Summary
Using an elaborately evolved language of cytokines and chemokines as well as cell-cell interactions, the different components of the immune system communicate with each other and orchestrate a response (or wind one down). Immunological synapses are a key feature of the system in the ways in which they can facilitate and direct these responses. Studies analyzing the structure of an immune synapse as it forms between two cells have provided insight into how the stability and kinetics of this interaction ultimately affect the sensitivity, potency, and magnitude of a given response. Furthermore, we have gained an appreciation of how the immunological synapse provides directionality and contextual cues for downstream signaling and cellular decision-making. In this review, we discuss how using a variety of techniques, developed over the last decade, have allowed us to visualize and quantify key aspects of the dynamic synaptic interface and have furthered our understanding of their function. We describe some of the many characteristics of the immunological synapse that make it a vital part of intercellular communication and some of the questions that remain to be answered.
Keywords: T-cell receptor, peptide–major histocompatibility complex, immunological synapse, central supramolecular activation cluster, cytokines
Introduction
Like social networks, the cellular components of the immune system interact with each other using a language or operating system to activate and coordinate its many functions. Indeed, as with any language, we can trace the ancestral origins of different arms of the immune system and their molecular constituents. We see this in the roots of innate pathogen recognition by Toll receptors in Drosophila, to the birth of more diverse lymphocyte receptors for surveillance in jawless fish, and the subsequent ‘big bang’ development of RAG-mediated receptor diversity as the centerpiece of the adaptive immune system in higher vertebrates (1). Immunologists and microbiologists alike have used this immune evolution across species to help understand the functions of the various arms of the immune system, akin to the work of a linguist reaching back to ancient language to appreciate modern syntax. One could therefore argue that the immune system has evolved the capacity for acquiring and using a system of communication to share information and to educate lymphocytes for successful immune surveillance and defense mechanisms.
Similar to words, the coding system of immune cell language resides in the genes that give rise to soluble protein ‘morphemes’ that can be combined together into cytokine ‘lexicons’, for example, in the form of Th1 or Th2 polarizing cytokines, that specifically educate target cells bearing the appropriate surface receptors for ‘listening’. Like the symbols of language, there are a finite number of cytokines, but that can be grouped in a variety of combinations to infer manifold meanings (2). The manner in which these cytokine signals are interpreted is also predicated on both temporal and spatial aspects that will set in motion a variety of downstream mechanisms. For example, local or autocrine cytokine secretion will increase the sensitivity to the initial stimulus [in our case an antigen-presenting cell (APC) displaying a peptide-MHC complex] to promote a pathogen-specific response. In contrast, lymphocytes can also secrete cytokines in a paracrine manner to instruct the recruitment and differentiation of other cells. Without proper contextual cues such as these, the meaning can be misinterpreted and lead to immune-pathology as is the case with autoimmunity. Consequently, the ability to communicate regulatory signals to modify or attenuate a response is just as important as initiating a response. This is especially important when one considers that the immune system is constantly multitasking, initiating some responses while winding down others. Thus, calibrating each action must be crucial to conserving energy and the myriad inhibitory mechanisms reinforce that likelihood.
Here, we define immunological synapses (ISs) as at least transiently stable, concerted interactions between two or more cells in the immune system, that have consequences for at least one of the cells involved. In their different forms, they are an essential component of the immune system in integrating signals, directing soluble factors, and coordinating molecular interactions for the formation of an appropriate and specific immune response. The IS promotes language competency, the ability to share or transfer ‘knowledge,’ through its structure, kinetics, and mechanics. Structure (or ‘syntax’) involves how molecular components are brought together to form the supramolecular activation cluster (SMAC). Kinetics (or ‘semantics’) comprises the temporal and spatial components that dictate the ‘meaning’ of cellular interaction. Finally, mechanics (or ‘pragmatics’) encompasses how context contributes to the intended meaning. In the case of the immune system, the ‘learning’ or transfer of knowledge can take the form of T cells being educated by an APC bearing their cognate antigen, B cells receiving T-cell help, or CD8+ T cells carrying out a kill-order.
In this review, we focus on the different ways in which immunological synapses foster communication between the cells of the immune system, in particular between T cells and APCs. Other lymphocytes, such as B cells and natural killer (NK) cells, can also form synapses with cells that they recognize and have been reviewed elsewhere (3, 4).
Synaptic structure
The structure of the IS is both highly ordered and dynamic. Just as how the order of words in a sentence can change the intended meaning, so can the arrangement of the synapse alter the ‘message’ that is conveyed between the cells that are engaged. The use and adaptation of new two-dimensional (2D) and three-dimensional (3D) technologies to visualize in situ and in vitro IS formation have changed our original ideas of not only the role of the IS, but also what constitutes a functional IS. It is traditionally thought that the surface molecules are rearranged upon TCR engagement in an ordered manner to form a focal point between two conjugated cells that facilitates the exchange of information required for amplifying and terminating activation signals. Likewise, the cognate interaction between two cells is also a dynamic process that is highly dependent on this reorganization of surface molecules. As a T cell becomes activated, TCR complexes form the central SMAC (cSMAC), adhesion molecules form the peripheral SMAC (pSMAC), and F-actin is concentrated in the distal SMAC (dSMAC) area to help stabilize the cell to cell contact (5, 6). Certain molecules, such as the phosphatase CD45, are also enriched in the dSMAC during initial activation, likely to prevent premature cessation of signaling (7–9). Furthermore, as we discuss, it is clear that the original ‘bull’s eye’ model of the IS is not the only type of synapse that can form when cells are engaged.
Using electron microscopy (EM) and 3D EM tomography, currently the highest possible resolution achievable, we have visualized the process of synapse formation and maturation in CD4+ T cells (10), complementary to work with CD8+ T cells by the Griffiths group (11). Using these techniques, we observed T cells making contact with a B-cell line bearing their cognate antigen through the formation of pseudopodia that reached deep into the other cell, almost to the nuclear envelope, but without any apparent damage. This first of four distinct stages occurred within 30 min (10) (Fig. 1). Similar observations were made some time ago in CD8+ T cells, but were thought to relate to cytotoxicity (12, 13). Clearly this is not the case with CD4+ T cells, and so it must be that they serve some other purpose, such as increasing the surface area that a T cell can survey by up to 10-fold by our estimate. It is important to note that this phenomenon cannot be seen with planar bilayer activation of T cells. Stage 2 is a transitional stage where the appearance of microtubule initiating sites is observed between the centrioles and the membrane. Stage 3 occurs after about 1–2 h, and the centrioles could be seen moving under the contact zone along with the Golgi complex while other organelles remain randomly scattered. Stage 4 occurs after approximately 4 h, when the Golgi complex becomes greatly enlarged, and this is also correlated with cytokine secretion. At this stage, the plasma membranes of each cell are pressed flat against each other, with no evidence of pseudopodia at this stage.
Fig. 1. Four stages of the immune synapse.
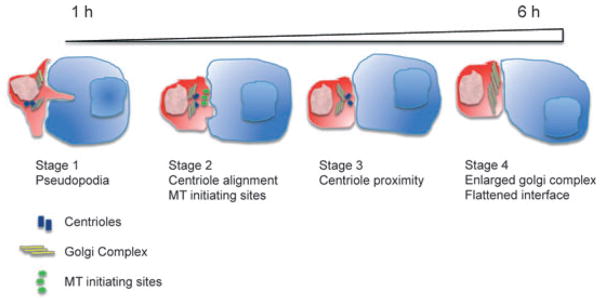
In Stage 1, CD4+ T cells (red cell) extend pseudopodia causing deep invagination of the antigen-presenting cell (APC) (blue cell) cell membrane within 1 h of recognition of its cognate antigen. During Stage 2, centrioles (blue rectangles) realign themselves toward the IS and MT initiating sites (green bursts) form along the membrane that is in contact with the APC. In Stage 3, centrioles move within close proximity to the IS and the Golgi complex (yellow lines) migrates centrally to the contact site, while other organelles such as mitochondria are pushed away from it. During Stage 4, an enlarged Golgi complex is observed directly beneath the IS and the cell membrane at the T/APC contact site becomes smooth and flat.
These four stages of IS formation may not necessarily be seen for all cell types undergoing cognate interaction with an APC. We and others have observed less mature synapse formation between CD8+ T cells and their targets (14, 15) as well as none at all in thymocytes undergoing positive selection (16). CD8+ T cells themselves can form not only the more traditional stimulatory synapse but also a lytic synapse at a distinctly different location in the plasma membrane (14). This may potentially relate to the lower activation threshold of CD8+ T-cell lytic activity, as it has been shown that CD8+ T cells can be activated to kill targets after engagement of as few as 1–10 pMHC molecules (17). It is clear from these observations that not only the structural components of the IS, but also the kinetics of engagement will influence the information that is exchanged and how that information is interpreted.
TCR/CD3 preclustering
A controversy in the field is whether TCR-CD3 complexes are preclustered on the T-cell surface. Previously, Schamel et al. (18) used Blue Native polyacrylamide gel electrophoresis and electron microscopy to investigate the stoichiometry of TCRs on T-cell surfaces. They found that TCRs were a mixture of monovalent (αβγεδεζζ) and multivalent complexes, the latter containing varied number (from 2 to >20) of TCRα/β subunits.
Using super-resolution fluorescence microscopy and electron microscopy, Lillemeier et al. (19) showed that in a resting T cell, CD3ζ, and linker for activation of T cells (LAT) are organized into separate clusters termed as ‘protein islands’ around the cell plasma membrane. This organization allows the physical separation of these molecules, which are only brought together under the right conditions. Specifically, once the T cell is activated through TCR engagement of its cognate antigen presented by an MHC, these islands come together into microclusters, which then allow CD3 and LAT to associate at the edges of their respective islands. These aggregated clusters of molecules constitute the microclusters that form initially in the periphery of the IS and then migrate through to the cSMAC by inducing cytoskeletal changes (20, 21). Preclustering of TCR and LAT seems independent of the T-cell activation history and was found to be a constitutive quality of the resting T cells.
T-cell activation and TCR/CD3 dimerization/multimerization
Another persistent debate in T-cell activation is whether TCR/CD3 dimerization/multimerization is necessary for activation (22, 23). Many early observations suggest that TCR/pMHC complexes oligomerize in T-cell activation and that a monomeric pMHC in solution is not sufficient to stimulate T cells (22, 24, 25). Using the erythropoietin receptor dimerization reporter system, we recently found that the two CD3ε subunits that associate with αβ TCR are juxtaposed and that TCRβ associates with CD3γε and TCRα associates with both CD3δε and CDζζ (26). This localizes the CD3 molecules in a cluster on one side of the TCR, in contrast with other suggestions that they are docked on opposite sides of the TCR (27). This suggests that the dimerization should occur on the side of the TCR opposite from where the CD3 heterodimers are located (28).
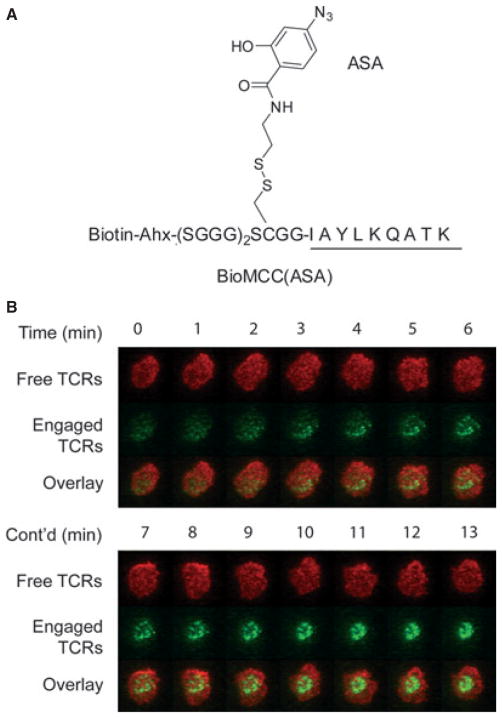
There are also studies showing that monomeric pMHC ligands in solution are stimulatory (23, 29). We recently addressed this controversy by crosslinking pMHC complexes onto TCRs in solution and examined whether the permanent engagement of monomeric pMHC ligands in solution would activate T cells. This was done by first developing a photocrosslinkable pMHC ligand that can covalently bind to cognate TCRs on live T-cell surfaces under ultraviolet (UV) irradiation. In particular, we introduced a photocrosslinker 4-azidosalicyclic acid (ASA) to a cysteine residue at P-3 position of the agonist MCC peptide, which is outside the core MHC-binding and TCR-recognition region of the peptide and thus will not interfere with TCR recognition (Fig. 2A). We were able to crosslink such pMHC complexes to more than 6,000 TCR molecules on the T-cell surface (i.e., >15% of total TCR molecules per cell), yet we did not observe any calcium influx in T cells. Subsequent aggregation of crosslinked pMHC-TCR complexes would then activate the T cell. This result showed unambiguously that monomeric pMHC binding alone was not stimulatory. It has been shown that productive TCR engagement would induce the exposure of a proline-rich motif in the intracellular domain of CD3ε chain and recruit the adapter protein Nck (30). Our finding that even a covalently bound pMHC monomer could not activate T cells suggested that either the monomeric ligand binding (and hence no TCR aggregation) could not induce the conformational change in TCR/CD3 or the ligand-induced conformational change (if any) was not sufficient for T-cell activation.
Fig. 2. A photocrosslinkable pMHC ligand revealed that ligand-bound TCRs were preferentially transported to the cSMAC.
(A) A photocrosslinker azidosalicyclic acid (ASA) was introduced to a cysteine residue at P-3 position of the MCC peptide. Loading this MCC derivative to murine class II MHC I-Ek led to a photocrosslinkable pMHC, which could covalently bind to 5C.C7 TCRs on live T-cell surfaces under ultraviolet irradiation with excellent specificity and efficiency. (B) Video fluorescent imaging showed that ligand-bound TCRs were preferentially transported to the cSMAC, while free TCRs remained randomly distributed on the T-cell surface in the initial phase of T-cell activation. Ligand-bound TCRs (in green) were labeled with Alexa Fluor 555 conjugates of monovalent streptavidin (via the biotin on the associated pMHC molecules); free TCRs (in red) were labeled with Alexa Fluor 647–conjugated antigen-binding fragments of antibody KJ25 to Vβ3.
It has been shown that T cells are able to initiate intracellular signaling responses to as few as one agonist pMHC ligand (31). To count the number of pMHC molecules required for T-cell activation, we labeled pMHC complexes with phycoerythrin and visualize individual pMHC at the interface of APC and T cells using video fluorescence microscopy. Irvine et al. (31) first developed this method to study the activation of T-helper cells and found that even a single agonist peptide–MHC ligand could stop T-cell motility and induced a transient increase in cytoplasmic calcium, and as few as 10 agonist pMHC molecules could lead to a sustained calcium flux and the formation of a mature immunological synapse. Further studies by Purbhoo et al. (17) showed that cytotoxic T cells could also detect a single pMHC ligand and only required three ligands for killing, even without the formation of a mature synapse. Ebert et al. (16) then used this peptide counting technique to examine the ligand sensitivity of T cells in thymic negative selection and found that as few as two agonist ligands in the contact area between APC and immature CD4+CD8+ double positive (DP) thymocytes could lead to the apoptosis of the latter. The extremely high sensitivity of immature DP thymocytes has also been shown in many earlier studies. For example, low affinity antigenic peptides that were unable to activate mature effector T cells were found to be sufficient to induce strong activation and clonal deletion (32); antagonists that were normally inhibitory to effector T cells could induce positive selection (33). These observations demonstrate that T-cell sensitivity is intrinsically regulated to ensure proper development of specificity and sensitivity to foreign antigens while avoiding self-recognition. The study by Li et al. (34) found that the microRNA miR-181a was expressed at high levels in DP thymocytes and low levels in mature T cells. Furthermore, increasing miR-181a expression in mature T cells augmented the sensitivity to peptide antigens, even enabled T cells to recognize antagonists. In contrast, inhibiting miR-181a expression in the immature T cells reduced sensitivity and impairs both positive and negative selection. These effects were in part achieved by the downregulation of multiple phosphatases, which led to elevated steady state levels of phosphorylated intermediates and a reduction in the T-cell receptor signaling threshold. These results suggest that T lymphocytes are able to adjust their antigen sensitivity to fit the need of different development stages, and miR-181a is an intrinsic ‘rheostat’ for that purpose.
So with the now substantial evidence that TCR multimerization is necessary for TCR-mediated activation and the evidence discussed above that even one peptide-MHC ligand is sufficient to initiate T-cell activation, how can TCRs dimerize or multimerize around a single agonist peptide-MHC? A possible explanation stems partially from the work of Stefanova et al. (35), who found that class II MHC molecules, presumably loaded with endogenous peptides, were important for the maintenance and responsiveness of CD4+ T cells in mice. This was followed by Krogsgaard et al. (36), who found that soluble class II MHC heterodimers, in which one MHC contained an agonist peptide while the other contained a particular endogenous peptide, could stimulate T cells of the appropriate specificity in vitro. This supports a ‘psuedodimer model’ of T-cell activation in which a TCR could use compatible endogenous peptides (termed ‘coagonists’) together with CD4 to form a five-membered activating complex (two TCRs, one CD4, and two pMHCs) as proposed earlier by Irvine et al. (31). This could explain how T cells could become activated when only one agonist pMHC was present and also gives a molecular rationale for positive selection, in that TCRs would be selected for their ability to weakly bind to particular endogenous peptide-MHC complexes that could serve as coagonists in the periphery [and also provide tonic stimulation to T cells as per Stefanova et al. (35)]. Consistent with this later postulate is work showing that thymocytes are very specific for what peptides stimulate them in vivo to positively select into CD4+ T cells (37, 38). This model has been disputed by Finkel et al. (39), who were unable to see cooperativity between agonists and one of coagonist peptides described by Krogsgaard et al. (36) in a lipid bilayer stimulation.
The TCR movement during synapse formation
Numerous earlier studies (5, 40, 41) have shown that TCRs form microclusters upon T-cell activation and are quickly transported to cSMAC of the immunological synapse. Such centripetal movement of TCRs is believed to be caused by the association between TCRs and the cytoskeleton (42–46). This was first shown by a study from the Finkel group, in which they used antibodies against TCRs to activate T cells, and found by immunoprecipitation as well as cell-free reconstitution experiments that tyrosine-phosphorylated TCR/CD3 complexes were associated with actin cytoskeletons (42, 47). They found that tyrosine phosphorylation of the third immunoreceptor tyrosine-based activation motifs (ITAM) of CD3ζ played a critical role in such association and that the process was driven by Lck, suggesting that TCR/CD3 linkage to cytoskeleton was a result of TCR activation. There have been many other reports supporting the similar notion.
What we do not know yet, however, is whether ligand engagement is required for TCR to form linkages with the cytoskeleton. This is due to a lack of technology to directly visualize the movement of a ligand-engaged TCR and unengaged TCR, because a pMHC-engaged TCR usually unbinds from its pMHC ligand within a few seconds and can no longer be discerned from a TCR that has not seen antigens. We recently used the photocrosslinking technique described above to fix a pMHC ligand to its cognate TCR, which enabled us to visualize the dynamics of ligand-bound TCRs and free TCRs separately (48) (Fig. 2B). Specifically, we visualized engaged TCRs using fluorescently labeled photocrosslinked pMHC ligands, and simultaneously visualized unengaged TCRs using fluorescently labeled antigen binding fragments (Fab) against TCR-Vβ3, which could not bind to engaged TCRs. We found that all or most engaged TCRs moved rapidly to the cSMAC upon T-cell activation, whereas most unengaged TCRs remained randomly distributed on the T-cell surface. This was also supported by the observation that the number of TCR molecules in the cSMAC region was only slightly in excess of the pMHC molecules in that same region. These findings indicate that ligand engagement is necessary for the formation of linkage between TCR/CD3 complexes and cytoskeleton, which are subsequently transported to the cSMAC.
The TCR/CD3 and actin cytoskeleton are likely linked by Lck, as suggested by early studies from the group of Finkel (42, 47). The movement to the cSMAC is likely driven by some motor proteins. Recent studies have suggested that myosin IIA (49) and/or dynein (50) are responsible for this process, but it is not clear which one is more critical or whether there are other motor proteins involved. Interestingly, Valitutti et al. (51) recently proposed that calcium flux could induce actin cytoskeleton to bind or to trap TCRs. Notably, TCRs in their experimental system were untriggered, because the T cell was activated by ionomycin rather than ligand engagement. Since our result has suggested a link between ligand engagement and TCR-cytoskeleton association, the decrease in TCR mobility in their study is more likely due to diffusion trapping by actin cytoskeleton rather than TCR association with actin cytoskeleton.
It has also been proposed that the T-cell activation signal is sustained by newly formed TCR clusters in the initial contact site and the periphery of synapses, and that once in the cSMAC, TCRs are internalized, presumably to terminate signaling (20, 52). However, recent studies of Shaw et al. (53, 54) have shown that the cSMAC can either downregulate TCRs or enhance signaling, depending on the quality of ligands. In our system, most engaged TCRs have moved to the cSMAC within 5 min, yet the signaling is sustained for a considerable period beyond that, as indicated by calcium flux and interleukin-2 (IL-2) secretion assays. These TCRs were not internalized as the fluorescent intensity of dyes conjugated to TCR–pMHC complexes in the synapse did not decrease over 25 min, probably because the TCRs were tethered to the lipid bilayer through the tags on the MHCs. This result indicates that the synapse is not a ‘dead zone’ for TCR signaling, which is partially consistent with the work of Shaw et al. (53, 54). In fact, beyond the TCR, the synapse itself is a rich source of extracellular crosstalk that works to sustain or abrogate cognate interactions.
Other surface molecules in synapse formation
The utility of co-receptors in a productive IS is also a topic in need of more clarity. Although considered to be specialized helpers of TCR activation, their exact role in transducing activation signals is not clear. CD8 binds to MHC class I, while CD4 binds to MHC class II and are thought to stabilize antigen receptor activation and its association with the pMHC complex. We and others have postulated that the role of CD4 may change depending on the cells forming the synapse, for example, T-DC synapse versus a thymocyte synapse during selection (55). Recent data revealed that blockade of CD4 did not affect the stability of the TCR-pMHC complex (56), but had an attenuating effect on downstream LAT phosphorylation and calcium flux. Chakraborty et al. (57) have modeled CD4 and CD8 activities in the context of T-cell activation. They conclude that in general, the binding of either molecule is not crucial for TCR stability and activation (even with the greater affinity of CD8 for its class I MHC ligand), but that their main purpose is to direct the kinase lck to productive TCR-pMHC complexes. This hypothesis is supported by early data suggesting that CD4 binding is subsequent to the initial TCR-pMHC engagement (58) and particularly by the recent data of Jiang et al. (59).
A number of other surface receptors, such as adhesion molecules and costimulatory receptors, also play a significant role in both the formation of the SMAC and in cellular crosstalk. Interaction between leukocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule (ICAM) precedes mature synapse formation and facilitates signaling events that promote T-cell activation (40). However, it is also known that the quality of the pMHC that is presented to the TCR will influence the clustering of these molecules into the cSMAC, independently of integrins such as LFA-1/ICAM interaction in the pSMAC (60). We have shown that MHC bearing antagonist peptides can compete for TCR engagement with MHC bearing agonist peptides and prevent full realization of the ‘stop signal’ and cell arrest, despite LFA-ICAM1 ligation (60). This exemplifies how adhesion molecules can work independently of TCR/pMHC interaction, despite the fact that they are an integral part of the SMAC. On the other hand, costimulatory (and in turn inhibitory) molecules can influence the quality of the TCR/pMHC interaction either positively or negatively, respectively (61– 63). Particularly important in synapse formation are the findings (61, 64) that stimulation through both TCR and the CD28 or LFA-1 costimulatory molecules trigger an active transport of TCRs and other molecules into the synapse. Previously it had been thought that passive diffusion and co-capping, as it was called, was sufficient to generate the characteristic IS structure. In addition, CD28 is also important in response functions, such as proliferation and IL-2 production, but cannot overcome the effect of antagonizing peptide interference (60). Engagement of CD2 has also been found to promote efficient downstream signaling after TCR engagement (65). In turn, CD2 is also involved with the internalization of the TCR after productive formation of the IS, which serves to disrupt sustained signaling by an APC displaying agonist peptides. Thus, CD2 plays an integral part in resetting the activation threshold for a cell.
Timing the earliest events during T-cell activation
One of the very early signaling steps downstream of antigen recognition is the activation of the Src family kinase Lck and subsequent phosphorylation of immunoreceptor tyrosine-based activation motifs on the CD3 signaling molecules. Phospho-CD3ζ chains recruit another protein kinase ZAP-70, which in turn phosphorylates the adapter protein LAT anchored on the plasma membrane. The resulting phospho-LAT proteins recruit phospholipase C-γ (PLC-γ), which hydrolyzes phosphatidylinositol-4,5-bisphosphate to yield the second messengers diacylglycerol (DAG) and inositol-1,4,5-triphosphate (IP3). DAG recruits other signaling proteins to the membrane, while IP3 triggers the influx of calcium into the cytoplasm (66, 67). These will lead to other downstream events, such as the reorientation of the microtubule organizing center (MTOC) to the T cell-APC contact site (68, 69), the formation of immunological synapses, and the secretion of cytokines, etc.
These events are known to be remarkably fast, but exactly how fast they are and how they change over time had not been clear until recently. Timing these early events of T-cell signaling is of great interest, because it would provide new insights into the organization of T-cell signaling network and also help to understand how T cells are so sensitive and specific to antigens. Earlier efforts to timing the T-cell signaling events using video microscopy have noted the rapidity of TCR signaling, but these studies were not able to precisely define the timescale of T-cell activation, except to within about 15 min. Thus, our group (70) and that of Jay Groves (71) worked independently to synthesize a photoactivatable pMHC reagent, which was intrinsically nonstimulatory to the TCR, but would turn into an agonist pMHC upon irradiation by UV light. These studies used the antigenic peptide from moth cytochrome c (MCC) (amino acids 88–103, ANERADLIAYLKQATK) bound to the murine MHC class II molecule I-Ek, which is specifically recognized by 5C.C7 and 2B4 TCRs. Specifically, Lys99 of the MCC peptide, a key TCR-recognition residue, was protected by a photocleavable 1-ortho-nitrophenyl-ethyl urethane (NPE) moiety on its ε-amino group (NPE-MCC) (Fig. 3A). The resulting pMHC reagent, NPE-MCC-I-Ek, was immobilized on glass coverslips. 5C.C7 or 2B4 T cells carrying fluorescent signaling probes (e.g. green fluorescent protein fusion proteins or calcium dyes) were then loaded on to the surface, and the signaling events were imaged by either epifluorescence or total internal reflection fluorescence (TIRF) microscopy. NPE-MCC-I-Ek was non-stimulatory to 5C.C7 or 2B4 T-cell blasts even though T cells could still touch the surface. This was to be expected, because the centrally located Lys99 (p5) of the MCC peptide is critical for agonist activity with most T cells specific for this pMHC, and adding a bulky, hydrophobic NPE group to its side chain should block recognition. Subsequent irradiation with UV light at 365 nm cleaves off the NPE group to yield native MCC peptide (Fig. 3A), restoring its stimulation potency. Since the photoactivation reaction occurs within 1 ms after exposure to UV lights (72), this approach enabled one to achieve precise temporal control over the TCR–pMHC recognition. Since NPE-MCC-I-Ek reagents in any micron-scale region of the surface beneath the T cell can be specifically activated using a source of focused UV light, one can achieve precise spatial control of T-cell activation as well. The intracellular responses to this stimulatory event would then be recorded. In particular, the use of TIRF microscopy was very useful for visualizing TCR proximal signaling dynamics that occur at the membrane, because it would only record high-resolution images of the plasma membrane in contact with the glass.
Fig. 3. A photoactivatable pMHC ligand enabled the precise temporal determination of early events in TCR signaling.
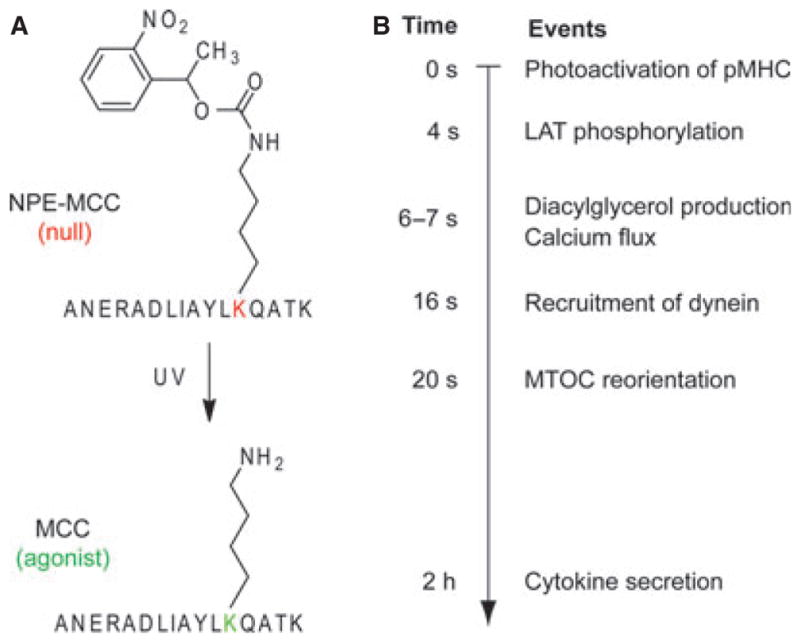
(A) A photoactivatable agonist peptide NPE-MCC was generated by attaching the 1-ortho-nitrophenyl-ethyl urethane (NPE) moiety to the ε-amino group of Lys99, the key TCR-recognition residue, in the MCC peptide. Irradiation with ultraviolet light would cleave off the NPE group to yield the native MCC peptide. The NPE-MCC/I–Ek complex was produced and coated to the glass surface and 5C.C7 T-cell blasts were attached to this surface. A brief UV-laser illumination would expose MCC/I–Ek in situ and enable the measurement of early events of T-cell activation with subsecond time resolution as well as micron-scale spatial resolution. (B) Summary of the timing of several key events in T-cell activation, as determined using the above technology in two studies (70, 75).
This photoactivatable pMHC ligand combined with the rapid sampling of subsequent responses (100 ms intervals) enabled us to define the moment of agonist engagement and to time a number of early TCR signaling events with unprecedented precision (Fig. 3B). We found that LAT was phosphorylated within 4 s of TCR stimulation, DAG and calcium signaling were induced after 6–7 s, and stimulated MTOC translocation occurred in less than 2 min. The offset times we observed were substantially shorter than those in earlier reports, likely due to the fact that we were able to separate the time interval required for cell-surface contact formation from the interval that encompasses the signaling response. Blocking the coreceptor CD4 reduced the magnitude of LAT phosphorylation and the speed of calcium flux. DAG production desensitized quickly after initial TCR triggering, but LAT phosphorylation and MTOC reorientation remained sensitive to repeated stimulation. These results suggested that some molecular events behaved like incidence detectors, whereas others acted more like signal integrators. The above data also gave us new insights into the ‘kinetic proofreading’ process. The kinetic proofreading model proposes that T cells must complete a series of signaling events to be activated, thus the recognition of an antigen with a too short half-life (t1/2) will not be able to activate T cells (73). To achieve optimal discrimination between an agonist pMHC and a null pMHC, the length of this ‘proofreading delay’ would need to be several times longer than the t1/2 of a typical agonist ligand, which is about 1 s at 37 °C (74). The offset times for LAT phosphorylation and DAG production both satisfy these criteria, although it remains unclear which delay plays the proofreading role in this system. In any case, our results suggest that T cells discriminate between agonists and endogenous ligands within the first 6 –7 s, by using signaling steps upstream of calcium flux.
Huse et al. (75) continued to use this UV-induced T-cell activation system and video fluorescent microscopy to study the signaling cascade leading to the MTOC reorientation in T-cell activation. They demonstrated that PLC-γ activity is required for MTOC reorientation. On further examination, they found that the localized accumulation of DAG, but not the influx of calcium, links PLC-γ activity to MTOC reorientation. MTOC reorientation was preceded by synaptic accumulation of DAG (by 14 s) and the motor protein dynein (by 4 s) (Fig. 3B). Using a photoactivatable version of DAG, they found that DAG accumulation alone was sufficient to drive the MTOC reorientation.
The kinetics of pMHC-TCR interactions: 2D (in situ) versus 3D (in vitro)
T cells can detect as few as one single antigenic pMHC ligand among an abundance of endogenous pMHC ligand. It is widely believed that the kinetics of pMHC-TCR interaction plays a critical role in determining T cell’s activity and specificity, but there are still many debates about which kinetic parameters are most important. Some studies showed that the potency of pMHC ligands correlate with its affinity (Kd) to cognate TCRs, while many others argued that the half-life (t1/2) of pMHC-TCR interaction was the most important factor. This discrepancy was even more pronounced as two recent articles (76, 77) proposed the concept of effective or aggregate t1/2 to account for the possibility that a pMHC ligand may rebind to the same TCR multiple times. Surface plasmon resonance (SPR) and microcalorimetric methods were two most popular methods used in these types of studies to analyze the pMHC-TCR interaction. Using these methods, the TCR–pMHC interaction was found to be usually very weak, with affinities (Kd value) in the range of 1–100 μM and half-lives (t1/2) on the order of seconds. However, these methods used purified pMHC and TCR in solution. Thus, the resulting kinetic parameter, which is referred as the 3D kinetic parameter, might not account for what occurred in situ where the pMHC and TCR are anchored on two opposite cell membranes in the context of cellular interface. Many factors within the cell-cell interface might have been overlooked, such as the restricted intercellular volume, the favorable molecular alignment of TCR and MHC, and molecular preclustering on cell membranes, etc.
Our group recently devised a single molecule fluorescence resonance energy transfer (FRET) method to measure the 2D kinetic parameters of pMHC-TCR interactions in immunological synapses (56) (Fig. 4). The results were vastly different from what we had obtained earlier using SPR. FRET is a distance-dependent physical process in which the excited state energy from a fluorescent dye molecule (a FRET donor) is transferred to another fluorescent dye molecule (a FRET acceptor). Since its efficiency is dependent on the inverse sixth power of the distance between two fluorescent molecules, FRET is a powerful technique to determine the distance between two molecules in close proximity (typically 10–100 Å). We used CD4+ T-cell blasts from 5C.C7 TCR transgenic mice, which specifically recognize MCC–I-Ek. We labeled TCRs on the T-cell surface with Cy3-conjugated single-chain variable fragments (scFv) of antibody H57, and labeled peptide–MHC complexes with Cy5 and presented them on the planar lipid bilayer. If the 5C.C7 TCR contacted MCC–I-Ek, the distance between Cy5 and Cy3 was estimated to be about 41 Å and would generate FRET signals. As expected, we observed the FRET signal when the 5C.C7 T cell contacted the bilayer that presented agonist MCC–I-Ek, but not when the bilayer presented a null pMHC (Fig. 4B,C). By real-time FRET analysis of Cy3-labeled TCRs on T-cell surfaces and Cy5-labeled pMHC ligands on planar lipid bilayers, we were able to calculate the 2D t1/2 and off-rate (koff) of agonist pMHC–TCR interactions in immunological synapses. We found that 2D t1/2 values were consistently four- to 12-fold shorter than 3D t1/2. Treatment of cells with actin-depolymerizing drugs cytochalasin D and latrunculin A reversed this effect, making the 2D and 3D t1/2 values almost identical. These findings indicate that cytoskeletal dynamics act to destabilize pMHC-TCR interactions, perhaps to favor the more stable of these (56). We also calculated the density of synaptic TCRs, pMHCs, and TCR-pMHC complexes on the basis of the average fluorescent intensity of single molecules, from which we were able to estimate the 2D Kd, and on-rate (kon) as well. We found that 2D Kd was 8.3-fold lower than that measured in 3D by SPR. This was a result of a large (about 100-fold) increase in 2D on-rates, a likely consequence of complementary molecular orientation and clustering.
Fig. 4. A real-time FRET system for analysis of TCR–pMHC interactions in situ.
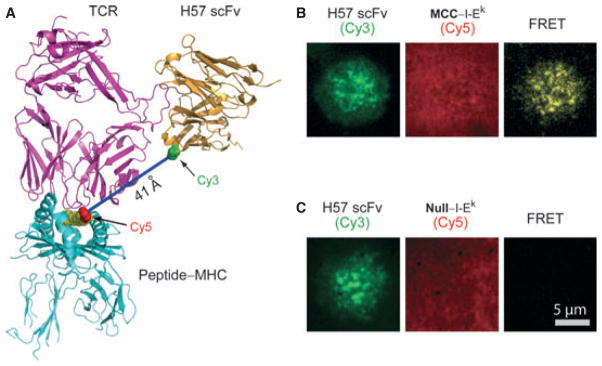
(A) A fluorescence resonance energy transfer (FRET) system was developed to analyze the in situ kinetic parameters of TCR binding to pMHC in synapses. TCRs on the T-cell surface were labeled with Cy3-conjugated H57-scFv, and peptide–MHCs on the planar lipid bilayer were labeled with Cy5 (at the C-terminal extension of bound peptides). The distance between Cy5 and Cy3 was about 41 Å. (B) FRET signals were observed when 5C.C7 transgenic T cells contacted the bilayer presenting agonist MCC–I-Ek ligands. (C) FRET signals were not observed when the bilayer presented null pMHC ligands.
Concurrently, Huang et al. (78) studied 2D kinetics of pMHC-TCR interactions using a novel mechanical assay. They immobilized a naive OT-1 CD8+ T cell to a micropipette, and then manipulated it to touch a red blood cell or a bead coated with cognate pMHC ligands. They then measured the adhesion frequency or the thermal fluctuation arisen from the pMHC-TCR interaction, from which they were able to calculate the 2D affinities, on-rates, and offrates. Similar to our findings, they found that the 2D affinity between a TCR and its antigenic pMHC was higher than the 3D affinity, which was driven by a vastly increased on-rate. Furthermore, they found that the 2D affinities and on-rates showed far greater differences from strong to weak pMHC ligands compared with those measured in solution, and that the 2D kinetics matched better with the functional outcome of pMHC ligands than the 3D kinetics did. Finally, 2D offrates were 30–8,300 fold faster than 3D off-rate, with the agonist pMHC dissociating the fastest. Recently, Adams et al. (79) found that a mutant pMHC that has a high affinity to its cognate TCR could not stimulate T cells expressing that TCR. Utilizing another approach using the mechanic assay described above, they found that this particular pMHC had a low 2D affinity to its cognate TCR. This result further underlined the importance of 2D in situ kinetics in determining T cells’ functional responses.
The accelerated kinetics of pMHC-TCR interaction in the cellular interface as observed in both studies was likely due to the cytoskeletal dynamics, because the effect could be reversed by the addition of the actin-depolymerizing drugs cytochalasin D and/or latrunculin A. Recently, Robert et al. (80) used a laminar flow chamber to monitor the 2D kinetics of pMHC-TCR interaction and found that the 2D dissociation rate was comparable to the 3D dissociation rate. Since it was a cell-free system, this result was consistent with the previous notion that the accelerated kinetics was due to cytoskeletal dynamics. In any case, the faster kinetics at the cellular interface may enable an individual pMHC ligand to successively engage many TCRs or engage the same TCR multiple times in a short period of time, which was consistent with the serial engagement model (107). Recently, we found that covalently bound pMHC ligands in large numbers (>2,600 per T cell) was more stimulatory than standard pMHC ligands, which suggested that serial TCR engagement was dispensable for T-cell activation when a large portion of TCRs were engaged (48). Nevertheless, serial engagement will still occur due to the fast in situ kinetics, and it may be critical for activation when ligand density is limited.
Cytokine secretion and communication through the synapse
As discussed above, the cytoskeleton is a key element for proper IS formation from the initial stage of engagement, to the rearrangement of organelles and the progression to a mature synapse, to the final stage where dissolution of the TCR-pMHC complex abolishes continued signaling. In a resting state, the cytoskeleton helps to form and maintain the protein islands in a T cell, to prevent premature activation through non-specific contact between signaling proteins (81). Upon activation or initial TCR-pMHC ligation, cytoskeletal cues promote both downstream activation and cell arrest to stabilize cell to cell contact at the IS (82–84).
The signaling machinery downstream of the TCR after a productive IS formation depends on the previous experience of the T cell. Both naive and antigen-experienced cells have different requirements for full activation. As such, naive cells were found to preferentially phosphorylate ERK after TCR-pMHC engagement that, in turn, works to attenuate the influx of calcium into the cell (85). Alternatively, memory or antigen-experienced T cells will induce phosphorylation of p38 instead and quickly amplify the activation signal (85). In either case, as these signaling pathways are fired and transcription factors are phosphorylated for translocation to the nucleus, the cells are thus positioned to relay important information about the type of immune response that is required.
While the cytoskeleton is important for the overall structure of an IS during cell-cell interaction, it is believed that it also plays a vital role in directed cell secretion of proteins toward the engaged APC. Recent studies have shown that microtubules reorganize directly beneath the synaptic junction, positioning them perfectly for the transport of both cytokines and apoptotic granules through the IS (10, 11, 14, 15, 86). This positioning forms a natural route for secretory products to leave the cell.
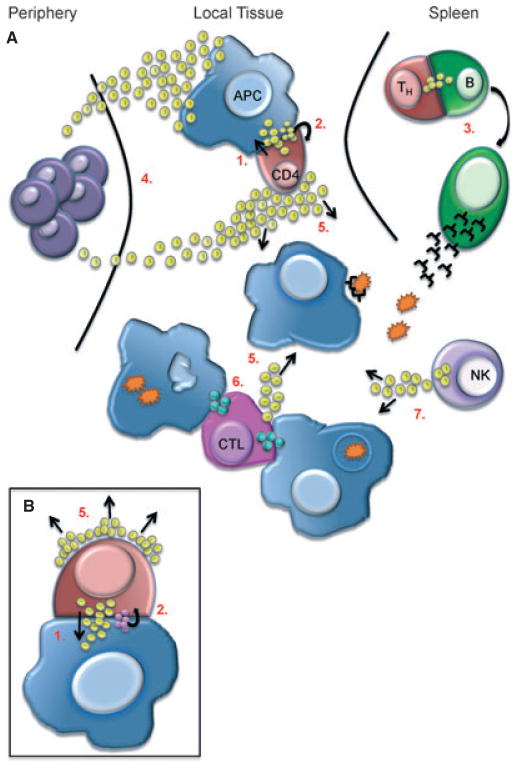
A cognate interaction between a CD4+ T cell and a B cell will achieve a mature IS and contact will be maintained over several hours (10). During this time, information is transferred between the cells via direct or indirect communication which likely happens at all stages of IS formation. Directed communication describes instances where cells can either talk to themselves in an autocrine fashion or to another cell through local, paracrine communication via the synapse (Fig. 5). Because the IS provides a very confined space that allows for the concentration of cytokine secreted into it, it is the perfect conduit for this kind of self- and cross-communication. For example, formation of an immature IS is enough to allow IL-2 production in naive cells and their activation in an autocrine manner, due to expression of the low affinity IL-2 receptor. Subsequent upregulation of the high affinity receptor on the T cell helps promote the full activation potential and expression of other surface receptors for T-cell differentiation (87). Alternatively, T cells can instruct another cell, such as a B cell bearing an agonist pMHC, to differentiate and mature (88). In yet a third example, they can initiate a death program to kill an infected cell by secreting lytic granules via the synapse (89). Importantly, the IS is not necessarily a tightly sealed interface. Communications may be ‘overheard’ by cells not directly engaged in an IS, and this is known as bystander activation. For example, IFNγ secreted via a synapse has been shown to promote bystander activation of cells that are in close proximity to but not directly targeted by the effector cell (90).
Fig. 5. Immune cell communication.
The IS promotes the exchange of information by directing secretion of soluble proteins, such as cytokines (yellow spheres) or cytolytic granules (blue spheres) between two specific cells. (A) As in the case of CD4/APC interaction upon TCR recognition of a specific pMHC complex secretion is directed in a paracrine (1), as well as autocrine (2) fashion (See also inset B) which helps promote CD4 activation and polarization to a T-helper (Th) cell. Differentiated Th cells migrate to the spleen where they can promote B-cell activation and plasma cell formation, also through directed secretion of cytokine via the synapse (3). Secretion of cytokines and chemokines directed away from the IS help to recruit innate immune cells from the periphery for immediate host defense (4) or promote bystander activation during an immune response (5). Cytotoxic T lymphocytes (CTL) are able to form synapses with multiple target cells for simultaneous killing via directed secretion of cytolytic granules (6). Finally, activated immune cells, such as NK cells, can secrete cytokine in the absence of a stable IS to amplify the immune response (7).
Cells can also communicate indirectly via a long distance paracrine mechanism directed away from the IS. Huse et al. (91) discovered that synapsing murine CD4+ T cells secrete specific cytokines, especially chemokines, away from the synapse upon activation using a separate secretory pathway. Similar bidirectional secretion was also observed in human CD8+ T cells (Davis MM, et al., unpublished results). In this way, cells can relay broader messages in a non-specific manner through the release of proinflammatory cytokines like IL-6 and TNFα. Likewise, changing a chemokine gradient will recruit immune cells to a target tissue to help with and amplify the immune response. Taken together, these studies show how a T cell, upon recognition of its cognate antigen, is capable of simultaneously directing secreted protein toward and away from the IS.
These cellular messages are only as effective as the ability of the target cells to interpret them by expressing the appropriate receptors. In turn, it is also important to remember that IS formation and cellular communication is often a two-way process. It has been shown that CD8+ T cells will polarize toward activated cells to kill those that they are in contact with specifically and not others in the vicinity (92). In addition, dendritic cells forming an IS with a T cell will polarize their MTOC for directed secretion of IL-12 at the synapse (93). Interestingly, both naïve and preactivated B cells will orient their MTOC toward an activated T cells even before a mature synapse has formed (92). This two-way communication helps to enhance cognate interaction between the engaged cells by forming a productive or cooperative IS on both sides. As further evidence of this, dendritic cells have been shown to cluster costimulatory ligands and adhesion molecules at the SMAC via their own cytoskeletal changes to increase T-cell stimulation and facilitate differentiation (94–97).
However, a large part of cellular communication depends not only on spatial context but also situational context, or the conditions of engagement. For example, CTLs can acquire functional competency and polarize their lytic machinery toward target cells independently of the usual molecular rearrangement that occurs during traditional synapse formation (15). In this way, one CTL is able to kill multiple targets around it simultaneously and quickly, without the need to form molecular clusters of surface receptors at each point of contact. The ability of CTLs to polarize their secretory machinery toward multiple cells allows for directed and efficient killing without the requirement of a tight synaptic interface that is seen with CD4+ T cells. Thus, we can also discuss cellular communication and information exchange as it relates to the physical synaptic framework.
T cells can be functional in the absence of synapse formation
For mature T cells, antigen recognition is often characterized by the formation of an immunological synapse between the T cell and the cell it is recognizing. In the case of T-B interactions, the synapse has the characteristic bull’s-eye pattern which was first discovered by Monks et al. (5). But there are also notions that T cells do not always require a mature synapse for becoming functional. Gunzer et al. (98) found that in a collagen matrix cell culture system, naive T-helper (Th) cells had only very transient interactions with dendritic cells (DC) presenting antigens and did not form a stable synapse. Rather, brief contacts with one DC and then another over many hours were sufficient to stimulate Th cells to proliferate and to provide help. We found that a cytotoxic T cell required about 10 pMHC complexes to form a mature synapse, but it only needed three pMHC complexes for killing (17). This finding suggested that formation of a stable and mature synapse was not required for cytotoxicity. Reports from other groups have led to the same conclusion (99, 100). A related finding was that a gene knockout of the adapter molecule CD2AP prevented T cells from forming a mature T-B synapse, and yet the CD4+ T cells were able to proliferate and produce IL-2 relatively normally (53).
Our group recently investigated the synapse formation in immature T cells during thymus selection, and the findings are intriguing. In thymus, immature T cells, i.e., DP thymocytes, interact with APCs presenting self peptide-MHCs and are subjected to both positive and negative selections. Thymocytes that strongly respond to self pMHCs are likely to undergo programmed cell death (the negative selection), and thymocytes that fail to achieve a sufficient threshold of TCR signaling against self pMHC are also dead by neglect (the positive selection). Only 1–5% thymocytes are able to survive both selections to become mature T cells and leave the thymus and circulate in the body. These mature T cells are sensitive to pMHC ligands, but not autoreactive. Earlier reports have showed that thymocytes are extremely sensitive to their cognate peptide-MHC ligands, even more than mature T cells (101, 102). Negative selection of TCR transgenic thymocytes has also been shown to occur in response to far fewer peptides per APC (on average) than mature T cells bearing the same transgenic TCRs (103). Thus, it would be of great interest to investigate whether thymocytes would form the same synapses as mature T cells do.
To investigate synapse formation during thymic selections, we used a reaggregate system to mimic the thymic microenvironment (104). Specifically, fetal thymic stromal cells were mixed with thymocytes under conditions that promote the growth of a multicellular reaggregate entity in which thymic positive and negative selection occurs readily. Using such a reaggregate thymus organ culture (RTOC) system, we transduced immature thymocytes with retroviral constructs containing CD3-green fluorescent protein (GFP) or Lck-GFP and characterized the synapses that these cells formed with thymic stromal cells when the appropriate negatively selecting peptide was introduced into the culture. It was found that cell conjugates formed very rapidly upon peptide introduction. In addition, CD3-GFP did not form stable, central accumulations as in mature T cells, but instead tended to accumulate on the periphery of the synapse. Lck-GFP was also accumulated in the periphery of the synapse, but it behaved the same way in both mature and immature T cells. This different synapse architecture is likely partly due to a lack of costimulation and may lead to different signals downstream of the TCR.
Using the same RTOC system to study the positive selection, we showed that productive signaling for positive selection did not involve formation of a synapse between thymocytes and selecting epithelial cells (16). Indeed, they did not even adhere tightly enough to thymic epithelium presenting such ligands to remain in contact with those cells over the course of minutes in a RTOC. Nonetheless, thymocytes could maintain signaling, as gauged by nuclear translocation of a GFP-labeled nuclear factor of activated T cells c (NFATc) construct. Furthermore, antibody blockade of endogenous positively selecting ligands prevented NFAT nuclear accumulation in such cultures and reversed NFAT accumulation in previously stimulated thymocytes. From these findings, we inferred that thymocytes achieve the duration of signaling required for maturation through many transient encounters, potentially with many different epithelial cells, rather than the prolonged contact with a single epithelial cell that immune-synapse formation might promote. As such, we proposed a ‘gauntlet’ model that speculates that thymocytes mature by continually acquiring and reacquiring positively selecting signals without sustained contact with epithelial cells. A lack of synapse-sustained contact would allow thymocytes to continually scan many epithelial cells for pMHCs that promote positively selecting signals. This might ensure a lower error rate in this critical transition, because it prevents the maturation of thymocytes that are ‘restricted’ only to very rare peptide-MHC.
Juang et al. (105) also studied the role of dimers and monomers in thymic selection using the dimerization system of Stern et al. (108). Consistent with the earlier discussion here of T-cell activation, no biological effect of peptide-MHC monomers (using the OT-1 TCR system) could be seen in fetal thymic organ cultures. Dimers of the Ova peptide (SIINFEKL) bound to H-2Kb induced negative selection very robustly, as did heterodimers of this agonist combined with any endogenous peptide [as identified by Hogquist et al. (106)]. Interestingly, positive selection also required dimers of pMHC, especially those previously described as biologically active (106). Thus, dimers are the minimal activation entity for both mature T-cell activation and thymic selection.
Conclusion
The discovery and intensive study of immunological synapses over the past 12 years have taught us a great deal about how this form of communication is structured and the kinds of information that are transmitted. Still, we obviously have much to learn about the mechanisms behind much of what we can ‘see’ about the processes, such as the role of microclusters migrating to the center of the synapse or the precise functions and organization of the cytoskeletal components. Most of our knowledge is also confined to T:B synapses, and not much about other types of stable cell-cell interactions that are going on, especially those that might require specialized in vivo environments such as in the thymus or the lymph node. While multi-photon imaging has made great strides in visualizing those interactions, we still do not have the technology to say much about their molecular underpinnings.
We also have deliberately framed this review as a lead-in to the larger question of how the immune system communicates with itself, with synapses playing a major, but still partial role. This question of how the immune system works as a system is surely one of the next big challenges before us. Thus, the emerging field of systems immunology will be key for keeping track of so many moving parts (350 CD antigens alone! 100 s of cytokine combinations possible!) (2). While computational models will be critical, we also suspect that there are many communication strategies yet to be discovered.
Acknowledgments
The authors are grateful for the funding from the Howard Hughes Medical Institute (to M. M. D.), the US National Institutes of Health (to M. M. D.), and the Cancer Research Institute (to J. X.).
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Schluter SF, Bernstein RM, Bernstein H, Marchalonis JJ. ‘Big Bang’ emergence of the combinatorial immune system. Dev Comp Immunol. 1999;23:107–111. doi: 10.1016/s0145-305x(99)00002-6. [DOI] [PubMed] [Google Scholar]
- 2.Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012;36:142–152. doi: 10.1016/j.immuni.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harwood NE, Batista FD. Early events in B cell activation. Annu Rev Immunol. 2010;28:185–210. doi: 10.1146/annurev-immunol-030409-101216. [DOI] [PubMed] [Google Scholar]
- 4.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 6.Freiberg BA, et al. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 7.Sperling AI, Sedy JR, Manjunath N, Kupfer A, Ardman B, Burkhardt JK. TCR signaling induces selective exclusion of CD43 from the T cell-antigen- presenting cell contact site. J Immunol. 1998;161:6459–6462. [PubMed] [Google Scholar]
- 8.Leupin O, Zaru R, Laroche T, Muller S, Valitutti S. Exclusion of CD45 from the T-cell receptor signaling area in antigen-stimulated T lymphocytes. Curr Biol. 2000;10:277–280. doi: 10.1016/s0960-9822(00)00362-6. [DOI] [PubMed] [Google Scholar]
- 9.James JR, Vale RD. Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature. 2012;487:64–69. doi: 10.1038/nature11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueda H, Morphew MK, McIntosh JR, Davis MM. CD4+ T-cell synapses involve multiple distinct stages. Proc Natl Acad Sci USA. 2011;108:17099–17104. doi: 10.1073/pnas.1113703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 12.Sanderson CJ, Glauert AM. The mechanism of T-cell mediated cytotoxicity. VI. T-cell projections and their role in target cell killing. Immunology. 1979;36:119–129. [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen D, Fishelson Z, Berke G. The role of cytotoxic T lymphocyte projections in target cell lysis. Transplant Proc. 1981;13:1073–1078. [PubMed] [Google Scholar]
- 14.Faroudi M, et al. Lytic versus stimulatory synapse in cytotoxic T lymphocyte/target cell interaction: manifestation of a dual activation threshold. Proc Natl Acad Sci USA. 2003;100:14145–14150. doi: 10.1073/pnas.2334336100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiedemann A, Depoil D, Faroudi M, Valitutti S. Cytotoxic T lymphocytes kill multiple targets simultaneously via spatiotemporal uncoupling of lytic and stimulatory synapses. Proc Natl Acad Sci USA. 2006;103:10985–10990. doi: 10.1073/pnas.0600651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert PJ, Ehrlich LI, Davis MM. Low ligand requirement for deletion and lack of synapses in positive selection enforce the gauntlet of thymic T cell maturation. Immunity. 2008;29:734–745. doi: 10.1016/j.immuni.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 18.Schamel WW, et al. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J Exp Med. 2005;202:493–503. doi: 10.1084/jem.20042155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci USA. 2007;104:20296–20301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boniface JJ, et al. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands. Immunity. 1998;9:459–466. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 23.Delon J, et al. CD8 expression allows T cell signaling by monomeric peptide-MHC complexes. Immunity. 1998;9:467–473. doi: 10.1016/s1074-7613(00)80630-5. [DOI] [PubMed] [Google Scholar]
- 24.Cochran JR, Cameron TO, Stern LJ. The relationship of MHC-peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity. 2000;12:241–250. doi: 10.1016/s1074-7613(00)80177-6. [DOI] [PubMed] [Google Scholar]
- 25.Cebecauer M, et al. CD8+ cytotoxic T lymphocyte activation by soluble major histocompatibility complex-peptide dimers. J Biol Chem. 2005;280:23820–23828. doi: 10.1074/jbc.M500654200. [DOI] [PubMed] [Google Scholar]
- 26.Kuhns MS, et al. Evidence for a functional sidedness to the alphabetaTCR. Proc Natl Acad Sci USA. 2010;107:5094–5099. doi: 10.1073/pnas.1000925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun ZY, Kim ST, Kim IC, Fahmy A, Reinherz EL, Wagner G. Solution structure of the CD3epsilondelta ectodomain and comparison with CD3epsilongamma as a basis for modeling T cell receptor topology and signaling. Proc Natl Acad Sci USA. 2004;101:16867–16872. doi: 10.1073/pnas.0407576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhns MS, Davis MM. TCR signaling emerges from the sum of many parts. Front Immunol. 2012;3:159. doi: 10.3389/fimmu.2012.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Randriamampita C, Boulla G, Revy P, Lemaitre F, Trautmann A. T cell adhesion lowers the threshold for antigen detection. Eur J Immunol. 2003;33:1215–1223. doi: 10.1002/eji.200323844. [DOI] [PubMed] [Google Scholar]
- 30.Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 31.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 32.Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 33.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 34.Li QJ, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 36.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 37.Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10:1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009;10:1155–1161. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Z, Sharp KA, Janmey PA, Finkel TH. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol. 2008;6:e43. doi: 10.1371/journal.pbio.0060043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wulfing C, Sjaastad MD, Davis MM. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc Natl Acad Sci USA. 1998;95:6302–6307. doi: 10.1073/pnas.95.11.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 42.Rozdzial MM, Malissen B, Finkel TH. Tyrosine-phosphorylated T cell receptor zeta chain associates with the actin cytoskeleton upon activation of mature T lymphocytes. Immunity. 1995;3:623–633. doi: 10.1016/1074-7613(95)90133-7. [DOI] [PubMed] [Google Scholar]
- 43.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Billadeau DD, Nolz JC, Gomez TS. Regulation of T-cell activation by the cytoskeleton. Nat Rev Immunol. 2007;7:131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 46.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 47.Rozdzial MM, Pleiman CM, Cambier JC, Finkel TH. pp 56Lck mediates TCR zeta-chain binding to the microfilament cytoskeleton. J Immunol. 1998;161:5491–5499. [PubMed] [Google Scholar]
- 48.Xie J, et al. Photocrosslinkable pMHC monomers stain T cells specifically and cause ligand-bound TCRs to be ‘preferentially’ transported to the cSMAC. Nat Immunol. 2012;13:674–680. doi: 10.1038/ni.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol. 2009;10:531–539. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashimoto-Tane A, et al. Dynein-driven transport of T cell receptor microclusters regulates immune synapse formation and T cell activation. Immunity. 2011;34:919–931. doi: 10.1016/j.immuni.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Dushek O, et al. Effects of intracellular calcium and actin cytoskeleton on TCR mobility measured by fluorescence recovery. PLoS ONE. 2008;3:e3913. doi: 10.1371/journal.pone.0003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokosuka T, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 53.Lee KH, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 54.Cemerski S, et al. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–422. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis MM, Krogsgaard M, Huse M, Huppa J, Lillemeier BF, Li QJ. T cells as a self-referential, sensory organ. Annu Rev Immunol. 2007;25:681–695. doi: 10.1146/annurev.immunol.24.021605.090600. [DOI] [PubMed] [Google Scholar]
- 56.Huppa JB, et al. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463:963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Artyomov MN, Lis M, Devadas S, Davis MM, Chakraborty AK. CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc Natl Acad Sci USA. 2010;107:16916–16921. doi: 10.1073/pnas.1010568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hampl J, Chien YH, Davis MM. CD4 augments the response of a T cell to agonist but not to antagonist ligands. Immunity. 1997;7:379–385. doi: 10.1016/s1074-7613(00)80359-3. [DOI] [PubMed] [Google Scholar]
- 59.Jiang N, et al. Two-stage cooperative T cell receptor-peptide major histocompatibility complex-CD8 trimolecular interactions amplify antigen discrimination. Immunity. 2011;34:13–23. doi: 10.1016/j.immuni.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sumen C, Dustin ML, Davis MM. T cell receptor antagonism interferes with MHC clustering and integrin patterning during immunological synapse formation. J Cell Biol. 2004;166:579–590. doi: 10.1083/jcb.200404059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wulfing C, Davis MM. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- 62.Diehn M, et al. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci USA. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 65.Singleton K, et al. A large T cell invagination with CD2 enrichment resets receptor engagement in the immunological synapse. J Immunol. 2006;177:4402–4413. doi: 10.4049/jimmunol.177.7.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huse M. The T-cell-receptor signaling network. J Cell Sci. 2009;122:1269–1273. doi: 10.1242/jcs.042762. [DOI] [PubMed] [Google Scholar]
- 67.Lin J, Weiss A. T cell receptor signalling. J Cell Sci. 2001;114:243–244. doi: 10.1242/jcs.114.2.243. [DOI] [PubMed] [Google Scholar]
- 68.Geiger B, Rosen D, Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol. 1982;95:137–143. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kupfer A, Dennert G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol. 1984;133:2762–2766. [PubMed] [Google Scholar]
- 70.Huse M, et al. Spatial and temporal dynamics of T cell receptor signaling with a photoactivatable agonist. Immunity. 2007;27:76–88. doi: 10.1016/j.immuni.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 71.DeMond AL, Starr T, Dustin ML, Groves JT. Control of antigen presentation with a photoreleasable agonist peptide. J Am Chem Soc. 2006;128:15354–15355. doi: 10.1021/ja065304l. [DOI] [PubMed] [Google Scholar]
- 72.McCray JA, Trentham DR. Properties and uses of photoreactive caged compounds. Annu Rev Biophys Biophys Chem. 1989;18:239–270. doi: 10.1146/annurev.bb.18.060189.001323. [DOI] [PubMed] [Google Scholar]
- 73.McKeithan TW. Kinetic proofreading in T-cell receptor signal transduction. Proc Natl Acad Sci USA. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krogsgaard M, et al. Evidence that structural rearrangements and/or flexibility during TCR binding can contribute to T cell activation. Mol Cell. 2003;12:1367–1378. doi: 10.1016/s1097-2765(03)00474-x. [DOI] [PubMed] [Google Scholar]
- 75.Quann EJ, Merino E, Furuta T, Huse M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat Immunol. 2009;10:627–635. doi: 10.1038/ni.1734. [DOI] [PubMed] [Google Scholar]
- 76.Aleksic M, et al. Dependence of T cell antigen recognition on T cell receptor-peptide MHC confinement time. Immunity. 2010;32:163–174. doi: 10.1016/j.immuni.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Govern CC, Paczosa MK, Chakraborty AK, Huseby ES. Fast on-rates allow short dwell time ligands to activate T cells. Proc Natl Acad Sci USA. 2010;107:8724–8729. doi: 10.1073/pnas.1000966107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang J, et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464:932–936. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adams JJ, et al. T cell receptor signaling is limited by docking geometry to peptide-major histocompatibility complex. Immunity. 2011;35:681–693. doi: 10.1016/j.immuni.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robert P, Aleksic M, Dushek O, Cerundolo V, Bongrand P, van der Merwe PA. Kinetics and mechanics of two-dimensional interactions between T cell receptors and different activating ligands. Biophys J. 2012;102:248–257. doi: 10.1016/j.bpj.2011.11.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lillemeier BF, Pfeiffer JR, Surviladze Z, Wilson BS, Davis MM. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc Natl Acad Sci USA. 2006;103:18992–18997. doi: 10.1073/pnas.0609009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 83.Dustin ML. Coordination of T cell activation and migration through formation of the immunological synapse. Ann NY Acad Sci. 2003;987:51–59. doi: 10.1111/j.1749-6632.2003.tb06032.x. [DOI] [PubMed] [Google Scholar]
- 84.Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat Immunol. 2004;5:531–538. doi: 10.1038/ni1065. [DOI] [PubMed] [Google Scholar]
- 85.Adachi K, Davis MM. T-cell receptor ligation induces distinct signaling pathways in naive vs. antigen-experienced T cells. Proc Natl Acad Sci USA. 2011;108:1549–1554. doi: 10.1073/pnas.1017340108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 87.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. Molecular programming of B cell memory. Nat Rev Immunol. 2012;12:24–34. doi: 10.1038/nri3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jenkins MR, Griffiths GM. The synapse and cytolytic machinery of cytotoxic T cells. Curr Opin Immunol. 2010;22:308–313. doi: 10.1016/j.coi.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanderson NS, et al. Cytotoxic immunological synapses do not restrict the action of interferon-gamma to antigenic target cells. Proc Natl Acad Sci USA. 2012;109:7835–7840. doi: 10.1073/pnas.1116058109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 92.Duchez S, Rodrigues M, Bertrand F, Valitutti S. Reciprocal polarization of T and B cells at the immunological synapse. J Immunol. 2011;187:4571–4580. doi: 10.4049/jimmunol.1100600. [DOI] [PubMed] [Google Scholar]
- 93.Pulecio J, et al. Cdc42-mediated MTOC polarization in dendritic cells controls targeted delivery of cytokines at the immune synapse. J Exp Med. 2010;207:2719–2732. doi: 10.1084/jem.20100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tseng SY, Waite JC, Liu M, Vardhana S, Dustin ML. T cell-dendritic cell immunological synapses contain TCR-dependent CD28-CD80 clusters that recruit protein kinase C theta. J Immunol. 2008;181:4852–4863. doi: 10.4049/jimmunol.181.7.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hiltbold EM, Poloso NJ, Roche PA. MHC class II-peptide complexes and APC lipid rafts accumulate at the immunological synapse. J Immunol. 2003;170:1329–1338. doi: 10.4049/jimmunol.170.3.1329. [DOI] [PubMed] [Google Scholar]
- 96.de la Fuente H, et al. Synaptic clusters of MHC class II molecules induced on DCs by adhesion molecule-mediated initial T-cell scanning. Mol Biol Cell. 2005;16:3314–3322. doi: 10.1091/mbc.E05-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Benvenuti F, et al. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J Immunol. 2004;172:292–301. doi: 10.4049/jimmunol.172.1.292. [DOI] [PubMed] [Google Scholar]
- 98.Gunzer M, et al. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 99.O’Keefe JP, Gajewski TF. Cutting edge: cytotoxic granule polarization and cytolysis can occur without central supramolecular activation cluster formation in CD8+ effector T cells. J Immunol. 2005;175:5581–5585. doi: 10.4049/jimmunol.175.9.5581. [DOI] [PubMed] [Google Scholar]
- 100.Fleire SJ, Goldman JP, Carrasco YR, Weber M, Bray D, Batista FD. B cell ligand discrimination through a spreading and contraction response. Science. 2006;312:738–741. doi: 10.1126/science.1123940. [DOI] [PubMed] [Google Scholar]
- 101.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 103.Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Cutting edge: negative selection of immature thymocytes by a few peptide-MHC complexes: differential sensitivity of immature and mature T cells. J Immunol. 1999;162:3117–3120. [PubMed] [Google Scholar]
- 104.Richie LI, Ebert PJ, Wu LC, Krummel MF, Owen JJ, Davis MM. Imaging synapse formation during thymocyte selection: inability of CD3zeta to form a stable central accumulation during negative selection. Immunity. 2002;16:595–606. doi: 10.1016/s1074-7613(02)00299-6. [DOI] [PubMed] [Google Scholar]
- 105.Juang J, Ebert PJ, Feng D, Garcia KC, Krogsgaard M, Davis MM. Peptide-MHC heterodimers show that thymic positive selection requires a more restricted set of self-peptides than negative selection. J Exp Med. 2010;207:1223–1234. doi: 10.1084/jem.20092170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hogquist KA, et al. Identification of a naturally occurring ligand for thymic positive selection. Immunity. 1997;6:389–399. doi: 10.1016/s1074-7613(00)80282-4. [DOI] [PubMed] [Google Scholar]
- 107.Valitutti S. The Serial Engagement Model 17 Years After: From TCR Triggering to Immunotherapy. Front Immunol. 2012;3:272. doi: 10.3389/fimmu.2012.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cochran JR, Cameron TO, Stern LJ. The relationship of MHC-peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity. 2000;12:241–250. doi: 10.1016/s1074-7613(00)80177-6. [DOI] [PubMed] [Google Scholar]