Abstract
The hepatocyte nuclear factor 4α (HNF4α) is a liver-enriched nuclear receptor that plays a critical role in early morphogenesis, fetal liver development, liver differentiation and metabolism. Human HNF4α gene mutations cause maturity on-set diabetes of the young type 1, an autosomal dominant non-insulin-dependent diabetes mellitus. HNF4α is an orphan nuclear receptor because of which the endogenous ligand has not been firmly identified. The trans-activating activity of HNF4α is enhanced by interacting with co-activators and inhibited by corepressors. Recent studies have revealed that HNF4α plays a central role in regulation of bile acid metabolism in the liver. Bile acids are required for biliary excretion of cholesterol and metabolites, and intestinal absorption of fat, nutrients, drug and xenobiotics for transport and distribution to liver and other tissues. Bile acids are signaling molecules that activate nuclear receptors to control lipids and drug metabolism in the liver and intestine. Therefore, HNF4α plays a central role in coordinated regulation of bile acid and xenobiotics metabolism. Drugs that specifically activate HNF4α could be developed for treating metabolic diseases such as diabetes, dyslipidemia and cholestasis, as well as drug metabolism and detoxification.
Keywords: bile acid metabolism, cytochrome P450, diabetes, drug metabolism, lipid metabolism, nuclear receptors
1. Introduction
The hepatocyte nuclear factor 4α (HNF4α) is a liver-enriched transcription factor that belongs to the NR2A subfamily of the nuclear receptor superfamily, which has 48 genes in the human genome and 49 in the mouse genome [1]. There are two mammalian members in the NR2A family, HNF4α (NR2A1) and HNF4γ (NR2A2) [1]. HNF4α was originally identified in rat liver extracts that bind to the promoter sequences of transthyretin and apolipoprotein CIII (apoCIII) genes [2]. HNF4α is the most abundant nuclear receptor expressed in the liver [3]. It has been reported that fatty acyl–CoA thioesters are endogenous ligands of HNF4α [4,5]. Recent studies of the crystal structure of the ligand-binding domain of HNF4α reveal that the ligand-binding pocket contains a mixture of saturated and cis-monounsaturated C14 – 18 fatty acids [6,7]. However, the bound fatty acids do not readily exchange with exogenously radioisotope-labeled fatty acids and attempts to remove fatty acids without denaturing the proteins have failed. It is thought that fatty acids may bind to the ligand-binding domain of HNF4α as cofactors rather than ligands. Therefore, HNF4α is still considered an orphan nuclear receptor.
HNF4α target genes are involved in lipid transport, fatty acid oxidation, bile acid synthesis and transport, lipoprotein metabolism, steroid metabolism, glucose metabolism, amino acid metabolism, blood coagulation and viral genome replication [8]. A recent chromatin immunoprecipitation combined with promoter microarray (ChiP-on-chip) analysis has revealed that HNF4α binds to about 12% of human liver genes (1555) and 11% of human pancreatic islets gene represented on the Hu13K DNA microarray [9]. About 42% of the genes (1262) occupied by RNA polymerase II are also bound by HNF4α in hepatocytes. These results are consistent with the findings that HNF4α is an abundantly expressed transcription factor that is highly active in transcriptional regulation of various metabolic pathways in the liver and pancreatic islet.
There are two recent reviews on HNF4α. Readers are referred to a book chapter on a comprehensive review of the HNF4α structure, functions and human diseases by Sladek in 2002 [8] and a 2008 review of the role of HNF4α in gene transcription by Gonzalez [10]. This review briefly overviews HNF4α expression and regulation, followed by a summary of recent advances in understanding the roles of HNF4α in coordinated regulation of CYPs involved in bile acid synthesis and drug metabolism.
2. Regulation of HNF4α expression
Expression of HNF4α is a marker of early liver development and is essential for liver morphogenesis [11–13], differentiation [14] and metabolism [8,10]. HNF4α expression is synergistically regulated by several liver-enriched transcription factors, including HNF4α, HNF1s (pou-homeodomain transcription factors), HNF3s (forkhead transcription factors) and HNF6 (one-cut homeodomain transcription factor) [15,16]. It has been proposed that HNF4α is in the center of a transcription factor regulatory circuitry including HNF1s, HNF3s and GATAs. These transcription factors cooperatively regulate gene transcription during development and differentiation of the liver and pancreas [8,9,17].
2.1 HNF4α expression
Nine HNF4α isoforms may exist in mammalian livers [8]. These isoforms are derived from alternative splicing events and/or alternative promoter usage. The HNF4α gene is located in human chromosome 20q13.1 – 13.2 [3], and has 13 exons spanning about 70 kb of the human genome. The HNF4α gene has two promoters, which are differentially utilized in hepatocytes and islets [17]. The promoter 1 (P1 promoter) initiates transcription of the ‘adult’ isoforms containing exon 1A (HNF4α1 – α6) in liver cells, whereas P2 promoter initiates transcription of the ‘embryonic’ isoforms containing exon 1D (HNF4α7 – α9) in pancreatic cells [17,18]. HNF4α2 and α8 are the alternative spliced variants of α1 and α7, respectively. The expression levels of these isoforms vary with development and differentiation stages in different tissues. In early liver development, HNF4α7 is transcribed from the P2 promoter. In adult liver, the P1 promoter actively transcribes HNF4α1 and HNF4α7 expression is very low. In pancreatic islets, the P2 promoter is preferentially utilized to transcribe HNF4α7 and HNF4α8 [19]. HNF4α7 and HNF4α8 have much lower trans-activating activity than HNF4α1 and HNF4α2.
Ablation of the Hnf4α gene is embryonic lethal [20]. Studies of tissue specific deletion of the Hnf4α gene in mice have provided valuable information on the physiological functions of HNF4α in different tissues. Liver-specific deletion of the hnf4α gene in adult mice reveals phenotypes of increased serum cholesterol, triglycerides and bile acids, and accumulation of lipids in mouse livers suggesting that HNF4α plays a critical role in maintaining lipid homeostasis [21,22]. In hepatocyte-specific hnf4α null mice, hepatic gluconeogenesis is impaired during fasting [23]. Expression of a dominant negative HNF4α mutant in pancreatic cells impaired insulin secretion and altered insulin, glucose transporter 2, L-pyruvate kinase and other genes involved in glucose metabolism [24]. Pancreatic-specific Hnf4α null mice have impaired insulin secretion and maturity on-set diabetes of the young type 1 (MODY-1) phenotypes [25,26].
A recent study of intestine epithelial cell-specific Hnf4α null mice has discovered the phenotypes of inflammatory bowel disease (IBD) [27]. HNF4α expression is reduced in dextran sulfate sodium-induced IBD and in intestine biopsies of Crohn’s disease and ulcerative colitis patients [27]. These observations suggest that HNF4α may be involved in the expression of mucins and aquaporins in epithelial cells. Impairment of mucosal integrity may increase intestine inflammation by xenobiotics and endotoxins. It is not known whether Crohn’s disease and IBD patients have HNF4α gene mutations or HNF4α is inactivated by inflammation in epithelial cells.
Heterozygous mutations of the HNF4α gene cause MODY1, which has impaired glucose regulation of insulin secretion due to dysfunction of the pancreatic β-cells [28,29]. Heterozygous mutations in various domains of HNF4α and the gene promoter have been identified in MODY1 patients [18,30,31]. Mutations of the DNA binding and dimerization domains of the HNF4α gene cause impaired DNA-binding, protein stability, trans-activation activity or nuclear localization [29,32–35]. Haploinsufficiency of HNF4α reduces serum levels of ApoAII, ApoCIII and triglyceride levels in MODY-1 patients [36].
2.2 HNF4α co-activators
The Class I steroid hormone receptors interact with the ubiquitous corepressors, silencing mediator of retinoid acid and thyroid hormone receptors and nuclear receptor corepressor 1 in the absence of a ligand, and are inactivate. On ligand binding, corepressors are released to allow co-activator binding to stimulate transcriptional activity. HNF4α is a member of the Class II orphan nuclear receptors that are constitutively active. HNF4α is located predominantly in the nucleus. Unlike most Class II receptors, HNF4α forms homodimers that bind to a direct repeat of the AGGTCA-like hormone response element with one-base spacing (DR1) (Figure 1) [37]. The trans-activating activity of HNF4α is stimulated by co-activators through interaction with the activation function domain 1 (AF-1) and activation function domain 2 (AF-2) domains (Figure 1). The HNF4α co-activators include p160 family co-activators, steroid receptor co-activator-1 (SRC-1), SRC-2 and SRC-3, and p300/CBP (cAMP response element binding protein) family co-activators [38–40]. The AF-1 of HNF4α1 also interacts with nuclear receptor co-activator 6 [41]. The AF-2 domain has repressive activity that inhibits HNF4α interaction with co-activators. The 10-amino acid insert in the F domain of HNF4α2 abrogates such interaction [38]. CBP is a histone acetyltransferase that acetylates lysine residues in the histone tails to allow transcription factors to bind to chromatin and stimulates RNA polymerase II activity. The N- and C-terminals of CBP interact with AF-1 and AF-2 of HNF4α, respectively [39]. HNF4α interacts with peroxisome proliferator-activated receptor γ co-activator 1-α (PGC-1α) [23,42], which is a versatile nuclear receptor co-activator induced during fasting to stimulate gluconeogenesis, lipoprotein, triglyceride, energy and drug metabolism [23,42–48]. PGC-1α expression levels may determine HNF4α target gene expression in hepatocytes. It has been reported that HNF4α interacts with a co-activator PC4 and induces the inducible nitric oxide synthase expression during oxidative stress in hepatocytes [49].
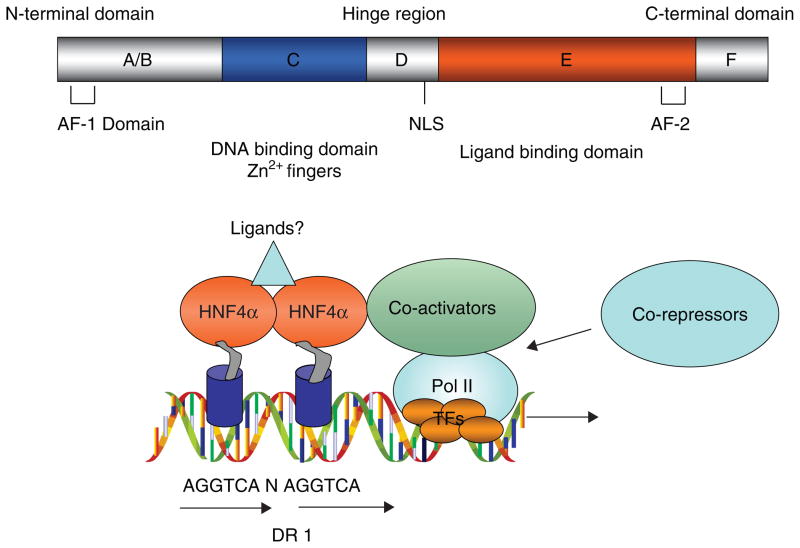
Figure 1. The structure of HNF4α.
The domain structure of a typical nuclear receptor is illustrated. HNF4α homodimer binds to a direct repeat of AGGTCA sequence separated by one base (DR1) in the gene promoter. The ligand-independent AF-1 is located in the N-terminus region (A/B). The DNA binding domain (C domain) has two cysteine-coordinated Zn2+ fingers and is directly involved in specific binding of nuclear receptors to the response element in the gene promoter. The hinge region (D) has an NLS. The ligand-binding domain is located in the E region, which contains the ligand-binding pocket and co-activator interacting sequences, and is also involved in dimerization of nuclear receptors. The endogenous ligand of HNF4α is not known. The ligand-dependent AF-2 is located within the ligand-binding domain and near the C-terminus. Co-activators interact with HNF4α through the C-terminal region and enhance trans-activating activity, whereas corepressors inhibit HNF4α activity.
AF-1: Activation function domain 1; HNF4α: Hepatocyte nuclear factor 4α; NLS: Nuclear localization sequence; TF: Transcription factor.
2.3 HNF4 corepressors
The transcriptional activity of HNF4α is inhibited by ubiquitous nuclear receptor corepressors, silencing mediator of retinoid acid and thyroid hormone receptors and nuclear receptor corepressor 1 [50]. Several novel transcription factors have been shown to inhibit HNF4α activity. Small heterodimer partner (SHP), an orphan nuclear receptor lacking a DNA binding domain, interacts with HNF4α and inhibits HNF4α target gene transcription [51,52]. Another orphan receptor chicken ovalbumin upstream transcription factor II has been shown to inhibit HNF4α gene transcription and activity by competing for binding to the DR1 sequence in the HNF4α and targets genes [53–57]. SHP is known to inhibit HNF4α by three distinct mechanisms: interaction with HNF4α and to directly inhibit HNF4α activity, interaction with HNF4α to prevent its binding to DNA, and interaction with HNF4α to block its recruitment of PGC-1α [51]. Interestingly, chicken ovalbumin upstream transcription factor II also can function as a transcriptional activator [56,58,59].
It has been reported recently that TGFβ1-activated transcription factors Smad3 and Smad4 specifically interact with HNF4α and inhibit HNF4α trans-activating activity [60,61]. The AF1 and C-terminal F domain of HNF4α interact with the Mad homology 1 domains of both Smad2 and Smad3. In contrast, TGFβ1/Smads signaling crosstalks with HNF4α and activates ApoCII gene transcription [61]. The ligand-binding domain of HNF4α interacts with a tumor suppressor p53 and recruits histone deacetylase to inhibit target gene transcription [62,63]. Inhibition of HNF4α expression by p53 may be a response to liver injury to inhibit liver gene expression and differentiation. Several studies show that Foxo1 interacts with HNF4α and inhibits HNF4α trans-activating activity [64–66]. Foxo1 mediates insulin signaling and regulates gluconeogenesis. Insulin signaling phosphorylates FoxO1 and excludes FoxO1 from the nucleus for degradation in proteosomes [67]. Another recent study reports that HNF4α is inhibited by GPS2, which is a component of the repressor complex [68].
2.4 Post-translational regulation of HNF4α
The trans-activating activity of HNF4α may be regulated by post-translational modifications. There are 13 potential serine/threonine phosphorylation sites on HNF4α [8]. Phosphorylation of HNF4α by protein kinase A [69,70], mitogen-activated protein kinases p38 [70] or JNK/cJun reduces DNA binding activity [49,70–72]. AMP kinase reduced HNF4α dimerization and protein stability [73] and reduces ApoCIII expression [71]. Phosphorylation of Serine78 by protein kinase C causes nuclear exclusion and degradation [74]. Tyrosine phosphorylation of the HNF4α affects its intranuclear compartmentation and DNA binding activity [75]. Furthermore, CBP acetylates HNF4α at a lysine residue causing HNF4α nuclear retention and increasing DNA binding and trans-activating activity [76,77]. These post-translational regulations have not been verified by an in vivo study, and thus the physiological relevance of HNF4α phosphorylation is not clear.
3. HNF4α regulation of bile acid metabolism
HNF4α plays a central role in regulation of bile acid synthesis. Bile acids are physiological detergents that facilitate biliary excretion of cholesterol and xenobiotic metabolites, and intestinal absorption of fats, steroids, nutrients, drug and xenobiotics [78]. Bile acids also are signaling molecules and inflammatory agents that activate the nuclear receptors and signaling pathways involved in lipid and glucose metabolism [79].
3.1 Bile acid synthesis
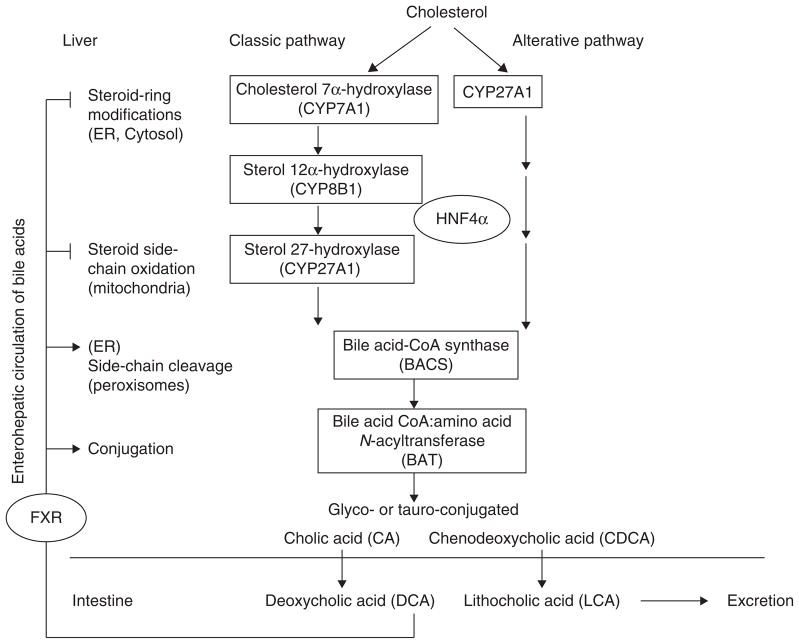
Bile acids are the end products of cholesterol catabolism. The bile acid biosynthetic pathways involve 15 enzyme-catalyzed reactions including steroid ring modifications, side chain oxidation and cleavage, and conjugation [80]. Figure 2 only shows known HNF4α regulated genes involved in bile acid synthesis in the liver [79,80]. Cholesterol 7α-hydroxylase (CYP7A1) initiates the classic bile acid biosynthetic pathway leading to formation of two primary bile acids, cholic acid (CA, 3α, 7α, 12α) and chenodeoxycholic acid (CDCA, 3α, 12α) (Figure 2). CYP7A1 is the only rate-limiting enzyme in the classic bile acid biosynthetic pathway. Sterol 12α-hydroxylase (CYP8B1) is required for the synthesis of CA. After modification of the sterol ring, the mitochondrial sterol 27-hydroxylase (CYP27A1) catalyzes the sterol side chain oxidation. CYP27A1 also can directly hydroxylate cholesterol to 27-hydroxycholesterol in liver and extrahepatic tissues. Bile acid–CoA synthase (BACS) then conjugates a Coenzyme-A to the C27-cholestanoic acids for cleavage of a propionyl group in peroxisomes to form C24-bile acids. To increase solubility of bile acids (bile salts), bile acid CoA:amino acid N-acyltransferase (BAT) conjugates a glycine or taurine to CA and CDCA to form glyco- or tauro-conjugated CA or CDCA. Conjugated-CA and CDCA are secreted into the bile through canalicular bile salt export pump (BSEP, or ABCB11). Bile acids form mixed micelles with phosphatidylcholine and cholesterol, and are stored in the gallbladder.
Figure 2. HNF4α regulation of bile acid synthesis.
Bile acids are the end products of cholesterol catabolism. The classic pathway is the major pathway in human liver. There are 15 enzyme-catalyzed reactions in the pathways. Only HNF4α regulated enzymes are shown. CYP7A1 is the first and rate-limiting enzyme of the classic pathway. CYP8B1 is involved in the synthesis of CA. Mitochondrial CYP27A1 catalyzes sterol side chain oxidation and also initiates the alternative pathway. CA and CDCA are two primary bile acids synthesized in human liver. BACS and BAT are involved in conjugation of glycine and taurine to bile acids. Glyco- or tauro-conjugated CA and CDCA are excreted into intestine, where bacterial 7α-dehydroxylase converts CA and CDCA to the secondary bile acids, DCA and LCA, respectively. CA, CDCA and DCA are re-circulated to the liver through portal blood to inhibit CYP7A1, CYP8B1 and CYP27A1, but stimulate BACS and BAT gene transcriptions.
BACS: Bile acid–CoA synthase; BAT: Bile acid CoA:amino acid N-acyltransferase; CA: Cholic acid; CDCA: Chenodeoxycholic acid; CYP7A1: Cholesterol 7α-hydroxylase; CYP8B1: Sterol 12α-hydroxylase; CYP27A1: Sterol 27-hydroxylase; DCA: Deoxycholic acid; ER: Endoplasmic reticulum; FXR: Farnesoid X receptor; HNF4α: Hepatocyte nuclear factor 4α; LCA: Lithocholic acid.
3.2 Regulation of bile acid synthesis
Bile acids stored in the gallbladder are released into the intestinal tract after each meal. In the intestine some CA and CDCA are converted to deoxycholic acid (DCA) and lithocholic acid (LCA), respectively, by 7-dehydoxylase in bacterial flora. CA, CDCA and DCA are quantitatively (95%) re-absorbed in the ileum by apical sodium-dependent bile salt transporter, and excreted into portal circulation through basolateral organic solute transporter α/β and organic anion transporting peptides (OATPs). Bile acids circulated to the liver are taken up by basolateral sodium-dependent taurocholate transporting peptide (NTCP). Bile acids inhibit bile acid synthesis by inhibiting CYP7A1 and CYP8B1 gene transcription [80].
3.2.1 Regulation by HNF4α
Several studies have implicated HNF4α as a critical transcriptional regulator of the CYP7A1 gene [79–81]. Chiang and Stroup first identified a putative HNF4α binding site (DR1) in the rat Cyp7a1 gene promoter [82]. Subsequent studies confirmed that the DR1 sequence bound HNF4α and was critical for basal and bile acid regulated transcription of the CYP7A1 gene [57,59,83]. The human CYP8B1 and CYP27A1 gene promoters also have HNF4α binding sites [52,84,85]. Conditional liver-specific knockout of the hnf4α gene in mice markedly reduced expression of CYP7A1 and CYP8B1, which confirmed the in vitro promoter study [21,22]. In mice lacking hepatic HNF4α, the BACS and BAT expression levels were reduced, and un-conjugated and glycine-conjugated bile acids are accumulated in the gallbladder [86]. The HNF4α binding sites have been identified in the BACS and BAT gene promoters. These studies support the discovery that HNF4α plays a central role in bile acid synthesis and conjugation [22,52,57,84,86].
3.2.2 Regulation by bile acid receptors
Bile acid synthesis is feedback inhibited by bile acids returning to the liver through enterohepatic circulation of bile acids [79]. Recent studies have discovered that bile acids are able to activate a nuclear receptor farnesoid X receptor (FXR), which plays a critical role in the regulation of bile acid metabolism and lipid homeostasis [59,79]. Among all bile acids tested, CDCA is the most efficacious endogenous FXR ligand [87]. Two FXR-mediated pathways have been proposed to inhibit CYP7A1 gene transcription. In the liver, bile acid-activated FXR induces SHP, which then inhibits trans-activation activity of HNF4α or orphan receptor liver-related homologue-1, and results in inhibiting CYP7A1 and CYP8B1 gene transcription [87,88]. The FXR–SHP pathway also inhibits NTCP gene transcription [89]. Deletion of the Fxr gene in mice impairs bile acid and lipid homeostasis supporting the critical role of FXR in the regulation of bile acid synthesis, transport and secretion [90]. In the intestine, FXR induces an intestine hormone, fibroblast growth factors 15 (or human orthologue FGF19) [91,92]. FGF15/FGF19 is transported to the liver to activate FGF receptor 4 signaling, which inhibits CYP7A1 gene transcription through an unknown mechanism [92].
On the other hand, FXR stimulates bile acid secretion into bile by inducing BSEP expression in the bile canalicular membrane. FXR also induces BACS and BAT gene expression by binding to FXR response elements located in the promoter of the BACS and intron 1 of the BAT gene [93]. Therefore, FXR regulates enterohepatic circulation of bile acids by inhibiting hepatic bile acid synthesis and uptake into hepatocytes, and stimulating bile acid excretion into bile, thus maintaining bile acid homeostasis.
Another bile acid-activated nuclear receptor PXR is also involved in regulation of bile acid homeostasis. LCA is a potent PXR ligand [94]. PXR inhibits CYP7A1 by interacting with HNF4α and preventing HNF4α from interacting with PGC-1α [95,96]. A recent study reports that feeding CA or a FXR agonist GW4064 induces PXR expression in mice suggesting that FXR binds to the Pxr promoter and induces Pxr gene transcription [97]. LCA also activates vitamin D receptor (VDR), which may protect against colon cancer promoted by bile acids [87,98].
4. HNF4α regulation of drug metabolism
Enterohepatic circulation of bile acids plays a critical role in biliary excretion and intestinal absorption of drugs and xenobiotics. There is a coordinated regulation of bile acid synthesis and drug metabolism [99]. HNF4α plays a key role in liver and intestine expression of CYP enzymes in drug metabolism.
4.1 Drug metabolizing CYPs in human hepatocytes
CYP superfamily enzymes play important roles in metabolism and detoxification of xenobiotics (drugs, carcinogens) and endobiotics (fatty acids, steroids) in the liver and other tissues. There are 12 drug metabolizing CYPs in the human liver: CYP1A1, CYP1A2, CYP1B1, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP2F1 and CYP3A4 [100].
HNF4α binding sites were first identified in the CYP2C genes [101]. A recent study using adenovirus-mediated antisense RNA to inhibit HNF4α mRNA expression in human hepatocytes has revealed that mRNA expression of CYP3A4, CYP3A5 (variant of CYP3A4) and CYP2A6, CYP2B6, CYP2C9 and CYP2D6 are downregulated suggesting that HNF4α plays a key role in basal expression of these major drug metabolizing CYPs in human hepatocytes [102]. HNF4α binding sites have been identified in the gene promoters of CYP2B6, CYP2C9, CYP2D6 and CYP3A4 [103–105]. CYP3A4 is the most abundant CYP expressed in the liver and intestine, and metabolizes > 50% of prescription drugs [106]. CYP3A4 is highly regulated by PXR, VDR and constitutive androstane receptor (CAR) [107–110].
4.2 HNF4α coordinates xenobiotic receptor regulation of drug metabolism
HNF4α, PXR, CAR and VDR crosstalk and regulate expression of CYP2B, CYP2C and CYP3A4 in liver and intestine [110,111]. Xenobiotic receptors PXR and CAR play critical roles in drug metabolism [111–113]. Human PXR is activated by 50% of drugs prescribed to humans and may be responsible for drug–drug interaction. CAR is induced by phenobarbital. PXR and CAR induce Phase I drug metabolizing enzymes CYP3A, CYP2B and CYP2C, Phase II drug conjugation enzymes glucuronosyltransferase (UGT1 and UGT2 family) and sulfotransferase 2A1 (SULT2A1), and Phase III drug transporters multi-drug resistant related proteins 2 (MRP2, or ABCC2/4, apical efflux transporter of glutathione, glucuronide, and sulfate conjugated bile acids, bilirubin) and MRP4 (basolateral efflux transporter of bile acids and conjugates), and multi-drug resistant protein 1 [108,114–118]. HNF4α is a key regulator of PXR expression during fetal liver development and in-activation of HNF4α in mice suppressed CYP3A11 and PXR expression [104]. Conditional deletion of the Hnf4α gene in fetal and adult mouse liver reduces CYP3A11 expression [105]. Subsequently, studies have revealed that HNF4α is involved in PXR and CAR induction of CYP3a4 in hepatocytes [45,105]. In human hepatocytes HNF4α and PXR interact and synergistically induce CYP3A4 in response to rifampicin, a potent PXR agonist [45]. Similarly, HNF4α synergizes with CAR and PXR to induce CYP2C9 and SULT2A1 expression [119,120]. HNF4α also controls CAR expression by binding to HNF4α response elements located in the CAR gene promoter [46]. HNF4α and PGC-1α interact to induce CAR and its target genes, CYP 2b10, UGT1a1, Oatp2 and Sult2a1 in mouse livers during fasting.
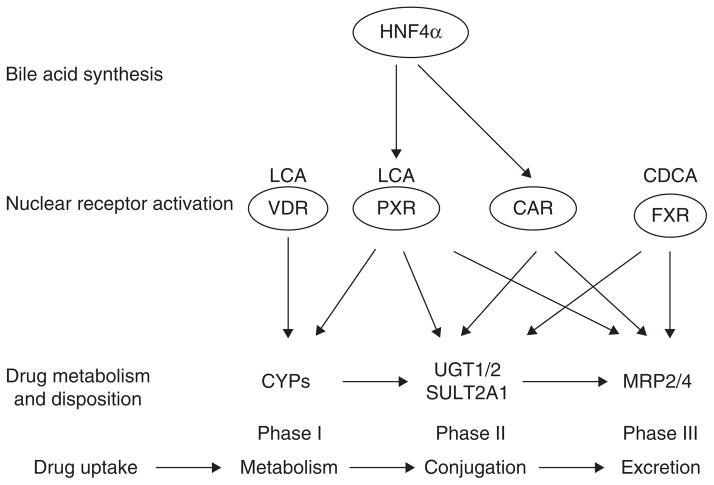
Interestingly, a recent analysis of mRNA expression levels of several nuclear receptors, co-regulators and CYPs has revealed that HNF4α mRNA expression levels show the highest colinearity to mRNA expression levels of CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, MRP2, OATP2, cytochrome P450 reductase and UGT1A1 in human liver [121]. These investigators suggest that HNF4α is probably a dominant regulator of basal xenobiotic metabolism in human livers. Their analysis also identified CAR as a regulator of basal expression of CYPs, MRP2, OATP2, UGT1A1 and cytochrome P450 reductase in human livers. It seems that a hierarchy of HNF4α, PXR and CAR determines the expression of xenobiotic metabolism. Figure 3 shows the hierarchy of HNF4α in drug metabolism. HNF4α regulates bile acid synthesis to produce primary bile acids CDCA and CA, which activate FXR to activate Phase II and III drug metabolism. The secondary bile acid LCA activates VDR and PXR. VDR induces CYPs in Phase I drug metabolism, whereas PXR induces all three phases of drug metabolism. HNF4α directly induces PXR, which induces all three phases of drug metabolism. HNF4α directly induces CAR to regulate Phase II and III drug metabolism. Therefore, HNF4α plays a predominant role in the coordinated regulation of drug metabolism, conjugation and secretion.
Figure 3. The hierarchy of HNF4α regulation of drug metabolism.
HNF4α either directly induces expression of CYPs in Phase I, UGTs and SULTs in Phase II drug conjugation, and MRP2 and MRP4 in Phase III drug transport, or through induction of PXR and CAR. CDCA activates FXR, whereas LCA activates VDR and PXR. These bile acid-activated receptors crosstalk with HNF4α and CAR to regulate Phase I, Phase II and Phase II drug metabolism as indicated by arrows.
CAR: Constitutive androstane receptor; CDCA: Chenodeoxycholic acid; FXR: Farnesoid X receptor; HNF4α: Hepatocyte nuclear factor 4α; LCA: Lithocholic acid; MRP: Multi-drug resistant related protein; PXR: Pregnane X receptor; SULT: Sulfotransferase; UGT: UDP-glucuronosyltransferase; VDR: Vitamin D receptor.
5. Expert opinion
HNF4α plays a critical role not only in liver development but also in liver metabolism. Because HNF4α regulates the key genes involved in lipid, glucose and drug metabolism, the expression levels of HNF4α in individuals may be critical in determining liver metabolic functions. To further understand the physiological functions of HNF4α in liver metabolism, the endogenous ligands of HNF4α need to be identified. Nuclear receptors are ideal targets for drug development [122–127]. The crystallographic structure of the ligand-binding domain of HNF4α provides critical information for designing specific and efficacious HNF4α agonists, which could be developed for treating metabolic diseases such as diabetes, dyslipidemia and cholestasis. Modulation of HNF4α activity may also regulate drug metabolism and detoxification in humans.
Acknowledgments
This research is supported by NIH grants DK44442 and DK58379.
Footnotes
Declaration of interest
The author states no conflict of interest and has received no payment in preparation of this manuscript.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Committee of Nuclear Receptor Nomenclature. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97(2):161–3. doi: 10.1016/s0092-8674(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 2.Sladek FM, Zhong WM, Lai E, Darnell JE., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990;4(12B):2353–65. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- 3.Drewes T, Senkel S, Holewa B, Ryffel GU. Human hepatocyte nuclear factor 4 isoforms are encoded by distinct and differentially expressed genes. Mol Cell Biol. 1996;16(3):925–31. doi: 10.1128/mcb.16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hertz R, Magenheim J, Berman I, Bar-Tana J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4α. Nature. 1998;392(6675):512–6. doi: 10.1038/33185. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder F, Huang H, Hostetler HA, et al. Stability of fatty acyl-coenzyme A thioester ligands of hepatocyte nuclear factor-4α and peroxisome proliferator-activated receptor-alpha. Lipids. 2005;40(6):559–68. doi: 10.1007/s11745-005-1416-y. [DOI] [PubMed] [Google Scholar]
- 6.Dhe-Paganon S, Duda K, Iwamoto M, et al. Crystal structure of the HNF4 alpha ligand binding domain in complex with endogenous fatty acid ligand. J Biol Chem. 2002;277(41):37973–6. doi: 10.1074/jbc.C200420200. [DOI] [PubMed] [Google Scholar]
- 7.Wisely GB, Miller AB, Davis RG, et al. Hepatocyte nuclear factor 4 is a transcription factor that constitutively binds fatty acids. Structure (Camb) 2002;10(9):1225–34. doi: 10.1016/s0969-2126(02)00829-8. [DOI] [PubMed] [Google Scholar]
- 8••.Sladek FM, Seidel SD. Hepatocyte nuclear factor 4alpha. In: Burris TP, McCabe E, editors. Nuclear Receptors and Genetic Diseases. Academic Press; London: 2001. A comprehensive review of HNF4 structure, function and human diseases. [Google Scholar]
- 9•.Odom DT, Zizlsperger N, Gordon DB, et al. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303(5662):1378–81. doi: 10.1126/science.1089769. ChIP-on chip Microarray analysis of HNF4 target genes in liver and pancreas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Gonzalez FJ. Regulation of hepatocyte nuclear factor 4α-mediated transcription. Drug Metab Pharmacokinet. 2008;23(1):2–7. doi: 10.2133/dmpk.23.2. An updated review of HNF4 regulation of gene transcription. [DOI] [PubMed] [Google Scholar]
- 11.Duncan SA, Manova K, Chen WS, et al. Expression of transcription factor HNF-4 in the extraembryonic endoderm, gut, and nephrogenic tissue of the developing mouse embryo: HNF-4 is a marker for primary endoderm in the implanting blastocyst. Proc Natl Acad Sci USA. 1994;91(16):7598–602. doi: 10.1073/pnas.91.16.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaret KS. Liver specification and early morphogenesis. Mech Dev. 2000;92(1):83–8. doi: 10.1016/s0925-4773(99)00326-3. [DOI] [PubMed] [Google Scholar]
- 13.Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev. 2002;3(7):499–512. doi: 10.1038/nrg837. [DOI] [PubMed] [Google Scholar]
- 14.Costa RH, Kalinichenko VV, Holterman AX, Wang X. Transcription factors in liver development, differentiation, and regeneration. Hepatology. 2003;38(6):1331–47. doi: 10.1016/j.hep.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 15.Xanthopoulos KG, Prezioso VR, Chen WS, et al. The different tissue transcription patterns of genes for HNF1, C/EBP, HNF3, and HNF4 protein factors that govern liver-specific transcription. Proc Natl Acad Sci USA. 1991;88:3807–11. doi: 10.1073/pnas.88.9.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rausa F, Samadani U, Ye H, et al. The cut-homeodomain transcriptional activator HNF-6 is coexpressed with its target gene HNF-3 beta in the developing murine liver and pancreas. Dev Biol. 1997;192(2):228–46. doi: 10.1006/dbio.1997.8744. [DOI] [PubMed] [Google Scholar]
- 17.Boj SF, Parrizas M, Maestro MA, Ferrer J. A transcription factor regulatory circuit in differentiated pancreatic cells. Proc Natl Acad Sci USA. 2001;98(25):14481–6. doi: 10.1073/pnas.241349398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas H, Jaschkowitz K, Bulman M, et al. A distant upstream promoter of the HNF-4α gene connects the transcription factors involved in maturity-onset diabetes of the young. Hum Mol Genet. 2001;10(19):2089–97. doi: 10.1093/hmg/10.19.2089. [DOI] [PubMed] [Google Scholar]
- 19.Eeckhoute J, Moerman E, Bouckenooghe T, et al. Hepatocyte nuclear factor 4α isoforms originated from the P1 promoter are expressed in human pancreatic beta-cells and exhibit stronger transcriptional potentials than P2 promoter-driven isoforms. Endocrinology. 2003;144(5):1686–94. doi: 10.1210/en.2002-0024. [DOI] [PubMed] [Google Scholar]
- 20.Chen WS, Manova K, Weinstein DC, et al. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Gen Dev. 1994;8(20):2466–77. doi: 10.1101/gad.8.20.2466. [DOI] [PubMed] [Google Scholar]
- 21•.Hayhurst GP, Lee YH, Lambert G, et al. Hepatocyte nuclear factor 4α (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21(4):1393–403. doi: 10.1128/MCB.21.4.1393-1403.2001. The first characterization of phenotypes of liver-specific HNF4α knockout study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Inoue Y, Yu AM, Yim SH, et al. Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4α. J Lipid Res. 2006;47(1):215–27. doi: 10.1194/jlr.M500430-JLR200. A more recent study of HNF4 regulation of bile acid synthesis in conditional knockout of HNF4α in mouse liver. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee J, Inoue Y, Yoon JC, et al. Regulation of hepatic fasting response by PPARγ coactivator-1α (PGC-1α): requirement for hepatocyte nuclear factor 4α in gluconeogenesis. Proc Natl Acad Sci USA. 2003;100(7):4012–7. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Maechler P, Antinozzi PA, et al. Hepatocyte nuclear factor 4α regulates the expression of pancreatic beta -cell genes implicated in glucose metabolism and nutrient-induced insulin secretion. J Biol Chem. 2000;275(46):35953–9. doi: 10.1074/jbc.M006612200. [DOI] [PubMed] [Google Scholar]
- 25.Miura A, Yamagata K, Kakei M, et al. Hepatocyte nuclear factor-4α is essential for glucose-stimulated insulin secretion by pancreatic beta-cells. J Biol Chem. 2006;281(8):5246–57. doi: 10.1074/jbc.M507496200. [DOI] [PubMed] [Google Scholar]
- 26.Gupta RK, Vatamaniuk MZ, Lee CS, et al. The MODY1 gene HNF-4α regulates selected genes involved in insulin secretion. J Clin Invest. 2005;115(4):1006–15. doi: 10.1172/JCI200522365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn SH, Shah YM, Inoue J, et al. Hepatocyte nuclear factor 4alpha in the intestinal epithelial cells protects against inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(7):908–20. doi: 10.1002/ibd.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamagata K, Furuta H, Oda N, et al. Mutations in the hepatocyte nuclear factors-4α gene in maturity-onset diabetes of the young (MODY1) Nature. 1996;384:458–60. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 29.Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4α regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sic USA. 1997;94:13209–14. doi: 10.1073/pnas.94.24.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lausen J, Thomas H, Lemm I, et al. Naturally occurring mutations in the human HNF4alpha gene impair the function of the transcription factor to a varying degree. Nucleic Acids Res. 2000;28(2):430–7. doi: 10.1093/nar/28.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryffel GU. Mutations in the human genes encoding the transcription factors of the hepatocyte nuclear factor (HNF)1 and HNF4 families: functional and pathological consequences. J Mol Endocrinol. 2001;27(1):11–29. doi: 10.1677/jme.0.0270011. [DOI] [PubMed] [Google Scholar]
- 32.Navas MA, Munoz-Elias EJ, Kim J, et al. Functional characterization of the MODY1 gene mutations HNF4(R127W), HNF4(V255M), and HNF4(E276Q) Diabetes. 1999;48(7):1459–65. doi: 10.2337/diabetes.48.7.1459. [DOI] [PubMed] [Google Scholar]
- 33.Sladek FM, Dallas-Yang Q, Nepomuceno L. MODY1 mutation Q268X in hepatocyte nuclear factor 4alpha allows for dimerization in solution but causes abnormal subcellular localization. Diabetes. 1998;47(6):985–90. doi: 10.2337/diabetes.47.6.985. [DOI] [PubMed] [Google Scholar]
- 34.Hani EH, Suaud L, Boutin P, et al. A missense mutation in hepatocyte nuclear factor-4 alpha, resulting in a reduced transactivation activity, in human late-onset non-insulin-dependent diabetes mellitus. J Clin Invest. 1998;101(3):521–6. doi: 10.1172/JCI1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokunaga A, Horikawa Y, Fukuda-Akita E, et al. A Common P2 Promoter Polymorphism of the Hepatocyte Nuclear Factor-4α Gene is Associated with Insulin Secretion in Non-Obese Japanese with Type 2 Diabetes. Endocr J. 2008 doi: 10.1507/endocrj.k08e-083. [DOI] [PubMed] [Google Scholar]
- 36.Shih DQ, Dansky HM, Fleisher M, et al. Genotype/phenotype relationships in HNF-4alpha/MODY1: haploinsufficiency is associated with reduced apolipoprotein (AII), apolipoprotein (CIII), lipoprotein(a), and triglyceride levels. Diabetes. 2000;49(5):832–7. doi: 10.2337/diabetes.49.5.832. [DOI] [PubMed] [Google Scholar]
- 37.Jiang G, Nepomuceno L, Hopkins K, Sladek FM. Exclusive homodimerization of the orphan receptor hepatocyte nuclear factor 4 defines a new subclass of nuclear receptors. Mol Cell Biol. 1995;15:5131–43. doi: 10.1128/mcb.15.9.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sladek FM, Ruse MD, Jr, Nepomuceno L, et al. Modulation of transcriptional activation and coactivator interaction by a splicing variation in the F domain of nuclear receptor hepatocyte nuclear factor 4alpha1. Mol Cell Biol. 1999;19(10):6509–22. doi: 10.1128/mcb.19.10.6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dell H, Hadzopoulou-Cladaras M. CREB-binding protein is a transcriptional coactivator for hepatocyte nuclear factor-4 and enhances apolipoprotein gene expression. J Biol Chem. 1999;274(13):9013–21. doi: 10.1074/jbc.274.13.9013. [DOI] [PubMed] [Google Scholar]
- 40.Wang JC, Stafford JM, Granner DK. SRC-1 and GRIP1 coactivate transcription with hepatocyte nuclear factor 4. J Biol Chem. 1998;273(47):30847–50. doi: 10.1074/jbc.273.47.30847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Surapureddi S, Rana R, Reddy JK, Goldstein JA. Nuclear receptor coactivator 6 mediates the synergistic activation of human cytochrome P-450 2C9 by the constitutive androstane receptor and hepatic nuclear factor-4α. Mol Pharmacol. 2008;74(3):913–23. doi: 10.1124/mol.108.048983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon JC, Puigserver P, Chen G, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–8. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 43.Rhee J, Ge H, Yang W, et al. Partnership of PGC-1α and HNF4α in the regulation of lipoprotein metabolism. J Biol Chem. 2006;281(21):14683–90. doi: 10.1074/jbc.M512636200. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Castellani LW, Sinal CJ, et al. Peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Gen Dev. 2004;18(2):157–69. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Li T, Chiang JY. Rifampicin induction of CYP3A4 requires pregnane X receptor cross talk with hepatocyte nuclear factor 4α and coactivators, and suppression of small heterodimer partner gene expression. Drug Metab Dispos. 2006;34(5):756–64. doi: 10.1124/dmd.105.007575. The first report of HNF4α and PXR interaction and synergy in induction of CYP3A4 in human hepatocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding X, Lichti K, Kim I, et al. Regulation of constitutive androstane receptor and its target genes by fasting, cAMP, hepatocyte nuclear factor alpha, and the coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha. J Biol Chem. 2006;281(36):26540–51. doi: 10.1074/jbc.M600931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116(3):615–22. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knutti D, Kralli A. PGC-1, a versatile coactivator. Trends Endocrinol Metab. 2001;12(8):360–5. doi: 10.1016/s1043-2760(01)00457-x. [DOI] [PubMed] [Google Scholar]
- 49.Guo H, Gao C, Mi Z, et al. Characterization of the PC4 binding domain and its interactions with HNF4α. J Biochem (Tokyo) 2007;141(5):635–40. doi: 10.1093/jb/mvm066. [DOI] [PubMed] [Google Scholar]
- 50.Torres-Padilla ME, Sladek FM, Weiss MC. Developmentally regulated N-terminal variants of the nuclear receptor hepatocyte nuclear factor 4α mediate multiple interactions through coactivator and corepressor-histone deacetylase complexes. J Biol Chem. 2002;277(47):44677–87. doi: 10.1074/jbc.M207545200. [DOI] [PubMed] [Google Scholar]
- 51.Lee YK, Dell H, Dowhan DH, et al. The orphan nuclear receptor SHP inhibits hepatocyte nuclear factor 4 and retinoid X receptor transactivation: two mechanisms for repression. Mol Cell Biol. 2000;20(1):187–95. doi: 10.1128/mcb.20.1.187-195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12α-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4α in mediating bile acid repression. J Biol Chem. 2001;276(45):41690–9. doi: 10.1074/jbc.M105117200. [DOI] [PubMed] [Google Scholar]
- 53.Hatzis P, Talianidis I. Regulatory mechanisms controlling human hepatocyte nuclear factor 4α gene expression. Mol Cell Biol. 2001;21(21):7320–30. doi: 10.1128/MCB.21.21.7320-7330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mietus-Snyder M, Sladek FM, Ginsburg GS, et al. Antagonism between apolipoprotein AI regulatory protein 1, Ear3/COUP-TF, and hepatocyte nuclear factor 4 modulates apolipoprotein CIII gene expression in liver and intestinal cells. Mol Cell Biol. 1992;12(4):1708–18. doi: 10.1128/mcb.12.4.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ktistaki E, Talianidis I. Chicken ovalbumin upstream promoter transcription factors act as auxiliary cofactors for hepatocyte nuclear factor 4 and enhance hepatic gene expression. Mol Cell Biol. 1997;17:2790–7. doi: 10.1128/mcb.17.5.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang S, Spann NJ, Hui TY, Davis RA. ARP-1/COUP-TF II Determines Hepatoma Phenotype by Acting as Both a Transcriptional Repressor of Microsomal Triglyceride Transfer Protein and an Inducer of CYP7A1. J Biol Chem. 2003;278(33):30478–86. doi: 10.1074/jbc.M304201200. [DOI] [PubMed] [Google Scholar]
- 57.Stroup D, Chiang JYL. HNF4 and COUP-TFII interact to modulate transcription of the cholesterol 7α-hydroxylase gene (CYP7A) J Lipid Res. 2000;41:1–11. [PubMed] [Google Scholar]
- 58.Malik S, Karathanasis S. Transcriptional activation by the orphan nuclear receptor ARP-1. Nucleic Acids Res. 1995;23(9):1536–43. doi: 10.1093/nar/23.9.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chiang JYL, Kimmel R, Weinberger C, Stroup D. FXR responds to bile acids and represses cholesterol 7α-hydroxylase gene (CYP7A1) transcription. J Biol Chem. 2000;275:10918–24. doi: 10.1074/jbc.275.15.10918. [DOI] [PubMed] [Google Scholar]
- 60.Chou WC, Prokova V, Shiraishi K, et al. Mechanism of a transcriptional cross talk between transforming growth factor-beta-regulated Smad3 and Smad4 proteins and orphan nuclear receptor hepatocyte nuclear factor-4. Mol Biol Cell. 2003;14(3):1279–94. doi: 10.1091/mbc.E02-07-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kardassis D, Pardali K, Zannis VI. SMAD proteins transactivate the human ApoCIII promoter by interacting physically and functionally with hepatocyte nuclear factor 4. J Biol Chem. 2000;275(52):41405–14. doi: 10.1074/jbc.M007896200. [DOI] [PubMed] [Google Scholar]
- 62.Maeda Y, Hwang-Verslues WW, Wei G, et al. Tumour suppressor p53 down-regulates the expression of the human hepatocyte nuclear factor 4alpha (HNF4alpha) gene. Biochem J. 2006;400(2):303–13. doi: 10.1042/BJ20060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maeda Y, Seidel SD, Wei G, et al. Repression of hepatocyte nuclear factor 4alpha tumor suppressor p53: involvement of the ligand-binding domain and histone deacetylase activity. Mol Endocrinol. 2002;16(2):402–10. doi: 10.1210/mend.16.2.0769. [DOI] [PubMed] [Google Scholar]
- 64.Hirota K, Daitoku H, Matsuzaki H, et al. Hepatocyte nuclear factor-4 is a novel downstream target of insulin via FKHR as a signal-regulated transcriptional inhibitor. J Biol Chem. 2003;278(15):13056–60. doi: 10.1074/jbc.C200553200. [DOI] [PubMed] [Google Scholar]
- 65.Li T, Kong X, Owsley E, et al. Insulin Regulation of Cholesterol 7α-Hydroxylase Expression in Human Hepatocytes: Roles of Forkhead Box O1 and Sterol Regulatory Element-Binding Protein 1c. J Biol Chem. 2006;281(39):28745–54. doi: 10.1074/jbc.M605815200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirota K, Sakamaki JI, Ishida J, et al. A combination of HNF-4 and Foxo1 is required for reciprocal transcriptional regulation of glucokinase and glucose-6-phosphatase genes in response to fasting and feeding. J Biol Chem. 2008;283(47):32432–41. doi: 10.1074/jbc.M806179200. [DOI] [PubMed] [Google Scholar]
- 67.Matsuzaki H, Daitoku H, Hatta M, et al. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA. 2003;100(20):11285–90. doi: 10.1073/pnas.1934283100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanyal S, Bavner A, Haroniti A, et al. Involvement of corepressor complex subunit GPS2 in transcriptional pathways governing human bile acid biosynthesis. Proc Natl Acad Sci USA. 2007;104(40):15665–70. doi: 10.1073/pnas.0706736104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Viollet B, Kahn A, Raymondjean M. Protein kinase A-dependent phosphorylation modulates DNA-binding activity of hepatocyte nuclear factor 4. Mol Cell Biol. 1997;17:4208–19. doi: 10.1128/mcb.17.8.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo H, Gao C, Mi Z, et al. Phosphorylation of Ser158 regulates inflammatory redox-dependent hepatocyte nuclear factor-4α transcriptional activity. Biochem J. 2006;394(Pt 2):379–87. doi: 10.1042/BJ20051730. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Reddy S, Yang W, Taylor DG, et al. Mitogen-activated protein kinase regulates transcription of the ApoCIII gene. Involvement of the orphan nuclear receptor HNF4. J Biol Chem. 1999;274(46):33050–6. doi: 10.1074/jbc.274.46.33050. [DOI] [PubMed] [Google Scholar]
- 72.Jahan A, Chiang JY. Cytokine regulation of human sterol 12α-hydroxylase (CYP8B1) gene. Am J Physiol. 2004;288:G685–95. doi: 10.1152/ajpgi.00207.2004. [DOI] [PubMed] [Google Scholar]
- 73.Hong YH, Varanasi US, Yang W, Leff T. AMP-activated protein kinase regulates HNF4alpha transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem. 2003;278(30):27495–501. doi: 10.1074/jbc.M304112200. [DOI] [PubMed] [Google Scholar]
- 74.Sun K, Montana V, Chellappa K, et al. Phosphorylation of a conserved serine in the deoxyribonucleic acid binding domain of nuclear receptors alters intracellular localization. Mol Endocrinol. 2007;21(6):1297–311. doi: 10.1210/me.2006-0300. [DOI] [PubMed] [Google Scholar]
- 75.Ktistaki E, Ktistaki T, Papadogeorgaki E, Talianidis I. Recruitment of hepatocyte nuclear factor 4 into specific intranuclear compartiments depends on tyrosine phosphorylation that affects its DNA-binding and transactivation potential. Proc Natl Acad Sci USA. 1995;92:9876–80. doi: 10.1073/pnas.92.21.9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soutoglou E, Talianidis I. Coordination of PIC assembly and chromatin remodeling during differentiation-induced gene activation. Science. 2002;295(5561):1901–4. doi: 10.1126/science.1068356. [DOI] [PubMed] [Google Scholar]
- 77.Soutoglou E, Katrakili N, Talianidis I. Acetylation regulates transcription factor activity at multiple levels. Mol Cell. 2000;5(4):745–51. doi: 10.1016/s1097-2765(00)80253-1. [DOI] [PubMed] [Google Scholar]
- 78.Chiang JY. Regulation of bile acid synthesis. Front Biosci. 1998;3:d176–93. doi: 10.2741/a273. [DOI] [PubMed] [Google Scholar]
- 79••.Chiang JY. Bile Acid regulation of gene expression: roles of nuclear hormone receptors. Endocr Rev. 2002;23(4):443–63. doi: 10.1210/er.2000-0035. The first review of the role of nuclear receptor in regulation of bile acid synthesis. [DOI] [PubMed] [Google Scholar]
- 80.Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol. 2004;40(3):539–51. doi: 10.1016/j.jhep.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 81.Crestani M, Sadeghpour A, Stroup D, et al. Transcriptional activation of the cholesterol 7α-hydroxylase gene (CYP7A) by nuclear hormone receptors. J Lipid Res. 1998;39:2192–200. [PubMed] [Google Scholar]
- 82.Chiang JY, Stroup D. Identification and characterization of a putative bile acid-responsive element in cholesterol 7α-hydroxylase gene promoter. J Biol Chem. 1994;269(26):17502–7. [PubMed] [Google Scholar]
- 83.Stroup D, Crestani M, Chiang JY. Identification of a bile acid response element in the cholesterol 7α-hydroxylase gene CYP7A. Am J Physiol. 1997;273(2 Pt 1):G508–17. doi: 10.1152/ajpgi.1997.273.2.G508. [DOI] [PubMed] [Google Scholar]
- 84.Chen W, Chiang JY. Regulation of human sterol 27-hydroxylase gene (CYP27A1) by bile acids and hepatocyte nuclear factor 4α (HNF4α) Gene. 2003;313:71–82. doi: 10.1016/s0378-1119(03)00631-0. [DOI] [PubMed] [Google Scholar]
- 85.Garuti R, Croce MA, Piccinini L, et al. Functional analysis of the promoter of human sterol 27-hydroxylase gene in HepG2 cells. Gene. 2002;283(1–2):133–43. doi: 10.1016/s0378-1119(01)00874-5. [DOI] [PubMed] [Google Scholar]
- 86.Inoue Y, Yu AM, Inoue J, Gonzalez FJ. Hepatocyte nuclear factor 4α is a central regulator of bile acid conjugation. J Biol Chem. 2004;279(4):2480–9. doi: 10.1074/jbc.M311015200. [DOI] [PubMed] [Google Scholar]
- 87.Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–5. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 88.Goodwin B, Jones SA, Price RR, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6(3):517–26. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 89.Denson LA, Sturm E, Echevarria W, et al. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121(1):140–7. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- 90.Sinal CJ, Tohkin M, Miyata M, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–44. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 91.Holt JA, Luo G, Billin AN, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17(13):1581–91. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2(4):217–25. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 93.Pircher PC, Kitto JL, Petrowski ML, et al. Farnesoid X receptor regulates bile acid-amino acid conjugation. J Biol Chem. 2003;278(30):27703–11. doi: 10.1074/jbc.M302128200. [DOI] [PubMed] [Google Scholar]
- 94.Staudinger JL, Goodwin B, Jones SA, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98(6):3369–74. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li T, Chiang JY. Mechanism of rifampicin and pregnane X receptor inhibition of human cholesterol 7α-hydroxylase gene transcription. Am J Physiol. 2005;288(1):G74–84. doi: 10.1152/ajpgi.00258.2004. [DOI] [PubMed] [Google Scholar]
- 96.Bhalla S, Ozalp C, Fang S, et al. Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1α. Functional implications in hepatic cholesterol and glucose metabolism. J Biol Chem. 2004;279(43):45139–47. doi: 10.1074/jbc.M405423200. [DOI] [PubMed] [Google Scholar]
- 97.Jung D, Mangelsdorf DJ, Meyer UA. Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem. 2006;281(28):19081–91. doi: 10.1074/jbc.M600116200. [DOI] [PubMed] [Google Scholar]
- 98.Makishima M, Lu TT, Xie W, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296(5571):1313–6. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 99.Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys. 2005;433(2):397–412. doi: 10.1016/j.abb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 100.Lewis DF. Human P450s in the metabolism of drugs: molecular modelling of enzyme-substrate interactions. Expert opinion Drug Metab Toxicol. 2005;1(1):5–8. doi: 10.1517/17425255.1.1.5. [DOI] [PubMed] [Google Scholar]
- 101.Chen D, Park Y, Kemper B. Differential protein binding and transcriptional activities of HNF4 elements in three closely related CYP2C genes. DNA Cell Biol. 1994;13:771–9. doi: 10.1089/dna.1994.13.771. [DOI] [PubMed] [Google Scholar]
- 102.Jover R, Bort R, Gomez-Lechon MJ, Castell JV. Cytochrome P450 regulation by hepatocyte nuclear factor 4 in human hepatocytes: a study using adenovirus-mediated antisense targeting. Hepatology. 2001;33(3):668–75. doi: 10.1053/jhep.2001.22176. [DOI] [PubMed] [Google Scholar]
- 103.Corchero J, Granvil CP, Akiyama TE, et al. The CYP2D6 humanized mouse: effect of the human CYP2D6 transgene and HNF4alpha on the disposition of debrisoquine in the mouse. Mol Pharmacol. 2001;60(6):1260–7. doi: 10.1124/mol.60.6.1260. [DOI] [PubMed] [Google Scholar]
- 104.Kamiya A, Inoue Y, Gonzalez FJ. Role of the hepatocyte nuclear factor 4alpha in control of the pregnane X receptor during fetal liver development. Hepatology. 2003;37(6):1375–84. doi: 10.1053/jhep.2003.50212. [DOI] [PubMed] [Google Scholar]
- 105.Tirona RG, Lee W, Leake BF, et al. The orphan nuclear receptor HNF4α determines PXR- and CAR-mediated xenobiotic induction of CYP3A4. Nat Med. 2003;9(2):220–4. doi: 10.1038/nm815. [DOI] [PubMed] [Google Scholar]
- 106•.Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23(5):687–702. doi: 10.1210/er.2001-0038. A comprehensive review of PXR in xenobiotic metabolism. [DOI] [PubMed] [Google Scholar]
- 107.Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999;56(6):1329–39. doi: 10.1124/mol.56.6.1329. [DOI] [PubMed] [Google Scholar]
- 108.Honkakoski P, Sueyoshi T, Negishi M. Drug-activated nuclear receptors CAR and PXR. Ann Med. 2003;35(3):172–82. doi: 10.1080/07853890310008224. [DOI] [PubMed] [Google Scholar]
- 109.Matsubara T, Yoshinari K, Aoyama K, et al. Role of vitamin D receptor in the lithocholic acid-mediated CYP3A induction in vitro and in vivo. Drug Metab Dispos. 2008;36(10):2058–63. doi: 10.1124/dmd.108.021501. [DOI] [PubMed] [Google Scholar]
- 110.Drocourt L, Ourlin JC, Pascussi JM, et al. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem. 2002;277(28):25125–32. doi: 10.1074/jbc.M201323200. [DOI] [PubMed] [Google Scholar]
- 111.Pascussi JM, Gerbal-Chaloin S, Drocourt L, et al. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta. 2003;1619(3):243–53. doi: 10.1016/s0304-4165(02)00483-x. [DOI] [PubMed] [Google Scholar]
- 112.Sonoda J, Rosenfeld JM, Xu L, et al. A nuclear receptor-mediated xenobiotic response and its implication in drug metabolism and host protection. Current Drug Metab. 2003;4(1):59–72. doi: 10.2174/1389200033336739. [DOI] [PubMed] [Google Scholar]
- 113•.Handschin C, Meyer UA. Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev. 2003;55(4):649–73. doi: 10.1124/pr.55.4.2. A comprehensive review of nuclear receptor regulation of drug metabolism. [DOI] [PubMed] [Google Scholar]
- 114.Moore LB, Parks DJ, Jones SA, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275(20):15122–7. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- 115.Ma X, Idle JR, Gonzalez FJ. The pregnane X receptor: from bench to bedside. Expert opinion Drug Metab Toxicol. 2008;4(7):895–908. doi: 10.1517/17425255.4.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kast HR, Goodwin B, Tarr PT, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277(4):2908–15. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 117.Xie W, Yeuh MF, Radominska-Pandya A, et al. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci USA. 2003;100(7):4150–5. doi: 10.1073/pnas.0438010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Staudinger JL, Madan A, Carol KM, Parkinson A. Regulation of drug transporter gene expression by nuclear receptors. Drug Metab Dispos. 2003;31(5):523–7. doi: 10.1124/dmd.31.5.523. [DOI] [PubMed] [Google Scholar]
- 119.Chen Y, Kissling G, Negishi M, Goldstein JA. The nuclear receptors constitutive androstane receptor and pregnane X receptor cross-talk with hepatic nuclear factor 4alpha to synergistically activate the human CYP2C9 promoter. J Pharmacol Exp Ther. 2005;314(3):1125–33. doi: 10.1124/jpet.105.087072. [DOI] [PubMed] [Google Scholar]
- 120.Fang HL, Strom SC, Ellis E, et al. Positive and negative regulation of human hepatic hydroxysteroid sulfotransferase (SULT2A1) gene transcription by rifampicin: roles of hepatocyte nuclear factor 4alpha and pregnane X receptor. J Pharmacol Exp Ther. 2007;323(2):586–98. doi: 10.1124/jpet.107.124610. [DOI] [PubMed] [Google Scholar]
- 121•.Wortham M, Czerwinski M, He L, et al. Expression of constitutive androstane receptor, hepatic nuclear factor 4α, and P450 oxidoreductase genes determines interindividual variability in basal expression and activity of a broad scope of xenobiotic metabolism genes in the human liver. Drug Metab Dispos. 2007;35(9):1700–10. doi: 10.1124/dmd.107.016436. Analysis of nuclear receptors in regulation of CYPs in human hepatocytes. [DOI] [PubMed] [Google Scholar]
- 122.Urquhart BL, Tirona RG, Kim RB. Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J Clin Pharmacol. 2007;47(5):566–78. doi: 10.1177/0091270007299930. [DOI] [PubMed] [Google Scholar]
- 123.Makishima M. Nuclear receptors as targets for drug development: regulation of cholesterol and bile acid metabolism by nuclear receptors. J Pharmacol Sci. 2005;97(2):177–83. doi: 10.1254/jphs.fmj04008x4. [DOI] [PubMed] [Google Scholar]
- 124.Fiorucci S, Rizzo G, Donini A, et al. Targeting farnesoid X receptor for liver and metabolic disorders. Trends Mol Med. 2007;13(7):298–309. doi: 10.1016/j.molmed.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 125.Westin S, Heyman RA, Martin R. FXR, a therapeutic target for bile acid and lipid disorders. Mini Rev Med Chem. 2005;5(8):719–27. doi: 10.2174/1389557054553802. [DOI] [PubMed] [Google Scholar]
- 126.Chen T. Nuclear receptor drug discovery. Current Opin Chem Biol. 2008;12(4):418–26. doi: 10.1016/j.cbpa.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Wipf P, Gong H, Janjic JM, et al. New opportunities for pregnane X receptor (PXR) targeting in drug development: Lessons from Enantio- and species-specific PXR ligands identified from a discovery library of amino acid analogues. Mini Rev Med Chem. 2007;7(6):617–25. doi: 10.2174/138955707780859404. [DOI] [PubMed] [Google Scholar]