Abstract
Objective
Outcomes in CREST did not differ between carotid artery stenting (CAS) and carotid endarterectomy (CEA) for the composite primary endpoint of stroke, myocardial infarction (MI), or death during the periprocedural period or ipsilateral stroke within four years. Rigorous credentialing and training of interventionists, including vascular surgeons, was required for the randomization phase of CREST. Because the lead-in phase of CREST had suggested higher perioperative risks after CAS performed by vascular surgeons, the purpose of this analysis was to examine differences in outcomes after randomization between CAS and CEA performed by vascular surgeons.
Methods
CREST is a prospective randomized controlled trial with blinded endpoint adjudication. Vascular surgeons performed 237 (21%) of the CAS procedures and 765 (65%) of the CEA procedures among 2320 patients who received their assigned treatment. Proportional hazards analyses were used to estimate the relative efficacy of CAS versus CEA for the composite primary endpoint and also for stroke and death.
Results
Among 2502 randomized patients, 1321 (53%) were symptomatic and 1181 (47%) asymptomatic. For procedures performed exclusively by vascular surgeons, the primary endpoint did not differ between CAS and CEA at four-year follow-up (6.2% vs 5.6%, respectively; hazard ratio [HR], 1.30; 95% confidence interval [CI], 0.70–2.41; P=0.41). In this subgroup, the periprocedural stroke and death rates were higher after CAS than CEA for symptomatic (6.1% vs 1.3%; P=0.01) patients. Asymptomatic patients also had slightly higher stroke and death rates after CAS (2.6% vs 1.1%; P=0.20), although this difference did not reach a level of statistical significance. Conversely, cranial nerve injuries (0.0% vs 5.0%; P<.001) were less frequent after CAS than CEA. MI rates were also slightly lower after CAS (1.3% vs 2.6%; P=0.24). In performing CAS, vascular surgeons had outcomes for the periprocedural primary endpoint comparable to outcomes of all interventionists (HR, 0.99; 95% CI, 0.50–2.00) after adjusting for age, sex, and symptomatic status. Vascular surgeons also had similar results after CEA for the periprocedural primary endpoint compared to other surgeons (HR, 0.73; 85%, 0.42–1.27).
Conclusion
CAS and CEA have similar net outcomes when performed by surgeons, although the periprocedural risks vary (lower stroke with CEA and lower MI with CAS). These data suggest that appropriately trained vascular surgeons may safely offer both CEA and CAS for the prevention of stroke. The remarkably low stroke and death rate after CEA performed by vascular surgeons in CREST, particularly among symptomatic patients, represent the best outcomes ever reported after carotid interventions from a randomized controlled trial.
INTRODUCTION
Both carotid endarterectomy (CEA) and carotid artery stenting (CAS) are effective interventions in preventing stroke and death among patients with significant carotid stenosis.1–7 Although CAS was initially reserved for patients with a high surgical risk for CEA,8, 9 recent clinical trials have revealed that CAS may also be an alternative for conventional-risk patients.6,10 In fact, comparison of standard risk patients in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST)6, 11 did not reveal any significant differences between CAS and CEA for the primary endpoint; periprocedural stroke, myocardial infarction (MI), or death and ipsilateral stroke up to four years were similarly low for both CAS and CEA, although a higher risk of stroke with CAS and a higher risk of MI with CEA were observed. A planned meta-analysis of European trials also failed to detect treatment differences among symptomatic patients younger than 70 years.10
Certification of surgeons and interventionists who performed CAS and CEA in CREST was required prior to randomizing patients.6, 12 Although some interventionists were certified after satisfactory evaluation of their endovascular experience and CAS results, most underwent rigorous hands-on training and auditing of their outcomes by participation in the lead-in phase. Interestingly, the results of the lead-in phase of CREST suggested higher perioperative risks after CAS performed by vascular surgeons.12 Stroke, death and MI rates at 30 days by specialty in the lead-in phase were 7.7% for vascular surgery, 6.7% for neurosurgery, 1.6% for neuroradiology, 6.6% for interventional radiology and 3.9% for interventional cardiology. After adjustment for age, vascular surgeons had a 2-fold higher event rate than interventional cardiologists (odds ratio, 2.05; 95% confidence interval, 1.18–3.56). Event rates did not differ significantly among other specialists.
The purpose of this study was to examine differences in outcomes between CAS and CEA performed by vascular surgeons in CREST. Specifically, data from the periprocedural period and up to four years were used to contrast the results of CEA and CAS performed by vascular surgeons and other specialists. Furthermore, the relative efficacy of the certification and training process for all interventionists was assessed.
METHODS
Details of the trial design and primary results of CREST have been reported.6, 11 CREST is a multicenter randomized clinical trial with blinded endpoint adjudication that compared the safety and efficacy of CAS versus CEA in patients with carotid stenosis. Patients were enrolled at 117 clinical centers in the United States and Canada. Ethics review boards at participating centers approved the protocol and informed consent and all patients gave written informed consent. To be eligible for the study, symptomatic patients required ≥50% ipsilateral carotid stenosis by angiography, ≥70% by duplex ultrasound, or ≥70% by computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) if the stenosis on ultrasonography was 50 to 69%, whereas asymptomatic patients needed ≥60% stenosis by angiography, ≥70% by ultrasound, or ≥80% by CTA or MRA if the stenosis on ultrasonography was 50% to 69%. Full eligibility criteria have been published.6, 11
Patients randomized to CAS were treated with aspirin and clopidogrel 48 hours before and for 30 days after the procedure. The ACCUNET and ACCULINK carotid stenting systems by Abbott Vascular Solutions, Inc. (formerly Guidant; Santa Clara, CA) were used for CAS procedures. Patients who underwent CEA received aspirin at least 48 hours before and for one year or more after the procedure. Full details of the procedures are provided elsewhere.13
Participating surgeons and interventionists were carefully selected by a well-documented process.12, 14 Certification was achieved by 477 surgeons, who documented that they had performed more than 12 procedures per year and that the rates of complications and death were less than 3% among asymptomatic patients and less than 5% among symptomatic patients. The 224 certified interventionists had to demonstrate experience in CAS with optimal results, receive hands-on experience with the RX ACCULINK stent and the RX ACCUNET embolic-protection device, or participate in a lead-in phase prior to randomizing patients. Most interventionists participated in the training program and the lead-in phase. Only 73 of the initial 427 potential applicants (17%) had clinical registry experience and satisfactory results with the devices used in CREST and were therefore exempt from training and approved for the randomization phase.
Periprocedural neurological evaluations were conducted pre-procedurally, at 24 to 48 hours post-procedurally, and at one month, three months, and annually; cardiac enzymes were obtained pre-procedurally, at 24 to 48 hours post-procedurally, and for chest pain lasting >15 minutes; ECGs were completed pre-procedurally and 24 to 48 hours post-procedurally, and for chest pain lasting >15 minutes. MI was defined as biomarker elevation plus either chest pain or ECG evidence of ischemia.
For the purpose of this study, similar statistical analyses to those for the CREST primary results were used.6 On-treatment endpoint analyses adjusting for major baseline covariates were conducted using standard time-to-event statistical modeling. In addition to the primary endpoint, outcome differences among specialists were also assessed for components of the composite endpoint and periprocedural risk. Since this analysis was performed for a subgroup of patients and only for those receiving therapy, the comparison of CEA to CAS is not protected by randomization. As such, differences between treatment efficacy for those patients treated by CAS or CEA could be due to the differential skills of the vascular surgeons for the two procedures, or alternatively because of differences in the type of patients receiving CAS/CEA treatment. In order to remove the potential effect of the latter source of differences, proportional hazards analysis was done, adjusting for age, sex, and symptomatic status, the primary factors shown to be associated with outcomes. Secondary aims were analyzed by including interaction terms in the proportional hazards models. For complication rates, the periprocedural period was defined according to the study protocol as the 30-day period after the procedure. The absolute differences in event proportions were calculated as the percentage of patients with events.
RESULTS
Between December 21, 2000 and October 16, 2008, 176 vascular surgeons performed 1002 (43%) of the carotid interventions among the 2320 patients who received their assigned treatment in the randomization phase. Of these interventions, vascular surgeons performed 237 (21%) of the 1136 CAS procedures and 765 (65%) of the 1184 CEAs. Among randomized patients who underwent carotid interventions by vascular surgeons, 467 (46.6%) were symptomatic and 535 (53.4%) were asymptomatic. As with the entire CREST cohort, there were no significant differences in baseline characteristics between the CAS and CEA patient groups, except for the percent asymptomatic CAS patients compared to CEA patients (65.4% vs 49.7%) and previous history of coronary artery disease or coronary artery bypass graft surgery (39.3% vs 47.5%) (Table 1). Among procedures performed by vascular surgeons, embolic protection was used in 229 (98.7%) of the CAS procedures, whereas for CEA general anesthesia and a patch were used in 647 (84.8%) and 616 (80.7%), respectively.
Table 1.
Baseline characteristics of the study population according to treatment group.
| Characteristic | CAS (N = 237) | CEA (N = 765) | P value |
|---|---|---|---|
| Age – y (mean ± SD) | 68.5 ± 8.0 | 69.4 ± 8.6 | 0.13 |
| Median | 68.5 | 70.3 | --- |
| Interquartile range | 11.1 | 12.7 | --- |
| Male gender (%) | 68.8 | 67.1 | 0.62 |
| White race (%) | 89.9 | 92.6 | 0.19 |
| Asymptomatic arteries (%) | 65.4 | 49.7 | <0.001 |
| Risk factors (%) | |||
| Hypertension | 82.6 | 85.2 | 0.33 |
| Diabetes | 26.7 | 29.5 | 0.40 |
| Dyslipidemia | 79.2 | 84.0 | 0.09 |
| Current tobacco smoking | 28.5 | 25.9 | 0.43 |
| Treatment with cholesterol meds | 93.0 | 90.0 | 0.22 |
| Coronary artery disease or CABG | 39.3 | 47.5 | 0.03 |
| Percent stenosis at randomization | |||
| Severe (≥70%) | 91.6 | 87.2 | 0.07 |
| Anatomic characteristics | |||
| Left carotid artery treated | 46.8 | 52.7 | 0.12 |
| Contralateral occlusion | --- | --- | --- |
| Procedural characteristics | |||
| Target lesion length (mm) (mean ± SD) | 19.5 ± 8.8 | --- | --- |
| Median | 19.5 | --- | --- |
| Interquartile range | 12.0 | --- | --- |
| Total length of stented segment (mm) (mean ± SD) | 34.7 ± 8.2 | --- | --- |
| Balloon angioplasty before stenting (%) | 73.4 | --- | --- |
| Embolic protection (%) | |||
| Was patient eligible for EPD | 99.6 | --- | --- |
| Was it successfully delivered | 98.7 | --- | --- |
| General anesthesia (%) | --- | 84.8 | --- |
| Surgical technique (%) | |||
| Patch | --- | 80.7 | --- |
| Shunt | --- | 59.1 | --- |
Sample sizes vary for specific characteristics (rows) because of missing data on specific items for a small number of patients. CAS=carotid artery stenting; CEA=carotid endarterectomy; SD=standard deviation; CAGB=coronary artery bypass graft surgery; EPD=embolic protection device.
Primary endpoint rates were not significantly different between CAS and CEA for procedures performed exclusively by vascular surgeons at four-year follow-up (6.2% vs 5.6%, respectively; hazard ratio (HR) =1.30; 95% confidence interval (CI), 0.70–2.41; P=0.41) (Table 2). These primary endpoint rates were slightly lower than originally reported for CAS versus CEA for the entire CREST cohort (7.2% vs 6.8%; HR=1.11; 95% CI, 0.81–1.51; P=0.51). Similarly, the periprocedural primary endpoint rates did not differ for CAS and CEA (4.2% vs 3.8%, respectively; HR=1.26; 95% CI, 0.61–2.60; P=0.54). After the periprocedural period, the incidence of ipsilateral stroke was similarly low after CAS and CEA performed by vascular surgeons (2.1% and 1.8%, respectively; P=0.63).
Table 2.
Treatment effect on time to first primary endpoint, components of the primary endpoint, and other events for Vascular Surgeons (n=1002).
| Periprocedural Period | Four-year Period | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CAS # events (rate ± SE) | CEA # events (rate ± SE) | Hazard Ratio (95% CI) | P-value | CAS # events (rate ± SE) | CEA # events (rate ± SE) | Hazard Ratio (95% CI) | P-value | ||
| MI Endpoint | Overall | 3 (1.3 ± 0.7) | 20 (2.6 ± 0.6) | 0.48a (0.14 – 1.62) | 0.24 | ||||
| Symptomatic | 2 (2.4 ± 1.7) | 10 (2.6 ± 0.8) | 0.93 a (0.20 – 4.25) | 0.93 | |||||
| Asymptomatic | 1 (0.6 ± 0.6) | 10 (2.6 ± 0.8) | 0.25 a (0.03 – 1.91) | 0.18 | |||||
| Stroke and Death Endpoint (any stroke or death within periprocedural period and post-procedural ipsilateral stroke) | Overall | 9 (3.8 ± 1.2) | 9 (1.2 ± 0.4) | 3.94 (1.53 – 10.10) | 0.004 | 14 (6.2 ± 1.6) | 20 (3.1 ± 0.7) | 2.76 (1.37 – 5.55) | 0.004 |
| Symptomatic | 5 (6.1 ± 2.6) | 5 (1.3 ± 0.6) | 4.84 a (1.40 – 16.74) | 0.013 | 6 (7.4 ± 2.9) | 13 (3.8 ± 1.1) | 2.45 (0.92 – 6.48) | 0.07 | |
| Asymptomatic | 4 (2.6 ± 1.3) | 4 (1.1 ± 0.5) | 2.50 a (0.63 – 9.99) | 0.20 | 8 (5.6 ± 2.0) | 7 (2.4 ± 1.0) | 3.10 (1.12 – 8.59) | 0.03 | |
| Primary Endpoint (any stroke, MI or death within periprocedural period and post-procedural ipsilateral stroke) | Overall | 10 (4.2 ± 1.3) | 29 (3.8 ± 0.7) | 1.26 (0.61 – 2.60) | 0.54 | 14 (6.2 ± 1.6) | 39 (5.6 ± 0.9) | 1.30 (0.70 – 2.41) | 0.41 |
| Symptomatic | 6 (7.3 ± 2.9) | 15 (3.9 ± 1.0) | 2.01 (0.78 – 5.20) | 0.15 | 6 (7.3 ± 2.9) | 22 (6.1 ± 1.3) | 1.36 (0.55 – 3.38) | 0.50 | |
| Asymptomatic | 4 (2.6 ± 1.3) | 14 (3.7 ± 1.0) | 0.75 (0.24 – 2.29) | 0.62 | 8 (5.6 ± 2.0) | 17 (5.1 ± 1.3) | 1.25 (0.54 – 2.89) | 0.61 | |
Univariate proportional hazards model employed because of the small number of events.
CAS=carotid artery stenting; CEA=carotid endarterectomy; SE=standard error; CI=confidence interval.
Among randomized patients who underwent the assigned intervention performed by vascular surgeons, the periprocedural stroke and death rates were higher after CAS than CEA among symptomatic patients (6.1% vs 1.3%; HR=4.84; 95% CI, 1.40–16.74 P=0.01) and among asymptomatic patients (2.6% vs 1.1%; HR=2.50; 95% CI, 0.63–9.99; P=0.20). Conversely, MI rates were lower for CAS compared to CEA (1.3% vs 2.6%; P=0.24). As expected cranial nerve injuries (0.0% vs 5.0%) were less frequent after CAS than CEA. Levels of significance merit conservative interpretation because of differences in number of events (10 periprocedural stroke and death events for symptomatic patients, 8 stroke and death events for asymptomatic patients, 12 MI events for symptomatic patients, and 11 MI events in asymptomatic patients).
When vascular surgeons were compared to all other specialties performing CAS, they had comparable outcomes for the periprocedural primary endpoint (HR, 0.99; 95% CI, 0.50–2.00) after adjusting for age, sex, and symptomatic status (Table 3). Vascular surgeons also had similar results after CEA for the periprocedural primary endpoint compared to other specialties performing CEA (HR, 0.73; 95% CI, 0.42–1.27).
Table 3.
Periprocedural endpoints by treatment group for vascular surgeons compared to all other specialties.
| Periprocedural Events | CAS Procedures | CEA Procedures | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Vascular Surgeons | Other Specialties | Hazard Ratio (95% CI) | P-value | Vascular Surgeons | Other Specialties | Hazard Ratio (95% CI) | P-value | ||
| Stroke and Death Endpoint (any stroke or death within periprocedural period) | Overall | 9 (3.8 ± 1.2) | 40 (4.5 ± 0.7) | 1.12 (0.54 – 2.35) | 0.76 | 9 (1.2 ± 0.4) | 16 (3.8 ± 0.9) | 0.32 (0.14 – 0.72) | 0.006 |
| Symptomatic | 5 (6.1 ± 2.6) | 31 (6.1 ± 1.1) | 1.11 (0.43 – 2.86) | 0.83 | 5 (1.3 ± 0.6) | 12 (5.0 ± 1.4) | 0.27a (0.09 – 0.76) | 0.013 | |
| Asymptomatic | 4 (2.6 ± 1.3) | 9 (2.4 ± 0.8) | 1.09 a (0.33 – 3.52) | 0.89 | 4 (1.1 ± 0.5) | 4 (2.2 ± 1.1) | 0.47 a (0.12 – 1.87) | 0.28 | |
| Primary Endpoint (any stroke, MI or death within periprocedural) | Overall | 10 (4.2 ± 1.3) | 48 (5.4 ± 0.8) | 0.99 (0.50 – 2.0) | 0.99 | 29 (3.8 ± 0.7) | 22 (5.3 ± 1.1) | 0.73 (0.42 – 1.27) | 0.26 |
| Symptomatic | 6 (7.3 ± 2.9) | 35 (6.9 ± 1.1) | 1.19 (0.50 – 2.84) | 0.70 | 15 (3.9 ± 1.0) | 16 (6.7 ± 1.6) | 0.59 (0.29 – 1.19) | 0.14 | |
| Asymptomatic | 4 (2.6 ± 1.3) | 13 (3.5 ± 0.9) | 0.74 (0.24 – 2.26) | 0.59 | 14 (3.7 ± 1.0) | 6 (3.4 ± 1.3) | 1.09 (0.42 – 2.84) | 0.86 | |
Univariate proportional hazards model employed because of the small number of events.
CAS=carotid artery stenting; CEA=carotid endarterectomy; CI=confidence interval; MI=myocardial infarction.
DISCUSSION
The results of this sub-study of CREST, the largest randomized clinical trial comparing carotid interventions for stroke prevention among conventional risk patients, failed to detect differences in the primary endpoint of periprocedural stroke, death and MI and ipsilateral stroke thereafter between CAS and CEA performed by appropriately trained vascular surgeons. As in the entire CREST cohort,6 the periprocedural risks vary (lower stroke with CEA and lower MI with CAS), which is predominantly seen among patients with symptomatic carotid stenosis. Of note, the periprocedural stroke and death rates after CEA performed by vascular surgeons for symptomatic carotid stenosis are the lowest ever reported for any carotid intervention among symptomatic patients.
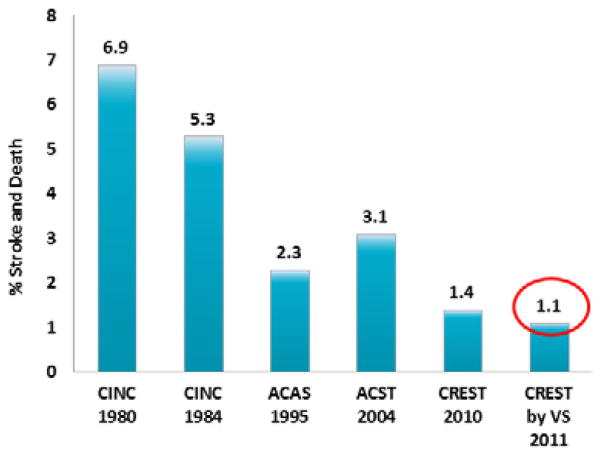
The periprocedural stroke and death rates for CAS and CEA performed by vascular surgeons in CREST are not only the lowest ever reported for interventions for symptomatic carotid stenosis, but also for asymptomatic carotid disease (Figures 1 and 2). Moreover, both stroke and death rates are well below the targets of 6% for symptomatic patients and 3% for asymptomatic patients suggested in recent treatment guideline statements.4, 5, 15
Figure 1.
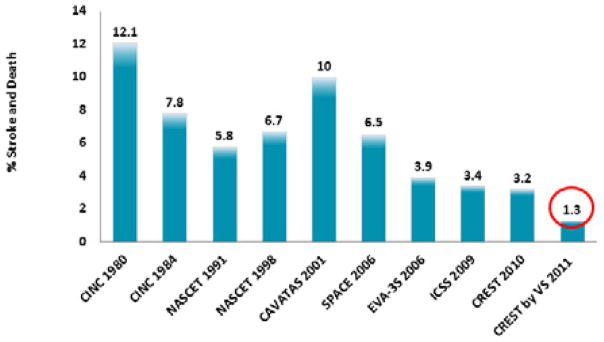
Perioperative stroke and death rate for carotid endarterectomy in symptomatic patients. CINC 1980=Cincinnati18; CINC 198418; NASCET 1991=North American Symptomatic Carotid Endarterectomy Trial19; NASCET 199822; CAVATAS=Carotid and Vertebral Artery Transluminal Angioplasty Study17; SPACE=Stent-protected Angioplasty versus Carotid Endarterectomy trial23; EVA-3S=Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis trial19; ICSS=International Carotid Stenting Study20; CREST=Carotid Revascularization Endarterectomy versus Stenting Trial6; CREST by VS=CREST by vascular surgeons.
Figure 2.
Perioperative stroke and death rate for carotid endarterectomy in asymptomatic patients. CINC 1980=Cincinnati18; CINC 198418; ACAS=Asymptomatic Carotid Atherosclerosis Study3; ACST=Asymptomatic Carotid Surgery Trial24; CREST=Carotid Revascularization Endarterectomy versus Stenting Trial6; CREST by VS=CREST by vascular surgeons.
As reported in this study and the original CREST publication,6 both CAS and CEA can be performed with optimal periprocedural outcomes by experienced surgeons and interventionists, including vascular surgeons. In many instances, vascular surgeons could potentially offer both procedures. Of note, CREST vascular surgeons were able to perform CEA with a significantly lower periprocedural risk of stroke and death as compared to the surgeons and other interventionists performing CAS. A higher MI rate with CEA and the added risk of postoperative cranial nerve palsies is still a matter of concern. Fortunately, cranial nerve palsies and MI did not have the same impact on physical and mental health as stroke based on quality of life assessment.6, 16 The higher rate of periprocedural stroke after CAS has fallen over time, as has the periprocedural risk of stroke after CEA. The periprocedural risk of CAS in our study appears to be comparable between vascular surgeons and interventionists from other specialties.
Interventionists’ training, experience, and specialty have been suggested as important factors for optimal outcomes after CAS procedures.12 In the CREST lead-in phase, higher periprocedural event rates were seen for procedures performed by vascular surgeons and marginally higher rates for interventional radiologists compared with cardiologists.12 These differences were attributed primarily to experience with catheter-based therapies and particularly CAS, and possibly to the complexity of the cases referred to specific specialties rather than to the specialty itself.
Multivariate analyses of lead-in phase data were performed in an attempt to adjust for potential confounders, including symptomatic status, degree of stenosis, and age. In these multivariate models, only age and interventionist specialty remained significant predictors of major adverse events after CAS. After adjustment for age, vascular surgeons had a higher event rate than interventional cardiologists, whereas event rates did not differ significantly among interventional radiologists, neurosurgeons, and interventional neuroradiologists. For the randomization phase, only those interventionists with a proven track record and optimal results in CAS techniques, irrespective of their specialty, were allowed to perform carotid stenting.6
The reduced stroke and death rates in CREST, particularly for procedures performed by vascular surgeons, as compared with previous trials and other specialists, may reflect the effective surgeon credentialing in CEA, assimilation of advanced endovascular technology, and rigorous training and credentialing of interventionists performing CAS. Although the certification requirements were important for patient safety, they limit the generalizability of the results and conclusions to similarly qualified operators, which constitutes one of the main limitations of CREST. Of the 427 stent operators who applied for the trial, only half (224; 52.4%) were ultimately approved for the randomization phase.12 The effects of experience in performing carotid interventions or the number of cases performed before the randomization on 30-day outcomes could not be defined with the available data and is beyond the scope of this sub-study. Total catheter experience, total endovascular treatment experience, or total carotid treatment experiences were not directly and objectively assessed during the trial. The more experienced interventionists were required to perform fewer cases in the lead-in phase than the less experienced ones, making the benefits of experience more difficult to detect from the lead-in phase results. The potential influence of patient and operator characteristics on the outcomes in CREST and in other randomized carotid intervention trials remains unknown and warrants further investigation.
The stroke and death rates after CAS performed by vascular surgeons were acceptable and within the targets suggested by the American Heart Association/American Stroke Association guidelines for the outcomes of carotid interventions.7 However, the outcomes after CEA performed by vascular surgeons were superior in terms of periprocedural stroke and death rates, 1.3% for symptomatic patients and 1.1% for asymptomatic patients. These improved outcomes after CEA in CREST may have several implications. First, the remarkably low stroke and death rates after CEA performed by vascular surgeons call for a revision of the accepted periprocedural outcomes and guidelines for carotid interventions. We suggest the guideline rates for periprocedural stroke and death of <6% for symptomatic patients and <3% for asymptomatic patients are too high. Second, vascular surgeons with current training and experience in both CAS and CEA are very well positioned to take care of patients with carotid disease as they may be able to choose from the different options of treatment impartially and without bias. Third, the improved outcomes with CEA in terms of stroke and death call for improved outcomes with CAS. Better outcomes following CAS may require improved systems for embolic protection and stent design.
In conclusion, CAS and CEA have similar net outcomes when performed by appropriately trained vascular surgeons, although the periprocedural risks vary (lower stroke with CEA and lower MI with CAS). Trained vascular surgeons may safely offer both CEA and CAS for the prevention of stroke. As for all interventionists/operators, focus on preventing periprocedural events is of high priority for vascular surgeons. The remarkably low stroke and death rate after CEA performed by vascular surgeons in CREST represents the best outcome ever reported after carotid interventions from a randomized controlled trial.
Acknowledgments
The study was supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH) (R01 NS 038384) with supplemental funding provided by Abbott Vascular Solutions, Inc., Santa Clara, CA (formerly Guidant).
Footnotes
Society Notation:
Presented at the Vascular Annual Meeting of the Society for Vascular Surgery, Chicago, IL, June 16–18, 2011.
ClinicalTrials.gov Identifier: NCT0000473
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolf PA, Kannel WB, Gee DL. Epidemiology of strokes in North America. In: Barnett HJM, Stein BM, Mohr JP, Yatsu FM, editors. Stroke: Pathophysiology, Diagnosis and Management. New York: Churchill Livingstone; 1986. pp. 19–29. [Google Scholar]
- 2.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 3.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–8. [PubMed] [Google Scholar]
- 4.Moore WS, Barnett HJ, Beebe HG, Bernstein EF, Brener BJ, Brott TG, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Circulation. 1995;91:566–79. doi: 10.1161/01.cir.91.2.566. [DOI] [PubMed] [Google Scholar]
- 5.Biller J, Feinberg WM, Castaldo JE, Whittemore AD, Harbaugh RE, Dempsey RJ, et al. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation. 1998;97:501–9. doi: 10.1161/01.cir.97.5.501. [DOI] [PubMed] [Google Scholar]
- 6.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the Prevention of Stroke in Patients With Stroke or Transient Ischemic Attack: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2011;42:227–76. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 8.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 9.Fairman R, Gray WA, Scicli AP, Wilburn O, Verta P, Atkinson R, et al. The CAPTURE registry: analysis of strokes resulting from carotid artery stenting in the post approval setting: timing, location, severity, and type. Ann Surg. 2007;246:551–6. doi: 10.1097/SLA.0b013e3181567a39. [DOI] [PubMed] [Google Scholar]
- 10.Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, Fraedrich G, et al. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet. 2010;376:1062–73. doi: 10.1016/S0140-6736(10)61009-4. [DOI] [PubMed] [Google Scholar]
- 11.Sheffet AJ, Roubin G, Howard G, Howard V, Moore W, Meschia JF, et al. Design of the Carotid Revascularization Endarterectomy vs. Stenting Trial (CREST) Int J Stroke. 2010;5:40–6. doi: 10.1111/j.1747-4949.2009.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkins LN, Roubin GS, Chakhtoura EY, Gray WA, Ferguson RD, Katzen BT, et al. The Carotid Revascularization Endarterectomy versus Stenting Trial: Credentialing of Interventionalists and Final Results of Lead-in Phase. J Stroke Cerebrovasc Dis. 2010;19:153–62. doi: 10.1016/j.jstrokecerebrovasdis.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silver FL, Mackey A, Clark WM, Brooks W, Timaran CH, Chiu D, et al. Safety of Stenting and Endarterectomy by Symptomatic Status in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) Stroke. 2011;42:675–80. doi: 10.1161/STROKEAHA.110.610212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hobson RW, II, Howard VJ, Roubin GS, Ferguson RD, Brott TG, Howard G, et al. Credentialing of surgeons as interventionalists for carotid artery stenting: Experience from the lead-in phase of CREST. J Vasc Surg. 2004;40:952–7. doi: 10.1016/j.jvs.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 15.Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1–31. doi: 10.1016/j.jvs.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 16.CREST trial. [Accessed December 27, 2010]; Supplementary Appendix http://www.nejm.org/doi/suppl/10.1056/NEJMoa0912321/suppl_file/nejmoa0912321_appendix.pdf.
- 17.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729–37. [PubMed] [Google Scholar]
- 18.Brott TG, Labutta RJ, Kempczinski RF. Changing patterns in the practice of carotid endarterectomy in a large metropolitan area. JAMA. 1986;255:2609–12. [PubMed] [Google Scholar]
- 19.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Eng J Med. 2006;355:1660–71. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 20.Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–97. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 22.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Eng J Med. 1998;339:1415–25. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 23.Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, et al. 30 day results from the SPACE trial of stentprotected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–47. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 24.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet. 2004;363:1491–502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]