Abstract
To address the complex nature of cancer occurrence and outcomes, approaches have been developed to simultaneously assess the role of two or more etiological agents within hierarchical levels including the: 1) macro-environment level (e.g., health care policy, neighborhood, or family structure); 2) individual level (e.g., behaviors, carcinogenic exposures, socioeconomic factors and psychological responses); 3) biological level (e.g., cellular biomarkers and inherited susceptibility variants). Prior multilevel approaches tend to focus on social and environmental hypotheses, and are thus limited in their ability to integrate biological factors into a multilevel framework. This limited integration may be related to the limited translation of research findings into the clinic. We propose a “Multi-level Biological And Social Integrative Construct” (MBASIC) to integrate macro-environment and individual factors with biology. The goal of this framework is to help researchers identify relationships among factors that may be involved in the multifactorial, complex nature of cancer etiology, to aid in appropriate study design, to guide the develop statistical or mechanistic models to study these relationships, and to position the results of these studies for improved intervention, translation, and implementation. MBASIC allows researchers from diverse fields to develop hypotheses of interest under a common conceptual framework, to guide transdisciplinary collaborations, and to optimize the value of multilevel studies for clinical and public health activities.
Keywords: Multilevel Model, Cancer Etiology, Translation, Implementation
Motivation
Cancer is etiologically complex and its causes are multifactorial. Risk factors associated with cancer development have been identified that represent a variety of levels of influence on health and disease (Table 1). Macro-environment factors including health system, neighborhood or community characteristics, have increasingly been linked to cancer incidence and mortality (1, 2). In addition, social determinants and processes (1, 3, 4) have been identified as cancer risk factors, including socioeconomic status or self-reported race (5-8). Environmental exposures at the level of the individual (5) including cigarette smoking (9), radon (10), asbestos (11), diet (12), and physical activity (13) are causally associated with some cancers. Applied and fundamental investigations have identified a wide array of biologic factors mechanistically involved in carcinogenesis including those of the tumor microenvironment, metabolome, proteome, transcriptome, and genome. For example, hundreds of novel genetic susceptibility loci have been identified through candidate and genome-wide association studies (GWAS (14)).
Table 1.
Hierarchical Level Definitions
| Level | Sub-Level | Factors at this Level Can Serve As: |
|---|---|---|
| Macro- Environment |
•Health Policy (National, State, Local) |
•Exposures that affect individual risk factors |
| •Community, Neighborhood | •Exposures that affect biological processes |
|
| •Social and Built Environment | •Contextual variables (87)Contextual variables (87) |
|
| •Practice Setting and Health Care Providers |
||
| •Family and Social Support | ||
|
| ||
| Individual | •Behaviors | •Exposures leading to disease |
| •Exposures | •Intermediates between the macro- environment and disease |
|
| •Psychological Determinants | ||
| •Socioeconomic Factors | ||
|
| ||
| Biologic | •Tissue | •Processes leading to disease |
| •Cell | •Intermediates and biomarkers reflecting the relationship between macro- environmental and individual factors |
|
| •Somatic Genome (SG) | ||
| •Inherited Genome (IG) | ||
Studies of factors at a single level have provided a great deal of insight into the etiology of disease. Despite successes in identifying cancer risk factors, these approaches are limited and at some point the information obtained from these single-level studies reach a saturation point, and have provided as much information as they can. It is clear that the factors reported to date do not fully explain cancer incidence in the general population. For example, while smoking is strongly associated with lung cancer (15), most smokers will not be diagnosed with lung cancer, whereas some non-smokers will (16). While BRCA1 or BRCA2 mutation carriers have a greatly increased lifetime risk of developing breast cancer (17), some BRCA1/2 mutation carriers are never diagnosed with breast or ovarian cancer, even at an advanced age. GWAS have identified a wealth of susceptibility genes, but the identification of novel genes using this approach is unlikely to continue ad infinitum. Therefore, risk factors studied in isolation and identified by standard approaches are unable to fully explain the complex, multifactorial causes of cancer. For this reason, cancer research has evolved from focusing on single factors to studies of complex relationships between social, behavioral, molecular, and environmental factors.
Overview of Current Multilevel Approaches
To address the complex nature of cancer etiology, multilevel approaches have been developed to simultaneously assess the role of two or more etiological agents within a hierarchical or nested structure (18). A number of conceptual frameworks have been proposed that integrate information across levels of disease etiology, including the “web of disease” of MacMahon and Pugh (19), the “wheel” of Mausner et al. (20), “systems epidemiology”(21), and more recent models of multifactorial etiology ((22-27), Multilevel approaches are generally characterized by three main levels: 1) macro-environment, referred to elsewhere as “eco-level” (23, 24); 2) individual; and 3) biology (Table 1). Each of these levels is further characterized by sub-levels (Table 1) that define domains of variables involved in cancer etiology or outcomes. Multi-level conceptual frameworks are based on the premise that factors affecting disease act within and across levels to collectively affect disease. These approaches generally hypothesized that cancer outcomes can result from the complex relationship of factors at multiple levels in at least two ways (Table 1). First, factors at the macro-environment and individual levels can directly affect the biological events and result in cancer. Second, factors may confer risk in a hierarchal fashion, such that biologic-level effects are affected by behaviors or exposures of the individual, and individual level effects are affected by the macro-environment (28).
The relationships described above in the context of a multilevel model refer to both statistical and biological interactions. Here, we use the term “interaction” generically to refer to any non-additive statistical structure that can be constructed between two or more factors. This concept includes that of effect modification, mediation(29), as well as biological structures that may be defined between two or more factors (e.g., epistasis among genetic loci). The goal of the multilevel framework we will present here is not to define a specific form for interaction. A variety of statistical approaches have been developed to guide implementation of hierarchal, longitudinal, or multilevel models (18, 30-32). Instead, we hope to provide a framework around which a researcher can generate hypotheses about the relationship among etiologic agents in a consistent manner. When results of these hypothesis tests are known, investigators using our proposed framework may be better able to compare and combine their results to form coherent multilevel inferences.
Most multilevel approaches lack a detailed focus on mechanisms that can be used to frame the relationships between macro-environment or individual-level factors. In part, the limited incorporation of mechanistic hypotheses stems from the early multilevel models having evolved from research focused on social factors. Thus, multi-level conceptual approaches have tended to take a “top-down” approach that is focused on the role of social determinants at the macro-environment level (Table 2). Only more recently has a detailed consideration of the biological level been included in multilevel studies. For instance, the model of Warnecke et al. (22) centers on health disparities as the outcome of interest and defines macro-environment level factors by policies, institutions, and social or physical factors. They also include a single level including biological factors. Similarly, the models of Taplin et al. (23) and Gorin et al. (24) focus on improved cancer care, sub-dividing the macro-environment level by national and state health policy, local community environment, organization or practice setting, health care provider teams, and family/social support. Across proposed multi-level frameworks, the traditional individual level risk factors for cancer (e.g., smoking, race, diet) are also considered, whereas biological factors in these constructs remain broadly defined by genes, proteins, enzymes and other somatic changes in the cellular environment (23, 24, 33). In these models, all biological processes are treated in a manner similar to those of other levels without accounting for the extensive knowledge of biological processes, pathways, and etiologic relationships that are involved in carcinogenesis.
Table 2.
Multilevel Framework Examples
| Level | Hiatt and Breen (3) |
Warnecke et al. (22) | Taplin et al. (23) Gorin et al. (24) |
Morrissey et al. [26] |
| Macro- Environment |
Defined by Factors: Social determinants and Health Care Systems |
Defined by sub-levels (from largest to smallest): Social conditions (e.g., discrimination), Institutions (e.g., Families), Neighbor- hood, Social Relationships |
Defined by sub-levels: National/state policy, local community, organization or practice setting, health care providers, family/ social support |
Defined by sub- levels from Taplin et al. (23) Gorin et al. (24) |
| Individual |
Defined by Factorsy: Social Determinants, Behavioral/ Psychological factors |
Defined by Factors: Age, Socioeconomic status, Education, Obesity, Tobacco Use, Acculturation, Diet, Race |
Defined by Factors: Biological Factors, Sociodemographics, insurance coverage, risk status, comorbidities, knowledge, attitudes, beliefs, decision- making preferences, psychological reaction/coping |
Similar to Taplin et al. (23) Gorin et al. (24) where individual is described as the patient. |
| Biologic |
Defined by Factors: genes and biomarkers |
Defined by Factors: Allostatic Load (e.g., combination of stress markers or other biomarkers), Metabolic Processes, Genetic Mechanisms |
Defined by sub- levels (largest to smallest level): Organ, Tissue, Cell, Gene, Molecule, Atom |
|
| Primary Outcome of Interest |
Cancer Control Continuum (Pre-disease, pre-clinical, incidence, morbidity/ survival, mortality) Interventions (Prevention, early detection, diagnosis/treat- ment, quality of life) |
Cancer Health Disparities |
Cancer Care Continuum (Risk assessment, primary prevention, detection, diagnosis, treatment, survivorship, end of life) |
Cancer Care Continuum |
Approaches that focus on macro-environment have had an impact on the conceptual advancement of our understanding of disease etiology. While the National Institutes of Health has increasingly recognized and encouraged the use of multilevel approaches to go beyond investigating individual level factors to include macro-environment level exposures (34, 35), many of the current models(34, 35), many of the current approaches come from the perspective of social and environmental research, and the full integration of biological level factors has yet to be realized. A search of PubMed for the term “multilevel” and “cancer” resulted in 55 articles published between 2002 and 2012, although the majority of these (26 of 55, 47%) were published since 2010. Most of these studies focused on individual-level and macro-environmental factors, and few incorporated biological factors. Thus, work is needed to improve the understanding which factors at each level are relevant to the disease, the hierarchical nature of the relationship of those factors, and the effective application of integrative multilevel approaches to achieve meaningful etiological inferences.
Multilevel Biologic and Social Integrative Construct (MBASIC)
To the degree that a researcher has knowledge of biological mechanisms of human cancer, multilevel models could be used to harness this information and to generate hypotheses that link macro-environment or individual level factors with mechanisms of carcinogenesis. Current multi-level conceptual approaches, while created to promote multi-disciplinary research, often lack detailed descriptions of the biological level that could be used to unite traditionally distinct fields (e.g., molecular biology and social epidemiology).
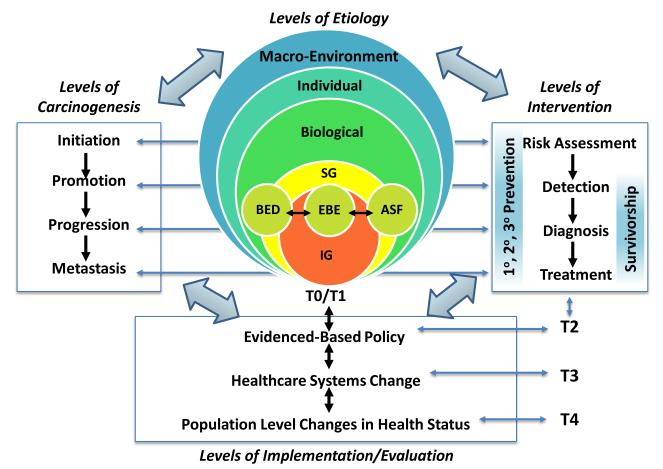
MBASIC defines the multilevel framework (construct) to include three main hierarchal levels that contribute to cancer etiology and levels of carcinogenesis (i.e., macro-environment, individual, and biological factors; Figure 1). This multilevel etiological model is then placed in the context of interventions, and translation/implementation (i.e., T0-T4; (36, 37); Figure 1). This framework allows researchers from the fields of public health, health policy, prevention, behavioral sciences, sociology, epidemiology, biology, clinical medicine, and others to test hypotheses of interest under a common conceptual framework, to address the dynamic nature of carcinogenesis, to facilitate translation of multilevel studies to clinical and public health strategies, and to support multi-disciplinary collaborations.
Figure 1. Multi-level Biological And Social Integrative Construct (MBASIC) (MBASIC).
This framework includes levels of etiology, carcinogenesis, intervention, and implementation/evaluation, as well as previously defined phases of translation (i.e., T0-T4; (36, 37). Biological levels include inherited genome (IG), somatic genome (SG), as well as related biomarkers of biologically effective dose (BED), biomarkers of early biological effect (EBE), and biomarkers of altered structure and function (ASF) (41).
The primary goal of MBASIC is to consistently and systematically frame complex hypotheses about cancer etiology. As may be expected with any comprehensive conceptual framework, the full range of MBASIC components is not meant to be implemented in any one study. Instead, MBASIC is meant to aid the researcher in stating hypotheses for individual studies that address a part of the complete framework. Thus, MBASIC provides the framework for hypotheses that allow comparison and compilation of individual study results using formal (e.g., meta-analytic) or ad hoc means. Individual studies built around the MBASIC framework could also motivate multidisciplinary collaborations and could rationalize single, large-scale multilevel studies in the future.
Predictive and Mechanistic Links Between and Among Hierarchical Levels of Etiology
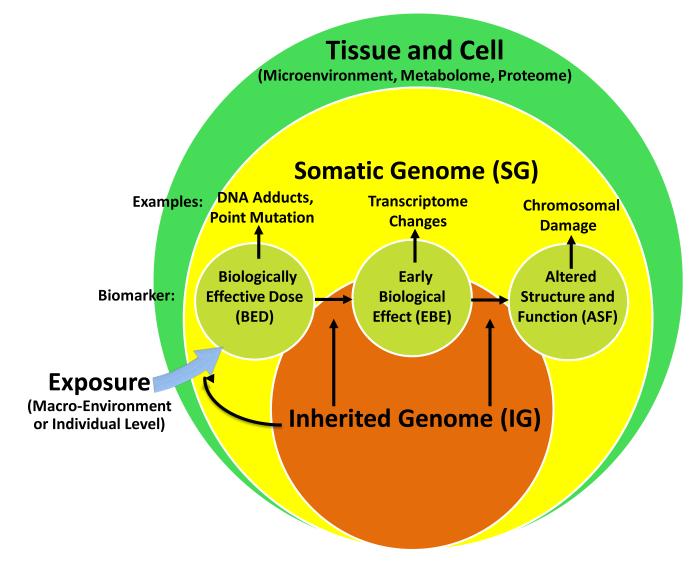
A primary goal of the MBASIC is to guide researchers to consistently and systematically incorporate biological mechanisms into a multilevel framework. Despite the substantial limitations in our ability to generate meaningful statistical or epidemiological models of mechanism and biological events (38, 39), knowledge of existing biological pathways emerging from animal, tumor, and other in vivo studies can be employed to improve generation of hypotheses about how each of the three hierarchal levels relates with the others in order to frame questions about the complexity of cancer etiology(40). The well-known molecular epidemiology paradigm (41-45) provides a useful structure into which biology can be incorporated into a multilevel framework. As shown in Figure 2 and defined below, the effect of exposures can be measured by biomarkers of biologically effective doses (BED), early biological effects (EBE), and altered structure and function (ASF) that are predictive of disease (42-45). The formation of these biomarkers can be influenced by inherited genotypes (IG). These factors can give rise to somatic genomic (SG) changes involved in carcinogenesis. Note that while prior constructs include markers of internal dose, which have great value as biomarkers for research, clinical or screening purposes, we exclude these in the present framework to emphasize biological and mechanistic effects in the multilevel etiology of cancer. While spontaneous mutation may give rise to the biomarkers of disease and effect shown in Figure 2, the multilevel construct assumes that each of the biomarkers occur in response an initial macro-environment or individual level exposure, even though that exposure may not be known or measurable.
Figure 2. Incorporating Molecular Epidemiology and Biomarkers in the Multilevel Framework.
Biological levels include inherited genome (IG), somatic genome (SG), as well as related biomarkers of biologically effective dose (BED), biomarkers of early biological effect (EBE), and biomarkers of altered structure and function (ASF) (41).
We adapt the traditional molecular epidemiology approach (42-45) in two ways: by considering the nested hierarchical nature of the multilevel model (Figure 2); and by expanding the definition of “exposure” to include both macro-environment level and individual level exposures. As noted in Table 1, relevant etiological factors can be measured by biomarkers (i.e., BED, EBE, ASF) of exposure or disease at the biological level. These biomarkers reflect somatic changes and are often measured at the tissue or cellular level. For example, biomarkers of exposure to cigarette smoking at the individual level can be measured by exposure biomarkers such as DNA adducts (42-45) in blood; prostate specific antigen (PSA) levels or chromosomal instability (45) measured in blood can serve as markers of disease. Thus, these factors may be framed as both processes leading to disease and as intermediates reflecting the relationship between macro-environmental and individual factors, separately, and disease (Table 1). For instance, macro-environment level variables can induce a psychological response, which can be directly measured at the biological level. Witnessing a crime in a neighborhood environment can lead to flight or fight cellular responses that cause increases in cortisol levels. Thus, cortisol is a biomarker of a macro-environment exposure. An example of a linkage between the individual level and the biological level is that of the human exposome (46). The exposome is defined by environmental exposures (including lifestyle factors) that represent combined exposures from all sources, from the prenatal period onwards (46). The exposome can be measured by biomarkers at the cellular level via bodily fluids or tissue that can serve as surrogates for exogenous or endogenous environmental exposures. For instance, exposure to organophosphate pesticides can be measured by certain metabolites, and dietary factors, like vitamin intake, can be measured by antioxidant metabolites. Like the GWAS approach, epidemiology has employed environment-wide association studies (EWAS) (46, 47) that use an agnostic approach to identifying environmental factors involved in disease. Future EWAS studies in cancer are warranted to provide practical evidence for a link between individual level exposures and the biological level. While EWAS and GWAS share some conceptual similarities, there are numerous methodological differences between the two approaches(48). However, the results of each can provide information that may promote the development of multilevel hypotheses in cancer etiology.
While the examples above demonstrate how macro-environment and the individual level factors can each separately affect the biological level as an exposure, we can also demonstrate the hierarchal effect among exposures at multiple levels on the biologic level. For example, exposure to a group of friends who smoke cigarettes could prompt an individual to change her behavior and also start to smoke cigarettes. This change in behavior at the individual level influences molecular carcinogenesis at the biologic level (i.e. DNA adducts; BED) and chromosomal damage (ASF). Despite symptoms of decreased lung function over the course of 15-20 years, the individual is genetically predisposed to nicotine dependence, is unable to quit smoking, and ultimately ends up developing lung cancer. Here, the behavior change served as an intermediate between the macro-environment and biological events involved in carcinogenesis. Thus, this example demonstrates the biological plausibility of how a macro-environmental factor can impact an individual, affecting her biological environment, ultimately resulting in disease. When the macro-environment, individual, and biological factors are collectively considered in order to predict or explain a cancer outcome, statistical methods will be needed to determine which levels or which risk factors within each level are most relevant to the cancer outcome under study. Thus, it is possible for intermediates to serve as surrogates of exposure and disease, but the importance of each level and each factor within each level will need to be determined statistically based on available methods.
Biology in a Multilevel Framework
Starting with the Levels of Etiology (Figure 1), the biological level can be subdivided into sub-levels with a hierarchical order based on our knowledge of biology and carcinogenesis: tissues are comprised of cells, which contain genes. Somatic mutations and cellular events (e.g. DNA replication) may be involved in carcinogenesis. In the following sections, we build the framework around which the biological level can be optimally incorporated into multilevel analysis (Figure 2).
Tissues
Tissues warrant consideration as a unique biological sub-level in a multilevel framework for two reasons. First, cellular markers and processes that are measured in normal tissue, pre-neoplasia, or malignant tumors could serve as potential markers of exposure, disease, or prognosis. Second, tumors occur at the tissue level. Most cancers are diagnosed and staged using tissue samples or by imaging techniques that may identify lesions in a particular organ. A growing area of research is focused on the tumor microenvironment, defined by normal cells, signaling molecules, matrices and blood vessels that surround and feed a tumor cell (49). A tumor can alter its microenvironment (as defined by cellular and genomic sub-level factors), and the microenvironment can affect how a tumor grows and spreads. Data about the role of the tumor microenvironment are rapidly becoming available via initiatives such as The Cancer Genome Atlas (TCGA; http://cancergenome.nih.gov).
Cells
The cellular sub-level is characterized by proteins, enzymes, and other biomarkers that can be detected in bodily fluids and tissues. In the context of our model, the cellular level includes the transcriptome, proteome, and metabolome, where biomarkers of exposure and disease can be measured (Figure 2). The transcriptome includes the various forms of RNA in the cell that affect gene expression and cellular function (50-52). The proteome includes the total set of proteins expressed in a given cell at a given time (51). Examples of factors measured in the proteome include prostate specific antigen (PSA) and CA-125 (45, 50, 53). Complex protein interactions are referred to as the metabolome (51, 54). Therefore, even within the cellular sub-level, there is an emerging hierarchy (51). Many approaches for disease biomarker discovery focus on a single biomarker at the cellular level, despite an emerging expectation that panels of biomarker analytes will be needed to provide sufficient sensitivity and specificity for cancer screening, diagnosis or prognosis (50, 54). Therefore, there is a shifting focus to the role of the pathway-based and statistical interactions among cellular factors, but progress in this area is limited by available, high-throughput technologies that can detect and organize the millions of proteins obtained from a given biological sample.
Somatic Genomics (SG)
The SG sub-level (Figures 1 and 2) is defined by acquired somatic genomic changes over the course of a person’s lifetime. The SG level is defined by factors that can be both markers of disease and markers of exposure. SG examples include mutations, copy number variants, and epigenetic changes occurring in DNA (50, 55). Early studies of SG used methods that identify potential susceptibly loci a priori, but this approach used a small number of genetic markers, rarely identified robust associations between candidate genes and cancer, and most findings were not replicable in other studies (14).
Inherited Genomics (IG)
The IG sub-level (Figures 1 and 2) is comprised of inherited susceptibility loci that serve as markers of disease risk and outcome. IG includes hereditary cancer syndromes (56), which confer a high risk of cancer development. IG research may use family-based linkage methods to identify important inherited, high-penetrance genes, such as BRCA1 and BRCA2. However, the mutations in these genes are rare in the general population (4, 14, 17), and only explain a small fraction of familial aggregation and cancer risk. GWAS have identified many dozens of cancer susceptibility loci (43, 57), most of which were not previously hypothesized to be involved in cancer susceptibility (14). Despite this success, genetic risk variants identified from GWAS, alone and in combination, explain a relatively minor proportion of disease risk, and have had limited translational value to the clinic. This has led to a focus on the identification of rare variants that may account for larger proportions of cancer genetic risk(58).
Given the limited clinical utility of SG and IG findings focused on single disease loci and statistical interactions thereof, there has been a renewed interest in studying epistasis, defined as genes at two or more loci that produce phenotype effects that are different than the expected effects of the individual loci (59). At both the SG and IG sub-levels, gene-gene interaction studies are being conducted to ascertain the independent and joint effects of risk loci on cancer outcomes (60). These studies may use multiple cancer risk susceptibility loci based on pathway or shared biological function, or be combined using statistical predictive models independent of biological knowledge.
Non-Hierarchical Effects Within and Across Levels of Etiology
Mechanisms and example methodologies have been proposed to build on the definition of the biological level and to illustrate how interactions between and among factors at each level relates to one another, assuming a hierarchal structure for levels of etiology (Figure 1). Hypotheses that consider the hierarchical framework of MBASIC are readily constructed from the discussion provided above. However, the effects of factors within each of these levels need not follow a strict hierarchy. In the context of predictive (as opposed to mechanistic) models, each level can dynamically affect another. Thus, statistical (causal) inferences need not be constrained in a linear hierarchical fashion (61). Concepts in social science and genetics support this assertion. According to the Social Ecological Perspective (62, 63), human health results from the complex interaction of personal factors (e.g., behaviors, biology, psychology) as well as physical and social environments (e.g., geography, built environment, culture, economics, politics, and social relationships) (62). For instance, a combination of geography, psychology, and behavior without a clear hierarchal or biological link could interact (statistically) and affect disease outcomes. Additionally, changes in eating habits at the individual level may affect social relationships at the macro-environment level since a person who is more conscious of their eating habits may prefer to be around other healthy eaters; the effect of each level on the other is not necessarily linear, top-down, or bottom-up. In the field of genetics, penetrance (64)In the field of genetics, penetrance (64) is defined by the probability of a phenotype given genotype. Even though a person is born with a disease genotype, lack of exposure to harmful environmental factors or carcinogens may prevent the disease from occurring. While it is likely that the disease genotype and exposure have some biological link, in the absence of this knowledge, specific methodologies aimed at analyzing gene-environment interactions, more recently, gene-environment interaction-wide association studies (GEIWAS) (65), can be developed to help elucidate statistical interactions across levels. Since it is clear that biological, social, and environmental factors interact in some way in cancer etiology, a multilevel framework is needed to both organize and guide traditionally separate fields of cancer research; however, these frameworks should also account for the dynamic nature of the disease.
Expanding MBASIC Levels of Intervention, Implementation and Evaluation
MBASIC expands the utility of the multilevel approach by including levels of etiology and carcinogenesis with levels of intervention and implementation/evaluation, all of which can influence one another in a nonlinear manner. Levels of intervention are characterized by primary, secondary, and tertiary prevention strategies and survivorship that range from risk assessment to detection to diagnosis and treatment (Figure 1). Previous multilevel studies have focused on assessing factors within the levels of intervention (3), particularly cancer care outcomes like detection or screening at the individual level or practice setting sub-level (23, 24).
The implementation/evaluation level is characterized by changes made through the application and translation of relevant interventions (66). Implementation/evaluation may occur through national, state, or local policy or health care systems changes, and the impact of interventions and implementation will ultimately be seen in changes to the health status of a population. The levels of implementation/evaluation are based on the translational model of Khoury et al. (36, 37), which describes five translational phases (Figure 1): T0/T1 (determination of mechanisms, etiology and development of interventional strategies); T2 (development of evidence-based policy and practice); T3 (implementing evidence-based guidelines to elicit health care system changes); and T4 (surveillance and monitoring the effect of changes on health outcomes in populations). Appropriate consideration of the dissemination, implementation, and evaluation of research findings into health systems is critical if the potential of multilevel models is to be realized. For instance, knowledge of the role of macro-environmental factors (e.g., residential location, social environment) in individuals with specific biological characteristics and risk factor profile could provide a resource-efficient approach to early detection or screening for cancer.
Simultaneous consideration of multiple levels in the MBASIC framework may impact a number of cancer outcomes. The levels or sublevels of inference (etiology, carcinogenesis, intervention, or implementation/evaluation; Figure 1) could serve as the outcome or exposure of interest. For instance, healthcare system changes (e.g., insurance coverage) can affect individual level behavior (e.g., participation in smoking cessation programs), which can affect the cellular environment (e.g., carcinogen levels and formation of DNA adducts). Therefore, interactions within and across levels can be modeled in a variety of ways, and extent to which the four levels of inference impact the trait of interest will vary depending on the etiological setting. For instance, during cancer initiation, living in a community that promotes cancer screening and having access to primary care may play a prominent role in cancer early detection. After cancer is diagnosed, the oncology provider and social support may become a predominant influence on clinical and psychosocial outcomes. Both of these scenarios may be imposed on a common biological context (e.g., a cancer having a specific mechanistic cause), but the relevant individual and macro-level factors may differ substantially.
MBASIC Example: Prostate Cancer
In the United States, prostate cancer (CaP) is the second leading cause of cancer death in men (67). CaP is of public health concern because it disproportionately affects different races. African American men are more likely to be diagnosed with and die from CaP than any other racial group, and this disparity is the largest observed for any cancer (68). Despite the burden of CaP, particularly for African American men, little is known about the etiology and predictors of poor prognosis for the disease. At present, the only widely agreed-upon risk factors for CaP are at the individual level: race, age, and family history of CaP (69). Tumor and patient characteristics used to identify men with a poor prognosis include tumor stage, Gleason score or grade, and prostate-specific antigen (PSA) level at diagnosis. However, these clinical characteristics are imperfect in their ability to determine long-term prognosis and appropriate treatment options. Thus, CaP is a good example of the potential value of the MBASIC framework.
PSA Screening: From the Cellular Level to T4 Implementation
In the 1980s, studies on the cellular level demonstrated that PSA levels could serve as markers for CaP recurrence (70). The use of PSA screening for patients undergoing treatment was approved in 1986 (70-72). Despite studies in the late 1980s suggesting that PSA might not be an ideal biomarker for screening and early detection of CaP (70-72), the FDA also approved PSA as an early detection screening test in 1994, and PSA became one of the first FDA approved early detection biomarkers for cancer (70). The FDA based its approval on a large clinic study whose results suggested that men with PSA values above 4.0mg/mL could be biopsied for cancer(73). As a result of this bench to beside clinical translation (T1 phase), screening guidelines with often conflicting recommendations from different organizations like the United States Preventive Services Task Force (74), the American Cancer Society (75), and the National Comprehensive Cancer Network (76), started to emerge. These guidelines affected clinical practice (T2 phase), resulting in more men being screened for and diagnosed with CaP (70). The health care system was also affected by these guidelines: insurance companies, particularly the Veterans Association and Medicare, incurred large costs covering routine PSA screening (T3 phase) (77). Continued research at both the population level (T4 phase) and levels of causation (cellular and individual levels) in more recent years have shown that PSA screening may not improve CaP mortality rates, that early detection of CaP can often lead to unnecessary treatment for some and insufficient treatment in others (70, 78, 79). As a result, researchers continue to developed enhanced PSA screening tests that are more sensitive and specific (70). Guidelines for routine PSA screening are continually being revised in the context of individual level factors. These include questioning the utility of screening for men under the age of 75 (74), focusing on screening high risk groups (5), and recommending baseline PSA measures in men under age 50 (58). Despite its limitations and controversies, PSA screening illustrates how cellular and individual levels of causation, resulting biomarker interventions, and health care implementation (Figure 1) can inform one another to optimize the early detection of CaP.
The PSA scenario also suggests that a comprehensive evaluation of PSA in early detection of CaP may benefit from the use of MBASIC to frame the hypotheses and approaches needed to improve screening and treatment for CaP. While macro-level factors have yet to be widely used in the context of PSA screening, it is not hard to imagine that screening strategies may be optimized by having a better understanding of those men who are most likely to have unfavorable CaP outcomes based on their socio-economic situation, access to health care, or other macro-environmental factors. The role of macro-environmental factors in CaP risk and mortality are beginning to emerge from the health disparity and PSA screening literature. Screening behaviors can be affected by economic, physical, and social characteristics of residential neighborhoods (80). Neighborhoods considered to be disadvantaged or low-income have been correlated with higher levels of pollutants, overcrowding, violence, less social cohesion, and less access to services (81).(81). Screening practices can affect CaP incidence, and low-income neighborhoods often have fewer medical facilities that are overburdened with indigent care to provide optimal screening(80). This can lead to differential screening practices by neighborhood (82) and differences in both the diagnosis and treatment of CaP, particularly among Caucasian versus African American men (83, 84). Therefore, neighborhood measures could serve as a surrogate for access to care in CaP, and appear to be a relevant macro-environment level measure to investigate for this cancer outcome. In the setting of an MBASIC approach, men with known biological risk profiles may therefore benefit from targeted intervention if they also reside in defined disadvantaged neighborhoods.
This concept is further illustrated by a multilevel analysis that investigated the role of individual level characteristics and census-tract neighborhood variables and stage of prostate cancer. Consistent with data showing an association between race, stage, and socioeconomic circumstances like living in a low income area, using geographical information systems technology, Xiao et al. (85) went beyond identifying factors associated with prostate cancer stage and suggest community education and outreach in areas with unfavorable neighborhood characteristics. In the context of MBASIC, discovery and early translation can be leveraged in a single study and can provide additional insights that would not be as readily apparent in studies focusing on a single etiological level.
Prostate Cancer Disparities: Piecing Together Studies on Biology and Neighborhood
Because of the complex etiology of CaP, an understanding of CaP disparities may benefit from a multilevel approach. A growing body of literature supports this hypothesis. Rundle and colleagues reported that neighborhood socioeconomic status (based on median income level of a census tract) modifies the association between individual smoking status and PAH–DNA adduct levels in prostate tissue(BED). We reported an interaction between CaP genetic susceptibility loci identified in GWAS and census-tract level neighborhood variables on time to PSA failure in men who had undergone radical prostatectomy (87). We identified no main effects of the genetic variants or neighborhood factors on PSA failure by themselves, but found statistically significant interactions between neighborhood variables and the susceptibility loci. Specifically, genotypes at MSMB and HNF1B/TCF2 predicted time to PSA failure in men from disadvantaged neighborhoods. This suggests that context-specific effects of genotype should be explored and may improve the ability to identify groups that may experience poor CaP outcomes. It is important to note that these studies represent predictive models that may have implications for implementation or translation, but themselves do not provide direct mechanistic conclusions. In general, these studies may motivate a continued focus on multi-level approaches and provide rationale for the utility of multilevel models in cancers like CaP, where typical single disciplinary approaches provide limited insight into disease etiology.
Charge to the Scientific Community
We have proposed a unifying conceptual framework that allows researchers from public health, policy, oncology, health services research, behavioral science, epidemiology, and the biomedical sciences to test hypotheses of interest under a common framework. The MBASIC framework allows researchers to generate common inferences from otherwise disparate individual research findings by using a common conceptual model. As illustrated by the prostate cancer example, taking a multilevel approach can help to expedite translation of etiologic findings into translational efforts, more than would occur in studies focused on single levels of etiology alone. By providing a stronger basis for inclusion of biological factors in a multilevel hierarchy, MBASIC bridges the gap between social science and biology in order to foster multidisciplinary collaboration and streamline intervention, implementation, and translation efforts. Emerging biomedical technologies enable population-based studies to include biomarker data such that the landscape of cancer research is changing and the lines between disciplines are increasingly blurring. MBASIC can serve as a road map for hypothesis generation and the development of emerging multidisciplinary teams. The MBASIC framework allows individual studies to more effectively piece together individual research findings under a common conceptual model. Knowledge gained from this integration can be used to rationalize the costs of future, large-scale, multilevel studies. Finally, MBASIC represents a framework around which transdisciplinary research (i.e., research that generates new fields of inquiry) can be built.
Acknowledgments
This research was supported by grants from the Public Health Service (P50-CA105641, P60-NM006900 and R01-CA85074 to TRR and F31-AG039986 to SML)
References
- 1.Krieger N. Defining and investigating social disparities in cancer: critical issues. Cancer Causes and Control. 2005;16:5–14. doi: 10.1007/s10552-004-1251-5. [DOI] [PubMed] [Google Scholar]
- 2.Krieger N, Quesenberry C, Peng T, Horn-Ross P, Stewart S, Brown S, et al. Social class, race/ethnicity, and incidence of breast, cervix, colon, lung, and prostate cancer among Asian, black, Hispanic, and white residents of the San Francisco Bay Area, 1988–92 (United States) Cancer Causes and Control. 1999;10:525–37. doi: 10.1023/a:1008950210967. [DOI] [PubMed] [Google Scholar]
- 3.Hiatt RA, Breen N. The Social Determinants of Cancer: A Challenge for Transdisciplinary Science. American journal of preventive medicine. 2008;35:S141–S50. doi: 10.1016/j.amepre.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes JH, Lehman A, Hade E, Ferketich AK, Gehlert S, Rauscher GH, et al. Challenges for Multilevel Health Disparities Research in a Transdisciplinary Environment. American journal of preventive medicine. 2008;35:S182–S92. doi: 10.1016/j.amepre.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute. National Institutes of Environmental Health Sciences . Cancer and the Environment. National Institutes of Health; Bethesda, MD: 2003. [Google Scholar]
- 6.Aarts MJ, Lemmens VEPP, Louwman MWJ, Kunst AE, Coebergh JWW. Socioeconomic status and changing inequalities in colorectal cancer? A review of the associations with risk, treatment and outcome. European journal of cancer (Oxford, England : 1990) 2010;46:2681–95. doi: 10.1016/j.ejca.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Bradley CJ, Given CW, Roberts C. Race, Socioeconomic Status, and Breast Cancer Treatment and Survival. Journal of the National Cancer Institute. 2002;94:490–6. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 8.Dayal HH, Polissar L, Dahlberg S. Race, socioeconomic status, and other prognostic factors for survival from prostate cancer. Journal of the National Cancer Institute. 1985;74:1001–6. [PubMed] [Google Scholar]
- 9.The United States Department of Health and Human Services . The health consequences of smoking: A Report of the Surgeon General. Washington, D.C.: 2004. [Google Scholar]
- 10.Lubin JH. Environmental Factors in Cancer: Radon. Rev Environ Health. 2010;25(1):33–38. doi: 10.1515/reveh.2010.25.1.33. [DOI] [PubMed] [Google Scholar]
- 11.Orenstein MR, Schenker MB. Environmental asbestos exposure and mesothelioma. Current Opinion in Pulmonary Medicine. 2000;6:371–7. doi: 10.1097/00063198-200007000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Gibson TM, Ferrucci LM, Tangrea JA, Schatzkin A. Epidemiological and Clinical Studies of Nutrition. Seminars in oncology. 2010;37:282–96. doi: 10.1053/j.seminoncol.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Na H-K, Oliynyk S. Effects of physical activity on cancer prevention. Annals of the New York Academy of Sciences. 2011;1229:176–83. doi: 10.1111/j.1749-6632.2011.06105.x. [DOI] [PubMed] [Google Scholar]
- 14.Varghese JS, Easton DF. Genome-wide association studies in common cancers—what have we learnt? Current Opinion in Genetics & Development. 2010;20:201–9. doi: 10.1016/j.gde.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Hecht SS. Tobacco Smoke Carcinogens and Lung Cancer. Journal of the National Cancer Institute. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 16.Thun MJ, Henley SJ, Burns D, Jemal A, Shanks TG, Calle EE. Lung Cancer Death Rates in Lifelong Nonsmokers. Journal of the National Cancer Institute. 2006;98:691–9. doi: 10.1093/jnci/djj187. [DOI] [PubMed] [Google Scholar]
- 17.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of Risk Reduction Estimates Associated With Risk-Reducing Salpingo-oophorectomy in BRCA1 or BRCA2 Mutation Carriers. Journal of the National Cancer Institute. 2009;101:80–7. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plewis I. Statistics in Education. Edward Arnold; London: 1997. [Google Scholar]
- 19.MacMahon B, Pugh TF, editors. Little, Brown and Company; Boston, MA: 1970. Epidemiology: principles and methods. [Google Scholar]
- 20.Mausner J, Kramer S, Bahn . Epidemiology: An Introductory Texts. Saunders; Philadelphia, PA: 1985. [Google Scholar]
- 21.Galea S, Riddle M, Kaplan GA. Causal thinking and complex system approaches in epidemiology. Int J Epidemiol. 2010;39:97–106. doi: 10.1093/ije/dyp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98:1608–15. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taplin SH, Anhang Price R, Edwards HM, Foster MK, Breslau ES, Chollette V, et al. Introduction: Understanding and Influencing Multilevel Factors Across the Cancer Care Continuum. JNCI Monographs. 2012;2012:2–10. doi: 10.1093/jncimonographs/lgs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorin SS, Badr H, Krebs P, Das IP. Multilevel Interventions and Racial/Ethnic Health Disparities. JNCI Monographs. 2012;2012:100–11. doi: 10.1093/jncimonographs/lgs015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med. 2006;62:1650–71. doi: 10.1016/j.socscimed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 26.Caporaso NE. Integrative study designs--next step in the evolution of molecular epidemiology? Cancer Epidemiol Biomarkers Prev. 2007;16:365–6. doi: 10.1158/1055-9965.EPI-07-0142. [DOI] [PubMed] [Google Scholar]
- 27.Spitz MR, Caporaso NE, Sellers TA. Integrative cancer epidemiology--the next generation. Cancer Discov. 2012;2:1087–90. doi: 10.1158/2159-8290.CD-12-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rappaport SM. Implications of the exposome for exposure science. J Expos Sci Environ Epidemiol. 2011;21:5–9. doi: 10.1038/jes.2010.50. [DOI] [PubMed] [Google Scholar]
- 29.Gelfand LA, Mensinger JL, Tenhave T. Mediation analysis: a retrospective snapshot of practice and more recent directions. J Gen Psychol. 2009;136:153–76. doi: 10.3200/GENP.136.2.153-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hox JJ. Applied Multilevel Analysis. TT-Publikaties; Amsterdam: 1994. [Google Scholar]
- 31.Kreft I, De Leeuw J. Introducing Multilevel Modelling. Sage; London: 1998. [Google Scholar]
- 32.Raudenbush SW, Willms JDE. Schools, Classrooms and Pupils. Academic Press; San Diego: 1991. [Google Scholar]
- 33.Morrissey JP, Lich KH, Price RA, Mandelblatt J. Computational Modeling and Multilevel Cancer Control Interventions. JNCI Monographs. 2012;2012:56–66. doi: 10.1093/jncimonographs/lgs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Office of Behavioral and Social Sciences Research. National Institutes of Health(NIH) Toward higher levels of analysis: progress and promise in research on social and cultural dimensions of health. NIH; Bethesda, MD: 2000. [Google Scholar]
- 35.Lam TK, Spitz M, Schully SD, Khoury MJ. Drivers of Translational Cancer Epidemiology in the 21st Century: Needs and Opportunities. Accepted article. 2012 doi: 10.1158/1055-9965.EPI-12-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khoury MJ, Gwinn M, Yoon PW, Dowling N, Moore CA, Bradley L. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med. 2007;9:665–74. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 37.Khoury MJ, Gwinn M, Bowen MS, Dotson WD. Beyond Base Pairs to Bedside: A Population Perspective on How Genomics Can Improve Health. American Journal of Public Health. 2011;102:34–7. doi: 10.2105/AJPH.2011.300299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siemiatycki J, Thomas DC. Biological models and statistical interactions: an example from multistage carcinogenesis. Int J Epidemiol. 1981;10:383–7. doi: 10.1093/ije/10.4.383. [DOI] [PubMed] [Google Scholar]
- 39.Thompson WD. Effect modification and the limits of biological inference from epidemiologic data. J Clin Epidemiol. 1991;44:221–32. doi: 10.1016/0895-4356(91)90033-6. [DOI] [PubMed] [Google Scholar]
- 40.Cortessis V, Thomas DC. Toxicokinetic genetics: an approach to gene-environment and gene-gene interactions in complex metabolic pathways. IARC Sci Publ. 2004:127–50. [PubMed] [Google Scholar]
- 41.Perera FP, Schulte PA. Chapter 1: A Conceptual and Historical Framework for Molecular Epidemiology. In: Schulte PA, Perera FP, editors. Molecular epidemiology: principles and practices. Academic Press; San Diego, CA: 1993. [Google Scholar]
- 42.Perera F, Weinstein IB. Molecular epidemiology and carcinogen-DNA adduct detection: new approaches to studies of human cancer causation. J Chronic Dis. 1982;35:581–600. doi: 10.1016/0021-9681(82)90078-9. [DOI] [PubMed] [Google Scholar]
- 43.Guy M, et al. Identification of new genetic risk variants for prostate cancer. Asian J Androl. 2009;11:49–55. doi: 10.1038/aja.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perera F. Molecular cancer epidemiology: a new tool in cancer prevention. J Natl Cancer Inst. 1987;78:887–98. [PubMed] [Google Scholar]
- 45.Schulte PA, Rothman N, Schottenfeld D. Chapter 6: Design Considerations in Molecular Epidemiology. In: Schulte PA, Perera FP, editors. Molecular epidemiology: principles and practices. Academic Press; San Diego, CA: 1993. [Google Scholar]
- 46.Patel CJ, Bhattacharya J, Butte AJ. An Environment-Wide Association Study (EWAS) on Type 2 Diabetes Mellitus. PLoS ONE. 2010;5:e10746. doi: 10.1371/journal.pone.0010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas DC, Siemiatycki J, Dewar R, Robins J, Goldberg M, Armstrong BG. The problem of multiple inference in studies designed to generate hypotheses. Am J Epidemiol. 1985;122:1080–95. doi: 10.1093/oxfordjournals.aje.a114189. [DOI] [PubMed] [Google Scholar]
- 48.Ioannidis JP, Loy EY, Poulton R, Chia KS. Researching genetic versus nongenetic determinants of disease: a comparison and proposed unification. Sci Transl Med. 2009;1:7ps8. doi: 10.1126/scitranslmed.3000247. [DOI] [PubMed] [Google Scholar]
- 49.Swartz MA, Iida N, Roberts EW, Sangaletti S, Wong MH, Yull FE, et al. Tumor Microenvironment Complexity: Emerging Roles in Cancer Therapy. Cancer Research. 2012;72:2473–80. doi: 10.1158/0008-5472.CAN-12-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tainsky MA. Genomic and proteomic biomarkers for cancer: A multitude of opportunities. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2009;1796:176–93. doi: 10.1016/j.bbcan.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dove A. Proteomics: Translating Genomics into products. Nature America Inc: 1999. [DOI] [PubMed] [Google Scholar]
- 52.Meltzer PS. Cancer genomics: Small RNAs with big impacts. Nature. 2005;435:745–6. doi: 10.1038/435745a. [DOI] [PubMed] [Google Scholar]
- 53.Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, Church TR, et al. Prostate Cancer Screening in the Randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: Mortality Results after 13 Years of Follow-up. Journal of the National Cancer Institute. 2012;104:125–32. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spratlin JL, Serkova NJ, Eckhardt SG. Clinical Applications of Metabolomics in Oncology: A Review. Clinical Cancer Research. 2009;15:431–40. doi: 10.1158/1078-0432.CCR-08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDermott U, Downing JR, Stratton MR. Genomics and the Continuum of Cancer Care. New England Journal of Medicine. 2011;364:340–50. doi: 10.1056/NEJMra0907178. [DOI] [PubMed] [Google Scholar]
- 56.Foulkes WD. Inherited susceptibility to common cancers. N. Engl. J. Med. 2008;359:2143–2153. doi: 10.1056/NEJMra0802968. [DOI] [PubMed] [Google Scholar]
- 57.Manolio TA. Genome-wide association studies and disease risk assessment. New England Journal of Medicine. 2010;363:166–76. doi: 10.1056/NEJMra0905980. [DOI] [PubMed] [Google Scholar]
- 58.Chen G, Wei P, DeStefano AL. Incorporating biological information into association studies of sequencing data. Genetic Epidemiology. 2011;35:S29–S34. doi: 10.1002/gepi.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cordell HJ. Epistasis: what it means, what it doesn’t mean, and statistical methods to detect it in humans. Human Molecular Genetics. 2002;11:2463–8. doi: 10.1093/hmg/11.20.2463. [DOI] [PubMed] [Google Scholar]
- 60.Fehringer G, Liu G, Briollais L, Brennan P, Amos CI, Spitz MR, et al. Comparison of Pathway Analysis Approaches Using Lung Cancer GWAS Data Sets. PLoS ONE. 2012;7:e31816. doi: 10.1371/journal.pone.0031816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiner BJ, Lewis MA, Clauser SB, Stitzenberg KB. In Search of Synergy: Strategies for Combining Interventions at Multiple Levels. JNCI Monographs. 2012;2012:34–41. doi: 10.1093/jncimonographs/lgs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stokols D. Establishing and maintaining healthy environments: Toward a social ecology of health promotion. American Psychologist. 1992;47:6–22. doi: 10.1037//0003-066x.47.1.6. [DOI] [PubMed] [Google Scholar]
- 63.Stokols D. Translating social ecological theory into guidelines for community health promotion. Am J Health Promot. 1996;10:282–98. doi: 10.4278/0890-1171-10.4.282. [DOI] [PubMed] [Google Scholar]
- 64.Lobo I. [accessed November 30, 2012];Same genetic mutation, different genetic disease phenotype. Nat Educ. 2008 1(1) Available from: http://www.nature.com/scitable/topicpage/same-genetic-mutation-different-genetic-disease-phenotype-938. [Google Scholar]
- 65.Cornelis MC, Tchetgen Tchetgen EJ, Liang L, Qi L, Chatterjee N, Hu FB, et al. Gene-Environment Interactions in Genome-Wide Association Studies: A Comparative Study of Tests Applied to Empirical Studies of Type 2 Diabetes. American Journal of Epidemiology. 2012;175:191–202. doi: 10.1093/aje/kwr368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yano EM, Green LW, Glanz K, Ayanian JZ, Mittman BS, Chollette V, et al. Implementation and Spread of Interventions Into the Multilevel Context of Routine Practice and Policy: Implications for the Cancer Care Continuum. JNCI Monographs. 2012;2012:86–99. doi: 10.1093/jncimonographs/lgs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.American Cancer Society [cited 2011; retrieved 2011 Apr 19];Cancer facts and figures 2010 tables and figures. 2010 http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/most-requested-tables-figures-2010.
- 68.Lansdorp PM. Stress, Social Rank, and leukocyte telomere length. Aging Cell. 2006;5:583–4. doi: 10.1111/j.1474-9726.2006.00247.x. [DOI] [PubMed] [Google Scholar]
- 69.Crawford ED. Epidemiology of Prostate Cancer. Urology. 2003;62:3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 70.De Angelis G, et al. Twenty Years of PSA: From Prostate Antigen to Tumor Marker. Rev Urol. 2007;9:113–23. [PMC free article] [PubMed] [Google Scholar]
- 71.Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-Specific Antigen as a Serum Marker for Adenocarcinoma of the Prostate. New England Journal of Medicine. 1987;317:909–16. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 72.Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJJ, et al. Measurement of Prostate-Specific Antigen in Serum as a Screening Test for Prostate Cancer. New England Journal of Medicine. 1991;324:1156–61. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 73.Catalona W, et al. Selection of optimal prostate specific antigen cutoffs for early dection of prostate cancer: receiver operating characteristic curves. J Urol. 1994;152:2037–42. doi: 10.1016/s0022-5347(17)32300-5. [DOI] [PubMed] [Google Scholar]
- 74.United States Preventive Task Force [accessed December 1, 2012];Screening for prostate cancer. 2012 Available from: http://www.uspreventiveservicestaskforce.org/uspstf/uspsprca.htm.
- 75.American Cancer Society . Guidelines for the early detection of cancer (prostate cancer) American Cancer Society; Atlanta, Georgia: 2012. [Google Scholar]
- 76.National Comprehensive Cancer Network(NCCN) NCCN Clinical Practice Guidelines in Oncology. Fort Washington, PA: 2007. Prostate cancer early detection guidelines, version 2. [Google Scholar]
- 77.Vickers AJ, Roobol MJ, Lilja H. Screening for Prostate Cancer: Early Detection or Overdetection? Annual Review of Medicine. 2012;63:161–70. doi: 10.1146/annurev-med-050710-134421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 79.Andriole GL, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeigler-Johnson C, Tierney A, Rebbeck TR, Rundle A. Prostate Cancer Severity Associations with Neighborhood Deprivation. Prostate Cancer. 2011 doi: 10.1155/2011/846263. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Subramanian SV, et al. Neighborhood effects on the self-rated health of elders: uncovering the relative importance of structural and service-related neighborhood environments. Journals of Gerontology-Series B. 2006;61:S153–S60. doi: 10.1093/geronb/61.3.s153. [DOI] [PubMed] [Google Scholar]
- 82.Fowke J, et al. Prostate cancer screening between low-income African-American and Caucasian Men. Urologic Oncology: Seminars and Original Investigations. 2005;23:333–40. doi: 10.1016/j.urolonc.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 83.Berglund A, Garmo H, Robinson D, Tishelman C, Holmberg L, Bratt O, et al. Differences according to socioeconomic status in the management and mortality in men with high risk prostate cancer. European journal of cancer (Oxford, England : 1990) 2012;48:75–84. doi: 10.1016/j.ejca.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 84.Carpenter W, Howard D, Taylor Y, Ross L, Wobker S, Godley P. Racial differences in PSA screening interval and stage at diagnosis. Cancer Causes and Control. 2010;21:1071–80. doi: 10.1007/s10552-010-9535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao H, Gwede CK, Kiros G, Milla K. Analysis of prostate cancer incidence using geographic information system and multilevel modeling. J Natl Med Assoc. 2007;99:218–25. [PMC free article] [PubMed] [Google Scholar]
- 86.Rundle A, Richards C, Neslund-Dudas C, Tang D, Rybicki BA. Neighborhood socioeconomic status modifies the association between individual smoking status and PAH-DNA adduct levels in prostate tissue. Environmental and Molecular Mutagenesis. 2012 doi: 10.1002/em.21693. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rebbeck TR, Weber AL, Walker AH, Stefflova K, Tran TV, Spangler E, et al. Context-Dependent Effects of Genome-Wide Association Study Genotypes and Macroenvironment on Time to Biochemical (Prostate Specific Antigen) Failure after Prostatectomy. Cancer Epidemiology Biomarkers & Prevention. 2010;19:2115–23. doi: 10.1158/1055-9965.EPI-10-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]