Abstract
Astroglia encompass a subset of versatile glial cells that fulfill a major homeostatic role in the mammalian brain. Since any brain disease results from failure in brain homeostasis, astroglial cells are involved in many, if not all, aspects of neurological and/or psychiatric disorders. In this article, the roles of astrocytes as homeostatic cells in healthy and diseased brains are surveyed. These cells can mount the defence response to the insult of the brain, astrogliosis, when and where they display hypertrophy. Interestingly, astrocytes can alternatively display atrophy in some pathological conditions. Various pathologies, including Alexander and Alzheimer's diseases, amyotrophic lateral sclerosis, stroke and epilepsy, to mention a few, are discussed. Astrocytes could represent a novel target for medical intervention in the treatment of brain disorders.
Keywords: Alexander disease, Alzheimer's disease, amyotrophic lateral sclerosis, astroglia, astroglial atrophy, astrogliosis, ischemia, neurodegeneration, neuropathology, stroke
Neuroglia are a central element of neuropathology
The nervous system is composed of an inter laced network of neurons and neuroglial cells. The term neuroglia covers several highly heterogeneous populations of cells, which, fundamentally, are responsible for all aspects of brain homeostasis. Neuroglia in the CNS are broadly divided into astroglia (which also include ependymal cells), oligodendroglia, NG-2 glia (all are of neural crest origin – i.e., all being the scions of neuroepithelial cells acting as universal neural progenitors [1–4]) and microglia (being of myeloid origin; microglial precursors enter the CNS early in development and scatter more or less evenly through the nervous tissue, where they undergo remarkable metamorphosis that converts these cells into ramified or resting microglia, the phenotype of which is dissimilar to their myeloid precursors [5–7]). The neuroglia of the peripheral nervous system comprises several types of myelinating and nonmyelinating Schwann cells and satellite cells localized in the peripheral ganglia, as well as highly diverse and numerous classes of enteric glia [4,8,9]. Despite a high degree of morphological and functional diversity, all neuroglial cells are primarily involved in the regulation of homeostasis of the nervous tissue. Conceptually, astroglia oversee multiple homeostatic matters in the CNS; oligodendrocytes provide connectome maintenance, while microglial cells are responsible for defensive homeostasis.
Neurological diseases are, in essence, failures in neural tissue homeostasis that can be triggered by acute (mechanical or chemical) lesions or result from chronic processes (developmental abnormalities or neurodegeneration). Therefore, it is perfectly logical to assume that homeostatic cells of the nervous tissue assume the primary role in the progression of neuropathologies. Incidentally, when Rudolf Virchow developed the concept of neuroglia (which, for him, was a true acellular connective tissue), he was deeply convinced in the central role of neuroglia in pathology [10,11]. However, for a long time, the neuron-centric views dominated neuropathological thinking. Only recently has the role of glia been reassessed and it appears that neuroglia, to a very large extent, define the progression and outcome of most (if not all) neurological diseases [12–16].
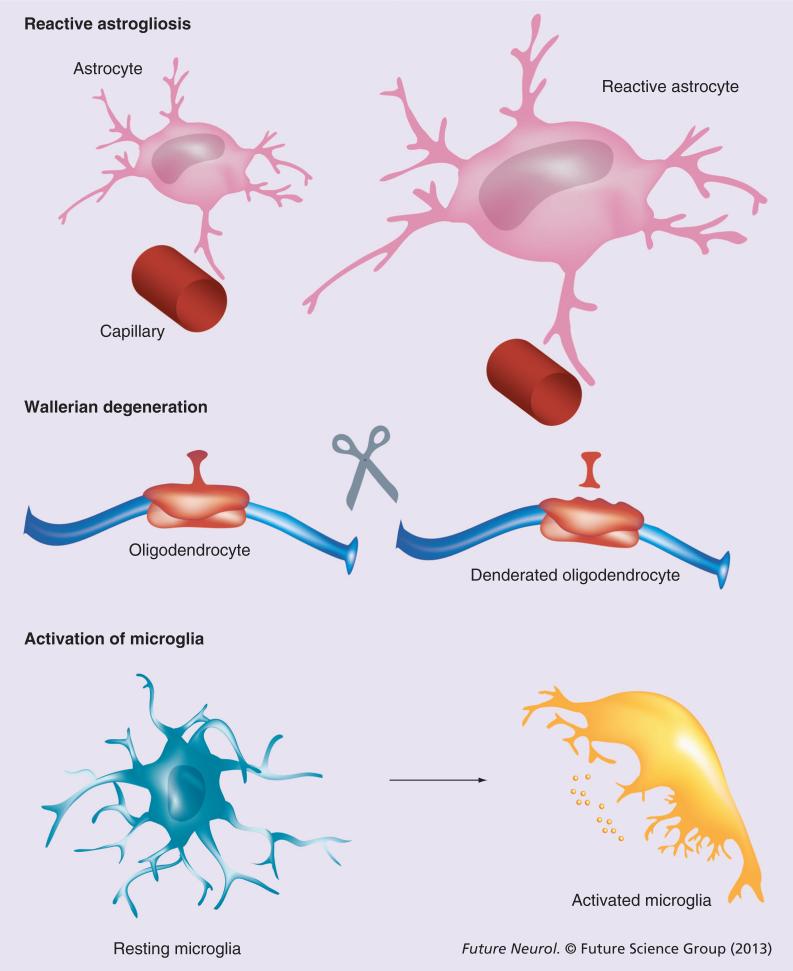
Neuroglial cells are generally neuroprotective; they contain the damage and facilitate neuronal survival. Lesions to the nervous tissue trigger evolutionarily conserved glial defensive reactions represented by astrogliosis, Wallerian degeneration and activation of microglia (Figure 1) [2,4]. In many pathological conditions, neuroglia can be neurotoxic. However, this can be regarded as a defensive and survivalist mechanism aimed at a rapid clean up of damaged elements and sealing of the lesion area.
Figure 1.
General pathophysiology of neuroglia.
Astrocytes: main homeostatic cells of the CNS
Astrocytes fulfill a remarkable range of homeostatic functions. Protoplasmic astrocytes define the brain microarchitecture by dividing the gray matter into relatively independent territorial domains that are the structural basis for neurovascular units (which, in fact, should be named glio–neuron–vascular units because these are astrocytes that physically integrate neuronal structures with brain capillaries [17,18]). Within its territorial domain, a protoplasmic astrocyte enwraps a substantial proportion of neuronal synaptic contacts. This specific coverage initiated the hypothesis of the tripartite synapse that highlights the contribution of preand post-synaptic neuronal compartments, as well as the astroglial perisynaptic compartment in making the synapse [19,20]. The perisynaptic astroglial membranes isolate synapses and, thus, provide for spatial specificity of synaptic inputs, control homeostasis of ions and neurotransmitters in the synaptic cleft and care for local metabolic support, thus acting as a synaptic cradle maintaining synaptic connectivity [21]. Astrocytes synthesize neurotransmitters (e.g., glutamate can only be produced de novo by astroglia [22]; neurons lack the relevant enzyme pyruvate carboxylase [but see [23]] and neurotransmitter precursors are generally involved in homeostasis of many neurotransmitters and neuromodulators [the most important being glutamate, GABA and adenosine]. Astroglia are fully responsible for the extracellular homeostasis of K+ ions [critical for neuronal excitability] and extracellular pH and both functions are supported by a multitude of plasmalemmal transporters, such as Na+/K+ ATPase, Na+/H+ exchanger and Na+/HCO3- exchanger [24]).
Astrogliosis: defensive astroglial reaction
Astrocytes provide neuroprotection through multiple mechanisms that are generally aimed at preserving brain homeostasis. Astrocytes also express a specific defensive program; this is known as a reactive astrogliosis and it is initiated in response to various brain lesions. The reactive astrogliosis generally appears as hypertrophy and proliferation of astrocytes associated with upregulation of GFAP, which is generally considered to be a specific marker for astrogliotic response. Astrogliosis is commonly regarded as a pathological glial reaction with negative outcomes; often astrogliosis is regarded as a sign of neuroinflammation. These views are factually incorrect. In reality, astrogliosis represents an evolutionarily conserved (astrogliotic changes are characterized already in arthropods and annelids) defensive response of astroglia, which develops in a multistage and heterogeneous fashion. In essence, astrogliosis encompasses a wide spectrum of changes that are specific for various pathological contexts and resolve in various ways [12,13,25–27]. Functionally, reactive astrogliosis provides for: increased neuroprotection and trophic support of insult-stressed neurons; isolation of the damaged area from the rest of the CNS tissue; reconstruction of the compromised blood–brain barrier; and regeneration of the lesion region. The overall result of astrogliosis is clearly beneficial for the nervous tissue, the suppression of which exacerbates tissue damage [27,28].
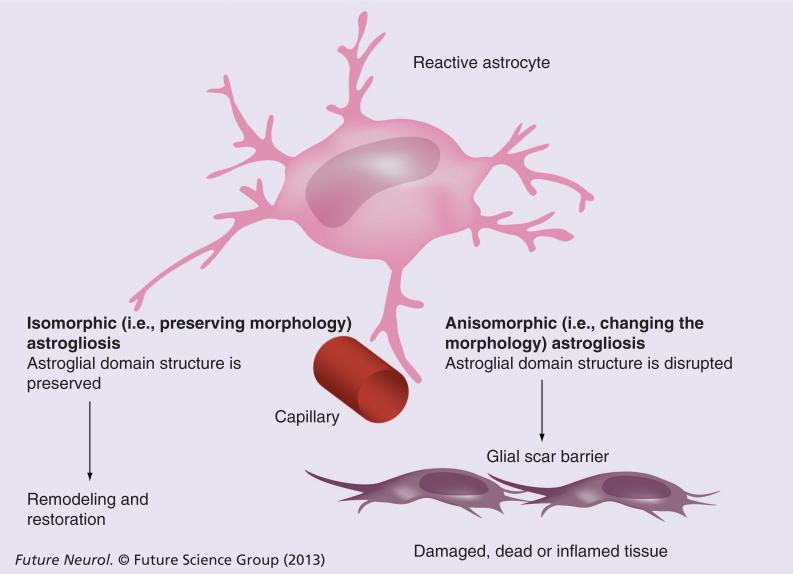
Morphologically reactive astrogliosis is classified into isomorphic (i.e., preserving morphology) and anisomorphic (i.e., changing the morphology; Figure 2) astrogliosis. Inisomorphic gliosis, astroglial cells become hypertrophic and undergo multiple biochemical and immunological changes without altering normal astroglial domain organization. Astroglial changes in isomorphic gliosis facilitate the growth of neurites and synaptogenesis, thus contributing to the regeneration of neuronal networks. Isomorphic gliosis is fully reversible and, after the resolution of pathology, astrocytes return to a healthy state. In anisomorphic gliosis, astroglial hypertrophy is complemented with a proliferative response and the disappearance of normal domain organization. Anisomorphic gliosis ultimately results in the formation of a permanent glial scar; this process prevents any axonal growth due to the presence of chondroitin and keratin, which are secreted by anisomorphic reactive astrocytes [2,12,27].
Figure 2.
Types of astrogliosis (see text for further details).
Astroglia in neurological diseases
Alexander disease: primary astrogliopathology
Alexander disease (AxD), described by a Scottish neuropathologist Stewart Alexander [29], is a leukodystrophy that results in severe white matter deficits. At the core of AxD is the expression of sporadically mutated GFAP with an apparent gain-of-function. AxD is classified into the early-onset type I and the late-onset type II, with a negative prognosis and the complete absence of treatments for both types [30,31]. The histopathological feature of AxD is the appearance of Rosenthal fibers in astrocytes; these are cytoplasmic inclusions, formed by GFAP and stress proteins. The majority of mutations of the GFAP gene that are linked to AxD occur de novo and are not conserved (because AxD patients rarely reach a reproductive age). The pathogenesis of AxD remains unknown; most likely it is associated with systemic astroglial failure that translates into white matter lesions.
Ischemia & stroke
The pathogenesis of ischemic damage to CNS tissue, which ultimately results in cell death and severe neurological deficits, involves astroglia, which, in the case of focal ischemia or stroke, very much determines its progression and outcome (see [12,32–34] for details). Astroglia in ischemia may either reduce or exacerbate neuronal damage, depending on the depth and duration of the ischemic insult.
Astrocytes are generally (when compared with neurons and oligodendrocytes) more resilient to ischemia, which is most likely due to their utilization of anerobic metabolic pathways. The ischemic insult, which rapidly causes ATP deficiency associated with a failure in ion homeostasis and depolarization of neurons, triggers a massive release of glutamate that further depolarizes neurons and instigates excitotoxicity [35]. Astrocytes, being the main sink of glutamate in the brain [36], form the first line of defence against glutamate overload. Removal of astrocytes from astroglial–neuronal cultures, for example, increases the neuronal vulnerability to glutamate by approximately 100-times. In addition, astrocytes (and especially reactive astrocytes) are capable of maintaining anerobic glycolysis and lactate production, with which they feed damaged neurons and thus support their survival. Astroglia are the main producers of glutathione and ascorbic acid, both being the main scavengers of reactive oxygen species in the CNS; indeed, astrocytes effectively prevent reactive oxygen species-mediated neuronal death [37]. Astrocytes are also central elements for K+ buffering [38], and by removing extracellular K+ they contain depolarization and thus ameliorate a vicious cycle of excitotoxicity. These neuroprotective functions are of particular importance for the penumbra that surrounds the ischemic core. In general, the penumbra (of which cells are compromised, but still surviving) is substantially larger than the infarction core, and it is the extent of propagation of cell death within the penumbra that often defines the degree of final neurological deficit.
At the same time, astrocytes may exacerbate neuronal damage during an ischemic attack, especially in conditions of severe insults. First, astrocytes can contribute to a build up of extracellular glutamate; astroglial glutamate can be released through several mechanisms that involve: reversed plasma membrane glutamate transporters (which may occur upon the massive overload of astrocytes with Na+ in combination with a severe increase in extracellular K+); through glutamate diffusion via astroglial ‘maxi’ (i.e., with a large pore) channels such as volume-regulated Cl- channels, purinergic P2X7 receptors with dilated pores and unpaired connexions/hemichannels; or increased exocytosis following an abnormal and sustained increase in astroglial cytosolic Ca2+ (see [39–41] for further details). Second, astrocytes can contribute to the overall acidosis by adding protons produced by anerobic glycolysis (incidentally, increasing glucose levels can exacerbate the ischemic neuronal damage, most likely by stimulation of astroglial anerobic metabolism). Third, astrocytes may contribute to the propagation of cell death through the penumbra, possibly via propagating aberrant astroglial Ca2+ waves causing the distal (to the infarction core) release of glutamate and other neurotoxic factors [42]. At the final stages of stroke, astrogliosis becomes apparent and astrocytes form the scar, which isolates the site of damage and replaces the dead tissue.
Migraine & spreading depression
Migraine often results from the specific phenomenon of cortical pathological excitation known as spreading depression, which was discovered in 1944 [43]. Spreading depression is a propagating wave of depolarization (with a speed between 1.5 and 7 mm/min) through cortical neuronal networks that renders these neurons nonexcitable for a period of time (for a detailed review see [44,45]). Spreading depression is associated with a propagating wave of increasing extracellular K+ concentration up to 80 mM, a very substantial (up to tenfold) decrease in extracellular Ca2+ concentration and a propagating wave of shrinkage of the extracellular space by up to 50%. A direct link between spreading depression and migraine was demonstrated using transgenic mice carrying the W887R mutation of the human a2 subunit of Na+/K+ATPase, encoded by the ATP1A2 gene, associated with familial hemiplegic migraine type II [46]. These mice had a higher susceptibility to spreading depression; the α2 subunit of Na+/K+ATPase is highly expressed in astrocytes.
The role of astroglia in spreading depression is supported by many experimental observations. Some of the seminal discoveries included the fact that spreading depression is associated with circulating currents passing through the glial syncytium [47] and that spreading depression can be blocked by inhibitors of gap junctions [48]. The expression of mutated Na+/K+ ATPase can affect K+ buffering [46], which is one of the causal factors for spreading depression.
Metabolic disorders
Astrocytes are directly implicated in cognitive/mental complications of liver failure, generally known as a hepatic encephalopathy. The liver failure leads to an increase in blood ammonia, and this ammonia in turn poisons the brain, characterized by neuropsychiatric symptoms such as confusion, memory deficits, lethargy, somnolence and, in the terminal stages, coma. In the brain, ammonia is metabolized either to glutamate or to glutamine; the latter reaction, catalyzed by glutamine synthetase, is the most important. Glutamine synthetase is specifically expressed in astrocytes and, in essence, hepatic encephalopathy represents toxic astrogliopathology (see [49–51] for detailed discussion). Increased activity of glutamine synthetase overloads astrocytes with glutamine, which results in osmotic shock and cell swelling. This subsequently compromises astroglial glutamate uptake, thus opening a pathway for excitotoxicity, reducing astroglial metabolic support and inducing brain edema, all of which lead to coma and ultimately death.
The congenital glutamine synthetase deficiency (which results from mutations in the relevant gene) is another type of astrogliopathology [52]. This inherited disease causes severe white matter deficiency and abnormal gyration, and most likely results from the inability of astrocytes to maintain glutamine/glutamate metabolism. Similarly, pyruvate carboxylase deficiency also manifests as astrogliopathy. In the CNS, pyruvate carboxylase is predominantly expressed in astrocytes [22,23]. Deficiency in this enzyme is an autosomal recessive disorder, which results in impaired mental development, recurrent seizures and metabolic acidosis [16]. Another type of an astroglia-associated metabolic disorder is neurodegenerative disease, known as aceruloplasminemia, which is associated with loss-of-function mutations of ceruloplasmin. Ceruloplasmin (also known as ferroxidase) is synthesized in perivascular astrocytes and is a key component of iron metabolism. Aceruloplasminemia is characterized by astroglial lesions, appearance of foamy spheroid bodies at the vascular endfeet, iron depositions and neuronal death [53].
Heavy metal toxicity
Poisoning of the CNS with heavy metals almost invariably affects astrocytes as key pathogenic targets. Minamata disease, or poisoning with methylmercury, is manifested by visual abnormalities, sensory lesions, cerebellar ataxia, hearing loss, weakness, tremor and cognitive decline. Methylmercury, having entered the CNS, is primarily accumulated by astrocytes and affects glutamate uptake and the ability of astrocytes to produce glutathione, which increases glutamate excitotoxicity and decreases neuroprotection from reactive oxygen species [54]. Similarly, astrocytes accumulate lead, which downregulates glutamate uptake and affects astroglial water transport. These mechanisms contribute to the development of cytotoxic and vascular brain edema in patients with lead poisoning that ultimately causes acute neurodegeneration. Manganese toxicity is characterized by psychosis (following acute poisoning) or Parkinsonian symptoms (in chronic poisoning). Astrocytes are endowed with a high capacity manganese transport system and thus accumulate manganese, which, in turn, inhibits glutamate transport and even causes glial cell death. Compromised astroglial glutamate metabolism is also considered to be involved in the pathogenesis of aluminium toxic encephalopathy that triggers speech alterations, seizures, flapping wrist tremor (asterixis) and cognitive impairments [55,56].
Epilepsy
The cellular substrate of epilepsy is a slow depolarization of neurons, termed paroxysmal depolarization shift, which develops synchronously in all cells within an epileptic focus. This depolarization is mediated by ionotropic glutamate receptors, which became activated because of simultaneous glutamate release around many neurons, comprising an epileptic focus. Epilepsy is associated with prominent reactive astrogliosis, and often with an appearance of a glial scar. Reactive astrogliosis occurs at the very early stages of the disease, even before the clinical manifestation in the form of seizures. Reactive astrocytes in the epileptic tissue lose their domain organization; this feature is being observed in post-mortem human samples and in animal models [57]. Astrocytes from epileptic tissues have an increased expression of ionotropic and metabotropic glutamate receptors and a decreased presence of inward rectifier K+ channels and aquaporins. In addition, epilepsy causes a decrease in the expression and activity of astroglial plasma membrane glutamate transporters and glutamine synthetase, which results in the aberrant homeostasis of glutamate and GABA (for detailed review of astroglial changes see [12,58–62]). Changes in the astrocytic intra- and inter-cellular Ca2+ dynamics have been implicated in epilepsy [63].
Neurodegenerative diseases
Neurodegeneration is a chronic process manifested by a progressive loss-of-function, structure and number of neural cells, which ultimately causes atrophy of the brain and profound cognitive deficits. The role of neuroglia in neurodegeneration is not yet fully explored; nonetheless, profound homeostatic changes that accompany (and are most likely causative to) neurodegeneration implicate glial dysfunction. A wealth of evidence indicates that neuroglial cells are invariably already affected at the early stages of neurodegenerative processes and are an important element of pathophysiology of these diseases [12,13,64–66].
Alzheimer's disease
Alzheimer's disease (AD), named after Alois Alzheimer, who was the first to describe this pathology in 1907, is a form of dementia resulting from profound atrophy of brain tissue [67]. Histological signs of AD are the extracellular deposits of β-amyloid (Aβ) protein, known as senile plaques, and intraneuronal accumulation of abnormal tau-protein filaments, which can be observed in the form of neuronal tangles. Astroglial reactions in AD are yet to be fully characterized, although two main types of changes have been observed [64–66,68].
At the late stages of the disease, widespread astrogliosis is routinely detected in post-mortem human tissues and is also observed in animal models of the disease. This astrogliosis is characterized by cellular hypertrophy and upregulation of the expression of GFAP and astroglia-specific protein S100β. Reactive astrocytes in the late stages of AD have also diminished expression and activity of glutamine synthetase, indicating possible deficits in glutamate metabolism [69]. Hypertrophic astrocytes are also often present in the senile plaques together with activated microglia. Astrocytes associated with senile plaques in transgenic AD animal models have an abnormal Ca2+ signaling [70]. At the same time, astrocytes in AD preserve their domain organization.
At the early stages of AD, as was found in experiments in transgenic animals, astrocytes display signs of atrophy manifested by a decreased area of GFAP-positive profiles, a reduction in the size of the somata and a decrease in the number of primary processes [71–73]. This early atrophy of astrocytes may be pathologically relevant, as reduced astroglial synaptic coverage and support can be the primary mechanisms for a decrease in synaptic connectivity and abnormal synaptic transmission observed in the early stages of AD [74].
The role of astroglia in the metabolism of Aβ remains controversial. Reactive astrocytes have been suggested to contribute to the clearance and degradation of Aβ [68]. By stark contrast, there are indications that in AD, astrocytes can start to express β-secretase and thus may be an additional source for Aβ [75,76].
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS or ‘Lou Gehrig's disease’ in the USA, so named after a baseball player who suffered and died from this pathology) refers to a specific degeneration and ultimate death of motor neurons located in the cortex, brain stem and spinal cord. Death of these motoneurons determines the clinical picture of ALS that is manifested by progressive paralysis and muscle atrophy; death is usually caused by respiratory failure. In ALS, similarly to AD, both astrogliosis and astroglial atrophy have been described. During the early stages of the disease (i.e., before the appearance of clinical symptoms) astrocytes undergo degeneration and become atrophic, whereas reactive astroglia appear at the later stages of disease, and are probably activated by specific lesions. Astrocytes in ALS were also found to release neurotoxic factors and instigate activation of microglia [77,78]. Astrocytes in ALS were reported to have deficits in plasma membrane glutamate transport, which may result in exacerbated excitotoxicity. Genetic deletion of EAAT2 in mice, referred to as Glt-1 in rodents, led to a significant death of motor neurons, thus mimicking ALS [79]. Selective silencing of the SOD1 mutant gene (associated with a familial form of ALS) in astrocytes significantly slowed the progression of ALS in transgenic mice [80,81].
Wernicke encephalopathy
Wernicke encephalopathy is the combination of ataxia, ophthalmoplegia and mental confusion, which, in terminal stages, is manifested by Korsakoff psychosis (anterograde and retrograde amnesia, apathy and confabulation). This is an example of neurodegeneration primarily associated with the loss of astroglial homeostatic function. Wernicke encephalopathy is caused by functional atrophy of astroglial glutamate uptake, with the expression of EAAT1/EAAT2 transporters being decreased by 60–70% in human samples [82]. A very similar decrease in the expression of EAATs in astrocytes was detected in the rat thiamine deficiency model of the disease [82,83]. This profound failure in glutamate uptake causes excitotoxicity neuronal death and rapidly developing cognitive deficiency.
Astrocytes are also affected in other types of dementia including frontotemporal dementia, Pick's disease, frontotemporal lobar degeneration, thalamic dementia, HIV-associated dementia and other non-Alzheimer's-type dementia. In all these pathologies both astroglial atrophy and astrogliosis have been detected [84–86].
Niemann–Pick disease type C
Niemann–Pick disease type C (a progressive neurodegeneration with hepatosplenomegaly; not to be confused with Pick's disease) results from the loss-of-function mutations of genes encoding NPC-1 or NPC-2 proteins (which cooperatively appear to function as transporters in the endosomal–lysosomal system). These proteins are present in astroglial perisynaptic processes and are believed to contribute to synaptogenesis and synaptic maintenance [87].
Parkinson's disease
The role of astroglia in the pathogenesis of Parkinson's disease is virtually unknown. In the post-mortem samples of the brain stem, significant astrogliosis was detected. Incidentally, the substantia nigra (which is primarily affected by the disease) contains a low density of astrocytes compared with other brain regions, and this reduced astroglial support can have certain pathological relevance. Experiments in vitro demonstrated the neuroprotective effect of astrocytes on dopaminergic neurons [88,89].
Huntington's disease
Astroglial pathology in Huntington's disease is characterized by a decrease in the expression of EAAT2 and ascorbic acid [90], with these changes possibly contributing to neuronal damage.
Psychiatric diseases
In all three major psychiatric disorders – that is, schizophrenia, bipolar disorder and major depressive disorder – reduced numbers of astrocytes and signs for astroglial atrophy were obtained. Contrary to the majority of other neurological diseases, the psychiatric conditions are generally not associated with significant astrogliosis [91,92].
Demyelinating diseases
The main types of demyelinating diseases are represented by multiple sclerosis and neuromyelitis optica or Devic's disease. Astroglial reactions in multiple sclerosis can be both protective and toxic. Astrocytes can provide a link between oligodendroglia and vasculature and thus can be critical for maintaining energy support of the latter. At the same time, astrocytes in multiple sclerosis can contribute to myelin degradation and can release factors toxic to oligodendroglia [93]. Neuromyelitis optica is an autoimmune disease associated with an appearance of the so-called neuromyelitis optica immunoglobulin G autoantibody that binds to astroglial water channels of the aquaporin-4 type, and thus can be considered as a primary autoimmune astrogliopathology [16].
Conclusion
The intent of this review was to broadly summarize our understanding of the pathological potential of astroglia. Astrocytes, the main homeostatic cells in the CNS, are intimately involved in all types of neurological diseases and their reactions to pathological insults represent an important part of neuropathology. Future experimental approaches need to incorporate astrocytes as contributors to the etiology/pathology of neurological and psychiatric disorders in order to form a more complete picture of how to medically intervene. Thus, astrocytes could represent a fertile ground for novel interventions in prevention, curing and/or slowing the progression of various disorders.
Future perspective
The main future challenges lie in two domains. First, the pathological potential of astrocytes and the specificity of astroglial reactions in different diseases need to be characterised in detail. We need to clearly understand the role of glial atrophy (both morphological and functional) as an early (and possibly primary) mechanism of deficient homeostasis that initiates malfunctions of brain tissue and weakening of synaptic connectivity with obvious negative outcomes to the functional response. We also need to characterize the disease specificity of astrogliosis and distinguish its neuroprotective and neurotoxic actions.
Second, we need to recognize astrocytes as potential therapeutic targets and start a specific search for astroglia-related pharmacological agents. This area would also cover a variety of interventions that would correct to dysregulated astrocytic signaling in the diseased brain; a particularly fertile ground for intervention might present itself in astrocyte–microglia signaling.
Executive summary.
Neuroglia in neuropathology
■ Neuroglial cells are primarily responsible for the overall homeostasis of the nervous system and are key elements of all neurological diseases.
Astrocytes act as the main homeostatic cells in the CNS
■ Astroglia are represented by highly heterogeneous populations of cells residing in both gray and white matter.
■ Astrocytes assume overall responsibility for morphological and functional homeostasis and are fundamental for maintaining the architecture of gray matter, for ion, water and neurotransmitter homeostasis and for activity-dependent energy supply to neurons.
Astrogliosis as a defensive reaction of astroglia
■ Brain lesions initiate an evolutionarily conserved multistage defensive program defined as reactive astrogliosis.
■ Reactive astrogliosis is fundamental for the neuroprotection and trophic support of insult-stressed neurons, isolation of the damaged area, reconstruction of the compromised blood–brain barrier and regeneration of the lesioned region.
Astroglia & neurological diseases
■ Astrocytes are involved in all neuropathologies. The genetic primary neuropathology known as Alexander disease stems from the expression of mutant GFAP that affects astroglial function and results in severe damage to white matter.
■ In ischemia, astrocytes are critically involved in the spread of damage through after-stroke penumbra, thus defining the neurological deficit. Similarly, astrocytes are critical for the development of spreading depression and migraine status.
■ Deficits in astroglial metabolic cascades determine numerous metabolic disorders such as hepatic encephalopathy, congenital glutamine synthetase deficiency or aceruloplasminemia.
■ Brain poisoning by heavy metals results from their primary action on astroglial homeostatic function.
■ Astrocytes are also affected in epilepsy.
■ Astroglial morphofunctional dystrophy, combined with astrogliosis, determines the pathogenesis of neurodegenerative diseases.
Conclusion
■ Astrocytes, as main homeostatic cells in the CNS, are intimately involved in all types of neurological diseases and their reactions to pathological insults represent an important part of neuropathology.
Acknowledgments
The authors’ research was supported by the Alzheimer's Research Trust (UK) Programme Grant (ART/PG2004A/1) to A Verkhratsky and J Rodríguez; by the National Science Foundation (CBET 0943343) grant to V Parpura; by the Grant Agency of the Czech Republic (GACR 309/09/1696) to J Rodríguez and (GACR 305/08/1384) to A Verkhratsky.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■■ of considerable interest
- 1.Verkhratsky A. Physiology of neuronal–glial networking. Neurochem. Int. 2010;57(4):332–343. doi: 10.1016/j.neuint.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 2■■.Verkhratsky A, Butt A, editors. Physiology and Pathophysiology of Neuroglia. Wiley; Chichester, UK: 2013. [Provides an introduction to neuroglial function in health and disease.] [Google Scholar]
- 3.Verkhratsky A, Parpura V, Rodriguez JJ. Where the thoughts dwell: the physiology of neuronal–glial ‘diffuse neural net’. Brain Res. Rev. 2011;66(1–2):133–151. doi: 10.1016/j.brainresrev.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 4■■.Kettenmann H, Ransom BR, editors. Neuroglia. 3rd Edition. Oxford University Press; Oxford, UK: 2012. [Most comprehensive reference book in the field.] [Google Scholar]
- 5.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 6.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 7.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 8.Hanani M. Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res. Rev. 2010;64(2):304–327. doi: 10.1016/j.brainresrev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2012;9(11):625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 10■■.Virchow R. Die Cellularpathologie in ihrer Begründung auf physiologische and pathologische Gewebelehre. Zwanzig Vorlesungen gehalten während der Monate Februar, März und April 1858 im pathologischen Institut zu Berlin. August Hirschwald; Berlin, Germany: 1858. [First description of the concept of neuroglia.] [Google Scholar]
- 11.Kettenmann H, Verkhratsky A. Neuroglia: the 150 years after. Trends Neurosci. 2008;31(12):653–659. doi: 10.1016/j.tins.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Verkhratsky A, Sofroniew MV, Messing A, et al. Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro. 2012;4(3):e00082. doi: 10.1042/AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parpura V, Heneka MT, Montana V, et al. Glial cells in (patho)physiology. J. Neurochem. 2012;121(1):4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedergaard M, Rodriguez JJ, Verkhratsky A. Glial calcium and diseases of the nervous system. Cell. Calcium. 2010;47(2):140–149. doi: 10.1016/j.ceca.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Giaume C, Kirchhoff F, Matute C, Reichenbach A, Verkhratsky A. Glia: the fulcrum of brain diseases. Cell Death Differ. 2007;14(7):1324–1335. doi: 10.1038/sj.cdd.4402144. [DOI] [PubMed] [Google Scholar]
- 16■■.De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J. Neurol. Sci. 2008;267(1–2):3–16. doi: 10.1016/j.jns.2007.08.044. [Comprehensive account of astroglial pathophysiology.] [DOI] [PubMed] [Google Scholar]
- 17.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 2004;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 18.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007;10(11):1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 19■■.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [Introduced the concept of the tripartite synapse.] [DOI] [PubMed] [Google Scholar]
- 20.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 2007;13(2):54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Nedergaard M, Verkhratsky A. Artifact versus reality – how astrocytes contribute to synaptic events. Glia. 2012;60(7):1013–1023. doi: 10.1002/glia.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: astrocytes as stars of the show. Trends Neurosci. 2004;27(12):735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Hassel B, Brathe A. Neuronal pyruvate carboxylation supports formation of transmitter glutamate. J. Neurosci. 2000;20(4):1342–1347. doi: 10.1523/JNEUROSCI.20-04-01342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirischuk S, Parpura V, Verkhratsky A. Sodium dynamics: another key to astroglial excitability? Trends Neurosci. 2012;35(8):497–506. doi: 10.1016/j.tins.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11(5):400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 26■■.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [Comprehensive description of astrogliosis as a multistage and complex defensive reaction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat. Rev. Neurosci. 2011;12(2):88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- 29.Alexander WS. Progressive fibrinoid degeneration of fibrillary astrocytes associated with mental retardation in a hydrocephalic infant. Brain. 1949;72(3):373–381. doi: 10.1093/brain/72.3.373. [DOI] [PubMed] [Google Scholar]
- 30■■.Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE. Alexander disease. J. Neurosci. 2012;32(15):5017–5023. doi: 10.1523/JNEUROSCI.5384-11.2012. [Up-to-date comprehensive description of Alexander disease and its cellular pathophysiology.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prust M, Wang J, Morizono H, et al. GFAP mutations, age at onset, and clinical subtypes in Alexander disease. Neurology. 2011;77(13):1287–1294. doi: 10.1212/WNL.0b013e3182309f72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takano T, Oberheim N, Cotrina ML, Nedergaard M. Astrocytes and ischemic injury. Stroke. 2009;40(Suppl. 3):S8–S12. doi: 10.1161/STROKEAHA.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vangeison G, Rempe DA. The Janus-faced effects of hypoxia on astrocyte function. Neuroscientist. 2009;15(6):579–588. doi: 10.1177/1073858409332405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Rempe DA. Targeting astrocytes for stroke therapy. Neurotherapeutics. 2010;7(4):439–451. doi: 10.1016/j.nurt.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell. Calcium. 2010;47(2):122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Danbolt NC. Glutamate uptake. Progr. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka J, Toku K, Zhang B, Ishihara K, Sakanaka M, Maeda N. Astrocytes prevent neuronal death induced by reactive oxygen and nitrogen species. Glia. 1999;28(2):85–96. doi: 10.1002/(sici)1098-1136(199911)28:2<85::aid-glia1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 38.Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience. 2004;129(4):1045–1056. doi: 10.1016/j.neuroscience.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parpura V, Grubisic V, Verkhratsky A. Ca(2+) sources for the exocytotic release of glutamate from astrocytes. Biochim. Biophys. Acta. 2011;1813(5):984–991. doi: 10.1016/j.bbamcr.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem. Int. 2008;52(1–2):142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding S, Wang T, Cui W, Haydon PG. Photothrombosis ischemia stimulates a sustained astrocytic Ca2+ signaling in vivo. Glia. 2009;57(7):767–776. doi: 10.1002/glia.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin JH, Weigel H, Cotrina ML, et al. Gap-junction-mediated propagation and amplification of cell injury. Nat. Neurosci. 1998;1(6):494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- 43.Leão AAP. Spreading depression of activity in the cerebral cortex. J. Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- 44.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat. Med. 2011;17(4):439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 45.Eikermann-Haerter K, Ayata C. Cortical spreading depression and migraine. Curr. Neurol. Neurosci. Rep. 2010;10(3):167–173. doi: 10.1007/s11910-010-0099-1. [DOI] [PubMed] [Google Scholar]
- 46.Leo L, Gherardini L, Barone V, et al. Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLoS Genet. 2011;7(6):e1002129. doi: 10.1371/journal.pgen.1002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugaya E, Takato M, Noda Y. Neuronal and glial activity during spreading depression in cerebral cortex of cat. J. Neurophysiol. 1975;38(4):822–841. doi: 10.1152/jn.1975.38.4.822. [DOI] [PubMed] [Google Scholar]
- 48.Nedergaard M, Cooper AJ, Goldman SA. Gap junctions are required for the propagation of spreading depression. J. Neurobiol. 1995;28(4):433–444. doi: 10.1002/neu.480280404. [DOI] [PubMed] [Google Scholar]
- 49.Brusilow SW, Koehler RC, Traystman RJ, Cooper AJ. Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics. 2010;7(4):452–470. doi: 10.1016/j.nurt.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Butterworth RF. Altered glial–neuronal crosstalk: cornerstone in the pathogenesis of hepatic encephalopathy. Neurochem. Int. 2010;57(4):383–388. doi: 10.1016/j.neuint.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 51.Butterworth RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. 2011;53(4):1372–1376. doi: 10.1002/hep.24228. [DOI] [PubMed] [Google Scholar]
- 52.Haberle J, Gorg B, Rutsch F, et al. Congenital glutamine deficiency with glutamine synthetase mutations. N. Engl. J. Med. 2005;353(18):1926–1933. doi: 10.1056/NEJMoa050456. [DOI] [PubMed] [Google Scholar]
- 53.Oide T, Yoshida K, Kaneko K, Ohta M, Arima K. Iron overload and antioxidative role of perivascular astrocytes in aceruloplasminemia. Neuropathol. Appl. Neurobiol. 2006;32(2):170–176. doi: 10.1111/j.1365-2990.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 54.Yin Z, Milatovic D, Aschner JL, et al. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131(1):1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Struys-Ponsar C, Guillard O, van den Bosch de Aguilar P. Effects of aluminum exposure on glutamate metabolism: a possible explanation for its toxicity. Exp. Neurol. 2000;163(1):157–164. doi: 10.1006/exnr.2000.7355. [DOI] [PubMed] [Google Scholar]
- 56.Suarez-Fernandez MB, Soldado AB, Sanz-Medel A, Vega JA, Novelli A, Fernandez-Sanchez MT. Aluminum-induced degeneration of astrocytes occurs via apoptosis and results in neuronal death. Brain Res. 1999;835(2):125–136. doi: 10.1016/s0006-8993(99)01536-x. [DOI] [PubMed] [Google Scholar]
- 57.Oberheim NA, Tian GF, Han X, et al. Loss of astrocytic domain organization in the epileptic brain. J. Neurosci. 2008;28(13):3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carmignoto G, Haydon PG. Astrocyte calcium signaling and epilepsy. Glia. 2012;60(8):1227–1233. doi: 10.1002/glia.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coulter DA, Eid T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia. 2012;60(8):1215–1226. doi: 10.1002/glia.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heinemann U, Kaufer D, Friedman A. Blood–brain barrier dysfunction, TGFβ signaling, and astrocyte dysfunction in epilepsy. Glia. 2012;60(8):1251–1257. doi: 10.1002/glia.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seifert G, Carmignoto G, Steinhauser C. Astrocyte dysfunction in epilepsy. Brain Res. Rev. 2010;63(1–2):212–221. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Seifert G, Steinhauser C. Neuron–astrocyte signaling and epilepsy. Exp. Neurol. 2011 doi: 10.1016/j.expneurol.2011.08.024. doi:10.1016/j.expneurol.2011.08.024 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 63.Reyes RC, Parpura V. Models of astrocytic Ca dynamics and epilepsy. Drug Discov. Today Dis. Models. 2008;5(1):13–18. doi: 10.1016/j.ddmod.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heneka MT, Rodriguez JJ, Verkhratsky A. Neuroglia in neurodegeneration. Brain Res. Rev. 2010;63(1–2):189–211. doi: 10.1016/j.brainresrev.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Rodriguez JJ, Verkhratsky A. Neuroglial roots of neurodegenerative diseases? Mol. Neurobiol. 2011;43(2):87–96. doi: 10.1007/s12035-010-8157-x. [DOI] [PubMed] [Google Scholar]
- 66.Verkhratsky A, Olabarria M, Noristani HN, Yeh CY, Rodriguez JJ. Astrocytes in Alzheimer's disease. Neurotherapeutics. 2010;7(4):399–412. doi: 10.1016/j.nurt.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg. Z. Psychiat. Psych.-Gericht. Med. 1907;64:146–148. [Google Scholar]
- 68.Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC. Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol. Aging. 2004;25(5):663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 69.Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ. Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer's disease mouse model: mechanism for deficient glutamatergic transmission? Mol. Neurodegener. 2011;6:55. doi: 10.1186/1750-1326-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323(5918):1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Olabarria M, Noristani HN, Verkhratsky A, Rodriguez JJ. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer's disease. Glia. 2010;58:831–838. doi: 10.1002/glia.20967. [DOI] [PubMed] [Google Scholar]
- 72.Yeh CY, Vadhwana B, Verkhratsky A, Rodriguez JJ. Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer's disease. ASN Neuro. 2012;3(5):271–279. doi: 10.1042/AN20110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kulijewicz-Nawrot M, Verkhratsky A, Chvatal A, Sykova E, Rodriguez JJ. Astrocytic cytoskeletal atrophy in the medial prefrontal cortex of a triple transgenic mouse model of Alzheimer's disease. J. Anat. 2012;221(3):252–262. doi: 10.1111/j.1469-7580.2012.01536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Terry RD. Cell death or synaptic loss in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2000;59(12):1118–1119. doi: 10.1093/jnen/59.12.1118. [DOI] [PubMed] [Google Scholar]
- 75.Heneka MT, Sastre M, Dumitrescu-Ozimek L, et al. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflam. 2005;2(1):22. doi: 10.1186/1742-2094-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rossner S, Lange-Dohna C, Zeitschel U, Perez-Polo JR. Alzheimer's disease beta-secretase BACE1 is not a neuron-specific enzyme. J. Neurochem. 2005;92(2):226–234. doi: 10.1111/j.1471-4159.2004.02857.x. [DOI] [PubMed] [Google Scholar]
- 77.Rossi D, Brambilla L, Valori CF, et al. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ. 2008;15(11):1691–1700. doi: 10.1038/cdd.2008.99. [DOI] [PubMed] [Google Scholar]
- 78.Rossi D, Volterra A. Astrocytic dysfunction: Insights on the role in neurodegeneration. Brain Res. Bull. 2009;80:224–232. doi: 10.1016/j.brainresbull.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 79.Staats KA, Van Den Bosch L. Astrocytes in amyotrophic lateral sclerosis: direct effects on motor neuron survival. J. Biol. Phys. 2009;35(4):337–346. doi: 10.1007/s10867-009-9141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamanaka K, Chun SJ, Boillee S, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 2008;11(3):251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang L, Gutmann DH, Roos RP. Astrocyte loss of mutant SOD1 delays ALS disease onset and progression in G85R transgenic mice. Hum. Mol. Genet. 2011;20(2):286–293. doi: 10.1093/hmg/ddq463. [DOI] [PubMed] [Google Scholar]
- 82.Hazell AS, Sheedy D, Oanea R, et al. Loss of astrocytic glutamate transporters in Wernicke encephalopathy. Glia. 2009;58:148–156. doi: 10.1002/glia.20908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hazell AS. Astrocytes are a major target in thiamine deficiency and Wernicke's encephalopathy. Neurochem. Int. 2009;55(1–3):129–135. doi: 10.1016/j.neuint.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 84.Broe M, Kril J, Halliday GM. Astrocytic degeneration relates to the severity of disease in frontotemporal dementia. Brain. 2004;127(Pt 10):2214–2220. doi: 10.1093/brain/awh250. [DOI] [PubMed] [Google Scholar]
- 85.Kersaitis C, Halliday GM, Kril JJ. Regional and cellular pathology in frontotemporal dementia: relationship to stage of disease in cases with and without Pick bodies. Acta Neuropathol. 2004;108(6):515–523. doi: 10.1007/s00401-004-0917-0. [DOI] [PubMed] [Google Scholar]
- 86.Potts R, Leech RW. Thalamic dementia: an example of primary astroglial dystrophy of Seitelberger. Clin. Neuropathol. 2005;24(6):271–275. [PubMed] [Google Scholar]
- 87.Rosenbaum AI, Maxfield FR. Niemann–Pick type C disease: molecular mechanisms and potential therapeutic approaches. J. Neurochem. 2011;116(5):789–795. doi: 10.1111/j.1471-4159.2010.06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov. Disord. 2008;23(4):474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 89.Mena MA, Garcia de Yebenes J. Glial cells as players in parkinsonism: the ‘good’, the ‘bad’ and the ‘mysterious’ glia. Neuroscientist. 2008;14(6):544–560. doi: 10.1177/1073858408322839. [DOI] [PubMed] [Google Scholar]
- 90.Estrada-Sánchez AM, Rebec GV. Corticostriatal dysfunction and glutamate transporter 1 (GLT1) in Huntington's disease: interactions between neurons and astrocytes. Basal Ganglia. 2012;2(2):57–66. doi: 10.1016/j.baga.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bernstein HG, Steiner J, Bogerts B. Glial cells in schizophrenia: pathophysiological significance and possible consequences for therapy. Expert Rev. Neurother. 2009;9(7):1059–1071. doi: 10.1586/ern.09.59. [DOI] [PubMed] [Google Scholar]
- 92.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol. Disord. Drug Targets. 2007;6(3):219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miljkovic D, Timotijevic G, Mostarica Stojkovic M. Astrocytes in the tempest of multiple sclerosis. FEBS Lett. 2011;585(23):3781–3788. doi: 10.1016/j.febslet.2011.03.047. [DOI] [PubMed] [Google Scholar]