Introduction
The central event in the retroviral life cycle is integration of the DNA replica of the retroviral genome into the host cell’s chromosomal DNA. Integration enables the virus to replicate and express its genome to give rise to new virus generations. All events of the retroviral life cycle prior to integration are referred to as the early phase events, whereas all post-integration events represent late phase events. The early phase events include virus entering into target cell, conversion of its genome from RNA to DNA (reverse transcription), delivery of intact DNA to the site of integration and integration itself. Noteworthy, all these steps occur in the presence of an active counteraction of the target cell. Since relatively few virus particles initiate infection under natural conditions, the early phase of retroviral life cycle is particularly vulnerable to host anti-viral responses. Thus, it is not surprising that retroviruses acquired during their evolution specific mechanisms that allow them protect their genome and reverse transcription machinery and perform transportation of the viral genetic material towards the site of integration. From this standpoint, formation of multimolecular complexes termed reverse transcription complexes (RTCs) at a post-entry step of infection may be considered as one of key adaptations of retroviruses that accomplishes protective and transport functions.
Different groups of retroviruses are characterized by different mechanisms of intracellular transfer of their genomes to the site of integration. HIV as a representative of lentiviruses delivers its genome into the nucleus of target cells by translocating the RTC through the nuclear pore complex (NPC) [1–4]. Foamy viruses likely employ a similar strategy [5]. Onco-retroviruses, on the other hand, can get into the nuclear compartment only during cell division, when the nuclear envelope disappears. Their RTCs probably bind chromatin during mitosis and are subsequently retained in the nucleus upon formation of the nuclear envelope ([6–9]).
Technical difficulties associated with observation, isolation and analysis of RTCs at different steps of their trafficking in the cytoplasm and especially in the nucleus explain our poor understanding of the events in the early phase of the retroviral life cycle (reviewed in [10–12]) in comparison with the late phase events (reviewed in [13]). This gap in our knowledge is even more regrettable given that pre-integration events are preferable targets for chemotherapy (reviewed in [14] and understanding of the early events in the life cycle of HIV-1 should be helpful for drug development. Moreover, knowledge of the early phase events is necessary for understanding of the mechanisms of activity of host cell restriction factors, most of which block retroviral infection at pre-integration steps (reviewed in [15]). Finally, early phase events are critical for the ability of lentiviruses to infect non-dividing cells, and are therefore crucial for developing safe retroviral vectors for gene therapy (reviewed in [16]).
Post-fusion events: formation of RTC
The central function of RTC is accomplishment of reverse transcription. This process underlies formation of RTCs, re-arrangements of the structure of the complexes and their transformation into the integration-competent complexes termed pre-integration complexes (PICs) ([17]). The RTCs are multimolecular complexes which assemble shortly after entry of a retrovirus into the cytoplasm of a target cell. It is commonly accepted that activation of reverse transcription results from disassembly of the viral core (uncoating), and that this reaction is carried out within the RTC (reviewed in [10,18]). On the other hand, it has been reported that reverse transcription of HIV-1 can be initiated within the capsid core early after viral entry ([19,20]) or even within the intact virion ([21,22]). It was demonstrated that initial steps of the endogenous reverse transcription (ERT), which takes place before infection of the target cell, can increase replication of HIV-1 in quiescent T cells as well as non-proliferating cells such as macrophages, whereas completion of ERT within virions leads to morphologic changes in the viral particles that abrogate virion infectivity ([23]). These results suggest that activation of reverse transcriptase (RT) and initiation of reverse transcription are not triggered by RTC formation and do not require viral core disassembly. Thus, viral capsids, released into the cytoplasm of target cells after fusion of the viral envelope with the plasma membrane, actually represent pre-RTC (Fig. 1). Since it was demonstrated in numerous in vitro experiments that RT reaction itself does not need any special multimolecular complexes, and no viral or cellular proteins other than reverse transcriptase is required for this process, post-entry re-assembly of retroviral core particles is likely needed for optimization of RT activity, protection of reverse transcription machinery from destructive cellular factors and also for intracytoplasmic and nuclear transport of the viral genome.
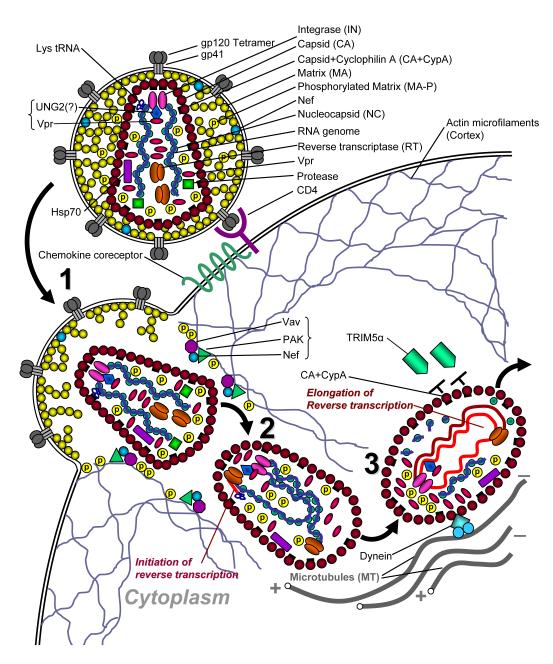
Figure 1.
Schematic view of the early phase events involving coated HIV-1 cores (pre-RTC). The significant events are numbered and highlighted. (1) Fusion of the viral envelope with the plasma membrane leads to release of the matrix protein (MA) localized outside of the core. Cores are released into the cell. Molecules of core-associated phosphorylated MA (MA-P) and Nef interact with microfilaments of the actin cortex, resulting in local rearrangement of the actin cytoskeleton. (2) Coated core particles (which may be called pre-RTCs) of Nef-containing virus traverse the submembrane cortex and get into the cytosol. This step usually correlates with the early step of reverse transcription (synthesis of negative-strand “strong-stop” DNA). (3) Core particles interact with dynein which provides directed trafficking of the particles along microtubules (MT) to their minus (−) ends. Reverse transcription occurs within encapsidated RTCs. Cyclophilin A (CypA) bound to some molecules of CA (CA+CypA) (~10% of all CA molecules) likely protects RTCs from attack by the host restriction factor TRIM5α and hence from rapid capsid disassembly (uncoating). Encapsidated molecules of the heat shock protein 70 (Hsp70) probably also stabilize RTCs. Molecules of the viral protein R (Vpr) within RTCs facilitate higher fidelity of reverse transcription.
Eukaryotic cells have various defense mechanisms targeting early events in replicative cycle of viruses and intracellular parasitic organisms. The cortical actin network which lies directly below the plasma membrane constitutes a physical barrier ([24,25]; reviewed in [26,27]), whereas proteasomal and lysosomal activity in target cell as well as action of restriction factors preventing early steps of viral infection, such as TRIM5, may be considered as functional barriers (reviewed in [15]). The cascade of events leading to the formation of reverse transcription-competent RTC requires passing of the entering core particles through the cortical dense layer of actin ([25]) which is capable of preventing virus trafficking into the cytoplasm. Campbell and co-authors [25] demonstrated that HIV-1 Nef incorporated into cores of infectious virions is crucial for local actin depolymerization and disruption of normal composition of the actin network (Fig. 1, step 1). This is likely accomplished through known interactions of Nef with proteins involved in actin cytoskeleton dynamics, such as Vav and PAK [25]. This effect is essential for successful traversing of the submembrane cortex by incoming subviral particles and for subsequent reverse transcription [28], which is facilitated by engagement of microtubules ([29]. Moreover, the large subunit of HIV-1 reverse transcriptase interacts with β-actin ([30]), although the role of this interaction in RT function has not been demonstrated.
Very little is known about disassembly of the viral matrix in incoming virions, which is an obvious step in releasing the viral capsid. Since the amino terminal glycine residue of the matrix protein (MA p17Gag) is myristoylated [31,32], this protein directly binds the viral envelope and, when virions fuse with the plasma membrane, remains associated with the inner surface of the membrane of infected cell [33] (Fig. 1, step 1). However, some MA molecules tightly associate with the RTC where they subsequently function at the stage of the nuclear import (reviewed in [10,12,34,35]). Currently, there is no information about the nature of these RTC-associated MA molecules or when and how they get into the core. Similar to the main bulk of MA, MA molecules within RTC are myristoylated [33]. However, membrane targeting property of myristoylated MA is regulated by phosphorylation ([36,37]). Non-phosphorylated MA remains predominantly at the plasma membrane, whereas phosphorylated protein may incorporate into the RTC. MA protein is phosphorylated at serine residues by protein kinase C ([38]). Bukrinskaya and colleagues reported that al least five of ten serine residues in MA are phosphorylated during HIV-1 entry into susceptible cells [33]. Consistent with the role of phosphorylation in MA incorporation into RTC, phosphorylated molecules of MA associate with actin filaments in infected cells [28] and phosphorylation of serine residues 9, 67, 72 and 77 was critical for implementing the early post-entry steps of infection [38]. Thus, distribution of MA between the plasma membrane and RTCs may occur during the traversing of incoming core particles through the actin cortex. In other words, formation of the RTC may begin early after the entry, before the cores reach the deeper layers of the cytoplasm. However, at this step of trafficking, viral RNA molecules as well as RT and other RNA-associated proteins subsequently involved in RTC formation, are encircled by a dense capsid shell composed of p24Gag capsid protein (CA). The capacity of MA molecules to traverse this dense layer is unknown, making this model of MA incorporation into RTC highly speculative.
Another, more attractive, possibility is that some MA molecules are incorporated into the viral core during its assembly shortly after virus budding from a producer cell. Indeed, some of serine residues of MA are phosphorylated in virus-producing cell before virion budding when MA protein is still not processed and exists as an MA domain of Pr55Gag or Pr160GagPol polyprotein precursors [38]. Release of the matrix protein is the first step of the polyprotein precursor processing and occurs before the p24 CA and sp2 spacer proteins are released [39,40]. Thus, MA protein appears in the virion before capsid assembly, suggesting that some of phosphorylated MA molecules can get into the RNA-containing compartment of the virion prior to CA shell formation (Fig. 1). These MA molecules can subsequently incorporate into the RTC.
Taken together, the available data reveal several novel features of the early steps of RTC formation. Initial steps of reverse transcription as well as initiation of capsid disassembly can take place in intact virions prior to fusion. The release of viral core particles following fusion of the virions with the plasma membrane of a target cell and traversing of the cores through the cortical actin network likely associates with a rearrangement of the viral matrix and, probably, with changes in the capsid morphology. Thus, the beginning of uncoating, a key step in formation of the functional RTC, can not be definitively separated from the preceding events.
Uncoating and RTC assembly
Viral core particles incoming into susceptible cell are covered by a shell composed of CA. Proportion of the CA protein in RTCs varies depending on both the method of RTC isolation (capsid is relatively unstable and easily disassembles in the presence of detergents or in low salt conditions of hypotonic lysis [41–43]) and the time post-entry when the complexes are analyzed [44–46]. McDonald and co-workers, using immunofluorescence technique, determined that 67±6% of the early cytoplasmic RTCs contained the amount of CA similar to that found in extracellular virions [47]. On the other hand, in integration-competent complexes (PICs), which are the end result of RTC maturation, CA protein was not detected [29,48,49]. Electron microscopic studies of acutely infected cells did not reveal visible conical cores [50]. Our immunopreciritation analysis of HIV-1 PICs isolated from the nuclei of infected cells identified most of RTC proteins, but not CA, whereas in the early cytoplasmic RTCs this protein was detected at a 50% ratio relative to MA protein [44]. These data confirm the hypothesis that disintegration of the capsid correlates with accomplishment of reverse transcription and is required for carrying out the subsequent steps of replication [44,45,51,52] (Fig. 1, steps 2-4). In contrast to HIV-1, the RTCs of onco-retrovirus MLV, which is not capable of overcoming the nuclear envelope barrier, contain CA protein throughout the whole time of RTC/PIC persistence in the cell [7,9,53]. This likely ensures a long-term protection of the MLV RTC (see below) and is possible because the size and protein composition of its RTC are not limited by requirement to interact with the cellular transport machinery and traverse through the tight nuclear pore.
Studies by McDonald and co-workers [47] demonstrated that reverse transcriptase activity is not suppressed in HIV-1 RTCs encircled by the capsid shell. Analysis of the structure of the HIV capsid suggested the presence of pores sufficient to provide entry of the nucleotides into the core [54]. Moreover, according to several reports [45,51,52,55,56], the presence of capsid is necessary for stability of the RTC and rapid disassembly of the capsid shell blocks HIV infection [52]. Thus, perseverance of CA in RTCs provides stability of the complexes and facilitates the process of reverse transcription.
The capacity of the lentiviral RTC to enter the nuclear compartment of a non-dividing cell implies that it can recruit cellular nuclear import machinery, most likely via interaction of viral protein(s) contained in the RTC with the cellular nuclear import factors. A number of studies revealed at least three HIV proteins capable of performing this activity, MA, IN and Vpr (reviewed in [12,14,35,57]). The presence of a capsid shell would likely mask the nuclear localization signals (NLSs) on these proteins and prevent or make difficult their interaction with components of the nuclear import machinery. Consistent with this notion, mutations within HIV-1 CA have profound effects on nuclear import. For example, substitutions in positions 63 and 67 of CA (Q63A/67A) resulted in elevated levels of CA in the RTC and impaired RTC nuclear import and integration [45]. These results suggest that lentiviruses need to disassemble their capsid shell for successful nuclear import, and uncoating may be considered as a necessary attribute of the strategy of infection adopted by this group of retroviruses.
The mechanism of retroviral uncoating is poorly understood, but there are experimental data suggesting that this mechanism is multifactorial and may require cellular activation. This knowledge comes from analysis of HIV infection of quiescent T cells. It has been found that HIV replication in quiescent CD4+ T cells is blocked at the pre-integration step of infection [58,59], before translocation of the HIV genome into the nucleus [2]. Later studies demonstrated that reverse transcription of HIV-1 RNA was also inefficient in resting cells [60,61] and took significantly longer time (2-3 days) to proceed to completion than in activated T cells [62]. Another report demonstrated that efficient reverse transcription requires transition of the infected cell from G0/G1a to G1b phase of the cell cycle, implying that cellular activation is needed [63]. Experiments using inhibitors of cell cycle progression demonstrated that cell cycle arrest in the G2/M phase also strongly increased HIV-mediated gene transduction [64]. Since this boost in transduction efficiency did not depend strongly on whether the HIV envelope or vesicular stomatitis virus G protein was used to deliver HIV into cells, the stimulation of transduction by cell cycle arrest was likely not due to increased entry but rather to action at a later step, such as uncoating. Recently, Auewarakul and co-authors demonstrated a critical role of G0/G1a to G1b cell cycle transition in uncoating of the HIV-1 core particles [65], suggesting an alternative interpretation for earlier results. Indeed, a block to uncoating would affect later steps of the viral life cycle, such as reverse transcription and nuclear import. Therefore, both G1 and G2/M phases of the cell cycle may be associated with activation of some cellular factors facilitating uncoating and subsequent early events of HIV replication.
In vitro analysis of uncoating, RTC formation and reverse transcription of avian sarcoma and leucosis virus (ASLV) in a cell-free system demonstrated dependence of these early events on cellular factors and ATP hydrolysis [66]. A cytoplasmic factor was required for initiation of reverse transcription, whereas late DNA synthesis was dependent on recruitment of a factor from the nuclear fraction. It was suggested that the cytoplasmic factor could stimulate uncoating events required for initiation of reverse transcription [66]. Requirement of a nuclear factor for enhancement of the late DNA synthesis suggests that uncoating and reverse transcription may occur via discrete intermediates involving different cellular factors. ATP hydrolysis was also required for initiation of ASLV early DNA synthesis [66]. Thus, energy is needed to trigger the earliest steps of retroviral uncoating. Extending these observations to HIV-1, it appears possible that ATP is used as a substrate to phosphorylate viral proteins such as HIV-1 MA [38], an important event in HIV RTC formation (see above). Another possibility is that ATP is required for the function of a molecular chaperone that my play a role in viral uncoating [66]. As has been reported, family members of the heat shock proteins Hsp60, Hsp70 and Hsc70 are specifically incorporated into the cores of retroviral virions [67]. Treatment of HIV-1 virions with ATPγS, a nucleotide triphosphate analogue resistant to hydrolysis, did not decrease the capacity of virions to fuse with the target cell membrane, but dramatically impaired viral cDNA synthesis. Endogenous RT reaction in ATPγS-treated virions of SIV, HIV and MLV was also inhibited [68]. These inhibitory effects appeared to be indirect and resulted from disruption of virion core morphology observed using electron microscopy [68]. Thus, the ATPase activity of virion-incorporated heat-shock proteins, in particular Hsp70, appears critical for correct processing of the Gag precursor. This notion was further supported by inability of ATPγS-treated virus-like particles to saturate restriction factor TRIMCyp, which binds CA and blocks HIV-1 infection in owl monkey kidney cells [68].
Another indication that viral uncoating is an active and regulated process was obtained from the studies of HIV-1 CA mutants. These studies demonstrated that certain point mutations in CA could either reduce or increase the stability of the HIV-1 capsid without impairing formation of the cone-shaped capsid capsule in virions [56]. Alterations in capsid stability resulted in severely attenuated HIV-1 replication and impaired reverse transcription in target cells. The authors concluded that formation of the viral capsid of optimal stability is a prerequisite for efficient HIV-1 infection and suggested that capsid uncoating may be a temporally regulated process. Both a too rapid or a too slow disassembly of the capsid negatively affected viral infectivity [56].
As one of the critical points in the retroviral life cycle, viral uncoating is a target for cellular restriction factors acting in a species-specific manner (reviewed in [15]). For example, two alleles of the Fv1 gene, widely distributed in various strains of mice, provide resistance to infection by particular murine leukemia viruses. The determinant of viral sensitivity to Fv1 was found to lie in the CA protein of the virus [69], suggesting that uncoating might be involved. TRIM5α, a member of a large family of TRIM proteins, impairs accumulation of HIV-1 reverse transcripts in cells of Old World monkeys, such as rhesus macaques and African green monkeys [70]. The TRIM5α molecules from Old World monkeys specifically associate with the HIV-1 CA and promote its rapid premature disassembly [71]. This accelerated uncoating is likely not associated with proteasomal activity of the target cell, as inhibition of proteasomes did not significantly alter the anti-HIV-1 effect of TRIM5α [71] despite involvement of some TRIM proteins in ubiquitylation and proteasomal degradation [72]. Surprisingly, in HeLa cells stably expressing GFP-tagged rhesus TRIM5α, disruption of proteasomal function altered TRIM5α localization and allowed the normal generation of HIV-1 late reverse transcription products [73]. The authors suggested that rhesus TRIM5α restricts HIV-1 not at the phase of uncoating, but at the step of transport of reverse-transcribed viral genome to the nucleus by re-directing the RTCs to proteasomes. One possible explanation for these contradictory results may be the differences between cell types used for analysis, but more experiments would be required to resolve this seeming controversy.
It should be noted here that TRIM5α affects HIV-1 uncoating and dramatically restricts infection of HIV-1 only in Old World monkey cells. The human TRIM5α does not block HIV-1 infection, suggesting that HIV-1 has adapted to counteract TRIM5α activity in the natural target cells. Braaten and co-workers [74] found that the cellular peptidyl prolyl isomerase cyclophilin A (CypA), which is incorporated into virions via binding to the CA domain of Pr55Gag [75,76], is critical for correct disassembly of the HIV-1 cores early after infection. Recently, Towers and colleagues provided a direct demonstration that CypA incorporated into virions can counteract the restriction activity of a cellular factor and protect the viral capsid in human cells [77]. Interestingly, CypA has an inhibitory effect on HIV-1 infection in Old World monkey cells by rendering virus particles sensitive to restriction by TRIM5α, and inhibition of CypA activity rescued HIV-1 infectivity [78]. It appears that CypA-mediated isomerization of a proline residue in the TRIM5α-targeted site of the HIV-1 CA sensitizes it to restriction by the Old World monkey TRIM5α, whereas in human cells CypA protects CA from TRIM5α activity. Taken together, these results indicate that uncoating is a tightly regulated step of the retroviral life cycle which is rather vulnerable to cellular factors and can be considered as a target for anti-HIV therapy.
Structure and maturation of the RTC
Reverse transcription is the key characteristic of retroviruses (for review see [79]. This process is catalyzed by RT, which is constitutively present in RTCs. However, efficient implementation of this reaction, as well as related events (nuclear transport of RTC and integration), require the presence of certain viral and cellular proteins. Alterations in protein composition and structure of the RTC associated with reverse transcription and subsequent nuclear import is termed RTC maturation [44,80,81].
Extensive experimental data (reviewed in [12]) established that incoming HIV-1 core particles comprise diploid viral RNA, tRNALys primer, viral proteins MA, CA, nucleocapsid (NC), RT, integrase (IN), viral protein R (Vpr), and cellular proteins incorporated during virion assembly, such as uracil DNA glycosylase (UNG2) [82], CA-bound cyclophilin A [75,76], and Hsp70 [67,68] (Fig. 1, steps 1,2). Capsid disassembly, which probably proceeds hand-in-hand with reverse transcription, results in formation of uncoated, CA-deprived RTCs characterized by exposure of such viral NLS-containing proteins as MA, Vpr and IN [44,47,56,83,84]. These complexes were found to be associated with host cell proteins, such as non-homologous DNA end joining proteins Ku70 and Ku80 [85], lens epithelium-derived growth factor (LEDGF/p75) [86], nonhistone chromosomal protein HMGA [87], integrase interactor 1 (Ini1) and PML protein [88] (Fig. 1, steps 4,5).
Analysis of the protein composition of HIV-1 RTCs using immunoprecipitation established that mature RTCs are characterized by a three- and seven-fold increased proportion of NLS-containing Vpr and IN, respectively, compared to the early complexes [44]. Transmission electron microscopy (TEM) with gold-labeled antibodies demonstrated that these proteins were not distributed evenly along the nucleic acid-containing filaments of the early RTCs. Small clusters of gold particles were observed, presumably reflecting protein oligomerization and suggesting formation of nucleoprotein structures at the viral DNA ends that contain IN as well as cellular proteins involved in subsequent integration event [84,89–91]. Biochemical analisys revealed binding of RTC proteins to the ends of LTRs in complexes analyzed at 8.5 h post-infection, whereas in later RTCs these proteins were bound along the length of the cDNA [81]. These findings suggest rearrangement of RTC morphology in the course of maturation. This rearrangement is likely required for surface exposure of the proteins comprising NLSs [44] and for protection of the viral cDNA from nuclease activity in the host cell [81,84]. TEM analysis also indicated that NC and other small proteins coating the viral nucleic acids also contribute to protection from nuclease attack [84]. Remarkably, the viral nucleic acids within CA-shelled immature RTCs are sensitive to nuclease digestion, whereas mature RTCs which had lost CA protected cDNA from this enzymatic activity [81,83]. This phenomenon suggests that the predominant function of the capsid shell is to protect particle from the proteasomal attack rather than from activity of nucleases.
Studies of cytoplasmic HIV-1 RTCs using TEM [84] and scanning EM analysis [47] produced some intriguing results. Negative staining revealed relatively large structures (a few hundred nanometers in diameter) consisting of a mesh-like network of flexible filaments 6 nm thick. The size of the complexes was larger than what was previously determined using gel filtration chromatography (56 nm in diameter) [49]. Importantly, the size of RTCs isolated 3, 4, and 16 h after acute infection (mature RTCs) did not change, making it unclear how such big structures can translocate through the NPC with the maximal effective aperture of 45 nm [92,93]. Our recent data showed that migration of the RTC into the nucleus correlated with significant changes in the RTC protein composition. Nuclear RTCs were characterized by a dramatic decrease of RT molecules, which coincided with reduction of RTCs comprising incomplete reverse transcripts [44]. Interestingly, RTCs containing incomplete cDNA were unable to integrate into isolated chromatin even after in vitro completion of reverse transcription, suggesting that reverse transcription has to be completed in the cytoplasm to produce functional PICs [44]. Perhaps, completion of reverse transcription, as well as RT disassembly and, probably, interaction with importins prior to nuclear import can facilitate conversion of RTCs into more condensed particles. This conversion may occur at the nuclear membrane vicinity, immediately before the translocation through the nuclear pore.
Taken together, information about RTC structure and maturation obtained using morphological and biochemical methods suggests that maturation of the RTCs coincides with completion of reverse transcription, which in turn proceeds parallel to uncoating. The conformation and protein composition of mature RTCs is optimized to protect completed reverse transcripts from host nuclease activity and to recruit the cellular nuclear import machinery for RTC transport through the NPC.
Another important function of the RTC is to increase the fidelity of reverse transcription. At least one RTC protein, Vpr, has been implicated in reparation of RT errors. Mansky and Temin showed that the forward mutation rate for HIV-1 RTCs was about 20-fold lower than the error rate of purified HIV-1 reverse transcriptase [94], whereas Vpr-deficient HIV-1 had an overall mutation rate as much as 4-to-18-fold higher than that of the parental Vpr-positive virus [95,96]. It was suggested that this activity of Vpr couples with its capacity to bind UNG2 and incorporate this enzyme into HIV-1 particles (reviewed in [97]). Indeed, UNG2 catalyzes the base excision repair that specifically removes the RNA base uracil from DNA and thus can repair errors of reverse transcriptase [82,96]. However, recent experiments with cells expressing human UNG inhibitor UGI as well as with human UNG−/− cells demonstrated that UNG2 expression in both virus producer and target cells was dispensable for viral replication [98]. Moreover, Schrofelbauer and co-workers showed that Vpr can induce proteosomal degradation of UNG2, and UNG2 levels in Vpr-positive virions were significantly lower than in Vpr-deficient virus particles [99]. Thus, the contribution of UNG2 to reverse transcription fidelity in HIV-1 RTC remains uncertain, however, the presence of Vpr in incoming virions and RTC decreases the error rate of the HIV-1 RT.
Intracellular trafficking of RTCs
Efficient nuclear import of RTC requires interaction of its karyophilic components (MA, IN, Vpr and LEDGFp75) with factors of the host cell nuclear import machinery. These nuclear transport proteins called importins or karyopherins bind NLS-containing proteins in the cytosol and target them to the NPC [100]. An important step in RTC nuclear translocation is the interaction between RTC-importin complexes and nucleoporins, which is greatly facilitated by perinuclear accumulation of the cargo. Experiments with EGFP-Vpr labeled HIV-1 showed that RTCs localized to the perinuclear area as early as two hours after infection, whereas 30 min after infection the GFP signal was spread throughout the cell [47]. Numerous studies performed during the last decade established that HIV-1, as well as many other viruses replicating within the nucleus (herpes simplex virus type 1, adenovirus), utilize cellular cytoskeleton for their trafficking toward the nucleus [101–103].
Association of the viral Nef and MA proteins with actin microfilaments at early post-fusion steps of infection may be critical for trafficking of the RTCs from the peripheral region of the cell toward deeper layers of the cytoplasm [25] (Fig.1, step 1). Actin filaments have been implicated in short distance motility within the cell [27,104]. Actin can be used also to gain access of the cargo to the microtubule (MT) network [105], which is essentially a cytoskeletal highway system responsible for long distance transport of host and viral cargo [106]. Since in cells infected with the EGFP-Vpr-labeled HIV-1 more than 90% of intracellular particles were associated with MT network versus only a few percent co-localizing with filamentous actin, and since analysis of HIV-1 movement within the cell showed dependence of intracellular trafficking on both the actin and MT, actin microfilaments can be considered as a local transporter network that transfers coated subviral particles to the MT cytoskeleton [47] (Fig. 1 steps 2-8). The MT network provides a curvilinear movement of subviral particles in the cytoplasm with final accumulation in the perinuclear region, often near the microtubule-organizing center (MT minus end-directed movement). The presence of CA in the particles, as well as its disassembly, did not have any effect on RTC interaction with MT [47], suggesting that CA protein is not involved in this association.
Cytoplasmic dynein and kinesins represent the motor proteins mediating transport either to the minus-ends of MT at the MT-organizing center (dynein), or toward the plus-ends in the cell periphery (kinesins) [104]. Blocking of motor function of dynein by specific antibody resulted in the clustering of subviral particles at points most distant from the nucleus of infected cell, indicating dominance of the plus-end-directed transport [47]. A candidate motor for this transport can be KIF-4, a kinesin shown to bind MA protein of HIV, SIV and MMLV [107]. Since binding of the MA domain of the Gag precursor to KIF-4 is likely involved in membrane targeting of newly synthesized Gag molecules, the KIF-4-binding site should be masked on MA within RTCs for their successful transport towards the nucleus. We can speculate that suppression of this interaction may be associated with phosphorylation of the processed MA protein. On the other hand, proteins within the RTC should expose motives responsible for binding of dynein. Which proteins mediate this interaction is unclear, but CA and Vpr can be excluded as candidates as HIV RTCs lacking these proteins still accumulate in the perinuclear area.
Misfolded cellular proteins which can not be corrected by chaperone-mediated refolding aggregate into specialized “holding stations” called aggresomes [108]. The aggresomal pathway provides a mechanism by which aggregated proteins form particulate (approximately 200 nm) mini-aggregates that are transported on MTs towards the MT organizing center by a process mediated by the dynein. Aggresomes recruit various chaperones and proteasomes, presumably to aid in the disposal of the aggregated proteins. They also likely activate the autophagic clearance mechanism that terminates in lysosomal degradation [108]. Since RTCs represent particles a few hundred nanometers across [84], they can be driven into the aggresomal pathway [106] (Fig. 1, step 8). Indeed, treatment of HIV-infected cells with proteasomal and lysosomal inhibitors resulted in a dramatic increase of HIV infection in comparison to untreated cells [64,109,110]). About two thirds of intracellular subviral particles are destroyed by proteasomes already at 4 h post infection with HIV-1 [109]. These results suggest that most RTCs that engage dynein-MT system for intracytoplasmic traveling are destroyed by proteasomes and lysosomes shortly after infection. Therefore, to ensure its survival, the virus needs to employ highly efficient mechanisms governing subsequent early steps (nuclear import and integration). Our recently published quantitative analysis of the nuclear import of HIV-1 RTCs demonstrated that about 1.6% of total cytoplasmic RTC population was translocated into the nucleus at 5 h post-infection. However, when this calculation was performed for cytoplasmic RTCs comprising complete reverse transcription product, 14% of the complexes were imported into the nuclear compartment [44]. Thus, the efficiency of nuclear translocation of mature RTCs is relatively high, indicating effectiveness of the nuclear import mechanism used by these complexes.
The mechanism of nuclear import of lentiviral RTCs/PICs is the subject of many recent reviews [10,12,18,34,111]. Here we will briefly characterize the principal features of this process. As mentioned above, HIV-1 RTC/PIC is capable of translocating into the nucleus through the nuclear pore complex (NPC) by an active, energy-dependent mechanism. In order to gain access to the nucleus, the virus likely recruits the cellular nuclear transport machinery. Mature RTC/PIC is likely karyophilic itself, since at least four components of the complex (MA, IN, Vpr and LEDGF/p75) comprise nuclear targeting signals (NLSs) (Fig. 1, step 6).
The data about host nuclear import pathways engaged by these proteins are very controversial. Most of this controversy comes from the finding that nuclear import of HIV RTCs cannot be completely suppressed by inactivation of NLSs in any or even several karyophilic proteins within the RTC. However, this finding can simply indicate that the nuclear import of HIV PIC results from cumulative activity of several karyophilic factors. This would be a logical solution on the part of the virus to maximize efficiency of its nuclear import. However, this solution has been challenged in a recent paper that suggested a completely revised view on the nuclear transport of HIV-1 [46]. Because this paper illustrates the problems and misconceptions common to many other articles analyzing this issue, it is worth some consideration. In this paper, the authors attempted to answer the question what determines the difference between HIV and MLV in the ability to infect non-dividing cells. They approach this question by inactivating potential nuclear import signals within HIV proteins or replacing them with MLV-derived counterparts (which supposedly do not have karyophilic capacity), and then analyzing infectivity of such recombinant viruses on dividing and non-dividing (growth-arrested) HeLa cells. Infectivity of the wild-type HIV-1 was similar in both cell-types, whereas MLV could efficiently infect only dividing cells. These experiments demonstrated that even when all known HIV-1 nuclear localization signals where inactivated, infectivity of the resultant construct was similar in growth-arrested and dividing cells. The only construct that showed preferential infectivity for dividing cells, similar to what was observed with MLV, was the construct carrying the whole MLV Gag within the context of the HIV genome. Since the main feature of this construct is that it replaces the HIV-1 CA with the CA of MLV (in addition to other Gag proteins), and CA plays the central role in uncoating, the authors conclude that uncoating, but not the features of the HIV pre-integration complex, is the critical step regulating nuclear import.
There is a major problem with the proposed interpretation of these results. The authors suppose that HIV has acquired the ability to infect non-dividing cells by transporting its PIC through the intact nuclear envelope while retaining the capacity to enter the cell nucleus during mitosis, which is typical for onco-retroviruses such as MLV. However, evolution usually leads to development of the new pathways at the expense of the old ones, and it appears wasteful for a virus to preserve two replication mechanisms, when one is so much superior to the other, as it allows infection of both dividing and non-dividing cells. This notion is supported by our finding that HIV-1 PICs that get into the nucleus during mitosis are significantly attenuated in their integration capacity [44]. The dependence of HIV on transition through the nuclear pore may be due to modifications of the PIC, such as rearrangement or loss of proteins which occurs during this step. This would be consistent with the differences in protein composition observed between cytoplasmic and nuclear PICs [44,83]. If we assume that HIV replicates in both dividing and non-dividing cells by transporting its PIC through the nuclear envelope, it would not be of surprise that inactivation of nuclear import signals diminishes replication in both cell types. This is exactly what Yamashita and Emerman [46] observed: about a 4 log decrease in infectivity of the HIV clone with deletion or inactivation of known nuclear import signals. The fact that such inactivation did not completely kill the virus is interesting, however, given an extremely weak infectivity of this construct, a likely explanation for this result is the presence of yet another weak nuclear import signal. Therefore, results of Yamashita and Emerman do not argue against the role of nuclear import signals in regulating HIV-1 replication in non-dividing cells, but in fact provide a support for this notion. Therefore, nuclear import of HIV RTC is mediated most likely by a synergetic activity of several karyophilic factors, including MA, Vpr, IN, DNA flap and LEDGF.
Following entry into the nucleus, RTC/PIC has to get to the site of integration. Given a highly structured composition of the nuclear compartment, this step is unlikely to occur by passive diffusion. However, very little is known about intranuclear trafficking of PIC. Our studies showed that nuclear PICs are enriched in Ini1 and PML [44]. These and other host proteins (HMGA1, BAF and LEDGF) associated with RTC/PIC may facilitate interaction between the PIC and chromatin. Recently, a role for components of nuclear lamina in chromatin engagement by intranuclear PICs has been suggested [112]. The authors demonstrated that siRNA-induced knockout of emerin, an integral inner-nuclear-envelope protein, in primary macrophages altered cDNA integration into chromatin. BAF (barrier-to-autointegration factor), a binding partner of emerin, and viral IN were required for the association of viral cDNA with emerin. Emerin and BAF did not facilitate cDNA integration but were required for the appropriate localization of the viral cDNA before chromatin engagement [112]. These intriguing results await confirmation from other laboratories.
Conclusions and perspectives
The following principal events can be outlined in the life cycle of RTC/PIC: release of the viral capsids (pre-RTCs) into the cytoplasm of the target cell, uncoating, reverse transcription and RTC maturation, intracytoplasmic transport toward the nucleus, nuclear import, intranuclear transport and engagement of the chromatin, and integration (Figs. 1, 2). Despite numerous studies addressing individual events in the replication cycle of HIV, information about mechanisms of many of RTC-related processes, as well as the spatial and temporal organization of the principal events is very limited. While certain events are strongly dependent on preceding steps (e.g., successful nuclear import depends on completion of reverse transcription, maturation of RTCs and recruitment of nuclear import factors [35,44]), some events are relatively independent from each other. For instance, transport of RTCs on microtubules toward their minus-ends does not require uncoating and does not depend on completion of reverse transcription; reverse transcription itself can start at different steps of RTC formation: either in intact virion [21,22] or after release of the core particles into the cytoplasm [18]. Interdependence of reverse transcription and uncoating is still an open question. While TRIM5α-induced rapid uncoating results in disruption of reverse transcription and RTC degradation [71], reverse transcription itself can initiate capsid disassembly [23].
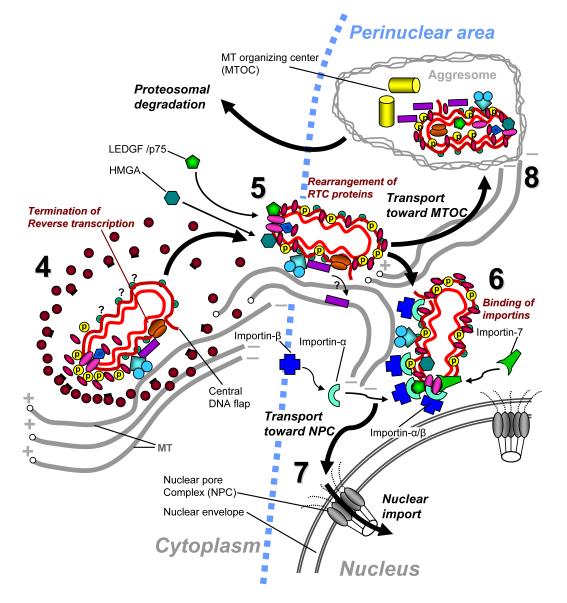
Figure 2.
Schematic view of the early phase events involving uncoated HIV-1 RTC. The numbering of events continues from Fig. 1. (4) Completion of reverse transcription results in double-strand cDNA comprising 99-nucleotide-long central DNA flap produced as a result of additional initiation of the (+) DNA synthesis at the second polypurine tract in the middle of the HIV genome. Newly synthesized DNA is probably covered by molecules of nucleocapsid (NC) protein. Progress of reverse transcription is likely associated with progressive capsid disassembly (uncoating). (5) RTCs undergo protein rearrangement which results in binding of MA and Vpr molecules along the length of cDNA. This RTC design protects cDNA from nuclease attack and provides proper exposure of the nuclear localization signals (NLSs) of MA, Vpr and IN to importins. RTC also incorporates host cell proteins: high-mobility group DNA-binding protein, HMGA, and the transcriptional co-activator LEDGF/p75. The latter protein binds IN molecules and contributes to protection of IN from proteolysis and to nuclear targeting of RTC. (6) During their intracytoplasmic transport toward the (−) ends of MT, uncoated RTCs interact with importin (karyopherin) α/β heterodimers which provide translocation of the RTC through the nuclear pore complex (NPC). NLS-comprising proteins of RTC (MA, IN, Vpr and LEDGF/p75) can bind importin α of the α/β heterodimer. IN also binds importin 7, however the role of this interaction in RTC nuclear import is unclear. (7) Cytoplasmic transport of the RTC-importin complexes in the productive pathway ends with interaction with nucleoporins of the NPC and subsequent translocation into the nucleus. (8) Most RTCs traveling to the (−) ends of MT end up in an aggresomal (non-productive) pathway. In this pathway, RTCs are transported on MTs towards the MT organizing center (MTOC) together with aggresomes, which recruit proteasomes. Thus, this pathway results in disposal of RTCs.
Certain events in RTC saga are practically unknown and await detailed analysis. One such event is incorporation of HIV MA into the viral capsid and RTC. Does this incorporation occur before or after fusion with the target cell? Where does processing of RTC-associated MA take place: in the producer cell or in the virion? Answers to these questions would help understand subsequent steps of RTC functioning. The role of MA in RTC life cycle in the cytoplasm, particularly its potential contribution to intracytoplasmic trafficking of the complexes is also unclear. Despite arising interest to the mechanism of uncoating in recent years, information about this event is still very scarce. We expect that future studies will identify new cellular factors involved in uncoating of HIV and other retroviruses. Another interesting problem is recruitment of the cytoskeleton by HIV-1 RTCs. While it was shown that cellular actin interacts with MA protein [25,28], with Nef [25], and with RT [30], the role of these interactions remains unknown. Exploitation of the microtubule network by RTCs is also open for future studies. Since RTCs of HIV-1 recruit dynein motor for directed transport toward the minus ends of MT, it would be interesting to determine how RTCs manage to avoid proteasomes recruited to the MT organizing center by aggresomes. Of course, since only a small percentage of RTCs reach the nucleus, it may be a fortuitous process. However, given a perfect adaptation of HIV to its host, a specific mechanism may be expected. Another interesting and highly controversial area is HIV nuclear import. Despite significant efforts of many laboratories, we still do not fully understand the mechanisms that allow HIV RTC to traverse the nuclear membrane. Finally, a lot of interesting findings can be expected from the studies of intranuclear transport of PICs. This area is now in its infancy.
Acknowledgements
The authors’ research is supported by grants from NIH.
Bibliography
- 1.Weinberg JB, Matthews TJ, Cullen BR, Malim MH. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J. Exp. Med. 1991;174:1477–1482. doi: 10.1084/jem.174.6.1477. ··The first demonstration that HIV-1 can infect and replicate in non-dividing cells.
- 2.Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc. Natl. Acad. Sci. U. S. A. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. ··The first article showing that nuclear import of HIV-1 is an active, energy-dependent process.
- 3.Li G, Simm M, Potash MJ, Volsky DJ. Human immunodeficiency virus type 1 DNA synthesis, integration, and efficient viral replication in growth-arrested T cells. J. Virol. 1993;67:3969–3977. doi: 10.1128/jvi.67.7.3969-3977.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann-Che J, Giron ML, Delelis O, Lochelt M, Bittoun P, Tobaly-Tapiero J, de The H, Saib A. Protease-dependent uncoating of a complex retrovirus. J. Virol. 2005;79:9244–9253. doi: 10.1128/JVI.79.14.9244-9253.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;11:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roe T, Reynolds TC, Yu G, Brown PO. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatziioannou T, Goff SP. Infection of Nondividing Cells by Rous Sarcoma Virus. J. Virol. 2001;75:9526–9531. doi: 10.1128/JVI.75.19.9526-9531.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fassati A, Goff SP. Characterization of intracellular reverse transcription complexes of Moloney murine leukemia virus. J. Virol. 1999;73:8919–8925. doi: 10.1128/jvi.73.11.8919-8925.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goff SP. Intracellular trafficking of retroviral genomes during the early phase of infection: viral exploitation of cellular pathways. J. Gene Med. 2001;3:517–528. doi: 10.1002/1521-2254(200111)3:6<517::AID-JGM234>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 11.Greene WC, Peterlin BM. Charting HIV’s remarkable voyage through the cell: Basic science as a passport to future therapy. Nat. Med. 2002;8:673–680. doi: 10.1038/nm0702-673. [DOI] [PubMed] [Google Scholar]
- 12.Nisole S, Saib A. Early steps of retrovirus replicative cycle. Retrovirology. 2004;1:9. doi: 10.1186/1742-4690-1-9. ··An excellent review of the early events in HIV replicative cycle.
- 13.Pornillos O, Garrus JE, Sundquist WI. Mechanisms of enveloped RNA virus budding. Trends Cell Biol. 2002;12:569–579. doi: 10.1016/s0962-8924(02)02402-9. [DOI] [PubMed] [Google Scholar]
- 14.Haffar O, Bukrinsky M. Nuclear translocation as a novel target for anti-HIV drugs. Expert Rev. Anti Infect. Ther. 2005;3:41–50. doi: 10.1586/14787210.3.1.41. [DOI] [PubMed] [Google Scholar]
- 15.Goff SP. Retrovirus restriction factors. Mol. Cell. 2004;16:849–859. doi: 10.1016/j.molcel.2004.12.001. ··Reviews the mechanisms of action of anti-retroviral restriction factors.
- 16.Romano G. Current development of lentiviral-mediated gene transfer. Drug News Perspect. 2005;18:128–134. doi: 10.1358/dnp.2005.18.2.886481. [DOI] [PubMed] [Google Scholar]
- 17.Farnet CM, Haseltine WA. Integration of human immunodeficiency virus type 1 DNA in vitro. Proc. Natl. Acad. Sci U. S. A. 1990;87:4164–4168. doi: 10.1073/pnas.87.11.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dvorin JD, Malim MH. Intracellular trafficking of HIV-1 cores: journey to the center of the cell. Curr. Top. Microbiol. Immunol. 2003;281:179–208. doi: 10.1007/978-3-642-19012-4_5. [DOI] [PubMed] [Google Scholar]
- 19.Khan M, Garcia-Barrio M, Powell MD. Treatment of human immunodeficiency virus type 1 virions depleted of cyclophilin a by natural endogenous reverse transcription restores infectivity. J. Virol. 2003;77:4431–4434. doi: 10.1128/JVI.77.7.4431-4434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu K, Dobard C, Chow SA. Requirement for integrase during reverse transcription of human immunodeficiency virus type 1 and the effect of cysteine mutations of integrase on its interactions with reverse transcriptase. J. Virol. 2004;78:5045–5055. doi: 10.1128/JVI.78.10.5045-5055.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trono D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J. Virol. 1992;66:4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. ··An early demonstration of endogenous reverse transcription.
- 22.Zhang H, Dornadula G, Pomerantz RJ. Natural endogenous reverse transcription of HIV type 1. AIDS Res. Hum. Retroviruses. 1998;14:S93–S95. [PubMed] [Google Scholar]
- 23.Zhang H, Dornadula G, Orenstein J, Pomerantz RJ. Morphologic changes in human immunodeficiency virus type 1 virions secondary to intravirion reverse transcription: evidence indicating that reverse transcription may not take place within the intact viral core. J. Hum. Virol. 2000;3:165–172. [PubMed] [Google Scholar]
- 24.Marsh M, Bron R. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J. Cell Sci. 1997;110:95–103. doi: 10.1242/jcs.110.1.95. [DOI] [PubMed] [Google Scholar]
- 25.Campbell EM, Nunez R, Hope TJ. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J. Virol. 2004;78:5745–5755. doi: 10.1128/JVI.78.11.5745-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruenheid S, Finlay BB. Microbial pathogenesis and cytoskeletal function. Nature. 2003;422:775–781. doi: 10.1038/nature01603. [DOI] [PubMed] [Google Scholar]
- 27.Smith GA, Enquist LW. Break ins and break outs: viral interactions with the cytoskeleton of Mammalian cells. Annu. Rev. Cell Dev. Biol. 2002;18:135–161. doi: 10.1146/annurev.cellbio.18.012502.105920. [DOI] [PubMed] [Google Scholar]
- 28.Bukrinskaya A, Brichacek B, Mann A, Stevenson M. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J. Exp. Med. 1998;188:2113–2125. doi: 10.1084/jem.188.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bukrinsky MI, Sharova N, McDonald TL, Pushkarskaya T, Tarpley WG, Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc. Natl. Acad. Sci. U. S. A. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. ··The first characterization of HIV-1 reverse transcription complex.
- 30.Hottiger M, Gramatikoff K, Georgiev O, Chaponnier C, Schaffner W, Hubscher U. The large subunit of HIV-1 reverse transcriptase interacts with beta-actin. Nucleic Acids Res. 1995;23:736–741. doi: 10.1093/nar/23.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pal R, Reitz MS, Jr., Tschachler E, Gallo RC, Sarngadharan MG, Veronese FD. Myristoylation of gag proteins of HIV-1 plays an important role in virus assembly. AIDS Res. Hum. Retroviruses. 1990;6:721–730. doi: 10.1089/aid.1990.6.721. [DOI] [PubMed] [Google Scholar]
- 32.Bouamr F, Scarlata S, Carter C. Role of myristylation in HIV-1 Gag assembly. Biochemistry. 2003;42:6408–6417. doi: 10.1021/bi020692z. [DOI] [PubMed] [Google Scholar]
- 33.Bukrinskaya AG, Ghorpade A, Heinzinger NK, Smithgall TE, Lewis RE, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc. Natl. Acad. Sci. U. S. A. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bukrinsky M. A hard way to the nucleus. Mol. Med. 2004;10:1–5. [PMC free article] [PubMed] [Google Scholar]
- 35.Piller SC, Caly L, Jans DA. Nuclear import of the pre-integration complex (PIC): the Achilles heel of HIV? Curr. Drug Targets. 2003;4:409–429. doi: 10.2174/1389450033490984. [DOI] [PubMed] [Google Scholar]
- 36.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 37.Jacque JM, Mann A, Enslen H, Sharova N, Brichacek B, Davis RJ, Stevenson M. Modulation of HIV-1 infectivity by MAPK, a virion-associated kinase. EMBO J. 1998;17:2607–2618. doi: 10.1093/emboj/17.9.2607. ··Demonstrates that MA phosphorylation influences HIV-1 infectivity.
- 38.Kaushik R, Ratner L. Role of human immunodeficiency virus type 1 matrix phosphorylation in an early postentry step of virus replication. J. Virol. 2004;78:2319–2326. doi: 10.1128/JVI.78.5.2319-2326.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Konvalinka J, Heuser AM, Hruskova-Heidingsfeldova O, Vogt VM, Sedlacek J, Strop P, Krausslich HG. Proteolytic processing of particle-associated retroviral polyproteins by homologous and heterologous viral proteinases. Eur. J. Biochem. 1995;228:191–198. doi: 10.1111/j.1432-1033.1995.tb20249.x. [DOI] [PubMed] [Google Scholar]
- 40.Krausslich HG, Facke M, Heuser AM, Konvalinka J, Zentgraf H. The spacer peptide between human immunodeficiency virus capsid and nucleocapsid proteins is essential for ordered assembly and viral infectivity. J. Virol. 1995;69:3407–3419. doi: 10.1128/jvi.69.6.3407-3419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganser BK, Li S, Klishko VY, Finch JT, Sundquist WI. Assembly and analysis of conical models for the HIV-1 core. Science. 1999;283:80–83. doi: 10.1126/science.283.5398.80. [DOI] [PubMed] [Google Scholar]
- 42.Kotov A, Zhou J, Flicker P, Aiken C. Association of Nef with the human immunodeficiency virus type 1 core. J. Virol. 1999;73:8824–8830. doi: 10.1128/jvi.73.10.8824-8830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Accola MA, Ohagen A, Gottlinger HG. Isolation of human immunodeficiency virus type 1 cores: retention of vpr in the absence of p6(gag) J. Virol. 2000;74:6198–6202. doi: 10.1128/jvi.74.13.6198-6202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iordanskiy S, Berro R, Altieri M, Kashanchi F, Bukrinsky M. Intracytoplasmic maturation of the human immunodeficiency virus type 1 reverse transcription complexes determines their capacity to integrate into chromatin. Retrovirology. 2006;3:4. doi: 10.1186/1742-4690-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dismuke DJ, Aiken C. Evidence for a functional link between uncoating of the human immunodeficiency virus type 1 core and nuclear import of the viral preintegration complex. J. Virol. 2006;80:3712–3720. doi: 10.1128/JVI.80.8.3712-3720.2006. ··An interesting report suggesting a link between the steps of uncoating and nuclear import.
- 46.Yamashita M, Emerman M. The Cell Cycle Independence of HIV Infections Is Not Determined by Known Karyophilic Viral Elements. PLoS Pathog. 2006;1:e18. doi: 10.1371/journal.ppat.0010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald D, Vodicka MA, Lucero G, et al. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol. 2002;159:441–452. doi: 10.1083/jcb.200203150. ··Provides visual analysis of intracellular trafficking of HIV RTC.
- 48.Farnet CM, Haseltine WA. Determination of viral proteins present in the human immunodeficiency virus type 1 preintegration complex. J. Virol. 1991;65:1910–1915. doi: 10.1128/jvi.65.4.1910-1915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller MD, Farnet CM, Bushman FD. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J. Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grewe C, Beck A, Gelderblom HR. HIV: early virus-cell interactions. J. Acquir. Immune Defic. Syndr. 1990;3:965–974. [PubMed] [Google Scholar]
- 51.Shi J, Aiken C. Saturation of TRIM5alpha-mediated restriction of HIV-1 infection depends on the stability of the incoming viral capsid. Virology. 2006;350:493–500. doi: 10.1016/j.virol.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Stremlau M, Perron M, Lee M, Li Y, et al. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5{alpha} restriction factor. Proc. Natl. Acad. Sci U. S. A. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. ··Suggests that anti-HIV activity of TRIM5alpha restriction factor is mediated at the step of uncoating.
- 53.Bowerman B, Brown PO, Bishop JM, Varmus HE. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 1989;3:469–478. doi: 10.1101/gad.3.4.469. [DOI] [PubMed] [Google Scholar]
- 54.Li S, Hill CP, Sundquist WI, Finch JT. Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature. 2000;407:409–413. doi: 10.1038/35030177. [DOI] [PubMed] [Google Scholar]
- 55.Yamashita M, Emerman M. Capsid Is a Dominant Determinant of Retrovirus Infectivity in Nondividing Cells. J. Virol. 2004;78:5670–5678. doi: 10.1128/JVI.78.11.5670-5678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forshey BM, von Schwedler U, Sundquist WI, Aiken C. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J. Virol. 2002;76:5667–5677. doi: 10.1128/JVI.76.11.5667-5677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersen JL, Planelles V. The role of Vpr in HIV-1 pathogenesis. Curr. HIV Res. 2005;3:43–51. doi: 10.2174/1570162052772988. [DOI] [PubMed] [Google Scholar]
- 58.Zack JA, Arrigo SJ, Weitsman SR, Go AS, Haislip A, Chen IS. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. ··The first analysis of HIV infection of quiescent T lymphocytes.
- 59.Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chou CS, Ramilo O, Vitetta ES. Highly purified CD25-resting T cells cannot be infected de novo with HIV-1. Proc. Natl. Acad. Sci. U. S. A. 1997;94:1361–1365. doi: 10.1073/pnas.94.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang S, Patterson B, Levy JA. Highly purified quiescent human peripheral blood CD4+ T cells are infectible by human immunodeficiency virus but do not release virus after activation. J. Virol. 1995;69:5659–5665. doi: 10.1128/jvi.69.9.5659-5665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pierson TC, Zhou Y, Kieffer TL, Ruff CT, Buck C, Siliciano RF. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J. Virol. 2002;76:8518–8531. doi: 10.1128/JVI.76.17.8518-8531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korin YD, Zack JA. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Groschel B, Bushman F. Cell cycle arrest in G2/M promotes early steps of infection by human immunodeficiency virus. J. Virol. 2005;79:5695–5704. doi: 10.1128/JVI.79.9.5695-5704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Auewarakul P, Wacharapornin P, Srichatrapimuk S, Chutipongtanate S, Puthavathana P. Uncoating of HIV-1 requires cellular activation. Virology. 2005;337:93–101. doi: 10.1016/j.virol.2005.02.028. ··One of the first studies of HIV uncoating.
- 66.Narayan S, Young JA. Reconstitution of retroviral fusion and uncoating in a cell-free system. Proc. Natl. Acad. Sci U. S. A. 2004;101:7721–7726. doi: 10.1073/pnas.0401312101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gurer C, Cimarelli A, Luban J. Specific incorporation of heat shock protein 70 family members into primate lentiviral virions. J. Virol. 2002;76:4666–4670. doi: 10.1128/JVI.76.9.4666-4670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gurer C, Hoglund A, Hoglund S, Luban J. ATPgammaS disrupts human immunodeficiency virus type 1 virion core integrity. J. Virol. 2005;79:5557–5567. doi: 10.1128/JVI.79.9.5557-5567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DesGroseillers L, Jolicoeur P. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J. Virol. 1983;48:685–696. doi: 10.1128/jvi.48.3.685-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–853. doi: 10.1038/nature02343. ··A ground-breaking paper describing restriction factor TRIM5alpha.
- 71.Stremlau M, Perron M, Lee M, et al. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proc. Natl. Acad. Sci U. S. A. 2006;103:5514–5519. doi: 10.1073/pnas.0509996103. ··Another report showing that TRIM5alpha works at the step of viral uncoating.
- 72.Hatziioannou T, Martin-Serrano J, Zang T, Bieniasz PD. Matrix-induced inhibition of membrane binding contributes to human immunodeficiency virus type 1 particle assembly defects in murine cells. J. Virol. 2005;79:15586–15589. doi: 10.1128/JVI.79.24.15586-15589.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu X, Anderson JL, Campbell EM, Joseph AM, Hope TJ. Proteasome inhibitors uncouple rhesus TRIM5alpha restriction of HIV-1 reverse transcription and infection. Proc. Natl. Acad. Sci U. S. A. 2006;103:7465–7470. doi: 10.1073/pnas.0510483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Braaten D, Franke EK, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J. Virol. 1996;70:3551–3560. doi: 10.1128/jvi.70.6.3551-3560.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luban J, Bossolt KL, Franke EK, Kalpana GV, Goff SP. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell. 1993;73:1067–1078. doi: 10.1016/0092-8674(93)90637-6. [DOI] [PubMed] [Google Scholar]
- 76.Thali M, Bukovsky A, Kondo E, et al. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994;372:363–365. doi: 10.1038/372363a0. [DOI] [PubMed] [Google Scholar]
- 77.Towers GJ, Hatziioannou T, Cowan S, Goff SP, Luban J, Bieniasz PD. Cyclophilin A modulates the sensitivity of HIV-1 to host restriction factors. Nat. Med. 2003;9:1138–1143. doi: 10.1038/nm910. ··Shows that CypA protects HIV RTC from restriction by TRIM5alpha.
- 78.Keckesova Z, Ylinen LM, Towers GJ. Cyclophilin A renders human immunodeficiency virus type 1 sensitive to Old World monkey but not human TRIM5 alpha antiviral activity. J. Virol. 2006;80:4683–4690. doi: 10.1128/JVI.80.10.4683-4690.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jonckheere H, Anne J, De Clercq E. The HIV-1 reverse transcription (RT) process as target for RT inhibitors. Med. Res. Rev. 2000;20:129–154. doi: 10.1002/(sici)1098-1128(200003)20:2<129::aid-med2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 80.Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 81.Khiytani DK, Dimmock NJ. Characterization of a human immunodeficiency virus type 1 pre-integration complex in which the majority of the cDNA is resistant to DNase I digestion. J. Gen. Virol. 2002;83:2523–2532. doi: 10.1099/0022-1317-83-10-2523. [DOI] [PubMed] [Google Scholar]
- 82.Mansky LM, Preveral S, Selig L, Benarous R, Benichou S. The interaction of vpr with uracil DNA glycosylase modulates the human immunodeficiency virus type 1 In vivo mutation rate. J. Virol. 2000;74:7039–7047. doi: 10.1128/jvi.74.15.7039-7047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fassati A, Goff SP. Characterization of intracellular reverse transcription complexes of human immunodeficiency virus type 1. J. Virol. 2001;75:3626–3635. doi: 10.1128/JVI.75.8.3626-3635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nermut MV, Fassati A. Structural analyses of purified human immunodeficiency virus type 1 intracellular reverse transcription complexes. J. Virol. 2003;77:8196–8206. doi: 10.1128/JVI.77.15.8196-8206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li L, Olvera JM, Yoder KE, Mitchell RS, Butler SL, Lieber M, Martin SL, Bushman FD. Role of the non-homologous DNA end joining pathway in the early steps of retroviral infection. EMBO J. 2001;20:3272–3281. doi: 10.1093/emboj/20.12.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cherepanov P, Maertens G, Proost P, Devreese B, Van Beeumen J, Engelborghs Y, De Clercq E, Debyser Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J. Biol. Chem. 2003;278:372–381. doi: 10.1074/jbc.M209278200. [DOI] [PubMed] [Google Scholar]
- 87.Farnet CM, Bushman FD. HIV-1 cDNA integration: requirement of HMG I(Y) protein for function of preintegration complexes in vitro. Cell. 1997;88:483–492. doi: 10.1016/s0092-8674(00)81888-7. [DOI] [PubMed] [Google Scholar]
- 88.Turelli P, Doucas V, Craig E, Mangeat B, Klages N, Evans R, Kalpana G, Trono D. Cytoplasmic recruitment of INI1 and PML on incoming HIV preintegration complexes: interference with early steps of viral replication. Mol. Cell. 2001;7:1245–1254. doi: 10.1016/s1097-2765(01)00255-6. ··The first demonstration that incoming HIV RTCs recruit SWI/SNF chromatin remodeling complex.
- 89.Chen H, Engelman A. The barrier-to-autointegration protein is a host factor for HIV type 1 integration. Proc. Natl. Acad. Sci U. S. A. 1998;95:15270–15274. doi: 10.1073/pnas.95.26.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen H, Wei SQ, Engelman A. Multiple integrase functions are required to form the native structure of the human immunodeficiency virus type I intasome. J. Biol. Chem. 1999;274:17358–17364. doi: 10.1074/jbc.274.24.17358. [DOI] [PubMed] [Google Scholar]
- 91.Wei SQ, Mizuuchi K, Craigie R. A large nucleoprotein assembly at the ends of the viral DNA mediates retroviral DNA integration. EMBO J. 1997;16:7511–7520. doi: 10.1093/emboj/16.24.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feldherr CM, Akin D. Regulation of nuclear transport in proliferating and quiescent cells. Exp. Cell Res. 1993;205:179–186. doi: 10.1006/excr.1993.1073. [DOI] [PubMed] [Google Scholar]
- 93.Feldherr CM, Akin D. Variations in signal-mediated nuclear transport during the cell cycle in BALB/c 3T3 cells. Exp. Cell Res. 1994;215:206–210. doi: 10.1006/excr.1994.1333. [DOI] [PubMed] [Google Scholar]
- 94.Mansky LM, Temin HM. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 1995;69:5087–5094. doi: 10.1128/jvi.69.8.5087-5094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mansky LM. The mutation rate of human immunodeficiency virus type 1 is influenced by the vpr gene. Virology. 1996;222:391–400. doi: 10.1006/viro.1996.0436. [DOI] [PubMed] [Google Scholar]
- 96.Chen R, Le Rouzic E, Kearney JA, Mansky LM, Benichou S. Vpr-mediated incorporation of UNG2 into HIV-1 particles is required to modulate the virus mutation rate and for replication in macrophages. J. Biol. Chem. 2004;279:28419–28425. doi: 10.1074/jbc.M403875200. [DOI] [PubMed] [Google Scholar]
- 97.Le Rouzic E, Benichou S. The Vpr protein from HIV-1: distinct roles along the viral life cycle. Retrovirology. 2005;2:11. doi: 10.1186/1742-4690-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaiser SM, Emerman M. Uracil DNA glycosylase is dispensable for human immunodeficiency virus type 1 replication and does not contribute to the antiviral effects of the cytidine deaminase Apobec3G. J. Virol. 2006;80:875–882. doi: 10.1128/JVI.80.2.875-882.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schrofelbauer B, Yu Q, Zeitlin SG, Landau NR. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J. Virol. 2005;79:10978–10987. doi: 10.1128/JVI.79.17.10978-10987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mattaj IW, Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. 265-306. ··An excellent review of the cellular nucleocytoplasmic transport events.
- 101.Lee GE, Murray JW, Wolkoff AW, Wilson DW. Reconstitution of herpes simplex virus microtubule-dependent trafficking in vitro. J. Virol. 2006;80:4264–4275. doi: 10.1128/JVI.80.9.4264-4275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Trotman LC, Mosberger N, Fornerod M, Stidwill RP, Greber UF. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nat. Cell Biol. 2001;3:1092–1100. doi: 10.1038/ncb1201-1092. [DOI] [PubMed] [Google Scholar]
- 103.Wisnivesky JP, Leopold PL, Crystal RG. Specific binding of the adenovirus capsid to the nuclear envelope. Hum. Gene Ther. 1999;10:2187–2195. doi: 10.1089/10430349950017176. [DOI] [PubMed] [Google Scholar]
- 104.Sodeik B. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 2000;8:465–472. doi: 10.1016/s0966-842x(00)01824-2. ··A comprehensive review of viral cytoplasmic transport along the microtubules.
- 105.Taunton J. Actin filament nucleation by endosomes, lysosomes and secretory vesicles. Curr. Opin. Cell Biol. 2001;13:85–91. doi: 10.1016/s0955-0674(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 106.Sodeik B. Unchain my heart, baby let me go--the entry and intracellular transport of HIV. J. Cell Biol. 2002;159:393–395. doi: 10.1083/jcb.200210024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang Y, Winkler U, Freed EO, et al. Cellular motor protein KIF-4 associates with retroviral Gag. J. Virol. 1999;73:10508–10513. doi: 10.1128/jvi.73.12.10508-10513.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garcia-Mata R, Gao YS, Sztul E. Hassles with taking out the garbage: aggravating aggresomes. Traffic. 2002;3:388–396. doi: 10.1034/j.1600-0854.2002.30602.x. [DOI] [PubMed] [Google Scholar]
- 109.Schwartz O, Marechal V, Friguet B, Arenzana-Seisdedos F, Heard JM. Antiviral activity of the proteasome on incoming human immunodeficiency virus type 1. J. Virol. 1998;72:3845–3850. doi: 10.1128/jvi.72.5.3845-3850.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wei BL, Denton PW, O’Neill E, Luo T, Foster JL, Garcia JV. Inhibition of lysosome and proteasome function enhances human immunodeficiency virus type 1 infection. J. Virol. 2005;79:5705–5712. doi: 10.1128/JVI.79.9.5705-5712.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Campbell EM, Hope TJ. Gene therapy progress and prospects: viral trafficking during infection. Gene Ther. 2005;12:1353–1359. doi: 10.1038/sj.gt.3302585. [DOI] [PubMed] [Google Scholar]
- 112.Jacque JM, Stevenson M. The inner-nuclear-envelope protein emerin regulates HIV-1 infectivity. Nature. 2006;441:641–645. doi: 10.1038/nature04682. ··The first report on intranuclear events preceding HIV integration.