Abstract
Epoxygenase activity and synthesis of epoxyeicosatrienoic acids (EETs) have emerged as important modulators of obesity and diabetes. We examined the effect of the EET-agonist 12-(3-hexylureido)dodec-8(2) enoic acid on mesenchymal stem cell (MSC) derived adipocytes proliferation and differentiation. MSCs expressed substantial levels of EETs and inhibition of soluble epoxide hydrolase (sEH) increased the level of EETs and decreased adipogenesis. EET agonist treatment increased HO-1 expression by inhibiting a negative regulator of HO-1 expression, Bach-1. EET treatment also increased βcatenin and pACC levels while decreasing PPARγ C/EBPα and fatty acid synthase levels. These changes were manifested by a decrease in the number of large inflammatory adipocytes, TNFα, IFNγ and IL-1α, but an increase in small adipocytes and in adiponectin levels. In summary, EET agonist treatment inhibits adipogenesis and decreases the levels of inflammatory cytokines suggesting the potential action of EETs as intracellular lipid signaling modulators of adipogenesis and adiponectin.
Keywords: MSC, EET-agonist, HO-1, Adipocyte
1. Introduction
Mesenchymal stem cells (MSCs) are multipotent cells which, under the appropriate culturing conditions, have the ability to differentiate into lineages of mesodermal tissue, that include skeletal muscle, adipocytes, bone, tendons and cartilage. MSCs are routinely isolated from several organs, including fetal liver, umbilical cord blood, and bone marrow [1–3]. Adipocytes differentiation plays a key role in the pathogenesis of diabetes. Adipogenesis begins with the commitment of MSCs to the adipocyte lineage, followed by terminal differentiation of preadipocytes to mature adipocytes. Cytokines and heme oxygenase (HO) activity have a regulatory role in mesenchymal stem cell microenvironment and hematopoiesis [4–6]. HO attenuates the overall production of reactive oxygen species (ROS) through its ability to degrade the pro-oxidant, heme, resulting in the production of carbon monoxide, biliverdin/bilirubin, and the release of free iron. These three products of heme degradation play an important role in signaling cascades, cell proliferation and differentiation [7–9]. HO exists as two isoenzymes, HO-1 (inducible) and HO-2 (constitutive) [10,11]. HO-1 is a stress response gene critical for bone marrow cell proliferation and differentiation [12]. Upregulation of HO-1 expression in obesity and type 2 diabetes results in a decrease in visceral and subcutaneous fat content, improved insulin sensitivity and increased insulin receptor phosphorylation [13–16]. MRI studies showed that up regulation of HO-1 decreased adiposity and adipocyte hypertrophy [13,15,17]. The decrease in HO-1 expression was associated with impairment in the MScs production of adiponectin and increased adipogenesis [3,13,18]. In addition, HO-1 gene expression has a differential effect on osteoblasts and adipocyte cell proliferation and differentiation [2,3]. EETs administration decreased adiposity and insulin resistance in mice and rat models of obesity and diabetes via an increase in HO-1 gene expression and signaling cascades including the activation of AMP activated kinase (AMPK) and pAKT [19]. EETs induce HO-1 protein and HO activity [18,20,21]. Human stromal-MSCs express CYP450 monooxygenase and form EETs and 20-HETE [22]. MSCs have the ability to metabolize arachidonic acid to HETE at comparable levels to that seen in endothelial cells [23]. Additionally, the EET agonist, inhibited soluble epoxide hydrolase (sEH), and reduced the rate of body weight gain in obese mice which was accompanied by an increase in HO-1 expression [19]. In the present study, we examined whether the EETs mediated decrease in adiposity is due to the direct effect of EETs on stem cell adipocytes via suppression of Bach-1 and subsequent increase in HO-1 expression and activity. EET also cause an increase in βcatenin and pACC and a decrease in the adipocyte differentiation markers PPARγ, C/EBPα and FAS.
2. Methods and procedures
2.1. Human bone marrow derived MSC differentiation into adipocytes
Frozen bone marrow mononuclear cells were purchased from Allcells (Emeryville, CA). After thawing, mononuclear cells were resuspended in an α-minimal essential medium (α-MEM, Invitrogen, Carlsbad, CA) supplemented with 10% heat inactivated fetal bovine serum (FBS, Invitrogen, Carlsbad, CA) and 1% antibiotic/antimycotic solution (Invitrogen, Carlsbad, CA). The cells were plated at a density of 1–5 × 106 cells per 100 cm2 dish. The cultures were maintained at 37 °C in a 5% CO2 incubator and the medium was changed after 48 h and every 3–4 days thereafter. When the MSCs were confluent, the cells were recovered by the addition of 0.25% trypsin/EDTA (Invitrogen, Carlsbad, CA). MSCs (Passage 2–3) were plated in a 75-cm2 flask at a density of 1–2 × 104 cells and cultured in α-MEM with 10% FBS for 7 days. The medium was replaced with adipogenic medium, and the cells were cultured for an additional 21 days. The adipogenic media consisted of complete culture medium supplemented with DMEM-high glucose, 10% (v/v) FBS, 10 µg/ml insulin, 0.5 mM dexamethasone (Sigma–Aldrich, St. Louis, MO), 0.5 mM isobutylmethylxanthine (Sigma–Aldrich, St. Louis, MO) and 0.1 mM indomethacin (Sigma–Aldrich, St. Louis, MO). Media were changed every 2 days. MSC-derived adipocytes were cultured in adipogenic differentiation media and the EET-agonist (AKR-1-27-28) was added every 3 days at a dose of 1 µM.
2.2. Oil Red O staining
For Oil Red O staining, 0.21% Oil Red O in 100% isopropanol (Sigma–Aldrich, St. Louis, MO) was used. Briefly, adipocytes were fixed in 10% formaldehyde, washed in Oil-red O for 10 min, rinsed with 60% isopropanol (Sigma–Aldrich, St. Louis, MO), and the Oil red O eluted by adding 100% isopropanol for 10 min and OD measured at 490 nm.
2.3. Cytokines Measurement
Adiponectin (high molecular weight, HMW) and the inflammatory cytokines TNF-α, INF-γ, IL-1α, IL-8 were determined in the culture supernatant. Multiple assays were conducted for quantification of the proteins (AssayGate, Inc., Ijamsville, MD). All measurements were performed in triplicate and normalized by cell numbers as previously described [17].
2.4. SiRNA knockdown of sEH gene expression
Adipocyte stem cells were treated with predesigned siRNAs of the gene in adipocyte culture media using a N-TER kit (Sigma-Aldrich, St. Louis, MO) according to manufacture’s protocol.
2.5. Western blot analysis
Cells were harvested using a cell lysis buffer as previously described [13,15,17]. The lysate was used to measure the protein levels of HO-1, CYP2J2, HO-2, PPARγ, FAS, pACC, βcatenin and C/EBPα. CYP2C23 antibodies has react with human CYP2C (antibodies were a gift from Dr. Jorge H. Capdevila, Nashville, TN). The phosphorylation of Acetyl-CoA carboxylase 1 (ACC1) was analyzed by immunoblotting with antibodies against phospho Ser79 ACC1 (Santa Cruz Biotechnology). Total ACC and β-actin were used as loading controls. Phosphorylation levels were quantified by scanning densitometry using an imaging densitometer normalized to the levels of total protein. The relative phosphorylation in each signaling molecule was calculated relative to the basal and/or control levels. Fatty acid synthase (FAS) antibodies, Wnt/βcatenin, PPARγ and C/EBPα were normalized to loading β-actin and presented as the ratio to the control. FAS, Wnt/βcatenin, PPARγ and C/EBPα (Santa Cruz Biotechnology), Bach 1, HO-1 and HO-2 were measured by immunoblotting with the corresponding antibodies and their levels were normalized to loading β-actin and the results are presented as relative to the basal or to the control levels as previously described [13,17].
2.6. Measurement of EETs and DHETs
MSCs were homogenized in 66% methanol containing a 500-pg mixture of internal standards [prostaglandin E2-d4, 8(9)-EET-d11, 11(12)-EET-d8, 12-hydroxyeicosatetraenoic acid-d8, 20-hydroxyeicosatetraenoic acid-d6, and 11,12-DHET-d11]. EETs and DHETs were extracted using solid phase C18-ODS AccuBond II 500-mg cartridges (Agilent Technologies, Santa Clara, CA).
All measurements were performed in triplicate and normalized by cell numbers as previously published [19].
2.7. mRNA isolation and real-time PCR quantification
Total RNA was isolated using Trizol® (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. First strand cDNA was synthesized with Roche reverse transcription reagents. Total RNA (1 µg) was analyzed by real-time PCR.
The quantitative real-time polymerase chain reaction (qRT-PCR) was performed with the TaqMan gene expression assay on an ABI Prism 7900 sequence analyzer according to the manufacturer’s recommended protocol (Applied Biosystems, Foster City, CA). Each reaction was run in triplicate. The comparative threshold cycle (CT) method was used to calculate the amplification fold as specified by the manufacturer. A value of 10 ng of reverse-transcribed RNA samples was amplified by using the TaqMan Universal PCR Master Mix and TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA).
2.8. Statistical analyses
Statistical significance between experimental groups was determined by the Fisher method of analysis of multiple comparisons (p < 0.05 was regarded as significant). For comparison between treatment groups, the null hypothesis was tested by either a single-factor ANOVA for multiple groups or the unpaired t-test for two groups. Data are presented as mean ± SEM.
3. Results
3.1. The expression of adipogenic proteins marker during adipogenesis
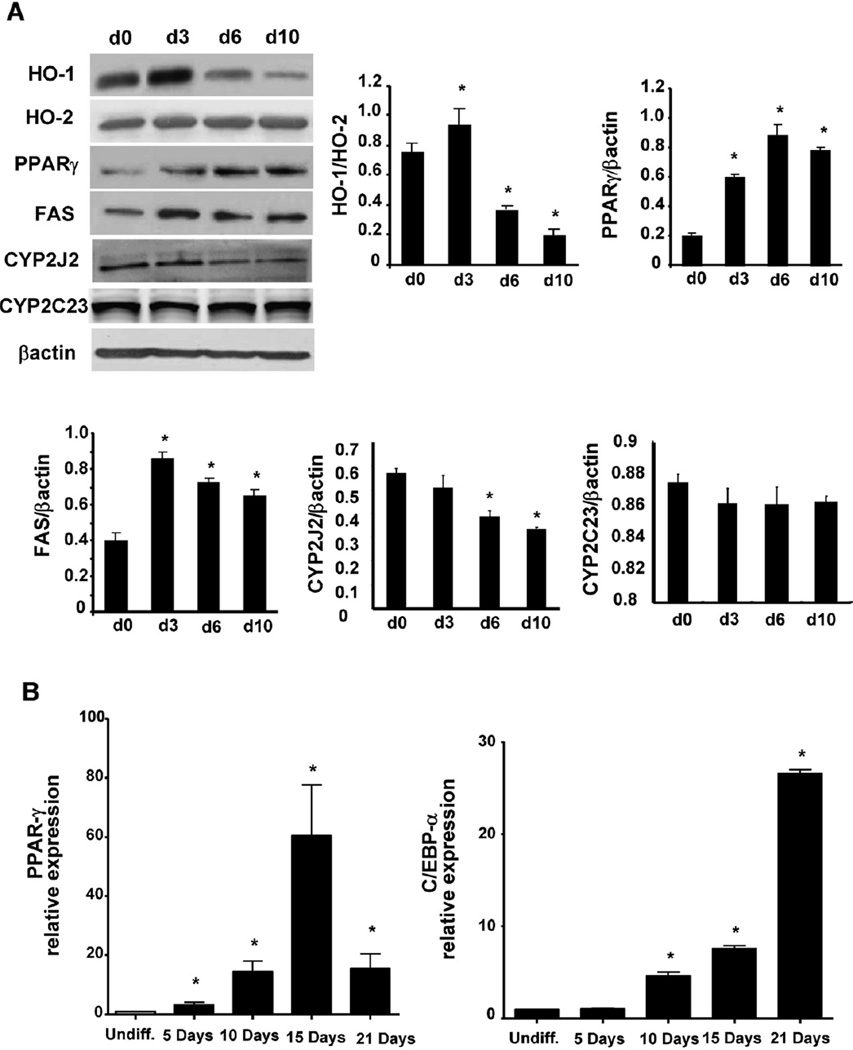
We examined the levels of HO-1, CYP2J2, PPARγ and FAS during adipogenesis. As seen in Fig. 1A, PPARγ is increased (p < 0.05) at day 3, plateaued at day 6 and remained elevated at day 10. The increase in PPARγ was associated with an increase in FAS (p < 0.05) which peaked at day 3 and remained elevated (p < 0.05) through day 10. HO-1 protein levels, but not HO-2, were significantly increased at day 3 (p < 0.05) but then decreased below starting values at day 6 (p < 0.05) and day 10 (Fig. 1A). Western Blot analysis showed that MSCs displayed a substantial level of epoxygenase CYP2J2 that was decreased in MSC-derived adipocytes (p < 0.05) in a time-dependent manner. In contrast CYP2C23 protein levels were not changed over the same period of time. Since PPARγ and C/EBPα are markers of adipocyte differentiation, we measured the mRNA of these two genes during MSC-differentiation to pre-adipocytes and adipocytes (5–21 days). PPARγ mRNA increased in a time dependent manner reaching a peak at day 15 before declining at day 21 where it remained elevated (p < 0.05) compared with undifferentiated cells. C/EBPα increased in a time dependent manner with significance (p < 0.05) attained at day 10 and a maximum at day 21 (Fig. 1B).
Fig. 1.
HO-1, PPARγ, FAS and CYP2J2, expression during adipogenesis in MSCs. (A) Expression of HO-1, HO-2, PPARγ, FAS, CYP2J2 and CYP2C in MSCs derived adipocytes on days 0, 3, 6 and 10 were measured by western blot (*p < 0.05 versus day 3, 6 and 10). (B) Expression of mRNA of PPARγ and C/EBPα with time of exposure, day 5, 10, 15 and 21. The results are 3 independent of experiments (*p < 0.05 versus undifferentiated cells).
3.2. The basal level of epoxygenase activity and the effect of soluble epoxide hydrolase inhibition on adipogenesis
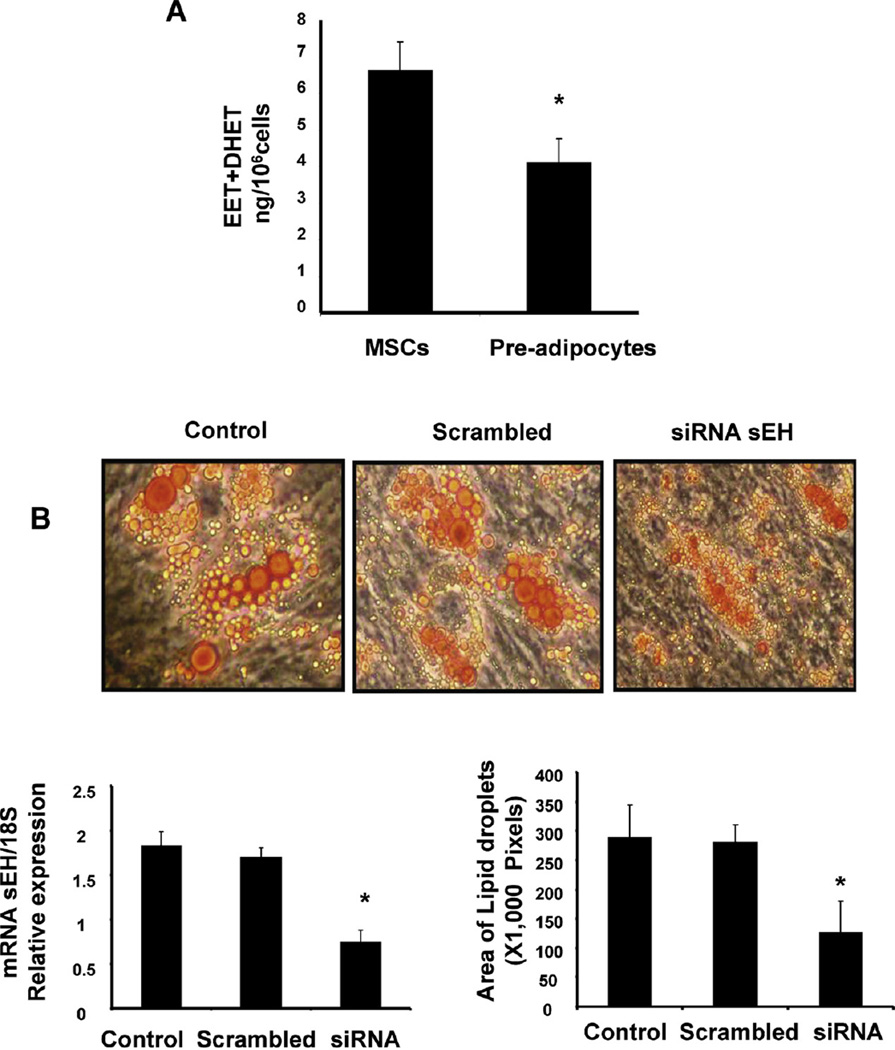
Since the levels of HO-1 and CYP2J2 decreased during differentiation we examined the levels of EET in undifferentiated and differentiated MSCs. As seen in Fig. 2A, the total level of EET + DHET is significantly (p < 0.05) decreased in pre-adipocytes. To elucidate the role of EETs in the regulation of adipogenesis during MSCs differentiation to adipocyte lineage, we measured the effect of suppression of sEH, on adipogenesis, using siRNAs (Fig. 2B). Quantitative PCR data 2 days after siRNAs delivery revealed a 60% decrease in sEH mRNA (Fig. 2B). As seen in Fig. 2B, the addition of siRNAs to sEH decreased lipid formation in MSC-derived adipocytes (p < 0.05). Additionally, droplet size was decreased in MSCs-derived adipocytes (p < 0.05).
Fig. 2.
(A). The total level of EET-DHET is significantly decreased in pre-adipocytes (*p < 0.05 versus undifferentiated cells). (B) siRNA-mediated decrease in sEH diminishes mRNA levels and decreased lipid droplet at 10 days of MSC-derived adipocytes differentiation cells transfected with siRNA for every 4 days. Data are expressed as mean ± SE (2B, left panel *p<0.01; 2B, right panel *p<0.05 vs siRNA scrambled or control treated n = 5).
3.3. Effect of EET agonist on FAS, PPARγ ACC and βcatenin
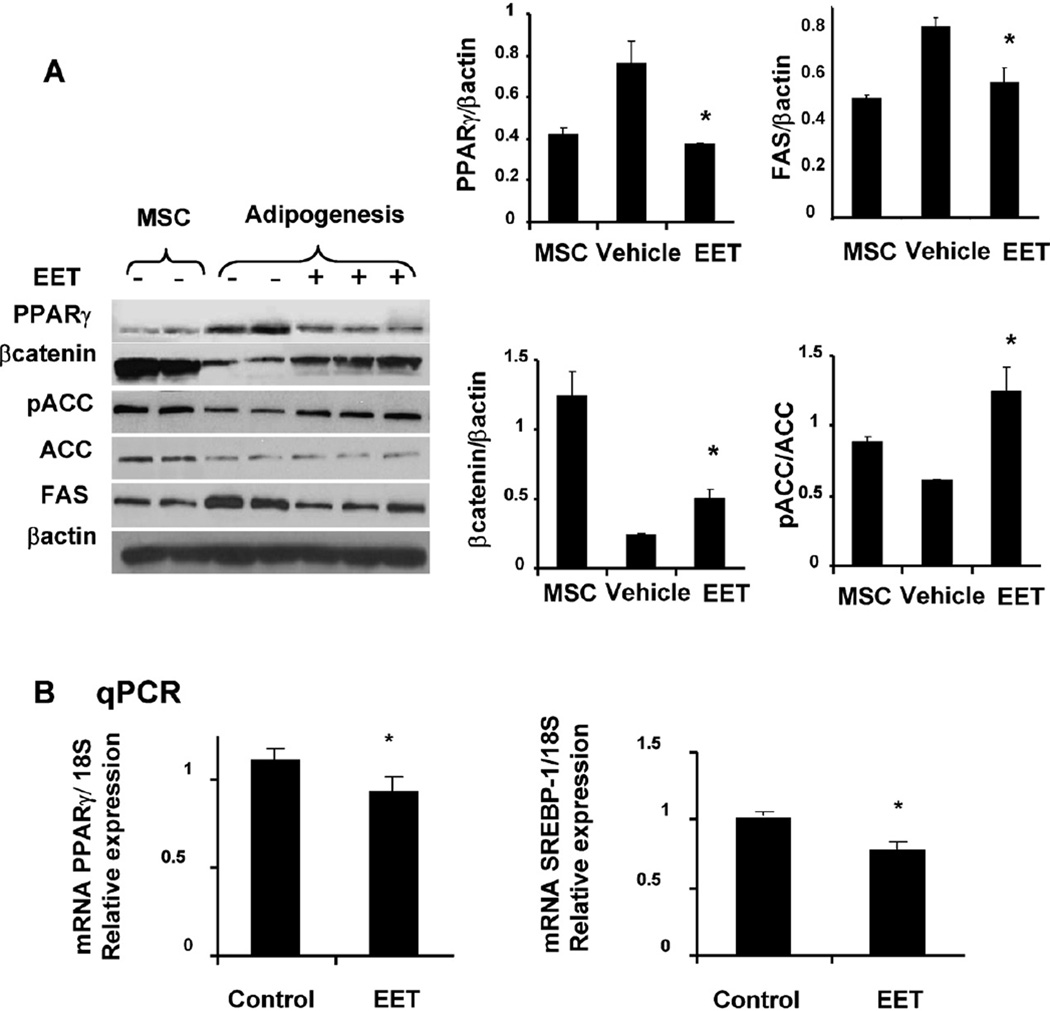
To further examine the mechanism by which EET-agonist regulates the adipogenic cell differentiation we measured PPARγ, βcatenin and FAS expression in adipocytes. As seen in Fig. 3A, expression of FAS and PPARγ levels was significantly (p < 0.05) increased in pre-adipocytes (14 days of MSC-derived adipocyte differentiation) and conversely, pACC and βcatenin were decreased (p < 0.05) in pre-adipocytes. The increase in FAS and PPARγ in pre-adipocytes was prevented by the EET-agonist 1 µM, Fig. 3A. The decrease in FAS and PPARγ was dose dependent in MSCs treated with EETs (data not shown). In contrast, the EET-agonist significantly increased both pACC and βcatenin (p < 0.05) compared to vehicle (Fig. 3A). MSC-derived adipocytes in adipogenic media for 14 days were used to determine the mRNA levels of PPARγ and SREBP-1(crucial in adipogenesis). MSCs-derived adipocytes exhibited a significantly (p < 0.05) higher expression of PPARγ and SREBP-1 compared to MSCs-adipocytes grown in the presence of 1 µM EET (Fig. 3B).
Fig. 3.
Effect of EET-agonist on the levels of PPARγ, FAS, Wnt/β-catenin and pACC. (A) hMSCs-derived adipocytes expressed elevated levels of PPARγ and FAS. Western blots showed that EET-agonist sustained decrease in PPARγ and FAS and simultaneous decrease in β-catenin and pACC. (B) Densitometric evaluations of protein were obtained from three different experiments. Data are expressed as mean ± SE (*p < 0.05 versus untreated 2 days MSCs-derived adipocyte growth). (C) adipogenic markers including PPARγ and SREBP-1 mRNA expression analyzed by quantitative PCR in 10 days culture treated and untreated with EET-agonist. Results for each condition are expressed as mean ± SE (n = 6, *p < 0.05 versus EET-agonist treated).
3.4. Effect of EET-agonist on HO-1, βcatenin, C/EBPα, PPARγ and Bach-1 levels
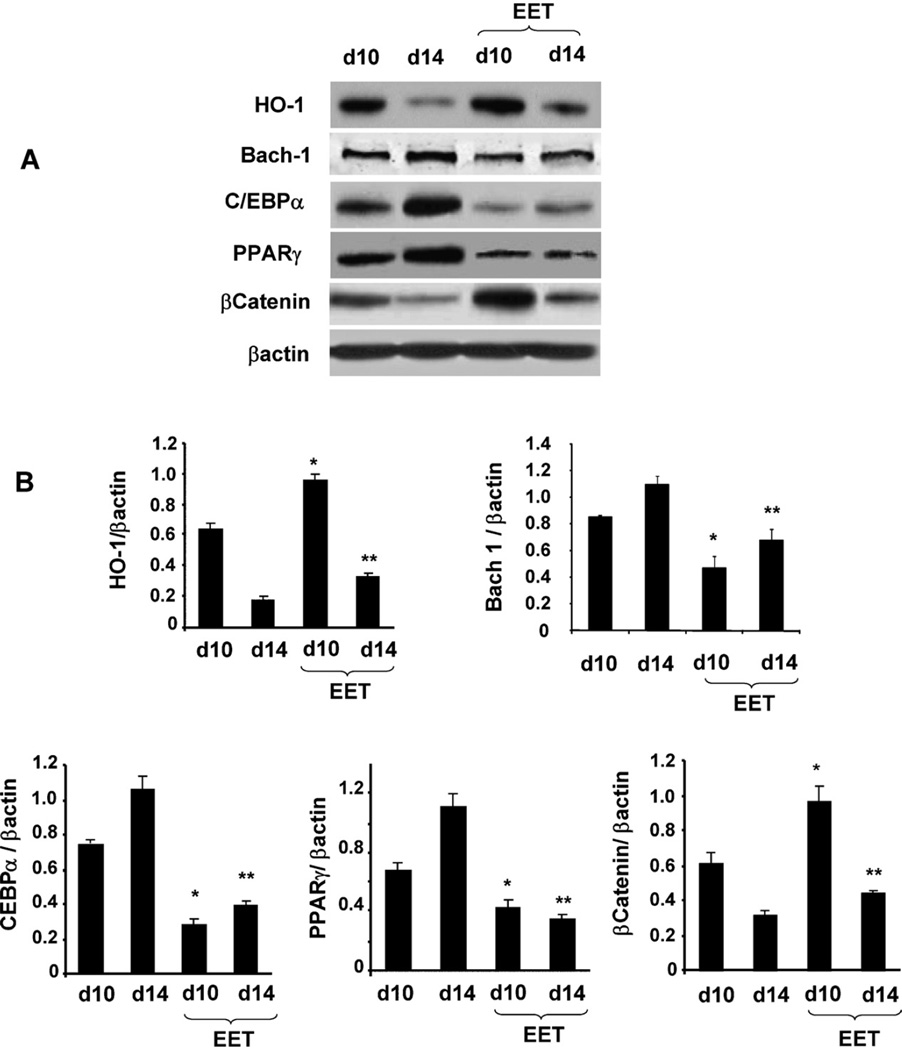
Since EET has been shown to increase HO-1 in animal model [19] we examined whether EET agonist increased HO-1 protein levels in MSC derived adipocytes. The protein levels of HO-1, β-catenin, PPARγ and C/EBPα were measured after 10 and 14 days in the presence and absence of the EET agonist. As seen in Fig. 4A and B, adipocytes cultured in the absence of the EET-agonist showed HO-1 levels that were decreased at day 10 and day 14 compared to adipocytes cultured in the presence of EET. In contrast PPARγ and C/EBPα protein pattern was the reverse of that of HO-1. PPARγ and C/EBPα levels were significantly increased (p < 0.05), while Wnt/βcatenin protein levels were decreased at 10 and 14 days of culture (p < 0.05) when compared with adipocytes cultured in the presence of EET. EET-agonist treated cells showed an increase in β-catenin (p < 0.05) at day 10 and 14 (Fig. 4B).
Fig. 4.
(A) Effect of EET-agonist on HO-1, Bach1 and adipogenic signaling. Western blots and densitometer analysis of C/EBPα, β-catenin, PPARγ Bach1 and HO-1 at d10 and 14 days in presence or absence of EET-agonist treatment. (B) Bars represent the mean ± SEM of four independent experiments (*p < 0.05 versus at d10 + EET-agonst, **p < 0.01 versus at 14 + EET-agonst days).
We examined whether EET increases HO-1 gene expression by a decrease in Bach-1 expression. As seen in Fig. 4, MSC-derived adipocytes treated with EET display a reduction in Bach-1 (p < 0.05). The mechanism which EET decrease Bach-1 is not clear. The decrease in Bach-1 was associated with an increase in HO-1 protein.
3.5. Effect of EET-agonist on inflammatory cytokines
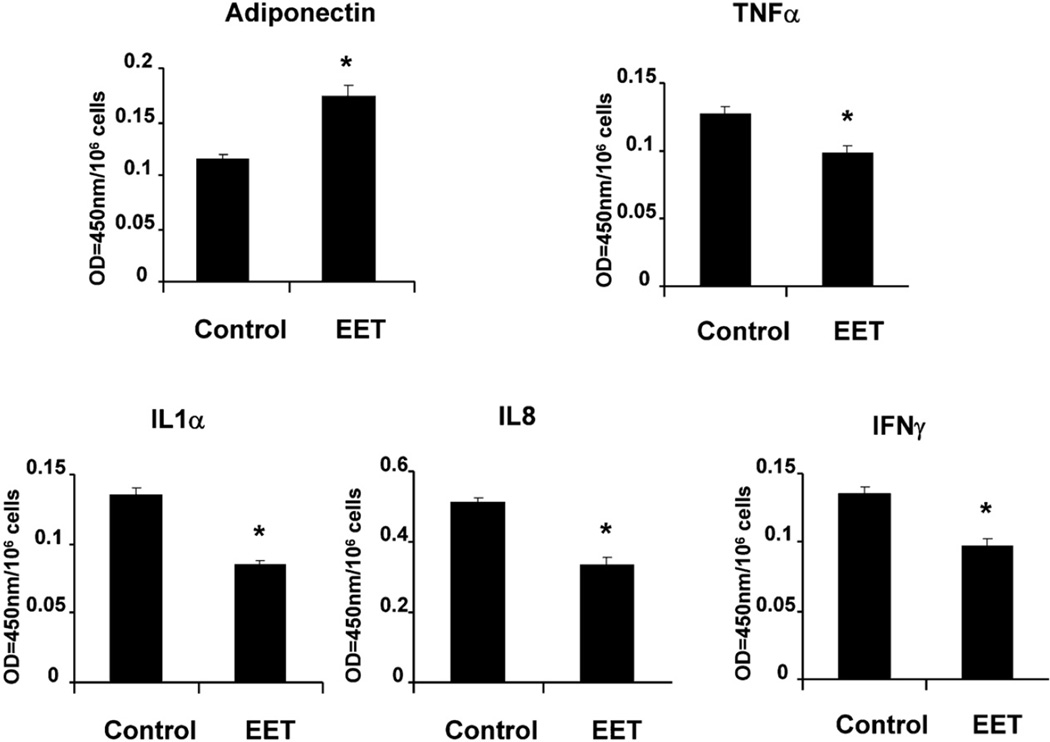
Since inhibition of sEH decreased MSC-derived adipocyte differentiation, we examined whether EET agonist, improved adipocyte function as, measured by the release of adiponectin and inflammatory cytokines. The levels of TNF-α, INF-γ, IL-1α, IL-8 were decreased (p < 0.05) in EET-agonist treated cells after samples were normalized by cell number (Fig. 5). As expected adiponectin levels in the conditioned media of MSC-derived pre-adipocytes were significantly lower compared to MSC-derived pre-adipocytes treated with EET agonist (Fig. 5).
Fig. 5.
Effect of EET-agonist on inflammatory cytokines levels in control and treated EET-agonist. EET-agonist was added every 2 days for 2 weeks, and cultured media samples were obtained immediately before the media was changed. The results are expressed as values at OD = 450 nm of cultured media. Results are calculated as pg/ml of cultured media for TNF-α, IFNγ, IL-1α, IL-8 and adiponectin (*p < 0.05 versus treated EET-agonist). Each cytokine value was normalized by cell number (values at OD = 450 nm/1000,000 cells ratio).
4. Discussion
This study demonstrates that, an increase in EETs levels suppresses adipogenesis and cell differentiation in MSC derived adipocytes. Three key findings substantiate this conclusion. Firstly, EETs are present in MSCs and their levels significantly decreased during differentiation to adipocytes. The fact that CYP2C is not affected but, reduction in CYP2J2 expression correlated with reduction in EET levels suggested that CYP2J2 is a primary source of EETs in MSCs; however, it does not exclude the possibility that other CYP epoxygenase contribute to EET production in MSCs. Additional studies are needed to fully characterize CYP epoxygenase activity in human MSCs. Our results also suggested that EET levels are regulated by sEH activity. Importantly, the increase in the levels of EETs by suppression of sEH, using siRNAs, led to diminished MSC-derived adipocyte stem cell differentiation. In agreement with previous reports the anti-adipogenic effect of an EET agonist, when combined with the inhibition of sEH, highlights the therapeutic potential of EETs in the management of cardiovascular disease [23,24]. An association of sEH gene polymorphism with insulin resistance has been reported implying that sEH is involved in the pathogenesis of insulin resistance [25]. Inhibition of sEH, decreased the degradation of EETs, leading to antiinflammatory and lipid lowering effects [26] and the amelioration of the inflammatory component of nephropathy associated with hypertension and type II diabetes [27].
Secondly, the treatment of MSCs-derived adipocyte stem cells with the EET-agonist led to increased HO-1 expression and decreased FAS, SREBP-1, PPARγ and increased pACC and βcatenin phosphorylation (Figs. 3 and 4). Although, EETs precise molecular mechanisms are not fully understood, one of the possible mechanisms via which epoxides enhance HO-1 expression may involve the transcriptional regulator Bach1. Previous studies demonstrated that Bach1, under baseline conditions, forms heterodimers, with small proteins of the Maf family, which repress the transcription of HO-1 gene [28]. Elevated intracellular concentration of heme increased binding of heme to Bach1 leading to a conformational change and a decrease in Bach-1 DNA binding activity. Bach1 suppresses HO-1 transcription and its inhibition has been shown to induce HO-1 [29,30]. In fact, silencing of Bach1 by siRNAs led to the up-regulation of HO-1 gene expression in human hepatocytes [31]. Disruption of the Bach1 gene in Apo E KO mice caused inhibition of atherosclerosis through upregulation of HO-1 [32]. Thus, by manipulating the expression of Bach1, it will be possible to induce or repress HO activity and the expression of HO-1. HO-1 and CYP2J2 expression were diminished during the adipogenic differentiation of MSCs, while FAS and PPARγ levels increased (Fig. 1). PPARγ is commonly referred to as the master regulator of adipogenesis [33] and ectopic expression and activation of PPARγ are sufficient to induce adipocyte differentiation. FAS mRNA levels increased during 3T3-L1 adipocyte differentiation [34].
In agreement with previous studies Wnt stimulation facilitated disruption of the axin-based complex [35,36]. This resulted in a decrease in the phosphorylation of βcatenin, which enhanced β-catenin accumulation and activation leading to an arrest in adipogenesis at the early progenitor stage through the blocking of PPARγ signaling [37,38]. The EET-mediated increase in Wnt signaling is considered a critical anti-adipogenic transcription factor of PPARγ [33]. Furthermore, EETs inhibited MSC-derived stem cell adipogenesis presumably through activation of HO-1/Wnt/β-catenin (illustrated in schematic diagram, Fig. 6) and the expression of C/EBPα, a marker of adipocyte differentiation [39]. In the present study, the increase of C/EBPα was prevented by treatment with an EET-agonist at day 10 and day 14. Our results provide direct evidence that EET-agonist induced increased expression of HO-1 led to the increases in adiponectin, phosphorylation/inactivation of ACC and consequently decreased levels of FAS. These perturbations occur in sequence, commencing with increased levels of HO-1 expression after EET-agonist treatment, suggesting that increased expression of HO-1 is a trigger for changes in lipid metabolism. Thirdly, the EET agonist decreased lipid size and both adipogenesis and the levels of inflammatory cytokines including TNFα, IFNγ and IL-1α [14,40]. This supports the concept that expansion of adipogenesis leads to an increased number of adipocytes of smaller cell size; smaller adipocytes are considered to be healthy, insulin sensitive adipocyte cells that are capable of producing adiponectin [41]. While increases in obesity and diabetes are considered a risk factor for cardiovascular complications [42], improvement in the diabetic phenotype, including increases in insulin sensitivity and glucose tolerance, may occur through increased pre-adipocyte differentiation and increased adiponectin secretion [41,43].
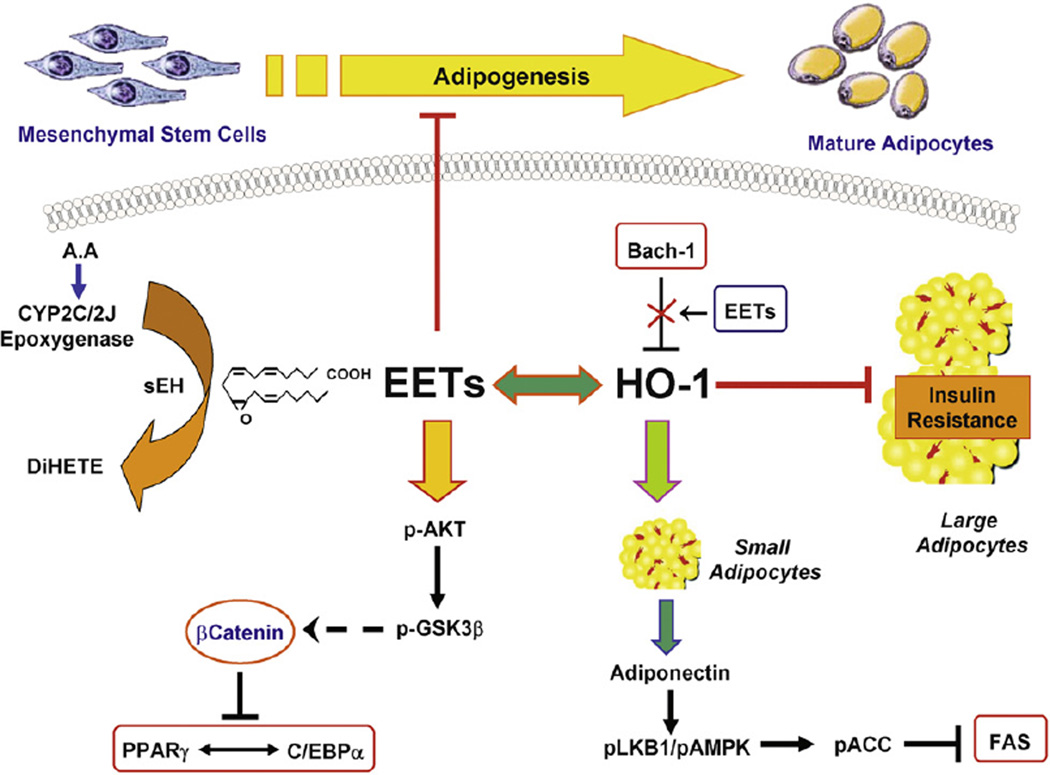
Fig. 6.
Proposed mechanism for the EET agonist-mediated suppression of MSCs-derived adipocyte differentiation and lipid accumulation. EET agonist-induced HO-1 expression, decreased superoxide inflammatory condition and activated pACC and Wnt/βcatenin leading to a decrease in adipogenic markers.
Obesity and metabolic syndrome are characterized by an increase in serum levels of inflammatory cytokines such as TNF-α and IL-1α, with a resultant decrease in insulin sensitivity [41,42]. This study demonstrates that the EETs-HO system participates in the regulation of the levels of inflammatory cytokines. Our results show that up-regulation of HO-1 by EET-agonist treatment was accompanied by a decrease in TNFα, IFNγ and IL-1α secretion. The latter, when considered with a decrease in adipogenesis, demonstrates that a favorable clinical outcome may be possible by increased levels of HO-1 expression in obesity. The acute induction of HO-1 has been shown to have a beneficial effect due to the rapid decrease in undesired heme [44]. Acute induction of HO-1 can have both positive and negative effects depending on the levels of induction. A modest increase in HO-1 increases EC-SOD, mitochondrial cytochrome and rapidly decreases iNOS and NOX-2 (NADPH-oxidase) [1]. Sacerdoti et al. [45] first reported the benefits of an acute effect, treatment with stannous chloride (SnCl2) prevented the development of high blood pressure. Small doses of heme arginate, or heme, which is used clinically for the treatment of porphyria [46], has been shown to have a beneficial effect on acute induction of HO-1 and lowers blood pressure in hypertensive rats [47,48]. However, chronic or super-induction of HO-1 protein can have both beneficial and detrimental effects on cellular metabolism. In cell cultures, the chronic induction of HO activity by SnCl2 resulted in a time- and dose-dependent decrease in cGMP levels, due to the limitations of heme and culture media and in heme synthesis [49]. However, chronic induction of HO-1, using iron as an inducer, did not affect cellular heme or cytochrome P450, content due to the rapid increase in heme turnover [1]. In contrast, inducers such as CoPP or CoCl2 will, at high concentrations, cause super induction of HO-1 and decrease cytochrome P450 [50,51]. Chronic administration of small doses of CoPP, attenuated the coronary constrictor response to ischemia-reperfusion [52]. The HO-1 mediated increase in EET and adiponectin provides the adipocyte system with tolerance and resistance to oxidative stress generated not only in hyperglycemias, but in other types of vascular stress [13]. One can speculate that when a genetic or environmental decrease in heme synthesis occurs; chronic induction of HO activity would exacerbate the disease state. Thus, careful review of the individual circumstances and the levels of HO-1 are necessary before embarking on the use of drugs targeting HO-1 for vascular and adipocyte protection. In conclusion, we have presented novel results which indicate the existence of molecular cross-talk between EET, HO-1 and adiponectin signaling in the regulation of MSCs-adipocyte stem cell differentiation and adipogenesis (Fig. 6). This suggests that EETs may suppress adipogenesis or the recruitment of stem cells in adipose tissue, prevent adipogenic lineage and ameliorate metabolic syndrome. In support of this conclusion, EET-agonist administration inhibited adiposity, increased insulin sensitivity and vascular function in an animal model of obesity [19]. Thus, EET-agonists may be developed as a class of compounds be employed therapeutically to address the metabolic derangements associated with the metabolic syndrome. In addition this study provides insights to the mechanism of action of the EETs as intracellular signaling molecules in lipid metabolism.
Acknowledgements
This work was supported by NIH grants DK068134, HL55601 (NGA), HL34300 (MLS), The Robert A. Welch Foundation, GM31278 (JRF) and The Beatrice Renfield Foundation (AK).
Abbreviations
- MSCs
mesenchymal stem cells
- EETs
epoxyeicosatrienoic acids
- HO-1
heme oxygenase-1
- PPARγ
peroxisome proliferator-activated receptor gamma
- SREBP-1
sterol regulatory element-binding protein 1
- FAS
fatty acid synthase
References
- 1.Abraham NG, Kappas A. Pharmacological. clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 2.Barbagallo I, Vanella A, Peterson S, et al. Overexpression of heme oxygenase-1 increases human osteoblast stem cell differentiation. J Bone Miner Metab. 2010;28:276–288. doi: 10.1007/s00774-009-0134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanella L, Kim DH, Asprinio D, et al. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46:236–243. doi: 10.1016/j.bone.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham NG. Molecular regulation—biological role of heme in hematopoiesis. Blood Rev. 1991;5:19–28. doi: 10.1016/0268-960x(91)90004-v. [DOI] [PubMed] [Google Scholar]
- 5.Chertkov JL, Jiang S, Lutton JD, Levere RD, Abraham NG. Hemin stimulation of hemopoiesis in murine long-term bone marrow culture. Exp Hematol. 1991;19:905–909. [PubMed] [Google Scholar]
- 6.Abraham NG, Chertkov JL, Staudinger R, et al. Long-term bone marrow stromal and hemopoietic toxicity to AZT: protective role of heme and IL-1. Exp Hematol. 1993;21:263–268. [PubMed] [Google Scholar]
- 7.Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Glatz JF, Vork MM, Cistola DP, van d V. Cytoplasmic fatty acid binding protein: significance for intracellular transport of fatty acids and putative role on signal transduction pathways. Prostaglandins Leukot Essent Fatty Acids. 1993;48:33–41. doi: 10.1016/0952-3278(93)90007-j. [DOI] [PubMed] [Google Scholar]
- 9.Chhikara M, Wang S, Kern SJ, et al. Carbon monoxide blocks lipopolysaccharide-induced gene expression by interfering with proximal TLR4 to NF-kappaB signal transduction in human monocytes. PLoS One. 2009;4:e8139. doi: 10.1371/journal.pone.0008139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abraham NG, Drummond GS, Lutton JD, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem. 1996;6:129–168. [Google Scholar]
- 11.Shibahara S, Yoshizawa M, Suzuki H, et al. Functional analysis of cDNAs for two types of human heme oxygenase and evidence for their separate regulation. J Biochem Tokyo. 1993;113:214–218. doi: 10.1093/oxfordjournals.jbchem.a124028. [DOI] [PubMed] [Google Scholar]
- 12.Abraham NG, Nelson JC, Ahmed T, Konwalinka G, Levere RD. Erythropoietin controls heme metabolic enzymes in normal human bone marrow culture. Exp Hematol. 1989;17:908–913. [PubMed] [Google Scholar]
- 13.Li M, Kim DH, Tsenovoy PL, et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 14.Kim DH, Burgess AP, Li M, et al. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines, tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J Pharmacol Exp Ther. 2008;325:833–840. doi: 10.1124/jpet.107.135285. [DOI] [PubMed] [Google Scholar]
- 15.Nicolai A, Li M, Kim DH, et al. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53:508–515. doi: 10.1161/HYPERTENSIONAHA.108.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson SJ, Drummond G, Kim DH, et al. L-4F treatment reduces adiposity, increases adiponectin levels and improves insulin sensitivity in obese mice. J Lipid Res. 2008;49:1658–1669. doi: 10.1194/jlr.M800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson SJ, Kim DH, Li M, et al. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J Lipid Res. 2009;50:1293–1304. doi: 10.1194/jlr.M800610-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DH, Vanella L, Inoue K, et al. EET-agonist regulates human mesenchymal stem cells-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma. Stem Cells Dev. 2010 doi: 10.1089/scd.2010.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sodhi K, Inoue K, Gotlinger K, et al. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacerdoti D, Bolognesi M, Di PM, et al. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am J Physiol Heart Circ Physiol. 2006;291:H1999–H2002. doi: 10.1152/ajpheart.00082.2006. [DOI] [PubMed] [Google Scholar]
- 21.Sacerdoti D, Colombrita C, Di PM, et al. 11,12-Epoxyeicosatrienoic acid stimulates heme-oxygenase-1 in endothelial cells. Prostaglandins Other Lipid Mediat. 2007;82:155–161. doi: 10.1016/j.prostaglandins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Abraham NG, Feldman E, Falck JR, Lutton JD, Schwartzman ML. Modulation of erythropoiesis by novel human bone marrow cytochrome P450-dependent metabolites of arachidonic acid. Blood. 1991;78:1461–1466. [PubMed] [Google Scholar]
- 23.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 24.Imig JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev. 2006;24:169–188. doi: 10.1111/j.1527-3466.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 25.Ohtoshi K, Kaneto H, Node K, et al. Association of soluble epoxide hydrolase gene polymorphism with insulin resistance in type 2 diabetic patients. Biochem Biophys Res Commun. 2005;331:347–350. doi: 10.1016/j.bbrc.2005.03.171. [DOI] [PubMed] [Google Scholar]
- 26.Zhang LN, Vincelette J, Cheng Y, et al. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler Thromb Vasc Biol. 2009;29:1265–1270. doi: 10.1161/ATVBAHA.109.186064. [DOI] [PubMed] [Google Scholar]
- 27.Olearczyk JJ, Quigley JE, Mitchell BC, et al. Administration of a substituted adamantyl urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats. Clin Sci (Lond) 2009;116:61–70. doi: 10.1042/CS20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oyake T, Itoh K, Motohashi H, et al. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol. 1996;16:6083–6095. doi: 10.1128/mcb.16.11.6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitamuro T, Takahashi K, Ogawa K, et al. Bach 1 functions as a hypoxia-inducible repressor for the heme oxygenase-1 gene in human cells. J Biol Chem. 2003 doi: 10.1074/jbc.M209939200. [DOI] [PubMed] [Google Scholar]
- 30.Reichard JF, Sartor MA, Puga A. BACH1 is a specific repressor of HMOX1 that is inactivated by arsenite. J Biol Chem. 2008;283:22363–22370. doi: 10.1074/jbc.M801784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shan Y, Lambrecht RW, Ghaziani T, Donohue SE, Bonkovsky HL. Role of Bach-1 in regulation of heme oxygenase-1 in human liver cells: insights from studies with small interfering RNAS. J Biol Chem. 2004;279:51769–51774. doi: 10.1074/jbc.M409463200. [DOI] [PubMed] [Google Scholar]
- 32.Watari Y, Yamamoto Y, Brydun A, et al. Ablation of the bach1 gene leads to the suppression of atherosclerosis in bach1 and apolipoprotein E double knockout mice. Hypertens Res. 2008;31:783–792. doi: 10.1291/hypres.31.783. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Farmer SR. Regulating the balance between peroxisome proliferator-activated receptor gamma and beta-catenin signaling during adipogenesis. A glycogen synthase kinase 3beta phosphorylation-defective mutant of beta-catenin inhibits expression of a subset of adipogenic genes. J Biol Chem. 2004;279:45020–45027. doi: 10.1074/jbc.M407050200. [DOI] [PubMed] [Google Scholar]
- 34.Moustaid N, Sul HS. Regulation of expression of the fatty acid synthase gene in 3T3-L1 cells by differentiation and triiodothyronine. J Biol Chem. 1991;266:18550–18554. [PubMed] [Google Scholar]
- 35.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Etheridge SL, Spencer GJ, Heath DJ, Genever PG. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells. 2004;22:849–860. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- 37.Ross SE, et al. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 38.Okamura M, Kudo H, Wakabayashi K, et al. COUP-TFII acts downstream of Wnt/beta-catenin signal to silence PPARgamma gene expression and repress adipogenesis. Proc Natl Acad Sci USA. 2009;106:5819–5824. doi: 10.1073/pnas.0901676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin FT, Lane MD. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 1992;6:533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- 40.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 41.Kim JY, van de WE, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- 43.Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 44.Abraham NG, Drummond G. CD163-mediated hemoglobin-heme uptake activates macrophage HO-1, providing an antiinflammatory function. Circ Res. 2006;99:911–914. doi: 10.1161/01.RES.0000249616.10603.d6. [DOI] [PubMed] [Google Scholar]
- 45.Sacerdoti D, Escalante B, Abraham NG, et al. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science. 1989;243:388–390. doi: 10.1126/science.2492116. [DOI] [PubMed] [Google Scholar]
- 46.Kordac V, Kozakova M, Martasek P. Changes of myocardial functions in acute hepatic porphyries. Role of heme arginate administration. Ann Med. 1989;21:273–276. doi: 10.3109/07853898909149205. [DOI] [PubMed] [Google Scholar]
- 47.Schwartzman ML, Martasek P, Rios AR, et al. Cytochrome P450-dependent arachidonic acid metabolism in human kidney. Kidney Int. 1990;37:94–99. doi: 10.1038/ki.1990.13. [DOI] [PubMed] [Google Scholar]
- 48.Martasek P, Schwartzman ML, Goodman AI, et al. Hemin and l-arginine regulation of blood pressure in spontaneous hypertensive rats. J Am Soc Nephrol. 1991;2:1078–1084. doi: 10.1681/ASN.V261078. [DOI] [PubMed] [Google Scholar]
- 49.Abraham NG, Mieyal PA, Quan S, et al. Modulation of cyclic GMP by retrovirus-mediated human heme oxgyenase-1 gene transfer in microvessel endothelial cells. Am J Physiol. 2002;283:L1117–L1124. doi: 10.1152/ajplung.00365.2001. [DOI] [PubMed] [Google Scholar]
- 50.Drummond GS, Kappas A. The cytochrome P-450-depleted animal: an experimental model for in vivo studies in chemical biology. Proc Natl Acad Sci USA. 1982;79:2384–2388. doi: 10.1073/pnas.79.7.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin JH, Villalon P, Martasek P, Abraham NG. Regulation of heme oxygenase gene expression by cobalt in rat liver and kidney. Eur J Biochem. 1990;192:577–582. doi: 10.1111/j.1432-1033.1990.tb19263.x. [DOI] [PubMed] [Google Scholar]
- 52.L’Abbate A, Neglia D, Vecoli C, et al. Beneficial effect of heme oxygenase-1 expression on myocardial ischemia-reperfusion involves an increase in adiponectin in mildly diabetic rats. Am J Physiol Heart Circ Physiol. 2007;293:H3532–H3534. doi: 10.1152/ajpheart.00826.2007. [DOI] [PubMed] [Google Scholar]