Acinetobacter baumannii typically causes infection among immunocompromised patients in intensive care units (ICUs) [1]. In an effort to improve detection of this organism and reduce transmission, we recently validated a sensitive screening method to identify patients carrying multidrug-resistant (MDR) A. baumannii using a combination of sponge and selective culture media [2]. In that study, screening isolates from all the patients were MDR, as defined by non-susceptibility to three or more classes of antimicrobials commonly used against this organism [3]. We subsequently implemented this method in the ICUs at one of our hospitals, with the following practical modifications to the original protocol: (1) only one sponge is used for sequentially swiping down the arm and leg, (2) broth enrichment is conducted for 4 hours before inoculation of the selective agar plate containing ceftazidime, and (3) the species is confirmed with Vitek2. Here we report data on the natural history of MDR A. baumannii carriage in this patient population that were obtained through this initiative.
The active screening program was implemented in three ICUs (medical, burn and cardiovascular ICUs; total of 54 beds) at UPMC Mercy Hospital in Pittsburgh, Pennsylvania between June 2010 and May 2011. The initiative was approved by the UPMC Quality Improvement Review Committee. All admissions to the ICUs underwent active screening for MDR A. baumannii upon admission and every seven days thereafter as prompted by the electronic ordering system. Patients who were newly identified as positive were placed in contact isolation, but attempts at decolonization were not made. All the screening culture results as well as clinical cultures that grew MDR A. baumannii were collected from the microbiology database and matched with the corresponding admission data. For the purpose of this analysis, MDR A. baumannii was defined as non-susceptibility to ceftazidime. When the culture results turned from positive to negative or vice versa for the same admission, the midpoint of the two dates was calculated and used to calculate the carriage-positive days. For cultures turning from positive to negative and then positive again, two consecutive negative cultures were required to define clearance and subsequent re-colonization, given the approximately 80% sensitivity of the screening method [2]. The minimum duration of carriage was calculated as time from the first positive culture (or the midpoint between the first positive culture and the last negative culture prior to it if present) to the last positive culture (or the midpoint between the last positive culture and the last negative culture after it if present). The estimated duration of carriage was calculated likewise, except the patients were considered carriers until discharge from the ICUs if the last positive culture was not followed by a negative culture. Fisher's exact test was used to determine statistical significance.
A total of 118 ICU admissions with at least one screening or clinical culture positive for MDR A. baumannii were identified during this period, consisting of 86 unique patients. Of the 118 admissions, 56 were identified by screening cultures only, 6 by clinical cultures only, and 56 by both screening and clinical cultures. Of the latter, 26 were identified by screening cultures first, and 17 on the same day. Overall, 82 of the 118 admissions (69.4%) were initially identified as carriage-positive by screening cultures. The mean and median lengths of stay in the ICUs were 15.4 and 10 days, respectively (range, 0 to 141 days). The mean and median lengths of stay until the first positive culture were 2.5 and 0 days (range, 0 to 40.5 days). Of the 118 admissions, 71.2% had the first culture positive for MDR A. baumannii within one day of admission. The rate was 80.1% for those with another ICU admission in the prior month, and 67.1% for those without (p = 0.19). The mean and median minimum duration of carriage was 8.5 and 3.5 days (range, 0 to 63 days). The mean and median estimated duration of carriage was 10.8 and 6.3 days (range, 0 to 63 days). The total minimum and estimated duration of carriage corresponded to 55.2 and 70.5% of the total ICU days, respectively (Figure). For over half of the admissions, the estimated duration of carriage exceeded 90% of the respective ICU days. Only 19.5% of the admissions had a negative screening culture documented before ICU discharge.
Figure.
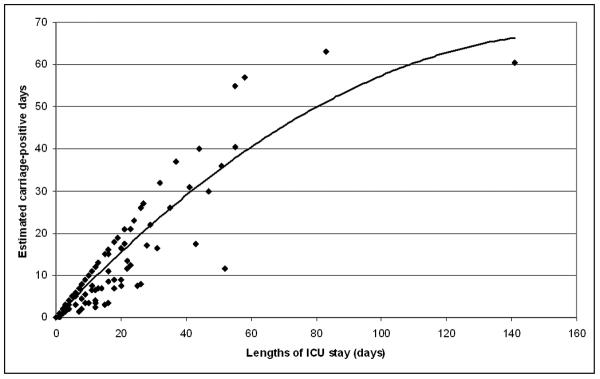
Lengths of ICU stay for admissions with positive MDR A. baumannii cultures and their estimated carriage-positive days. The curve represents second order polynomial regression.
While long-term carriage of MDR A. baumannii has been reported [4], this is the first study to quantify the duration of carriage of this organism in ICUs. Our data suggest that, at least in non-outbreak settings, importation by patients who were colonized elsewhere constitutes the main source of this organism in ICUs and thus screening cultures on admission are likely to be more cost-effective than subsequent screening cultures. Also, the carriage-positive days accounted for the majority of the total ICU days, with only 19.5% of the carriers apparently clearing carriage before ICU discharge.
Our study has several limitations. We could not define the carriage status upon discharge for all patients since discharge cultures were not routinely conducted. Also, the program was limited to ICUs and as such we do not have information on long-term carriage status of patients on other hospital units before and after ICU admission.
In summary, the majority of MDR A. baumannii carriers can be identified by active screening upon admission to ICUs, and they should be considered as carriers throughout their ICU admission at least in the absence of further interventions such as decolonization.
Acknowledgments
We thank the staff of intensive care units and clinical microbiology laboratory at UPMC Mercy Hospital for their contribution to this program.
Financial support. This study was funded by the Pennsylvania Department of Health (grant #4100047864). Y.D. and L.H.H. are supported by the National Institute of Allergy and Infectious Diseases research career awards (K22AI80584 and K24AI52788, respectively).
Footnotes
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.
References
- [1].Towner KJ. Acinetobacter: an old friend, but a new enemy. J Hosp Infect. 2009;73:355–363. doi: 10.1016/j.jhin.2009.03.032. [DOI] [PubMed] [Google Scholar]
- [2].Doi Y, Onuoha EO, Adams-Haduch JM, et al. Screening for Acinetobacter baumannii colonization by use of sponges. J Clin Microbiol. 2011;49:154–158. doi: 10.1128/JCM.01043-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Paterson DL. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43(Suppl 2):S43–48. doi: 10.1086/504476. [DOI] [PubMed] [Google Scholar]
- [4].Marchaim D, Navon-Venezia S, Schwartz D, et al. Surveillance cultures and duration of carriage of multidrug-resistant Acinetobacter baumannii. J Clin Microbiol. 2007;45:1551–1555. doi: 10.1128/JCM.02424-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


