Abstract
The concentration and composition of PAHs emitted from biomass cooking fuel were characterized in a rural non-smoking household in northern China. Twenty-two parent PAHs (pPAHs), 12 nitro-PAHs (nPAHs), and 4 oxy-PAHs (oPAHs) were measured in the kitchen, bedroom, and outdoors during both summer and winter. The most severe contamination occurred in the kitchen in the winter, where the daily mean concentrations of pPAHs, nPAHs, and oPAHs were 7500±4100, 38±29, and 8400±9200 ng/m3, respectively. Our results suggest that the nPAHs were largely from secondary formation in ambient air while oPAHs were either from primary emission of biomass burning or secondary formation from pPAHs in the kitchen. The daily mean benzo(a)pyrene equivalent exposure concentration was as high as 200±160 ng/m3 in the winter for the housewife who did the cooking compared to 59±37 ng/m3 for the control group that did not cook.
Keywords: rural indoor air, solid fuel, PAHs, nitro-PAHs, oxygenated-PAHs
1. Introduction
During the past three decades, China has experienced rapid economic development and environmental deterioration including severe air pollution (World Bank, 2007). Among many air pollutants, polycyclic aromatic hydrocarbons (PAHs) are one of the major public health concerns due to their carcinogenic potential (Xue and Warshawsky, 2005). It has been reported that inhalation exposure of the Chinese population to 16 PAHs in ambient air cause a significant risk of lung cancer morbidity (Zhang et al., 2009). Given that most people spend more time indoors and PAH concentrations in rural indoor air are higher than those in ambient air, the overall PAH exposure risk to rural residents from indoor air may be more severe than ambient air (Mumford et al., 1990; Lu et al., 2006). This is especially true in northern China in winter, when solid fuels are widely used for heating and windows and doors are usually closed. Given China’s large rural population of over 600 million and their heavy reliance on solid fuel for cooking and heating, more attention should be paid to rural indoor air pollution. In fact, among various sources, rural residential combustion of solid fuel accounted for a large fraction of the total PAH emission in China (Zhang et al., 2008).
To date, information on PAH indoor air pollution in rural China from indoor biomass combustion is limited. In the limited number of studies that exist, high PAH concentrations have been measured in several rural locations (Mumford et al., 1990; Lu et al., 2006). For example, in rural households in Xuanwei where firewood was used indoors, the measured benzo(a)pyrene in indoor air was as high 3249 ng/m3 (Mumford et al., 1990). For a regional assessment on indoor air quality and population exposure risk, large scale representative investigations are needed. However, smaller studies conducted under controlled conditions are also needed in order to characterize the pattern of PAH contamination, to understand the processes that change the PAH composition from emission to exposure, and to identify other factors affecting indoor air quality.
Although most studies have focused on the 16 USEPA priority PAHs, there are many other PAHs which are hazardous to human health but not included in this list. For example, in addition to the 16 parent PAHs, nitro-PAHs (nPAHs), oxy-PAHs (oPAHs), and high molecular weight (MW>302) PAHs are also a concern for human health (Purohit et al., 2000; Bolton et al., 2000; USEPA, 2010).
The aim of this research was to characterize the indoor air pollution from parent PAHs (pPAHs), nPAHs, and oPAHs in a typical rural household in northern China where biomass was used for heating and cooking. The specific objectives of this research were to investigate the differences and connections between the air pollution in the kitchen and the adjacent bedroom, indoor and outdoor air, winter and summer, and personal exposure to PAHs. Although the results from a single household cannot be generalized for population exposure assessment, this controlled exposure experiment provides important insight into the processes governing PAH indoor air pollution from biomass burning in rural Chinese households.
2. Experimental
2.1. Study home and experimental conditions
The study home was located in a typical northern Chinese rural household in Zhuanghu (116°52′E, 39°59′N), Hebei. The details of the village and the household were previously described (Zhong et al., submitted) and additional information is given in the Supplementary Material (Section SI1, Figure SI1). In the kitchen of this home, a traditional Chinese rural cook stove, fueled by biomass, is often used for cooking and a liquid petroleum gas (LPG) stove is also occasionally used. Both stoves appeared to be working properly. The household was heated in the winter by a hot-water-heating system with a boiler in a separate hut in the back yard. The controlled exposure experiment was conducted for 4 days in winter (Jan. 16, 17, 19, and 20) and 3 days in summer (June 13–15) in 2010. Corn residue was burned in the traditional stove during the first two winter days and the second summer day, while firewood was burned on the third and forth winter days and the last summer day. On the first summer day, the LPG stove was used for a comparison. Three meals were cooked each day by the housewife in a similar way by steaming, simmering, and very limited stir-frying. It has been reported that high BaP concentrations in kitchen air can result from frying (65 ng/m3) and roasting (45 ng/m3) (Zhu et al., 2003). Therefore, frying and roasting were avoided during the experiment. The housewife was asked to cook in the way she does everyday. The large variations in fuel consumed and time spent were due to the random way of cooking and change in weather conditions. No one in the household smoked cigarettes. Detailed information on fuel quantities and burning times is available in the Section SI2. Although the data were collected from a single household and can not represent all rural households in Northern China, they do provide useful information on PAHs from typical cooking practices and fuels in summer and winter.
2.2. Sample collection
For each of the 7 sampling days, 24-h gaseous and particulate phase PAH samples were collected using 6 stationary (2 in the kitchen, 2 in the bedroom adjacent to the kitchen, and 2 outdoors, 1~2 m above the ground) and 4 personal air samplers simultaneously. For the particulate phase, PM2.5, the particles with diameter of 2.5 μm or less were sampled. The stationary and personal air samplers were low-volume pumps (Libra Plus LP-5, Buck, USA, 4 L/min) with 37-mm diameter glass fiber filters (0.45 μm, BUCK, USA, PM2.5) and low density polyurethane foam (PUF) plug (Supelco, 22-mm diameter×7.6 cm) for particulate and gaseous phase PAHs, respectively. The filters were baked at 450°C for 6 h and equilibrated in a desiccator for 24 h prior to weighing and sampling. The PUFs were cleaned prior to sampling using acetone, dichloromethane, and n-hexane sequentially. The personal air samplers were worn by a cooking group (a housewife who did all of the cooking and a student who followed her activity closely) and a control group (a husband who did non-cooking chores and a student who followed his activity closely). More information on the air samplers is given in Section SI3.
2.3. Extraction and analysis
The PUFs were Soxhlet extracted for 8 h with 150 ml of hexane/acetone mixture (1:1). The filters were extracted with 25 ml of the same mixture using a microwave accelerated reaction system (CEM, Mars Xpress, USA) (Wang et al., 2007). The extracts were purified using silica/alumina chromatography. The following twenty two pPAHs, 12 nPAHs, and 4 oPAHs were quantified: naphthalene (NAP), acenaphthene (ACE), acenaphthylene (ACY), fluorene (FLO), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLA), pyrene (PYR), benzo(a)anthracene (BaA), chrysene (CHR), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), benzo(a)pyrene (BaP), dibenz(a,h)anthracene (DahA), indeno(l,2,3-cd)pyrene (IcdP), benzo(g,h,i)perylene (BghiP), retene (RET), benzo[e]pyrene (BeP), perylene (PER), dibenzo[a.l]pyrene (DalP), dibenzo[a,e]pyrene (DaeP), dibenzo[a.h]pyrene (DahP), 1-nitronaphthalene (1N-NAP), 2-nitronaphthalene (2N-NAP), 5-nitroacenaphthene (5N-ACE), 2-nitrofluorene (2N-FLO), 3-nitrophenanthrene (3N-PHE), 9-nitrophenanthrene (9N-PHE), 9-nitroanthracene (9N-ANT), 3-nitrofluoranthene (3N-FLA), 1-nitropyrene (1N-PYR), 7-nitrobenzo[a]anthracene (7N-BaA), 6-nitrochrysene (6N-CHR), 6-nitrobenzo[a]pyrene (6N-BaP), 9-fluorenone (9FO), anthracene-9,10-dione (ATQ), benz[a]anthracene-7,12-dione (BaAQ), and benzanthrone (BZO). Detailed procedures and conditions of the extraction and clean up are presented in Section SI4. pPAHs were analyzed using gas chromatographic mass spectrometry (GC, Agilent 6890, Agilent 5973, 30 m HP-5MS capillary column) in electron ionization mode. The oven temperature was programmed to 50 °C for 1 min, increased to 150 °C at a rate of 10 °C/min, to 240 °C at 3 °C/min, and then to 280 °C held for 20 min. nPAHs and oPAHs were analyzed using the gas chromatographic mass spectrometry (GC, Agilent 6890, Agilent 5975, 30 m HP-5MS capillary column) in negative chemical ionization mode. The oven temperature was programmed as: initial temperature at 60 °C, increased to 150 °C at a rate of 15 °C/min, and then to 300°C at 5° C/min held for 15 min. Carrier gas was He. Data scanned from 35 to 500 mass units. The target PAHs and their derivatives were identified based on retention time and qualifying ions of the standards in selected ion monitoring mode (SIM). Because DalP, DaeP, DahP, and other non-target dibenzopyrenes with a molecular weight of 302, may not be chromatographically resolved using a 30 m HP-5 column (Sauvain and Duc, 2004) and the molecular weights of the surrogates were less than 302, the dibenzopyrenes were measured qualitatively and not quantitatively.
2.4. Quality control
All samples were collected in duplicate and procedure-blank corrected. Method detection limits were 0.23 to 1.42 and 0.53 to 1.32 ng/mL for gaseous and particulate phase parent pPAHs, 0.17 to 3.07 and 0.15 to 2.61 ng/mL for gaseous and particulate phase NPAHs, and 0.33 to 1.33 and 0.28 to 1.14 ng/mL for gaseous and particulate phase OPAHs, respectively. Recoveries of spiked mixed standards were 70 to 121%, 45 to 108%, and 56 to 133 % for gaseous phase pPAHs, nPAHs, and oPAHs and 68 to 120%, 53 to 127%, and 66 to 114 % for particulate phase pPAHs, nPAHs, and oPAHs, respectively. Deuterated PAHs (NAP-d8, ANT-d10, ACY-d10, CHR-d12, and PER-d12, J&K Chemical, U.S.) were added as surrogates and surrogate recoveries were 75 to 127% and 59 to 100% for gaseous and particulate phase samples. The measured concentrations were reported without recovery correction. All solvents were from Beijing Reagent, China, re-distillated, and checked for purity. All glassware was cleaned in an ultrasonic cleaner and baked at 500 °C for at least 10 h.
2.5. Data analysis
All results are reported in ng/m3 of air. NAP is not included in the 15 USEPA pPAHs (pPAH15) reported in the present study because of its lower recovery and higher percentage in the total parent PAHs. When the summer PAH concentrations are compared to the winter PAH concentrations, the LPG results are excluded because LPG experiments were not conducted during the winter. SPSS (SPSS Co., USA) was used for the statistical tests, with a significance level of 0.05.
3. Results and discussion
3.1. Parent PAH concentrations
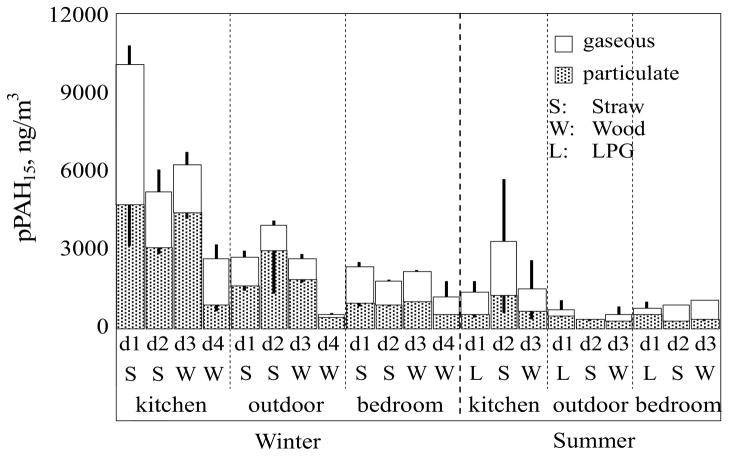
Figure 1 shows the daily mean gaseous and particulate phase pPAH15 concentrations in the kitchen, adjacent bedroom, and outdoors in the winter and summer. The measured individual pPAH concentrations are listed in Section SI-5 (Tables SI1 and SI2). The variations in the measured concentrations of the duplicate samples were relatively low with only a few exceptions. The daily mean pPAH15 concentrations in the kitchen were 6100±3100 and 2400±1600 ng/m3 in the winter and summer, respectively, which were 2.4–5.3 times higher than the pPAH15 concentrations in the bedroom and outdoors. In the summer, the daily mean pPAH15 concentrations in the kitchen were 1400, 3300, and 1500 ng/m3 on the days when LPG, straw, and wood were burned (PM2.5 were 0.42±0.02, 0.57±0.00, and 0.38±0.25, respectively). Previous studies have reported high PAH concentrations from burning biomass fuels in kitchens in other regions of China (Mumford et al., 1990; Lu et al., 2006) and in other developing countries (Bhargava et al., 2004). The significant difference (p < 0.05) in the pPAH15 concentrations in the kitchen between winter and summer was primarily due to minimal ventilation in the winter when both the kitchen door and window were kept closed. LPG is often considered a cleaner energy source than biomass (Deng et al., 1998). However, the kitchen pPAH15 concentration when LPG was used as the sole energy source (1400±490 ng/m3) was similar to the pPAH15 concentration when wood was burned (1500±1400 ng/m3). High PM2.5 and organic carbon concentrations were also measured in the kitchen when LPG was used (Zhong et al., submitted). Additional studies are needed to understand LPG emissions during cooking because more rural Chinese families are shifting from the use of biomass and coal to LPG as a “clean” fuel (Deng et al., 1998).
Figure 1.
Daily pPAH15 (total of 15 USEPA priority PAHs) concentrations in the kitchen, adjacent bedroom, and outdoors in the winter (four days from d1 to d4) and summer (three days from d1 to d3). The stacked bars represent gaseous (top) and particulate (bottom) phase PAH concentrations. The results are shown as arithmetic means and standard deviations (the whisker pointing up and down represent standard deviations for the particulate and gaseous phases, respectively, derived from the duplicate sampling).
Frying and roasting can generate relatively high PAH concentrations (Zhu et al., 2003). In this study, frying and roasting were avoided to focus on PAH emissions from the fuels used in the cooking. It was previously reported that the pPAH15 concentration in cooking fumes, measured 0.5 m above the cooking pan in a Chinese kitchen with no frying, was less than 50 ng/m3 (Zhu and Wang, 2003). The very high pPAH15 concentrations we measured in the kitchen were relatively unaffected by cooking fumes.
Retene (RET) is a major pyrolysis product of conifer trees and is often referred to as a marker of firewood combustion or forest fires (Ramdahl, 1983). However, there was no significant difference in RET concentrations in the kitchen air when firewood or corn residue were burned, suggesting that it is unlikely a unique tracer for firewood in this study. The emission of RET from rice and wheat straw combustion has also been reported in the literature (Hays et al., 2005). In addition, the median indoor RET concentrations in wood burning homes in Sweden during winter were comparable to homes without wood burning (p > 0.05) (Gustafson et al., 2008). There was also no significant difference in RET concentrations (p > 0.05) in the kitchen when LPG (4.73±1.23, n=2), straw (6.28±2.82, n=2), and wood (2.10±0.82, n=2) were burned during the summer experiment.
The pPAH15 concentration in outdoor air in winter (2500±1400 ng/m3) was 5.6 times higher than in the summer (450±210 ng/m3). However, both the winter and summer outdoor pPAH15 concentrations were significantly lower than the corresponding concentrations in the kitchen (p < 0.05). In terms of air pPAH15 concentration, the average indoor/outdoor ratios were 2.4 and 3.8 for kitchen and 0.8 and 1.8 for bedroom in the winter and summer, respectively, indicating heavy contamination in kitchen air. The daily variation in outdoor PAH concentrations was not correlated with the kitchen PAH concentrations because the outdoor PAH concentrations were strongly influenced by meteorological conditions (Liu et al., 2007). Although the outdoor PAH concentrations were lower than the kitchen concentrations, they were much higher than PAH concentrations reported for urban areas in China, including Taiyuan and Tianjin, during the same seasons (Wu et al., 2005; Fu et al., 2010). Ambient air PAH concentrations in the northern China Plain were as high as those in large cities in winter due to solid fuel combustion (Liu et al., 2007). BaP is the only PAH listed in the Chinese ambient air quality standard. In the winter, the mean ambient BaP concentration (124±83.2 ng/m3) in this study was more than one order of magnitude higher than China’s standard (10 ng/m3, daily average).
In the bedroom adjacent to the kitchen, the daily mean pPAH15 concentrations were 1900±520 and 980±110 ng/m3 in the winter and the summer, respectively, and the former was significantly higher than the latter (p < 0.05). In the winter, the daily variation in PAH concentration (d1 > d3 > d2 >d4) was similar between the kitchen and the adjacent bedroom (correlated significantly, p < 0.05), suggesting that the bedroom PAH concentrations were from the kitchen when all doors and windows in the bedroom were closed. In the summer, there was no correlation between the PAH concentrations in the bedroom and kitchen or between the bedroom and outdoors. It appears that the PAHs in the bedroom adjacent to the kitchen were likely from both outdoors and the kitchen during the summer.
3.2. Composition profiles of pPAHs
The composition profiles of 21 PAHs (in both the gas and particulate phases) in the kitchen, outdoors, and bedroom, measured during summer and winter, are shown in the Section SI6 (Figure SI2). It was found that the composition profiles were different between the winter and summer but were similar among individual days of the same season. In summer, the lower molecular weight PAHs, PYR, PHE, and FLO, dominated the PAH profile and contributed 66, 88, and 91% of the total pPAH concentration in the kitchen, bedroom, and outdoors, respectively. However, in the winter, the higher molecular weight PAHs on particulate matter made up a larger fraction of the PAH profile (8 PAHs from BaA to BghiP contributed 29, 32, and 31% of total pPAH concentrations in winter compared to 16, 1, and 1% in the summer in the kitchen, bedroom, and outdoors, respectively). Because the fuels and cooking methods used in summer and winter experiments were the same, the difference between winter and summer PAH profiles is likely due to outdoor air diluting indoor PAH emissions during the summer experiment. The composition profiles can also be affected by temperature, while temperatures were surely different between the two seasons and between indoor and outdoor in the winter.
The PAH emission factors (EFs) from the burning of corn residue were measured using a similar stove (Shen et al., 2011) and these emission profiles were compared to the air PAH profiles (Figure SI3) measured in this study when corn is used. A significant correlation existed (p < 0.05) between the PAH EFs and measured PAH air concentrations in the kitchen, adjacent bedroom, and outdoors when corn residue was used in both seasons for the gaseous phase pPAHs, but not the particulate phase pPAHs (Figure SI4). This suggests that the profile of gaseous phase pPAHs did not change significantly after emission given the relatively short distances from the stove to the kitchen, adjacent bedroom, or immediate outdoor environment. On the other hand, the profile of particulate phase pPAHs appears to have changed significantly from emission to air likely due to partition of PAHs and deposition of particulates after emission.
Ratios of PAH isomers can be used for source apportionment if the ratios do not change significantly from sources to receptors (Watson, 1984; Zuo et al., 2007). For example, the FlA/(FLA+PYR) ratio from coal and biomass burning is larger than 0.5 and this ratio is smaller than 0.5 from petroleum combustion (Yunker et al., 2002). However, this assumption may not always hold true and significant changes in the isomer ratios have been demonstrated in a field study (Zhang et al., 2005). Using the measured air PAH concentrations when corn residue was combusted in the winter and the EFs for corn residue measured under similar conditions (Shen et al., 2011), four commonly used isomer ratios, including ANT/(ANT+PHE), FLA/(FLA+PYR), BaA/(BaA+CHR), and IcdP/(IcdP+BghiP), were calculated and compared for the gaseous and particulate phases (see detailed results in the Section SI7 (Table SI3)). With all of windows closed in the winter, the PAHs in the bedroom were primarily from the kitchen. However, significant differences (p<0.05) existed between the measured gaseous phase ratios (0.10, 0.55, and 0.53) in the kitchen and reported corn residue EFs (0.17, 0.88, and 0.61) for ANT/(ANT+PHE), BaA/(BaA+CHR), and IcdP/(IcdP+BghiP), respectively. Significant differences (p<0.05) between the ratios in the kitchen and bedroom were also apparent for gaseous phase ANT/(ANT+PHE) (0.10 vs. 0.05) and particulate phase ANT/(ANT+PHE) (0.12 vs. 0.07), as well as for BaA/(BaA+CHR) (0.62 vs. 0.55) and IcdP/(IcdP+BghiP) (0.60 vs. 0.54) ratios. It appears that these ratios may change significantly during transport and should be used with care for source identification even within short distance indoors.
3.3. nPAH and oPAH concentrations
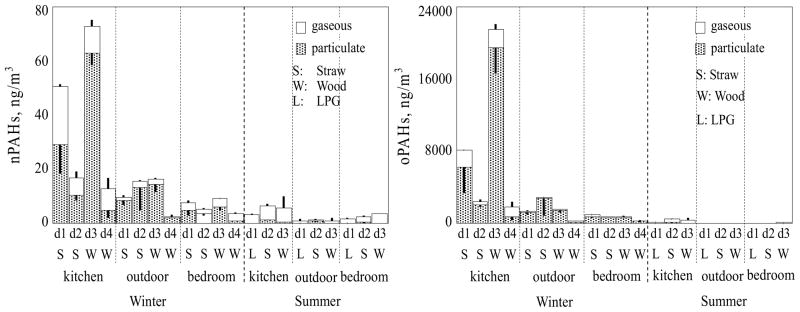
The measured nPAH and oPAH concentrations in the gaseous and particulate phases in the kitchen, outdoors, and bedroom, during winter and summer experiments, are shown in Figure 2. The detailed data are provided in Section SI5(Tables SI1 and SI2). The daily mean concentrations of 12 nPAHs were relatively low and varied from 2.8±0.7 (bedroom, summer) to 38±29 (kitchen, winter) ng/m3. On the other hand, the daily mean concentration of oPAHs in the kitchen in the winter was 8400±9200 ng/m3, more than two orders of magnitude higher than the nPAH concentrations and even higher than the pPAH15 concentrations (6100±3100 ng/m3). Like the pPAHs, there was no significant difference in oPAH or nPAH concentrations between corn residues (nPAHs 34±24, oPAHs 5200±4000 ng/m3, n=4) and firewood (nPAHs 45±42, oPAHs 12000±14000 ng/m3, n=4) experiments (p > 0.05) in the winter. The highest oPAH, nPAH, and PM2.5 concentrations (Zhong et al., 2010) were measured in the kitchen on the third day of the winter experiment when wood was burned.
Figure 2.
The measured nPAH (left panel) and oPAH (right panel) concentrations in the kitchen, adjacent bedroom, and outdoors in the winter (four days from d1 to d4) and summer (three days from d1 to d3). The stacked bars represent gaseous (top) and particulate (bottom) phase PAH concentrations. The results are shown as arithmetic means and standard deviations (the whiskers pointing up and down represent standard deviations for the particulate and gaseous phases, respectively).
There were significant correlations between pPAH, nPAH, and oPAH concentrations for the three locations and for both seasons, with r values of 0.942 (nPAHs-pAH15), 0.948 (oPAHs-pPAH15) and 0.960 (nPAHs-oPAHs) p < 0.001), respectively (Figure 3). This suggests that these compounds were from the same sources, giving the fact that the emission of these compounds is not location or season dependent.
Figure 3.
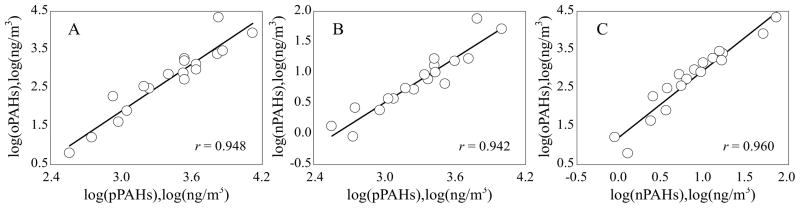
Correlations between oPAH and pPAH (A), nPAH and pPAH (B), and oPAH and nPAH (C) concentrations.
The nPAH and oPAH outdoor and bedroom concentrations were significantly lower than the kitchen concentrations. Like pPAHs, the nPAH and oPAH daily variation in concentration was similar between the kitchen and the adjacent bedroom in the winter, suggesting that the kitchen was an important source to the bedroom in winter. The summer oPAH concentration (420±170 ng/m3) in the kitchen was more than an order of magnitude lower than in the winter due to good ventilation with the window and door opened. The oPAH and nPAH concentrations in ambient air were 1500±1100 and 11±6 ng/m3 in the winter and 11±11 and 1.1±0.8 ng/m3 in the summer, respectively. Previous studies have also reported that nPAH concentrations in urban ambient air were 1~2 orders of magnitude lower than oPAH concentrations (Walgraeve et al., 2010). The summer PM-bound 1N-PYR concentrations in urban areas in Shenyang (0.029±0.024) and Beijing (0.010±0.002 ng/m3) (Tang et al., 2005; Wang et al., 2010) were similar to our outdoor 1N-PYR concentrations (0.016±0.019 ng/m3). However, in winter, our measured outdoor 1N-PYR concentrations (0.91±0.74 ng/m3) were significantly higher than previously reported for Shenyang (0.179±0.019 ng/m3) (Tang et al., 2005). Our winter outdoor oPAH concentrations were similar to those in urban areas of Beijing (Wang et al., 2010).
Of the PAH derivatives, 9FO, ATQ, and BZO contributed 95~99% of the total oPAH and nPAH concentration. The winter daily mean 9FO, ATQ, and BZO concentrations in the kitchen were 3400±4000, 2600±3300, and 2400±2100 ng/m3, respectively. It has been reported that both nPAHs and oPAHs can be formed during incomplete combustion or pPAH aging process (Albinet et al., 2008; Walgraeve et al., 2010). Although it was difficult to fully identify their sources, the ratio of individual nPAH or oPAH concentrations to their corresponding pPAH concentration provide some perspective. Figure 4 (left panel) shows these ratios for the three dominant oPAHs. The highest ratios were found in the kitchen and the ratios in the winter were significantly higher than those in the summer. This suggests oPAH formation from combustion source in the kitchen. The high oPAH concentrations in the kitchen during the winter, relative to summer, suggest that secondary formation of oPAHs in warm kitchen in the winter cannot be excluded although it was primarily due to limited ventilation. Higher time resolution sampling is needed to characterize the oPAH sources in the kitchen. For nPAHs (Figure 4, right panel), the ratios in the kitchen were low, with a single exception, and the highest ratios were measured in ambient air during the summer. High ambient temperatures are favorable for the formation of nPAHs due to enhanced reaction between pPAHs and reactive species (Wilson et al., 1995). Our data suggest that nPAHs could from secondary formation in ambient air during the summer.
Figure 4.
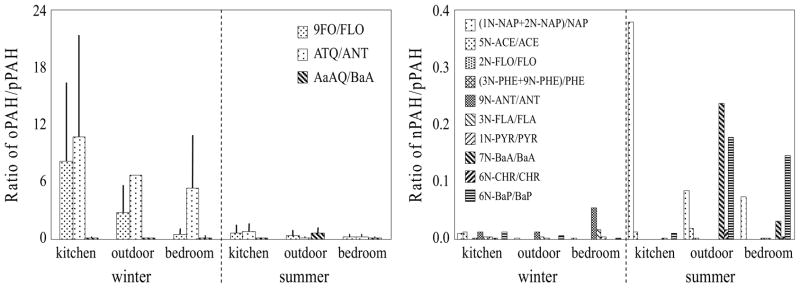
Ratios of individual oPAH/pPAH (left panel) and nPAH/pPAH (right panel) in the kitchen, adjacent bedroom, and outdoors in the winter (four day means) and summer (two day means). The results are shown as arithmetic means and standard deviations.
3.4. Gas-Particle partitioning
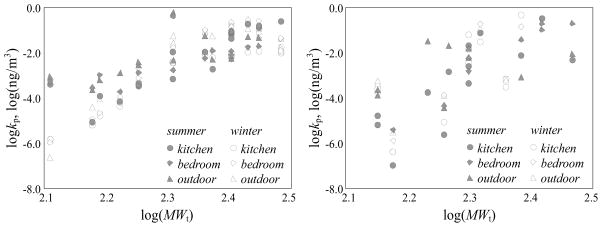
pPAHs and their derivatives are present in gaseous and/or condensed phases, depending on the properties of compounds and atmospheric conditions (Albinet et al., 2008). Gas-particle partitioning of organic compounds is often described by a partition coefficient (Kp, m3/μg), defined as the ratio of mass concentration of the particulate phase (ng/μg) and the volume concentration of gaseous phase (ng/m3) (Pankow, 1987). Figure 5 shows the log-transformed Kp of pPAHs (left panel) and derivatives (right panel) measured in the kitchen, outdoors, and bedroom in the winter and summer against the log-transformed molecular weight (MWt) of the compound. Molecular weight was used instead of vapor pressure because accurate vapor pressure data was not available all of the PAH derivatives. Similar to previous reports in the literature (Albinet et al., 2008), logKp increased as logMWt increased. For both seasons, the Kp values of pPAHs in ambient air (triangle in Figure 5) were higher than those in the kitchen and bedroom. This may be due to lower outside temperatures. In the winter, Kp values of intermediate molecular weight PAHs in the bedroom were significantly lower than those in the outdoors and kitchen. This may be because the diffusion of gaseous phase PAHs from the kitchen to the adjacent bedroom was faster than transport of particulate phase PAHs from the kitchen to the bedroom. The Kp values for the derivatives we0re generally higher than those of pPAHs, likely because that the vapor pressures of nPAHs and oPAHs are usually lower than those of corresponding pPAHs (Walgraeve et al., 2010). Although a similar relationship between Kp and MWt was observed for nPAHs and oPAHs, the seasonal differences were much larger than for pPAHs. A similar trend was found for pPAHs and their derivatives in ambient air in two French valleys (Albinet et al., 2008).
Figure 5.
Dependence of the log-transformed partition coefficient (Kp) of pPAHs (left panel) and derivatives (right panel) on the log-transformed molecular weight (MWt) in the kitchen (circle), outdoors (triangle), and adjacent bedroom (diamond) in the winter (white) and summer (grey).
Gas-particle partitioning of organic compounds is thought to be controlled by absorption and/or adsorption which is related to the particle surface area and black carbon content (Goss et al., 1998). These two mechanisms can be distinguished based on the slope of a scatter plot of the log-transformed Kp against log-transformed subcooled liquid vapor pressure (P0). A slope steeper than −1.0 (or shallower than −0.6) suggests adsorption (or absorption) domination (Goss et al., 1998). The calculated logKp values were plotted against logP0 for pPAHs and the slopes for the kitchen, bedroom, and ambient air varied from −0.2 to −0.5, implying that the gas-particle partitioning was governed by absorption (Section SI8-Figure SI5). Absorption was also the dominant mechanism for freshly emitted pPAHs from crop residues burned under similar conditions (Shen et al., 2011). The slopes for the bedroom and ambient air were shallower than the slope for the kitchen air. This difference may be explained by slow desorption kinetics when the PM from the kitchen entered less contaminated air, at lower temperatures (Tsapakis and Stephanou, 2003).
3.5. Exposure
The relatively high exposure in the kitchen in the winter was confirmed by the results obtained using the personal sampler. In the winter, the measured mean pPAH15 concentration was 2660±1120 ng/m3 for the cooking group, which was significantly higher than 1530±244 ng/m3 of the control group (p < 0.05). This is especially true for particulate phase PAHs (1610±980 and 684±258), corresponding to the relatively high level of particulate phase PAHs in kitchen air in the winter. However, there was no significant difference in the exposure concentrations of pPAH15 between the two groups in the summer (p > 0.05) likely due to the better ventilation with open window. It appears that kitchen ventilation is critical to the indoor inhalation exposure in rural household.
BaP is the only PAH regulated by Chinese air quality standards (daily average) for ambient (10 ng/m3) and indoor (1.0 ng/m3) air (General Administration of Quality Supervision, 2002; Bureau of Environmental Protection of P.R.C., 1996). In the winter, the measured BaP concentrations in the study home exceeded the standards by factors of 49 to 548, 31 to 187, and 1 to 19 times in the kitchen, adjacent bedroom, and outdoors, respectively. Even the average summer kitchen concentration exceeded the standard by 57 times. These data suggest that there is a high inhalation cancer risk for the residents of this study home. These high PAH concentrations were confirmed with personal air samplers (see detailed data in Section SI5 (Tables SI1 and SI2)). In the winter, the daily mean BaP exposure to the cooking group was 200±160 ng/m3, compared with 59±37 ng/m3 for the control group. The exposures in the summer were 2.0±0.41 and 1.6±0.27 ng/m3 for the cooking and control groups, respectively.
In addition to BaP, other PAHs were measured in the study. Using the concentrations of 17 pPAHs (total of gaseous and particulate phases excluding NAP, RET, and the three dibenzopyrenes) and associated relative potency factors (RPFs) (Purohit and Basu, 2000), total benzo(a)pyrene equivalent concentrations (BaPeq) of the pPAHs were calculated (Section SI9 (Table SI4)). The daily mean BaPeq concentrations were 710±540 and 12±1.3 ng/m3 for the cooking group in the winter and summer, compared to 230±140 and 13±2.0 ng/m3 for the control group, respectively. Because the RPF for DalP is 30 times higher than the RPF for BaP, the dibenzopyrene isomers should be quantified in future studies. A recent study found that the total excess inhalation cancer risk in Beijing would be underestimated by 23% if the dibenzopyrene isomers were not included in the risk calculation (Jia et al., 2011). In addition, it was previously estimated that the mean exposure of the Chinese population to BaPeq in ambient air was 7.64 ng/m3. This value is much lower than what we have estimated for the cooking group and even lower than that of the control group, leading to an excess annual lung cancer morbidity rate of 0.65×10−5 (Zhang et al., 2009). The cooking group was exposed to very high concentrations of oPAHs (1240±1100 ng/m3) in the winter. Because the carcinogenic effects of oPAHs and nPAHs are not well quantified, the inhalation cancer risk from these PAH derivatives was not determined. Although the results of this study cannot be generalized to the entire Chinese population, a high inhalation cancer risk to a housewife in northern China has been measured. In addition, PAHs other than the 16 USEPA priority PAHs should be included in future studies.
4. Conclusion
High PAH concentrations were measured in the indoor air of the household and the most severe contamination occurred in the kitchen in the winter. The measured concentrations of pPAHs, nPAHs, and oPAHs in the kitchen in the winter were as high as 7500±4100, 38±29, and 8400±9200 ng/m3, respectively. The PAHs in bedroom air were mainly from the kitchen in the winter and from ambient air in the summer. High level of oPAHs in the kitchen air suggests that oPAHs are primarily from the biomass burning. The daily mean benzo(a)pyrene equivalent exposure concentrations were 200±160 and 59±37 ng/m3 in the winter for those who cooked or not in this particular household.
Supplementary Material
Very high levels of parent PAHs, nitro-PAHs, and oxy-PAHs were detected in a rural non-smoking household in northern China;
The PAHs measured in the bedroom air were primarily from the kitchen in the winter and from ambient air in the summer;
The nPAHs were largely from secondary formation in ambient air, while oPAHs were either from primary emission of biomass burning or secondary formation from pPAHs in the kitchen;
The daily mean benzo(a)pyrene equivalent exposure concentration was as high as 200±160 ng/m3 in the winter for the housewife who did the cooking.
Acknowledgments
Funding for this study was provided by the National Natural Science Foundation of China (41001343, 41130754, 40703029), Beijing Municipal Government (YB20101000101), and NIEHS (P42 ES016465).
Appendix. Supplementary material
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.envpol.2010.01.021
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albinet A, Leoz-Garziandia E, Budzinski H, Villenave E, Jaffrezo J. Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two French alpine valleys Part 1: concentrations, sources and gas/particle partitioning. Atmos Environ. 2008;42:43–54. [Google Scholar]
- Bhargava A, Khanna RN, Bhargava SK, Kumar S. Exposure risk to carcinogenic PAHs in indoor-air during biomass combustion whilst cooking in rural India. Atmos Environ. 2004;38:4761–4767. [Google Scholar]
- Bolton JL, Trush MA, Penning TM, Dryhurst G, Monks TJ. Role of quinones in toxicology. Chem Res Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- Bureau of Environmental Protection of P.R.C. Ambient Air Quality Standard of RPC GB 3095–1996. Bureau of Environmental Protection of China; Beijing: 1996. [Google Scholar]
- Deng KY, Zhang LJ, He L. Study on energy strategy of sustainable development in rural area of China. I. Analysis of energy conditions in rural area of China. Trans CSAE. 1998;14:19–25. [Google Scholar]
- Fu S, Yang ZZ, Li K, Xu XB. Spatial characteristics and major sources of polycyclic aromatic hydrocarbons from soil and respirable particulate matter in a mega-city. China Bull Environ Contam Toxicol. 2010;85:15–21. doi: 10.1007/s00128-010-0026-9. [DOI] [PubMed] [Google Scholar]
- General Administration of Quality Supervision, Inspection and Quarantine of P.R.C. Ministry of Health of P.R.C. Bureau of Environmental Protection of P.R.C. 2002. Indoor Air Quality Standard of P.R.C. GB/T 1883–2002, Beijing.
- Goss K, Schwarzenbach RP. Gas/solid and gas/liquid partitioning of organic compounds: critical evaluation of the interpretation of equilibrium constants. Environ Sci Technol. 1998;32:2025–2032. [Google Scholar]
- Gustafson P, Östman C, Sällsten G. Indoor levels of polycyclic aromatic hydrocarbons in homes with or without wood burning for heating. Environ Sci Technol. 2008;42:5074–5080. doi: 10.1021/es800304y. [DOI] [PubMed] [Google Scholar]
- Hays MD, Fine PM, Geron CD, Kleeman MJ, Gullett BK. Open burning of agricultural biomass: physical and chemical properties of particle-phase emissions. Atmos Environ. 2005;39:6747–6764. [Google Scholar]
- Jia YL, Stong D, Wang WT, Schrlau J, Tao S, Simonich SLM. Reduction in Cancer Risk due to Source Control Measures during the 2008 Beijing Olympics. Environ Health Persp. 2011;119:815–820. doi: 10.1289/ehp.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YN, Tao S, Yang YF, Dou H, Yang Y, Coveney RM. Inhalation exposure of traffic police officers to polycyclic aromatic hydrocarbons (PAHs) during the winter in Beijing, China. Sci Total Environ. 2007;383:98–105. doi: 10.1016/j.scitotenv.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Liu SZ, Tao S, Liu WX, Liu YN, Dou H, Zhao JY, Wang LG, Wang JF, Tian ZF, Gao Y. Atmospheric Polycyclic Aromatic Hydrocarbons In North China: A Wintertime Study. Environ Sci Technol. 2007;41:8256–8261. doi: 10.1021/es0716249. [DOI] [PubMed] [Google Scholar]
- Lu CG, Gao X, Yu Q, Li CL, Chen LM. Indoor air polycyclic aromatic hydrocarbons in rural Tibetan residence and the depositions on human respiratory tract. J Fudan Univ (in Chinese) 2006;45:714–718. [Google Scholar]
- Mumford JL, Helmes CT, Lee X, Seidenberg J, Nesnow S. Mouse skin tumorigenicity studies of indoor coal and wood combustion emissions from homes of residents in Xuan Wei, China with high lung cancer mortality. Carcinogenesis. 1990;11:397–403. doi: 10.1093/carcin/11.3.397. [DOI] [PubMed] [Google Scholar]
- Pankow J. Review and comparative analysis of the theories on partitioning between the gas and aerosol particulate phases in the atmosphere. Atmos Environ. 1987;21:2275–2283. [Google Scholar]
- Purohit V, Basu AK. Mutagenicity of nitroaromatic compounds. Chem Res Toxicol. 2000;13:673–692. doi: 10.1021/tx000002x. [DOI] [PubMed] [Google Scholar]
- Ramdahl T. Retene - a molecular marker of wood combustion in ambient air. Nature. 1983;306:580–583. [Google Scholar]
- Sauvain JJ, Duc TV. Approaches to identifying and quantifying polycyclic aromatic hydrocarbons of molecular weight 302 in diesel particulates. J Sep Sci. 2004;27:78–88. doi: 10.1002/jssc.200301620. [DOI] [PubMed] [Google Scholar]
- Shen GF, Wang W, Yang YF, Ding JN, Xue M, Min YJ, Zhu C, Shen HZ, Li W, Wang B, Wang R, Wang XL, Tao S, Russell AG. Emission of PAHs from indoor crop residue burning in a typical rural stove: emission factors, size distributions and gas-particle partitioning. Environ Sci Technol. 2011;45:1206–1212. doi: 10.1021/es102151w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Hattori T, Taga R, Igarashi K, Yang XY, Tamura K, Kakimoto H, Mishukov VF, Toriba A, Kizu R, Hayakawa K. Polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons in urban air particulates and their relationship to emission sources in the Pan-Japan Sea countries. Atmo Environ. 2005;39:5817–5826. [Google Scholar]
- Tsapakis M, Stephanou EG. Collection of gas and particle semi-volatile organic compounds: use of an oxidant denuder to minimize polycyclic aromatic hydrocarbons degradation during high-volume air sampling. Atmos Environ. 2003;37:4935–4944. [Google Scholar]
- USEPA. Development of a Relative Potency Factor (RPF) Approach for Polycyclic Aromatic Hydrocarbon (PAH) Mixtures, an external review draft. Washington, DC: U.S. Environmental Protection Agency, Integrated Risk Information System (IRIS); 2010. [Google Scholar]
- Xue WL, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicology and Applied Pharmacology. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Walgraeve C, Demesstere K, Dewulf J, Zimmermann R, van Langenhove H. Oxygenated polycyclic aromatic hydrocarbons in atmospheric particulate matter: Molecular characterization and occurrence. Atmos Environ. 2010;44:1831–1846. [Google Scholar]
- Wang WT. PhD Dissertation. Peking University; Beijing, China: 2010. Regional distribution and air-soil exchange of polycyclic aromatic hydrocarbons (PAHs) and their derivatives in Beijing-Tianjin Area. [Google Scholar]
- Wang WT, Meng BJ, Lu XX, Liu Y, Tao S. Extraction of polycyclic aromatic hydrocarbons and organochlorine pesticides from soils: a comparison between Soxhlet extraction, microwave-assisted extraction and accelerated solvent extraction techniques. Anal Chim Acta. 2007;602:211–222. doi: 10.1016/j.aca.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Watson JG. Overview of receptor model principles. J Air Pollut Contr Assoc. 1984;34:619–623. [Google Scholar]
- Wilson NK, McCurdy TR, Chuang JC. Concentrations and phase distributions of nitrated and oxygenated polycyclic aromatic hydrocabrons in ambient air. Atmos Environ. 1995;29:2575–2584. [Google Scholar]
- World Bank report. Cost of Pollution in China. Economic Estimates of Physical Damages. Office of the Publisher of the World Bank; Washington, D.C: 2007. [Google Scholar]
- Wu SP, Tao S, Zhang ZH, Lan T, Zuo Q. Distribution of particle-phase hydrocarbons, PAHs and OCPs in Tianjin, China. Atmos Environ. 2005;39:7420–7432. [Google Scholar]
- Yunker MB, Macdonald RW, Vingarzan R, Mitchell HR, Goyette D, Sylvestre S. PAHs in the Fraser River basin: a critical appraisal PAH ratios as indicators of PAH source and composition. Org Geochem. 2002;33:489–515. [Google Scholar]
- Zhang YX, Dou H, Chang B, Wei ZC, Qiu WX, Liu SZ, Liu WX, Tao S. Emission of Polycyclic Aromatic Hydrocarbons from Indoor Straw Burning and Emission Inventory Updating in China. Ann NY Acad Sci. 2008;1140:218–227. doi: 10.1196/annals.1454.006. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Tao S, Liu WX, Yang Y, Zuo Q, Liu SZ. Source diagnostics of polycyclic aromatic hydrocarbons based on species ratios: A multimedia approach. Environ Sci Technol. 2005;39:9109–9114. doi: 10.1021/es0513741. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Tao S, Shen HZ, Ma JM. Inhalation exposure to ambient polycyclic aromatic hydrocarbons and lung cancer risk of Chinese population. Proc Nat Acad Sci USA. 2009;106:21063–21067. doi: 10.1073/pnas.0905756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong JJ. MS Thesis. Xingjian University; Wulumuqi, China: 2010. Indoor Particulate Matter Air Pollution and Human Exposure from Cooking Practices in a Rural Northern China Home. [Google Scholar]
- Zhu LZ, Wang J. Sources and patterns of polycyclic aromatic hydrocarbons pollution in kitchen air. China Chemosphere. 2003;50:611–618. doi: 10.1016/s0045-6535(02)00668-9. [DOI] [PubMed] [Google Scholar]
- Zuo Q, Duan YH, Yang Y, Wang XJ, Tao S. Source apportionment of polycyclic aromatic hydrocarbons in surface soil in Tianjin, China. Environ Pollut. 2007;147:303–310. doi: 10.1016/j.envpol.2006.05.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.