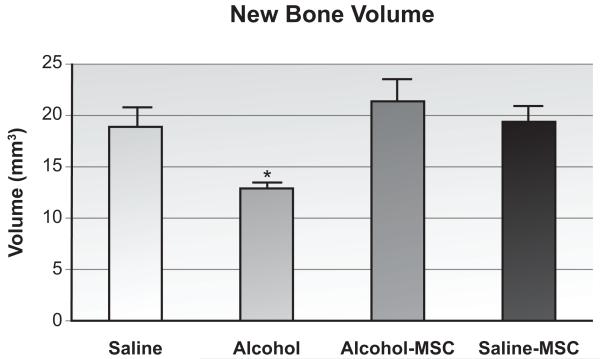
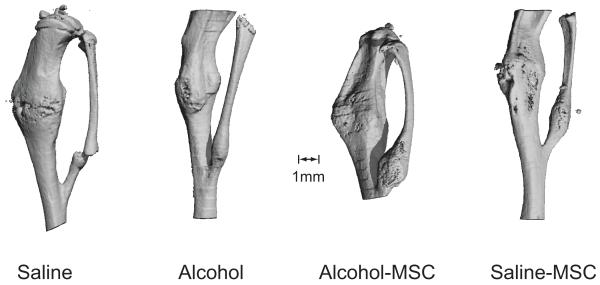
MicroCT was used to quantify the volume of callus formed following fracture. As shown in Figure 3, when compared with saline controls, binge alcohol exposure decreased callus volume (69% difference; p = 0.03, n=4/group). When compared with animals treated with alcohol alone, MSC administration to alcohol-exposed animals resulted in a significant improvement in new bone volume (165% difference; p = 0.01). When compared with saline controls, the saline-MSC specimens showed similar new bone volumes. Evaluation of callus tissue mineral density did not reveal any significant differences between any of the treatment groups. Reconstructions of fracture callus images are shown in Figure 4.
Figure 3. Fracture Callus Volume.
New bone volume throughout fracture callus was detected by microCT analysis as described in Methods (n=4/group). Volumes are given as cubic millimeters and were calculated as described in Methods. *p≤0.05, one-way ANOVA with Tukey’s posthoc.
Figure 4. Three Dimensional Micro-CT Reconstructions of Fracture Callus.
Relative fracture callus size and extent of bony bridging callus is observed above in MSC treatment specimens as compared with control- or alcohol-treatment groups.
Biomechanical Strength of Fracture Callus
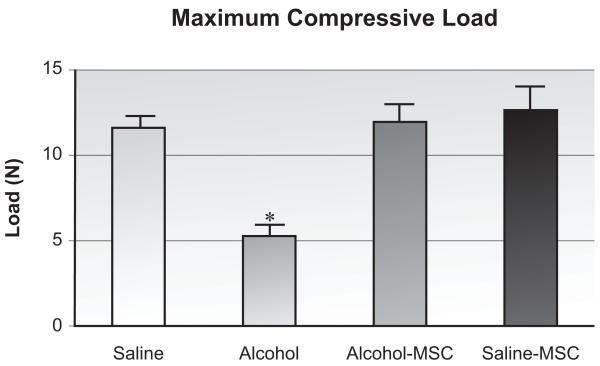
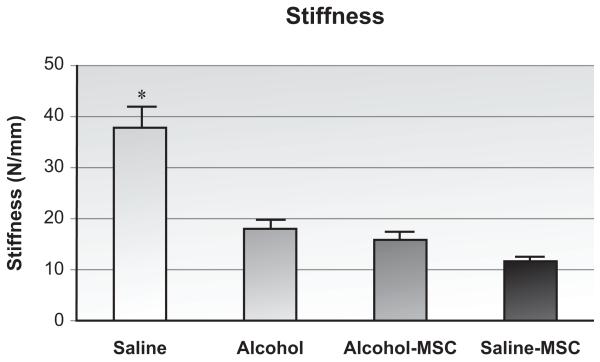
When compared with saline controls, binge alcohol exposure significantly impaired the maximum bending load borne by injured specimens (Figure 5, 45% difference; p < 0.01, n=8/group). Binge alcohol treatment also decreased callus stiffness (Figure 6, 47.7% difference; p < 0.01). When compared with specimens harvested from animals exposed to binge alcohol, MSC implant resulted in a significant improvement in maximum bending strength borne by injured specimens (238% increase; p < 0.01). MSC treatment in alcohol-exposed animals did not significantly change stiffness as compared to the alcohol group. Similar effects of MSC treatment were observed in the saline-MSC group.
Figure 5. Fracture Callus Biomechanical Strength Analysis.
Maximum bending load sustained by callus tissue at post-fracture day 14 as determined by biomechanical 4-point bending analysis. *p≤0.05, one-way ANOVA with Tukey’s posthoc, (n=10-12/group).
Figure 6. Fracture Callus Biomechanical Stiffness Analysis.
Stiffness of callus tissue at post-fracture day 14, determined using load versus displacement data from 4-point bending assay. *p≤0.05, one-way ANOVA with Tukey’s posthoc (n=10-12/group).
Histological Analysis of Fracture Callus
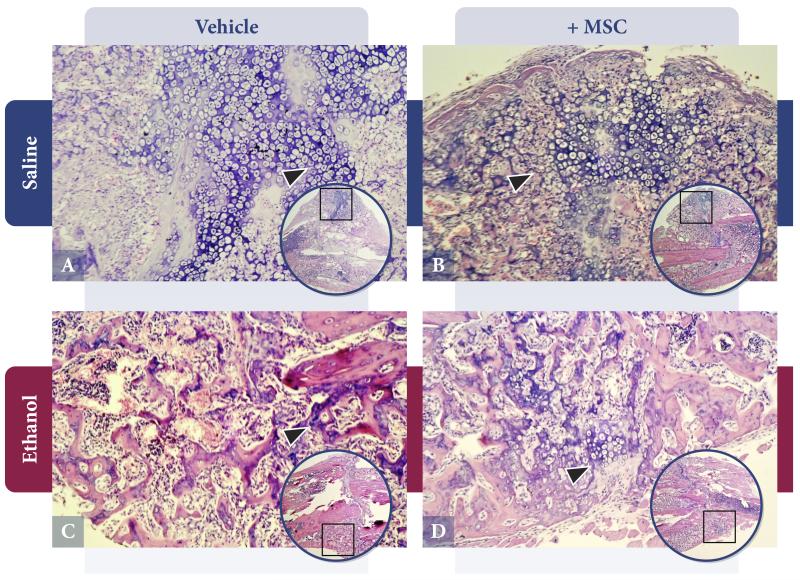
As shown in Figure 7, callus from saline control animals (Figure 7A) exhibited a normal cartilaginous external callus. Hypertrophic chondrocytes with associated endochondral ossification activity within a basophilic external callus are observed (arrow). Saline-treated animals given a MSC transplant (Figure 7B) have a more mature-appearing callus that has proceeded to active endochondral bone formation (arrow). In contrast, callus from alcohol-treated animals (Figure 7C) shows relative absence of cartilaginous external callus and endochondral bone formation, with peripheral callus derived solely from periosteum (arrow). In animals exposed to alcohol and MSC (Figure 7D), a partial restoration of cartilaginous external callus is observed (arrow) with associated endochondral ossification.
Figure 7. Histological Analysis of Fracture Callus Tissue Composition.
Callus specimens were harvested at 14 days following injury and processed as described in Methods. A. Saline-Vehicle: Normal external callus composition with cartilaginous content and endochondral bone formation. B. Saline-MSC: Mature callus with advanced endochondral ossification. C. Alcohol Treatment: Abnormal callus lacking in chondrocyte content and endochondral ossification. D. Alcohol-MSC: Partial restoration of cartilaginous callus and endochondral bone formation. Magnification 200x, inset 32x. (n=2/group).
DISCUSSION
This study demonstrates that autologous culture expanded MSC when administered intravenously following fracture can migrate to the site of injury and contribute to fracture repair in a model of alcohol-impaired healing. Transplanted MSC were detected in callus over a several day period and critical measures of callus volume strength, and composition were improved in alcohol-treated mice. While MSC homing to the site of fracture has been demonstrated [21], no studies to our knowledge have examined this phenomenon in a setting of fracture healing that was impaired by the clinically relevant co-morbidity of pre-injury alcohol exposure. In a study utilizing a similar alcohol exposure/fracture injury model in rats, our laboratory demonstrated that alcohol caused an impairment of external callus formation and endochondral ossification at 14 days following injury[7]. This was consistent with our preliminary observations in the mouse.
The use of binge alcohol exposure as a means of systemically impairing fracture repair in a rodent model has relevance to the adult trauma population. Alcohol has been shown to be a risk factor for sustaining injuries of all types[27, 28], and as many as 40% of patients admitted with fractures have positive blood alcohol screening on presentation[9, 10]. While both acute and chronic alcohol abuse has been shown to be prevalent among injured patients[11, 29], it is the binge pattern of alcohol consumption that confers the highest risk factor for injury[30] and this is the pattern of alcohol administration we selected for study.
Our model of impaired fracture healing is pharmacologically-induced and offers an alternative to the nonunion animal models in the literature, of which the “critical” defect is the most widely studied[6]. Critical defects are not based on existing pathologic processes. While these models may accurately represent the clinical situation of bone loss after high-energy fracture, they do not represent atrophic nonunion where reduced, juxtaposed bone ends show impaired healing. MSC-seeded scaffolds have shown promise in regenerating bone in these critical defect models[6] although mixed success has been encountered in recent studies[31]. Local placement of MSC-seeded scaffolds in these settings requires surgical intervention, and does not offer the potential for longitudinal MSC administration over the course of the fracture healing process. For these reasons, we studied the potential of noninvasive intravenous MSC administration. After MSC infusion, cells home to the site of fracture and show osteogenic potential[32]. Systemic MSC therapy is well tolerated[17, 33] and effective in the treatment of skeletal disorders such as osteogenesis imperfecta[18].
Our study evaluated the role of autologous MSC therapy where cells are isogenic and lack potential for host immunogenic reaction, removing a potentially confounding variable. However, allogeneic MSC transplants may be immune-privileged and do not appear to require immunosuppression of the host since they do not express major histocompatibility class II and co-stimulatory molecules[34] and do not induce a lymphocyte response[35]. Allogeneic MSC administration also avoids any potential harvest morbidity. The therapeutic potential of allogeneic MSC is promising and requires further investigation.
The mechanistic underpinning of the beneficial healing properties attributed to intravenous MSC therapy was not examined in our study. Although MSC preparations have been shown to induce osteogenesis when added locally to sites of nonunion in animal[6] and human[36, 37] studies, systemic MSC administration may also modulate inflammatory and oxidative stress pathways that contribute to the pathogenesis of nonunion. Several studies have demonstrated the ability of MSC to suppress the inflammatory response to injury[21, 32, 38]. In multiply injured patients, systemic MSC treatment may represent an opportunity to selectively curb the uncontrolled inflammatory response that contributes to nonunion.
The functional parameters of fracture healing that improved in alcohol-treated animals as a result of MSC treatment may have resulted, at least in part, from the rigorous methods we utilized for isolating and characterizing the MSC population used for exogenous administration. There is heterogeneity in the literature with regards to MSC isolation and expansion methodologies, making it difficult to compare study outcomes[39]. Some studies quantified CFU-F (colony forming unit – fibroblast) as a means of characterizing undifferentiated MSC; however, in murine systems, CFU-F are highly contaminated with hematopoietic cells[40, 41] and results must be interpreted with caution[31]. To remove any potentially contaminating hematopoietic and lymphocytic cells, we depleted our population of purified MSC from contaminating hematopoietic and lymphocytic cells.
One potential limitation in systemic MSC therapy is that a large fraction of these cells become trapped in the lungs[42, 43] and thus any migration to the site of fracture is dependent on systemic chemotactic signals. While this has not been evaluated in humans, animal studies have demonstrated that despite initial pulmonary localization, infused labeled MSC are capable of homing to the site of injury in a time-dependent manner[32], consistent with our observations. We were not able to quantify the fraction of cells initially localizing to the lungs in our study due to the unreliability of quantifying the GFP fluorescent reporter in deep tissues. MSC labeled with luminescent reporters may provide further insight into pulmonary and injury site localization.
In summary, this study provides evidence that binge alcohol exposure prior to injury causes deficiencies in fracture repair, leading to inhibition of callus strength, volume, and endochondral ossification. These deficiencies were largely reversed after systemic MSC administration, demonstrating these cells were capable of homing to the site of injury and of contributing to fracture repair. MSC therapy may have additional systemic therapeutic effects, which require further exploration. MSC administration following fracture may represent a novel noninvasive method to augment fracture repair in patients at risk for impaired fracture healing and nonunion.
ACKNOWLEDGEMENTS
The authors would like to credit funding from the AO Foundation, AO North America, and the NIH (NIAAA AA016138 to JJC). We would also like to credit Sherri Yong, PhD, Department of Pathology, Loyola University Medical Center, for her thoughtful contributions to the histological analysis of specimens.
Footnotes
This data has been presented at the following meetings: 2011 American Academy of Orthopaedic Surgeons Annual Meeting, San Diego, CA; 2011 Orthopaedic Trauma Association Annual Meeting, San Antonio, TX (Best Presentation, Basic Science Focus Forum); and at the 2012 Orthopaedic Research Society Annual Meeting, San Francisco, CA
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Einhorn TA. Enhancement of fracture-healing. J Bone Joint Surg Am. 1995;77(6):940–56. doi: 10.2106/00004623-199506000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Hayda RA BC, Esterhai JL., Jr. Pathophysiology of delayed healing. Clin Orthop Relat Res. 1998;355(Suppl:):S31–40. doi: 10.1097/00003086-199810001-00005. [DOI] [PubMed] [Google Scholar]
- 3.D M. Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat Res. 1998;355:Suppl:S22–30. doi: 10.1097/00003086-199810001-00004. [DOI] [PubMed] [Google Scholar]
- 4.Mirovsky Y, Neuwirth MG. The intracortical method of bone harvesting from the iliac crest did not reduce pain or bleeding at the donor site. The Journal of Bone and Joint Surgery (American) 2000;82(12):1809–1809. doi: 10.2106/00004623-200012000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Colterjohn NR, Bednar DA. Procurement of Bone Graft from the Iliac Crest. An Operative Approach with Decreased Morbidity*. The Journal of Bone and Joint Surgery (American) 1997;79(5):756–9. doi: 10.2106/00004623-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Tseng SS, Lee MA, Reddi AH. Nonunions and the potential of stem cells in fracture-healing. J Bone Joint Surg Am. 2008;90(Suppl 1):92–8. doi: 10.2106/JBJS.G.01192. [DOI] [PubMed] [Google Scholar]
- 7.Volkmer DL, et al. Antioxidant therapy attenuates deficient bone fracture repair associated with binge alcohol exposure. J Orthop Trauma. 2011;25(8):516–21. doi: 10.1097/BOT.0b013e31821f65cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sears BW, et al. Binge alcohol exposure modulates rodent expression of biomarkers of the immunoinflammatory response to orthopaedic trauma. J Bone Joint Surg Am. 2011;93(8):739–49. doi: 10.2106/JBJS.J.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy RS, et al. Drug and alcohol use in orthopedic trauma patients: a prospective study. J Orthop Trauma. 1996;10(1):21–7. doi: 10.1097/00005131-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Blake RB, et al. Alcohol and drug use in adult patients with musculoskeletal injuries. Am J Orthop (Belle Mead NJ) 1997;26(10):704–9. discussion 709-10. [PubMed] [Google Scholar]
- 11.Rivara FP, et al. The magnitude of acute and chronic alcohol abuse in trauma patients. Arch Surg. 1993;128(8):907–12. doi: 10.1001/archsurg.1993.01420200081015. discussion 912-3. [DOI] [PubMed] [Google Scholar]
- 12.Savola O, Niemela O, Hillbom M. Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol Alcohol. 2005;40(4):269–73. doi: 10.1093/alcalc/agh159. [DOI] [PubMed] [Google Scholar]
- 13.Savola O, Niemela O, Hillbom M. Blood alcohol is the best indicator of hazardous alcohol drinking in young adults and working-age patients with trauma. Alcohol Alcohol. 2004;39(4):340–5. doi: 10.1093/alcalc/agh064. [DOI] [PubMed] [Google Scholar]
- 14.Duckworth AD, et al. Fixation of intracapsular fractures of the femoral neck in young patients: risk factors for failure. J Bone Joint Surg Br. 2011;93(6):811–6. doi: 10.1302/0301-620X.93B6.26432. [DOI] [PubMed] [Google Scholar]
- 15.White TO, et al. The results of early primary open reduction and internal fixation for treatment of OTA 43.C-type tibial pilon fractures: a cohort study. J Orthop Trauma. 2010;24(12):757–63. doi: 10.1097/BOT.0b013e3181d04bc0. [DOI] [PubMed] [Google Scholar]
- 16.Mathog RH, et al. Nonunion of the mandible: an analysis of contributing factors. J Oral Maxillofac Surg. 2000;58(7):746–52. doi: 10.1053/joms.2000.7258. discussion 752-3. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, et al. Ex vivo expansion and in vivo infusion of bone marrow-derived Flk-1+CD31-CD34-mesenchymal stem cells: feasibility and safety from monkey to human. Stem Cells Dev. 2006;15(3):349–57. doi: 10.1089/scd.2006.15.349. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz EM, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99(13):8932–7. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novicoff WM, et al. Critical analysis of the evidence for current technologies in bone-healing and repair. J Bone Joint Surg Am. 2008;90(Suppl 1):85–91. doi: 10.2106/JBJS.G.01521. [DOI] [PubMed] [Google Scholar]
- 20.Alm JJ, et al. Circulating plastic adherent mesenchymal stem cells in aged hip fracture patients. J Orthop Res. 2010;28(12):1634–42. doi: 10.1002/jor.21167. [DOI] [PubMed] [Google Scholar]
- 21.Granero-Molto F, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27(8):1887–98. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shirley D, et al. Systemic recruitment of osteoblastic cells in fracture healing. J Orthop Res. 2005;23(5):1013–21. doi: 10.1016/j.orthres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Volkmer DL, et al. Antioxidant therapy attenuates deficient bone fracture repair associated with binge alcohol exposure. Journal of orthopaedic trauma. 2011;25(8):516–21. doi: 10.1097/BOT.0b013e31821f65cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauing KL, et al. Acute Alcohol Exposure Impairs Fracture Healing and Deregulates β-Catenin Signaling in the Fracture Callus. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiltunen A, Vuorio E, Aro HT. A standardized experimental fracture in the mouse tibia. J Orthop Res. 1993;11(2):305–12. doi: 10.1002/jor.1100110219. [DOI] [PubMed] [Google Scholar]
- 26.Baddoo M, et al. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89(6):1235–49. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- 27.Greiffenstein P, Molina PE. Alcohol-induced alterations on host defense after traumatic injury. J Trauma. 2008;64(1):230–40. doi: 10.1097/TA.0b013e318158a4ad. [DOI] [PubMed] [Google Scholar]
- 28.Lowenfels AB, Miller TT. Alcohol and trauma. Ann Emerg Med. 1984;13(11):1056–60. doi: 10.1016/s0196-0644(84)80070-0. [DOI] [PubMed] [Google Scholar]
- 29.Gentilello LM, et al. Alcohol interventions for trauma patients treated in emergency departments and hospitals: a cost benefit analysis. Ann Surg. 2005;241(4):541–50. doi: 10.1097/01.sla.0000157133.80396.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gmel G, et al. Alcohol-attributable injuries in admissions to a swiss emergency room--an analysis of the link between volume of drinking, drinking patterns, and preattendance drinking. Alcohol Clin Exp Res. 2006;30(3):501–9. doi: 10.1111/j.1530-0277.2006.00054.x. [DOI] [PubMed] [Google Scholar]
- 31.Cuomo AV, et al. Mesenchymal stem cell concentration and bone repair: potential pitfalls from bench to bedside. J Bone Joint Surg Am. 2009;91(5):1073–83. doi: 10.2106/JBJS.H.00303. [DOI] [PubMed] [Google Scholar]
- 32.Granero-Molto F, et al. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther. 2008;8(3):255–68. doi: 10.1517/14712598.8.3.255. [DOI] [PubMed] [Google Scholar]
- 33.Lazarus HM, et al. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557–64. [PubMed] [Google Scholar]
- 34.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32(5):414–25. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Klyushnenkova E, et al. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12(1):47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 36.Connolly JF, et al. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991;266:259–70. [PubMed] [Google Scholar]
- 37.Hernigou P, et al. The use of percutaneous autologous bone marrow transplantation in nonunion and avascular necrosis of bone. J Bone Joint Surg Br. 2005;87(7):896–902. doi: 10.1302/0301-620X.87B7.16289. [DOI] [PubMed] [Google Scholar]
- 38.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–99. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 39.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 40.Phinney DG, et al. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J Cell Biochem. 1999;72(4):570–85. [PubMed] [Google Scholar]
- 41.Anjos-Afonso F, Siapati EK, Bonnet D. In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci. 2004;117(Pt 23):5655–64. doi: 10.1242/jcs.01488. [DOI] [PubMed] [Google Scholar]
- 42.Schrepfer S, et al. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573–6. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 43.Gao J, et al. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]