Abstract
The organization of timing in mammalian circadian clocks optimally coordinates behavior and physiology with daily environmental cycles. Chronic consumption of a high-fat diet alters circadian rhythms, but the acute effects on circadian organization are unknown. To investigate the proximate effects of a high-fat diet on circadian physiology, we examined the phase relationship between central and peripheral clocks in mice fed a high-fat diet for 1 week. By 7 days, the phase of the liver rhythm was markedly advanced (by 5 h), whereas rhythms in other tissues were not affected. In addition, immediately upon consumption of a high-fat diet, the daily rhythm of eating behavior was altered. As the tissue rhythm of the suprachiasmatic nucleus was not affected by 1 week of high-fat diet consumption, the brain nuclei mediating the effect of a high-fat diet on eating behavior are likely to be downstream of the suprachiasmatic nucleus.
Keywords: C57BL/6J, hypothalamus, liver, luciferase reporter, mouse, obesity
Introduction
The mammalian circadian system is composed of multiple circadian oscillators: a central pacemaker in the suprachiasmatic nucleus (SCN) in the hypothalamus, and many oscillators in other regions of the brain and peripheral tissues. Real-time monitoring of circadian gene-driven luciferase expression in ex-vivo tissues has demonstrated that circadian clocks in central and peripheral tissues acquire a specific phase relationship with each other that coordinates behavior and physiology with environmental cycles of light–dark and fasting–feeding (Yamazaki et al., 2000; Stokkan et al., 2001; Yoo et al., 2004).
Recent studies have demonstrated that high-fat diets disrupt circadian rhythms. For example, whereas low-fat-fed mice displayed a periodicity of 23.6 h, mice fed a high-fat diet had lengthened periods of circadian activity rhythms (to ~23.8 h) (Kohsaka et al., 2007). After 6 weeks of high-fat diet consumption, daily rhythms of neuropeptides in the mediobasal hypothalamus and circulating factors, such as insulin, glucose, and leptin, were altered (with variable effects on amplitude, phase, and baseline expression) (Kohsaka et al., 2007). Furthermore, the amplitudes of the 24-h rhythms in core body temperature, corticosterone levels, and eating behavior were blunted in rodents fed high-fat diets (Kohsaka et al., 2007; Cano et al., 2008; Mendoza et al., 2008). A high-fat diet also impaired the ability of mice to synchronize their endogenous circadian rhythms with shifts in the light–dark cycle (a ‘jet-lag’ protocol) (Mendoza et al., 2008).
As a high-fat diet affects multiple facets of circadian rhythmicity, we hypothesized that it would alter the phase relationship between central and peripheral clocks. In this study, we examined the effects of short-term (1-week) consumption of a high-fat diet on circadian organization by measuring the phases of the circadian gene fusion protein reporter rhythms [PERIOD2::LUCIFERASE (PER2::LUC)] in explanted tissues. We found that high-fat diet consumption advanced the phase of the liver rhythm and immediately altered the daily rhythm of eating behavior.
Materials and methods
Animals
Male C57BL/6J heterozygous PER2::LUC mice (Yoo et al., 2004) (PER2::LUC mice were backcrossed to wild-type C57BL/6J mice from The Jackson Laboratory for 21–23 generations) were used to assess body weight, locomotor activity, and circadian organization (Figs 1–4; the same cohort was used for all measurements). Male wild-type C57BL/6J mice from our breeding colony were used to simultaneously analyze eating behavior and general activity (Fig. 5). All mice were bred in the Vanderbilt University animal facility in a 12-h light/12-h dark cycle (light intensity ~350 lux). Breeders were provided with standard chow (13.5% kcal from fat, LabDiet 5L0D) and water ad libitum. Genotyping for the presence of the PER2::LUC fusion protein was determined by measuring light emission from a fresh tail piece using a luminometer. For tissue explant experiments, mice were killed by cervical dislocation followed by decapitation. All experiments were carried out in accordance with the National Institutes of Health Guidelines regarding the care and use of animals for experimental procedures and were approved by the Institutional Animal Care and Use Committee at Vanderbilt University (M/08/096).
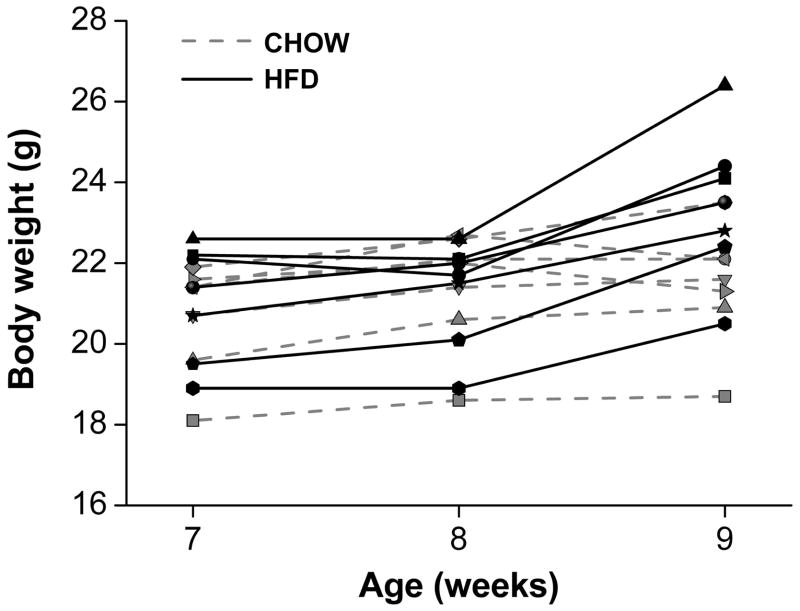
Fig. 1.
Body weight of mice fed a high-fat diet (HFD) for 1 week. Male heterozygous PER2::LUC mice were single-housed at 7 weeks old and provided with chow ad libitum until 8 weeks old. At 8 weeks old, mice were provided with fresh chow (gray symbols and dotted gray lines; n=7) or a HFD (black symbols and solid black lines; n=7). Body weights, measured at 7, 8, and 9 weeks old, from all individual mice are shown.
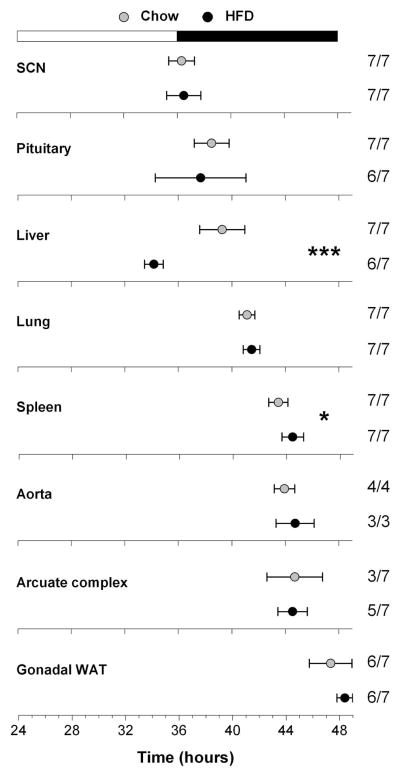
Fig. 4.
A high-fat diet (HFD) alters circadian organization. Male heterozygous PER2::LUC mice were fed either chow (gray circles) or HFD (black circles) for 1 week. The mean (±SD) phases were determined from the peaks of PER2::LUC expression during the interval between 12 and 36 h in culture and were plotted relative to the time of last lights on where 24 h is lights on and 36 h is lights off (black and white bar at top). The sample size is shown (number of rhythmic tissues/number of tissues tested). Tissues were taken from the mice shown in Fig. 1, except the aorta, which was collected from only half of the mice. *p=0.02; ***p=0.001. WAT, white adipose tissue.
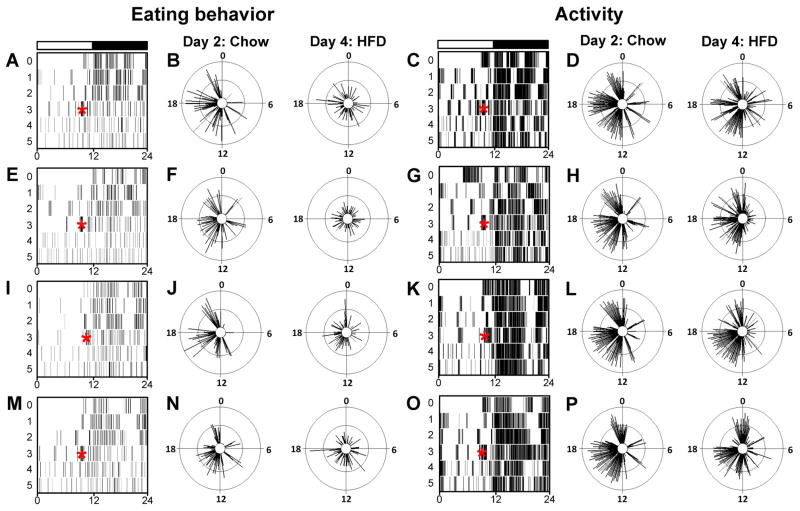
Fig. 5.
The eating behavior rhythm is altered by a high-fat diet (HFD). Single-plotted actograms (1-min bins; maximum 1 count/bin; x-axis: time in h; y-axis: days) of eating events (A, E, I and M) and locomotor activity (C, G, K and O) from male wild-type mice maintained in a 12-h light/12-h dark cycle (light and dark indicated by black and white bars, respectively, above actograms). Eating behavior (scored from video collected by infrared camera) and general activity (monitored with passive infrared sensors) were simultaneously collected from the same mouse. On day 0, the mouse was singly housed and provided with chow ad libitum. On day 3, chow was replaced with a HFD (~2.5 h before lights off, indicated by asterisks on the actograms). Circular histograms (plotted in 2.5° bins) show the distribution of eating events (B, F, J and N; scale: inner circle, 0; middle circle, 4.5; outer circle, 9) and activity events (D, H, L and P; scale: inner circle, 0; middle circle, 6.5; outer circle, 13) for each mouse during chow (left panels, day 2 of actogram) and a HFD (right panels, day 4 of actogram) consumption relative to the time of day (where ZT0 is lights on and ZT12 is lights off). The mean vectors are reported in
Experiment I. Experimental protocol for measuring body weight, food consumption, locomotor activity, and circadian organization
Male heterozygous PER2::LUC mice were weaned at 21 days old and group housed (two to four mice per cage) in a 12-h light/12-h dark cycle with standard chow and water provided ad libitum. At 7 weeks old, mice were singly housed in cages (33 × 17 × 14 cm) with locked wheels (wheels were present in the cages but they could not rotate) in light-tight boxes in a 12-h light/12-h dark cycle (light intensity 200–300 lux; temperature inside light-tight boxes: 25.5±1.5 °C) and standard chow was provided ad libitum. At 8 weeks old, the chow was replaced with either a high-fat diet (n=7; 45% kcal from fat; Research Diets D01060502) or with fresh chow (n=7) within 3 h before lights off. At 9 weeks old, PER2::LUC expression was measured in ex-vivo tissues. Body weight and food were measured at 7, 8, and 9 weeks old within 3 h before lights off. The percent change in body mass was calculated from body weights measured at 8 and 9 weeks old. The total food consumed (kcal) was calculated based on the grams of food consumed between 8 and 9 weeks (chow: 3.02 kcal/g metabolizable energy; 45% high-fat diet: 4.73 kcal/g). The general activity was monitored every minute with passive infrared sensors (sensors recorded a maximum of one count every 6 s; model 007.1, Visonic LTD). Activity data were double-plotted in actograms in 5-min bins using the normalized format in ClockLab (Actimetrics, Wilmette, IL, USA).
Luminescence recording
Cultures were prepared within 1.5 h before lights off, as previously described, except that CellGro (cat. no. 90–013PB plus L-glutamine) recording medium was used (Yamazaki & Takahashi, 2005). The gonadal white adipose tissue (surrounding the gonads), liver, lung, spleen, aorta, pituitary, SCN and arcuate complex [containing the arcuate nucleus of the hypothalamus and ependymal cell layer as described previously (Guilding et al., 2009)] were collected from the same mouse. Bioluminescence was monitored in real time (at 36.8±0.02 °C) with the LumiCycle (Actimetrics), and photon counts were integrated over 10-min intervals. LumiCycle software (Actimetrics) was used to subtract the 24-h moving average from the raw luminescence data and to smooth the data by 0.5-h adjacent averaging. To determine the period and phase, the detrended and smoothed data were exported to ClockLab (Actimetrics). The period was determined by fitting a regression line to the acrophase of at least 3 days of the PER2::LUC rhythm. The period of the rhythm in liver explants spontaneously changes after three to four cycles in culture. The periods reported for liver (Table 1) are the first periods measured in culture. The phase was determined from the peak of PER2::LUC expression during the interval between 12 and 36 h in culture. LumiCycle software was used to determine the amplitude of the same cycle used to determine phase for each tissue using the sine fit curve-fitting method. Only one cycle was analyzed for amplitude because the period and damping rate of PER2::LUC expression in liver are not constant (Pendergast & Yamazaki, 2012). Samples with a goodness of fit of less than 90% were excluded (the resulting number of samples analyzed for amplitude are reported in Table 1). The amplitudes of the rhythms from arcuate explants could not be analyzed because the goodness of fit was always less than 90% as the bioluminescence traces were noisy due to the low level of light emission from the tissue (see Fig. 3M).
Table 1.
Circadian parameters of bioluminescence rhythms in ex-vivo tissues
| Tissue | Chow (mean±SD) (n) | HFD (mean±SD) (n) | p | |
|---|---|---|---|---|
| Period (h) | SCN | 24.34 ± 0.25 (7) | 24.35 ± 0.11 (7) | t12= −0.10, p=0.92 |
| Pituitary | 23.37 ± 0.27 (6) | 23.73 ± 0.59 (7) | t11= −1.40, p=0.19 | |
| Liver | 21.59 ± 0.66 (5) | 21.28 ± 0.60 (5) | t8=0.77, p=0.46 | |
| Lung | 23.98 ± 0.35 (6) | 23.99 ± 0.45 (7) | t11= −0.06, p=0.95 | |
| Spleen | 24.40 ± 0.44 (6) | 24.34 ± 0.33 (7) | t11=0.31, p=0.76 | |
| Aorta | 24.54 ± 0.17 (4) | 24.53 ± 0.33 (3) | t5=0.09, p=0.94 | |
| Arcuate complex | 23.37 ± 0.88 (3) | 23.17 ± 0.51 (5) | t6=0.42, p=0.69 | |
| Gonadal WAT | 25.85 ± 0.54 (4) | 25.59 ± 0.77 (6) | t8=0.58, p=0.58 | |
| Amplitude | SCN | 36.03 ± 14.35 (7) | 27.61 ± 11.79 (7) | t12=1.20, p=0.25 |
| Pituitary | 37.61 ± 11.16 (7) | 29.15 ± 11.23 (6) | t11=1.36, p=0.20 | |
| Liver | 9.43 ± 5.38 (6) | 18.32 ± 13.82 (6) | t10= −1.47, p=0.17 | |
| Lung | 26.71 ± 8.31 (7) | 21.89 ± 8.68 (7) | t12= 1.06, p=0.31 | |
| Spleen | 23.58 ± 9.98 (7) | 21.57 ± 6.23 (7) | t12= 0.45, p=0.66 | |
| Aorta | 25.61 ± 13.18 (3) | 15.70 ± 2.59 (3) | t4= 1.28, p=0.27 | |
| Arcuate complex | ND | ND | - | |
| Gonadal WAT | 11.27 ± 6.3 (6) | 15.81±7.03 (3) | t7= −0.99, p=0.36 |
HFD, high-fat diet; ND, not determined; WAT, white adipose tissue.
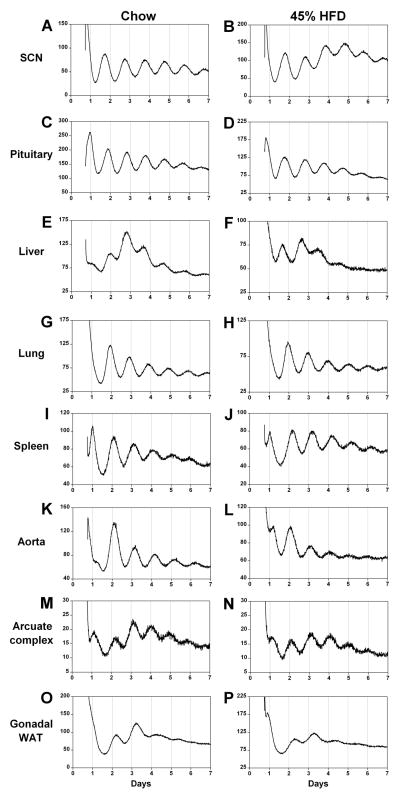
Fig. 3.
Effects of a high-fat diet (HFD) on tissue bioluminescence rhythms. Bioluminescence (x-axis: counts/s) recorded from tissue explants prepared from male heterozygous PER2::LUC mice fed either chow (A, C, E, G, I, K, M and O) or a HFD (45% kcal from fat; B, D, F, H, J, L, N and P) for 1 week. The lighting condition of the previous light/dark cycle is indicated for the first day; open bars are light and black bars are dark. WAT, white adipose tissue.
Experiment II. Eating behavior
The experimental conditions were identical to the previous experiment, except that mice (n=4) were provided with ad-libitum chow for 3 days, followed by a 45% high-fat diet for 3 days. The eating behavior and general activity were simultaneously monitored. An infrared video camera (PYLE PLCM22IR Flush Mount Rear View Camera with 0.5-lux Night Vision, Pyle Audio Inc., Brooklyn, NY, USA) was connected to a computer with the VideoSecu 4 Channel PCI DVR Card for CCTV Home Security Surveillance System C53 (VideoSecu, Carrollton, TX, USA). DVR software by EYEONET (AMETA International Co. Ltd, Markham, Ontario, Canada) was used to record and analyze the images. To reduce the size of the video file, images were recorded only when the mouse approached the feeder. Images were captured (one frame per second for 5 s) when the mouse entered a defined region around the feeder. Observers (K.L.B. and W.Y.) watched the videos and coded the eating behavior in 1-min bins. Eating behavior was defined based on the following criteria. The mouse: (i) took food from the feeder with its mouth or paws, (ii) moved food in the feeder with its mouth, or (iii) ate food in its paws away from the feeder, and this eating behavior persisted for 3 s or more (to distinguish eating from other behaviors such as sniffing). When eating behavior occurred, that 1 min was coded as “1” (a 1-min bin could only have a maximum value of “1” regardless of whether eating persisted for 3 s or 1 min), whereas 1-min bins without eating behavior were coded as “0.” Eating and activity (measured by passive infrared sensors) data (recorded simultaneously) were single-plotted in 1-min bins using the threshold format (threshold set to 1) in ClockLab software. For each mouse, behavior and activity events during 1 day of chow (day 2) and 1 day of high-fat diet (day 4) consumption were plotted in circular histogram plots in 2.5° bins (10-min) using Oriana 4.0 software (Kovach Computing Services, Wales, UK).
Statistical analysis
Statistical analysis was performed using SigmaStat 2.03 (Systat Software, San Jose, CA, USA). Independent t-tests (two-tailed) were used to compare two groups. The aorta, liver, and pituitary phase data were not normally distributed [as determined by the Kolmogorov–Smirnov test (with Lilliefors’ correction)], so the Mann–Whitney rank sum test was used for comparison. Circular data were analyzed and plotted using Oriana 4.0. The mean vector was calculated to indicate the angle (μ) and degree of clustering (length; r) of the samples with Rayleigh’s uniformity test. All data are the mean±SD. Significance was ascribed at p<0.05.
Results
High-fat diet alters the pattern of locomotor activity
We first measured the body mass (Fig. 1) and food intake in male mice that were single-housed and fed chow for 1 week and then fed either chow or a high-fat diet (45% kcal fat) for the subsequent week. Mice fed a high-fat diet for 1 week (Fig. 1, black symbols and lines) had a significantly greater percent change in body mass (10.15±3.73%) compared with mice fed chow (Fig. 1, gray symbols and dotted lines; 0.16±2.45%; t12=−5.93, p<0.001). Mice fed a high-fat diet ate a smaller amount of food by weight (20.82±2.28 g) than chow-fed mice (27.99±3.52 g; t11=4.27, p=0.001), but this equated to high-fat-diet-fed mice consuming more metabolizable energy (98.46±10.78 kcal) compared with chow-fed mice (84.52±10.62 kcal; t11=−2.34, p=0.04).
The pattern of locomotor activity changed immediately upon addition of the high-fat diet (Fig. 2). Whereas chow controls had five to six bouts of consolidated activity during the day (light; Fig. 2A), these bouts dissipated and were not consolidated in high-fat-fed mice (Fig. 2B).
Fig. 2.
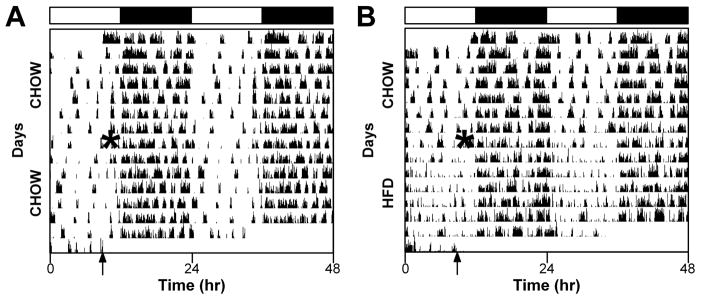
A high-fat diet (HFD) alters the pattern of locomotor activity. Representative double-plotted actograms (5-min bins) of male heterozygous PER2::LUC mice maintained in a 12-h light/12-h dark cycle (light and dark indicated by black and white bars, respectively, above actograms). General activity was continuously monitored with passive infrared sensors. All mice were provided with chow ad libitum for the first week. After 1 week, chow was replaced with either fresh chow (A) or a HFD (B). The times when food was replaced are indicated by asterisks on the left halves of the actograms. The arrows indicate when the mice were removed from the light-tight boxes for tissue culture. The actograms shown are typical examples from a total of chow (n=7, HFD: n=7 mice).
High-fat diet alters circadian organization
To assess the effect of a high-fat diet on circadian organization, we cultured tissues from mice fed chow or a high-fat diet for 1 week and recorded the PER2::LUC bioluminescence rhythms (Fig. 3). We analyzed the phases (defined as the peaks of the rhythms) of PER2::LUC expression (Fig. 4). We found that the phase of the liver rhythm was significantly advanced (by 5.1 h) when mice were fed a high-fat diet for 1 week compared with chow-fed mice (Mann–Whitney, T=21.00, p=0.001). The phase of the spleen rhythm was slightly, but statistically significantly, delayed (by 1.1 h) in mice fed a high-fat diet compared with chow (t12=−2.65, p=0.02). The phases of the SCN, pituitary, lung, aorta, arcuate complex, and gonadal white adipose tissue rhythms were not affected by a high-fat diet. In all tissues that we cultured, the periods and amplitudes of PER2::LUC expression rhythms did not differ between mice fed chow or a high-fat diet for 1 week (Table 1).
High-fat diet alters the eating behavior rhythm
To determine if the change in the phase of the liver caused by a high-fat diet was accompanied by a change in eating behavior, we used an infrared camera to monitor eating events (Fig. 5), which were defined as the mouse making physical contact with food. When mice were provided with chow, eating events were consolidated during the night, with several events occurring during the day (Fig. 5A, E, I and M, days 0–2 and Fig. 5B, F, J and N, left panels). When mice were given a high-fat diet, the eating events shifted from occurring largely in the dark cycle to being distributed across the day and night (Fig. 5A, E, I and M, days 3–5 and Fig. 5B, F, J and N, right panels). The mean vector length (Table 2) of eating behavior, which represents the distribution of eating events across the day, was decreased during high-fat diet consumption in all mice.
Table 2.
Vector properties of eating and activity rhythms
| Behavior | Diet | Mouse ID | Mean angle (μ) | Length (r) | p (Rayleigh uniformity test) |
|---|---|---|---|---|---|
| Eating | Chow | #6017 | 248.14 | 0.49 | Z=53.24, p<1E-12 |
| #6063 | 251.98 | 0.32 | Z=19.914, p=2.3E-09 | ||
| #6083 | 262.88 | 0.51 | Z=46.07, p< 1E-12 | ||
| #5927 | 226.34 | 0.39 | Z=26.196, p=4.2E-12 | ||
| HFD | #6017 | 250.12 | 0.22 | Z=5.32, p=0.005 | |
| #6063 | NS | NS | Z=1.35, p=0.26 | ||
| #6083 | 258.90 | 0.32 | Z=10.73, p=0.0000219 | ||
| #5927 | 198.32 | 0.28 | Z=9.065, p=0.000116 | ||
| Activity | Chow | #6017 | 256.58 | 0.38 | Z=89.06, p< 1E-12 |
| #6063 | 257.32 | 0.40 | Z=81.06, p< 1E-12 | ||
| #6083 | 261.47 | 0.43 | Z=103.09, p< 1E-12 | ||
| #5927 | 248.49 | 0.45 | Z=113.09, p< 1E-12 | ||
| HFD | #6017 | 241.56 | 0.29 | Z=42.51, p< 1E-12 | |
| #6063 | 267.04 | 0.43 | Z=81.29, p< 1E-12 | ||
| #6083 | 242.89 | 0.48 | Z=121.30, p< 1E-12 | ||
| #5927 | 255.15 | 0.26 | Z=33.82, p< 1E-12 |
HFD, high-fat diet.
Circular histogram plots of locomotor activity revealed that the pattern of activity changed when the mice were given a high-fat diet. More intense and discrete bouts of daytime activity (Fig. 5C, G, K and O, days 0–2 and Fig. 5D, H, L and P, left panels) became dispersed across the day during high-fat diet consumption (Fig. 5C, G, K and O, days 3–5 and Fig. 5D, H, L and P, left panels). The effect of a high-fat diet on the mean vector length of activity events (Table 2) varied from mouse to mouse, such that a high-fat diet increased the vector length in two mice and decreased the vector length in two other mice.
Discussion
To identify the proximate targets of high-fat diet consumption in the circadian system, we measured tissue rhythms in mice fed a high-fat diet for 1 week. We found that the phases of the rhythms in all of the central and peripheral tissues that we measured were resistant to 1 week of high-fat diet consumption, with the exception of the liver. The phase of the liver was markedly advanced by 5 h in mice eating a high-fat diet ad libitum compared with mice consuming chow. As the phase of the tissue liver clock is altered by meal timing (Stokkan et al., 2001), we hypothesized that the change in the timing of the liver clock during high-fat diet consumption resulted from a change in the eating behavior rhythm.
To measure the effect of high-fat diet consumption on the daily rhythm of eating behavior, we continuously monitored behavior with an infrared camera. Individual mice were first provided with ad-libitum access to standard chow and then were switched to ad-libitum high-fat diet. During chow consumption, the majority of mouse eating behavior was confined to the dark phase, with several consolidated eating bouts during the light phase. Eating behavior was immediately affected by consumption of a high fat diet, such that eating events became more evenly distributed across the day and night. In a previous study, Kohsaka et al. (2007) measured the daily rhythm of food intake (instead of eating behavior as measured in this study) in mice fed a high-fat diet. They found that 1 week of a high-fat diet caused an approximate 20% increase in food intake in the light phase compared with mice eating chow. Even with this change, the high-fat diet consumption of mice was clearly rhythmic, with ~30% of food intake occurring during the light phase and ~70% of food intake occurring during the dark phase. In this study, we found that the rhythm of eating behavior was drastically affected by consumption of a high-fat diet. By measuring different outputs (eating behavior vs. food intake), we may be distinguishing distinct mechanisms controlling different aspects of eating.
Regardless of whether eating behavior or food intake is measured, it is clear that a high-fat diet rapidly and robustly affects daily eating patterns. Furthermore, numerous studies have linked disruptions in the daily rhythm of food intake with weight gain (Mistlberger et al., 1998; Turek et al., 2005; Arble et al., 2009; Fonken et al., 2010; Hatori et al., 2012; Morales et al., 2012). Thus, understanding the mechanisms that regulate daily rhythms of eating, and how they are altered by the macronutrient content of food, is critical to understanding body weight regulation.
As eating behavior is immediately affected by consumption of a high-fat diet, a first step in investigating these mechanisms is to identify the brain regions that are acutely altered by eating a high-fat diet. We found that the phase, amplitude, and period of the tissue SCN rhythm were not affected by 1 week of high-fat diet consumption; thus it is likely that target nuclei are components of the homeostatic brain circuits downstream of the SCN. In this study, we measured the rhythm of the arcuate complex, which participates directly in regulating food intake. We found that the phase of the rhythm in the arcuate complex was not altered by 1 week of a high-fat diet, but we could not use statistics to analyze the amplitude of the rhythm due to the low level of light emitted from this tissue. An in-vivo technique, such as measuring multi-unit neural activity from freely moving mice (Nakamura et al., 2008, 2011), may be ideal for identifying neural substrates that are altered by high-fat consumption. Measuring the acute effects of a high-fat diet on other physiological outputs, such as body temperature rhythms, will also provide insight into the neural loci that are affected by a high-fat diet.
This study also provides insight into how a high-fat diet affects distinct regulatory processes controlling food intake. Eating is regulated by homeostatic mechanisms, which balance food intake with energy expenditure, and by the circadian system, so that the majority of food intake occurs during the active phase. The SCN is critical for circadian control of eating as the eating rhythm is abolished in SCN-lesioned rats (Nagai et al., 1978). Interestingly, circadian and homeostatic regulation of eating can be dissociated in certain conditions. For example, when rodents are provided with food for only several hours during the day (their inactive phase), they exhibit food anticipatory activity and readily consume food during the day (Mistlberger, 2009). This restricted feeding protocol does not change the phase of the SCN rhythm (Stokkan et al., 2001) (if the rodents are not calorically restricted), demonstrating that homeostatic and circadian control of eating are modulated by distinct neural circuitry. We observe a similar phenomenon with high-fat diet consumption; the timing of eating behavior is immediately and robustly affected by a high-fat diet, but the tissue rhythm in the SCN is unaffected. In contrast, the liver rhythm is acutely altered by a high-fat diet. Whether the liver rhythm shifts as a consequence of the change in eating behavior or due to a direct effect of high fat on the liver should be investigated in future studies. As a high-fat diet is highly palatable, the reward system also participates in the control of eating in this paradigm. By modulating the macronutrient content of food, we can distinguish the circadian, homeostatic, and reward circuits modulating eating behavior.
Acknowledgments
This research was supported by the MMPC MICROMouse program U24DK076169 (www.mmpc.org) from the National Institute of Diabetes and Digestive and Kidney Diseases and a National Science Foundation (grant IOS-1146908). K.L.J.E. was supported by a Young Investigator Award from the Vanderbilt University Digestive Disease Research Center (National Institutes of Health grant P30 DK058404) and the Vanderbilt Mouse Metabolic Phenotyping Center (National Institutes of Health grant U24 DK059637). K.D.N. was supported by resources of the Tennessee Valley Healthcare System and National Institutes of Health grants (DK085712) and the Diabetes Research and Training Center (DK20593). We thank Dr Nobuya Koike for technical expertise in setting up the camera system. We also thank the Research Experience for
Abbreviations
- PER2:LUC
PERIOD2::LUCIFERASE
- SCN
suprachiasmatic nucleus
Footnotes
High School Students (REHSS) program at Vanderbilt University. The authors declare no competing financial or non-financial interests.
References
- Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano P, Jimenez-Ortega V, Larrad A, Reyes Toso CF, Cardinali DP, Esquifino AI. Effect of a high-fat diet on 24-h pattern of circulating levels of prolactin, luteinizing hormone, testosterone, corticosterone, thyroid-stimulating hormone and glucose, and pineal melatonin content, in rats. Endocrine. 2008;33:118–125. doi: 10.1007/s12020-008-9066-x. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilding C, Hughes AT, Brown TM, Namvar S, Piggins HD. A riot of rhythms: neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol Brain. 2009;2:28. doi: 10.1186/1756-6606-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Pevet P, Challet E. High-fat feeding alters the clock synchronization to light. J Physiol. 2008;586:5901–5910. doi: 10.1113/jphysiol.2008.159566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. Food-anticipatory circadian rhythms: concepts and methods. Eur J Neurosci. 2009;30:1718–1729. doi: 10.1111/j.1460-9568.2009.06965.x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Lukman H, Nadeau BG. Circadian rhythms in the Zucker obese rat: assessment and intervention. Appetite. 1998;30:255–267. doi: 10.1006/appe.1997.0134. [DOI] [PubMed] [Google Scholar]
- Morales L, Del Olmo N, Valladolid-Acebes I, Fole A, Cano V, Merino B, Stucchi P, Ruggieri D, Lopez L, Alguacil LF, Ruiz-Gayo M. Shift of circadian feeding pattern by high-fat diets is coincident with reward deficits in obese mice. PLoS One. 2012;7:e36139. doi: 10.1371/journal.pone.0036139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Nishio T, Nakagawa H, Nakamura S, Fukuda Y. Effect of bilateral lesions of the suprachiasmatic nuclei on the circadian rhythm of food-intake. Brain Res. 1978;142:384–389. doi: 10.1016/0006-8993(78)90648-0. [DOI] [PubMed] [Google Scholar]
- Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD. Age-related decline in circadian output. J Neurosci. 2011;31:10201–10205. doi: 10.1523/JNEUROSCI.0451-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura W, Yamazaki S, Nakamura TJ, Shirakawa T, Block GD, Takumi T. In vivo monitoring of circadian timing in freely moving mice. Curr Biol. 2008;18:381–385. doi: 10.1016/j.cub.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Yamazaki S. The mammalian circadian system is resistant to dioxin. J Biol Rhythms. 2012;27:156–163. doi: 10.1177/0748730411434405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Takahashi JS. Real-time luminescence reporting of circadian gene expression in mammals. Methods Enzymol. 2005;393:288–301. doi: 10.1016/S0076-6879(05)93012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]